Abstract
Cisplatin is a platinum-based chemotherapeutic drug for treating various types of cancers. However, the use of cisplatin is limited by its negative effect on normal tissues, particularly nephrotoxicity. Various mechanisms such as DNA adduct formation, mitochondrial dysfunction, oxidative stress, and apoptosis are involved in the adverse effect induced by cisplatin treatment. Several studies have suggested that neuropeptide Y (NPY) is involved in neuroprotection as well as restoration of bone marrow dysfunction from chemotherapy induced nerve injury. However, the role of NPY in chemotherapy-induced nephrotoxicity has not been studied. Here, we show that NPY rescues renal dysfunction by reducing the expression of pro-apoptotic proteins in cisplatin induced nephrotoxicity through Y1 receptor, suggesting that NPY can protect kidney against cisplatin nephrotoxicity as a possible useful agent to prevent and treat cisplatin-induced nephrotoxicity. [BMB Reports 2016; 49(5): 288-292]
Keywords: Apoptosis, Chemotherapy-induced side effect, Cisplatin, Nephrotoxicity, Neuropeptide Y
INTRODUCTION
Cisplatin, an inorganic molecule, is one of the simplest and the most effective chemotherapeutic agent. It has been widely used to treat various types of solid tumors, including ovarian, testicular, head, neck, uterine cervical carcinoma, and nonsmall cell lung carcinoma. However, the use of cisplatin and its efficacy are limited by its various adverse effects, including ototoxicity, peripheral neuropathy, and particularly nephrotoxicity (1-5). The signaling mechanisms responsible for cisplatininduced nephrotoxicity appear to be multifactorial, including inflammation, oxidative stress, and apoptosis (4, 5). Approximately one out of three patients experience significant reduction in renal function following cisplatin treatment (5). Therefore, it is important to overcome the nephrotoxicity of cisplatin without diminishing its anticancer efficacy.
Neuropeptide Y (NPY) is a highly conserved peptide containing 36 amino acids. Its biological effect is mediated by six G-protein-coupled receptors (Y1R to Y6R). NPY is known to regulate a variety of physiological processes, including appetite, energy storage, and pain (6-9). It has been reported that p53 tumor suppressor gene can affect neuronal survival in sympathetic neurons (10). Several studies have reported that NPY is involved in cell death (7, 9, 10). It has also been reported that NPY can prevent bone marrow nerve injury by protecting sympathetic nervous system (SNS) fibers via Y1 receptor signaling in cisplatin-treated mice (10). Although the link between NPY and cell survival in diverse environments has been reported, it remains unclear whether NPY has any effect on renal cell survival. Therefore, the objective of this study was to determine the effect of NPY on renal cell survival associated with cisplatin-induced nephrotoxicity. We also determined whether NPY could protect renal cells against cisplatin-induced renal dysfunction and whether such effect was mediated by Y1 receptor signaling. Our results suggest a new role for NPY in renal dysfunction and have important relevance for preservation of renal function in cancer patients.
RESULTS
NPY prevents cisplatin-induced kidney injury in vivo
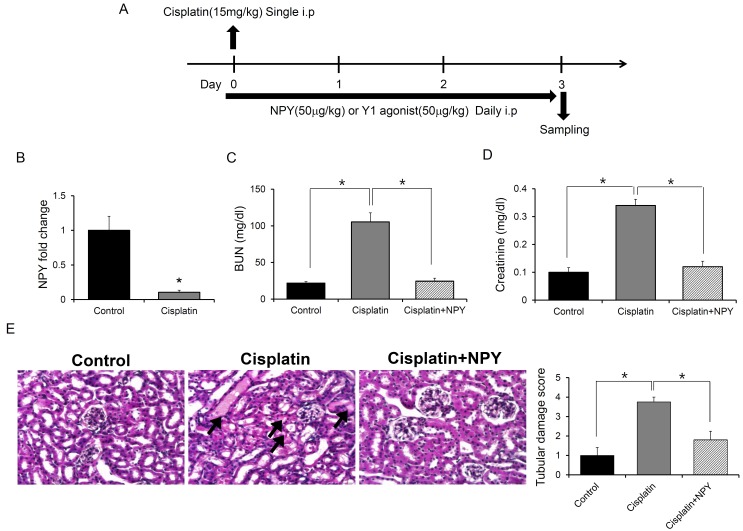
As mentioned above, cisplatin-induced nephrotoxicity is a common side effect of clinical chemotherapy for cancer patients (3-5, 11-13). To determine whether NPY could prevent cisplatin-induced nephrotoxicity, mice were injected with NPY or PBS after induction of renal injury by cisplatin (Fig. 1A). We first analyzed changes in NPY expression levels induced by cisplatin in the kidney. The mRNA levels of NPY in renal cells were significantly decreased in cisplatin-treated mice compared to those in PBS-treated controls (Fig. 1B). Plasma concentrations of blood urea nitrogen (BUN) and creatinine, indicators for loss of kidney function, were significantly (P < 0.05) elevated in cisplatin-treated mice. However, they were decreased by NPY treatment (Fig. 1C, D). Histologic analysis with hematoxylin and eosin (H&E) staining showed that the increase in apoptotic tubular cells in the renal cortex induced by cisplatin treatment was diminished by NPY treatment (Fig. 1E). These results suggest that NPY can protect renal cells against cisplatin-induced dysfunction.
Fig. 1. Effect of NPY treatment on cisplatin-induced renal injury. (A) Experimental design to determine the effect of NPY on cisplatin-induced renal injury. (B) Expression of NPY in the kidney of PBS- or cisplatin- treated mice (n = 5). (C) BUN level, (D) Creatinine level. Blood samples were collected on day 3 after cisplatin treatment (n = 5). scale bar: 20 μm. (E) Renal tissues were collected and processed for H&E staining to evaluate tubular damage and histology. *P < 0.05. All error bars indicate S.E.M. All expression levels were normalized against GAPDH mRNA expression.
NPY down-regulates p53-dependent apoptosis pathway in cisplatin-induced renal injury
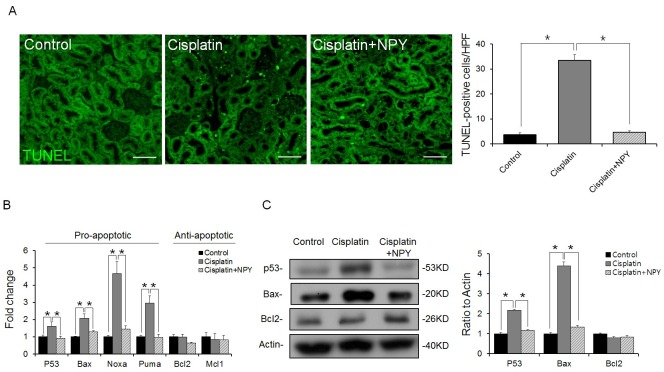
Renal apoptosis is a main pathogenesis in kidney injury caused by cisplatin treatment (1, 2, 14). To determine whether NPY diminished cisplatin-induced apoptosis in the kidney, we examined renal apoptosis by TUNEL assay. NPY treatment led to a decrease in apoptotic cells in the kidney (Fig. 2A). Next, we evaluated the expression levels of key modulators of the p53-dependent apoptosis pathway. We found that the expression levels of p53 and pro-apoptotic genes such as Bax, Noxa, and Puma were increased in cisplatin treated mice but decreased in NPY treated mice (Fig. 2B, C). Taken together, these results indicated that NPY could abrogate cisplatininduced renal apoptosis via down-regulating gene expression in the p53-dependent apoptosis pathway.
Fig. 2. Down-regulation of genes in the p53-dependent apoptosis pathway after NPY treatment. (A) Representative immunofluorescence images of kidneys showing apoptosis (TUNEL-positive nuclei, scale bar: 70 μm). (B) Quantitative real-time PCR analysis showing the expression levels of pro- or anti-apoptotic genes in the renal tissues of each group (n = 5). (C) Western blot analysis and quantification of p53, Bax, and Bcl2 in the renal tissues of each group (n = 6). *P < 0.05. All error bars indicating S.E.M. All expression levels were normalized against GAPDH mRNA expression.
The NPY/Y1 receptor pathway protects kidney against cisplatin-induced nephrotoxicity
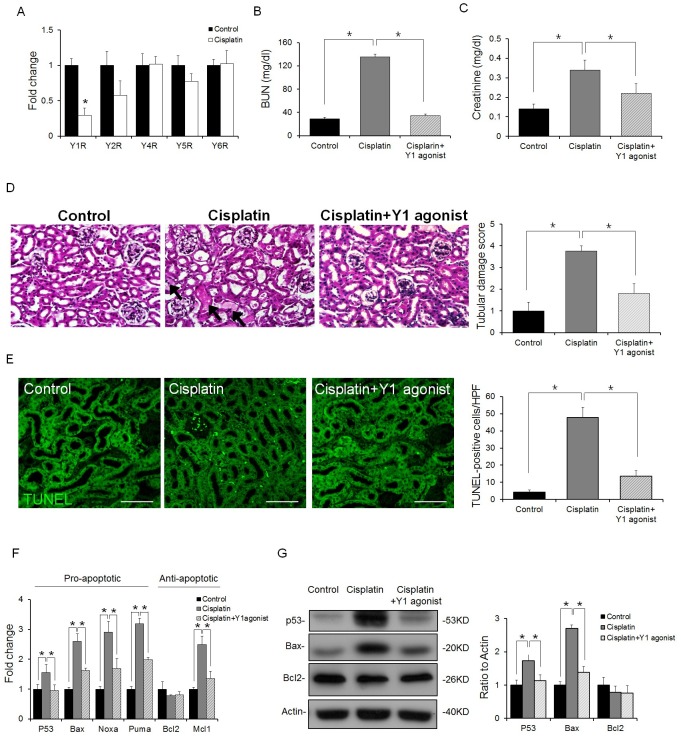
Next, we determined which receptor was associated with the protective effect of NPY in renal apoptosis. First, we examined the expression levels of NPY receptors in the kidney of cisplatin treated mice. Among different receptors, the expression level of Y1 receptor was significantly decreased after cisplatin treatment (Fig. 3A). Therefore, we focused on the Y1 receptor in order to examine the mechanism of NPY-mediated protection in cisplatin-induced renal injury. To confirm the renoprotection of NPY through Y1 receptor signaling, a Y1 agonist was injected into cisplatin-treated mice as described in Fig. 1A. Creatinine and BUN levels were reduced in Y1 agonist treated mice compared to those in cisplatin-treated mice (Fig. 3B, C). Mice treated with cisplatin demonstrated various injuries, whereas mice infused with the Y1 agonist showed significantly reduced renal injury (Fig. 3D). Moreover, the Y1 agonist decreased cisplatin induced renal injury (Fig. 3E). We also determined the expression levels of apoptosis-related genes and proteins. Similar to the results observed with NPY treatment, we found that the Y1 agonist suppressed the expression levels of p53 and pro-apoptotic genes such as Bax, Noxa, and Puma (Fig. 3F, G). Taken together, these results strongly suggest that NPY can provide renoprotection against cisplatin-induced kidney injury through Y1 receptors.
Fig. 3. NPY ameliorates cisplatin-induced renal injury through Y1 receptor signaling. (A) Expression levels of Y receptors in the kidney (n = 5), (B) BUN level, (C) Serum creatinine level (n = 5), (D) Kidney sections were stained with H&E (n = 5). scale bar: 20 μm; (E) Apoptosis in kidney tissues was examined by TUNEL assay. Representative images of TUNEL staining are shown (original magnification- 400X; scale bar, 70 μm). Quantification of TUNEL-positive cells in the tissues from each condition (n = 5). (F) Quantitative real-time PCR analysis for pro- or anti-apoptotic gene expression in the renal tissues of each group (n = 5). (G) Western blot analysis and quantification of p53, Bax, and Bcl2 levels in the renal tissues of each group (n = 6). *P < 0.05. All error bars indicating S.E.M. All expression levels were normalized against GAPDH mRNA expression.
DISCUSSION
Cisplatin is one of the most widely used and the most potent chemotherapeutic agent for different cancers, including testicular, ovarian, head and neck, lung, and other types of cancer. Unfortunately, its clinical use is limited by its severe side effect on normal tissues. Nephrotoxicity is one such side effect that limits its use in cancer therapy (1, 3-5, 11, 12). Many studies have suggested that cisplatin induces injury in renal vasculature and decreases blood flow and ischemic injury of kidneys, resulting in decreased glomerular filtration rate. These events will eventually culminate in the loss of renal function and trigger acute renal failure (11-13, 15). Recently, we have demonstrated that NPY is required for the maintenance of bone marrow function by protecting SNS fibers and cell survival within the bone marrow microenvironment from chemotherapyinduced bone marrow impairment through Y1 receptor signaling (10). However, the role of NPY in cisplatin-induced renal injury has not been fully characterized.
In this study, for the first time, we showed that NPY could protect renal dysfunction by regulating the pro-apoptotic pathway through Y1 receptor signaling. We demonstrated that NPY treatment could attenuate cisplatin-induced renal dysfunction by decreasing serum levels of creatinine and BUN (Fig. 1). It has previously been documented that the suppression of apoptosis can protect renal cells against cisplatin cytotoxicity and nephrotoxicity (2, 4, 12, 14). It has been reported that NPY can regulate downstream proteins of p53 and mediate apoptosis via Y receptors, thus preventing cell death (7, 9, 16). Here, we observed that the expression levels of p53 and pro-apoptotic genes such as Bax, Noxa, and Puma were decreased in cisplatin treated mice following NPY infusion (Fig. 2). In addition, we confirmed that NPY could attenuate renal dysfunction caused by cisplatin-induced nephrotoxicity via Y1 receptor signaling in the kidney (Fig. 3). Previous studies have demonstrated that some peptides such as Glucacon-Like Peptide-1 (GLP-1) can mediate renoprotection. GLP-1 or GLP-1 receptor agonist can protect renal dysfunction and tubular cell apoptosis by increasing the expression levels of anti-apoptotic genes such as Bcl-2 and Bcl-xL in cisplatin-induced renal injury mice model (17, 18). Although NPY did not significantly increase the expression levels of anti-apoptotic genes, the expression levels of pro-apoptotic genes were reduced by NPY treatment in this study. Especially, NPY down-regulated the levels of p53, an upstream protein of pro- and anti- apoptotic genes, suggesting that NPY could be used as an effective agent for renoprotection. NPY also has therapeutic benefit as a stable peptide synthesized in the human body (10). Therefore, NPY may be applicable in patients who have already received cisplatin or have experienced nephrotoxicity. Taken together, our results strongly indicate that NPY could be used as a potential strategy to prevent cisplatin-induced nephrotoxicity.
MATERIALS AND METHODS
Mice
Six- to eight- weeks old male or female C57BL/6 mice were purchased from the Jackson laboratory. A block randomization method was used to divide these animals into experimental groups. To eliminate bias, investigators were blinded during data collection and analysis. Mice were housed under a 12-hour day-night cycle. They were provided with free access to tap water and food pellets. Animal studies were approved by the Kyungpook National University Institutional Animal Care and Use Committee.
Drug treatment
Cisplatin (Sigma P4394) at 15 mg per kg body weight was used to induce renal injury. To assess renal protection from cisplatin, mice were intraperitoneally injected with NPY (Bachem; 50 μg per kg body weight, H-6375) or Y1 agonist (Bachem; 50 μg per kg body weight, H-3306) daily for 3 days (Fig. 1A). Three days later, kidneys were collected and analyzed.
Creatinine and BUN assay
To monitor renal function, plasma BUN and creatinine levels were determined using commercial kits as described previously (3, 19).
Histology, immunohistochemistry, and morphological assessment
Formaldehyde-fixed kidneys were dehydrated using graded alcohol series and paraffinized. Paraffin sections of 5 μm were stained with hematoxylin and eosin (H&E). Each parameter was determined for at least 6 different animals. TUNEL assays were performed using In Situ Cell Detection Kit, Fluorescein (Roche Diagnostic) according to the manufacturer's instructions. A pathologist quantified kidney damage in a doubleblinded manner using renal damage score described previously (14). Briefly, tissues were stained with H&E. The degree of morphological involvement in renal failure was determined using light microscopy. The following parameters were chosen as indicators of morphological damage to the kidney after cisplatin injection: brush border loss, red blood cell extravasation, tubule dilatation, tubule degeneration, tubule necrosis, and tubular cast formation. Each parameter was determined for at least five different animals.
Western blotting
Samples were immunoblotted as previously described (7, 20). Primary antibodies against the following proteins were used: p53 (mouse, 1 : 1000, Cell Signaling Technologies, 2524), Bax (rabbit, 1 : 1000, Cell Signaling Technologies, 2772), Bcl2 (rabbit, 1 : 1000, Cell Signaling Technologies, 5114S), and β-actin (1 : 1000, Santa Cruz, SC-1615). Densitometric quantification was performed using Image J software (US National Institutes of Health).
Quantitative Real-Time PCR
Real time RT-PCR was performed as previously described (21). RNA was extracted from bone marrow using RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA was synthesized from 5 μg of total RNA using RNA to cDNA EcoDryTM Premix (Oligo dT) from Clontech. Quantitative real-time PCR was performed using a Corbett research RG-6000 real-time PCR instrument. The following primers were used: Neuropeptide Y (NPY, Forward: 5'-AGATCCAGCCCTGAGACACT-3', Reverse: 5'-AGATGAG GGTGGAAACTTGG-3'), Y1R (Y1 receptor, Forward: 5'-TGTC ACCAACATTCTGATCG-3', Reverse: 5'-GATGAGAACCAGCG AGAAAA-3'), Y2R (Y2 receptor, Forward: 5'-TGCAGACCTCC CATTGTATT-3', Reverse: 5'-CAATCCAAGCATCGGTAATC-3'), Y4R (Y4 receptor, Forward: 5'-TAGTCGTGTCTGGGCTTTTC-3' Reverse: 5'-AGCAAAGGGCTAAACCATCT-3'), Y5R (Y5 receptor, Forward: 5'-GGGCTCTATACATTTGTAAGTCTTCTG-3', Reverse: 5'-CATGGCTTTGCCGAACATCCACTGATC-3'), Y6R (Y6 receptor, Forward: 5'-GGAGGGATGGTTATTGTGAC-3', Reverse: 5'-GTTGTTGCTCTTGCCACTGG-3'), P53 (Forward: 5'-TGAAACGCCGACCTATCCTTA-3', Reverse: 5'-GGCACA AACACGAACCTCAAA-3'), Bax (Forward: 5'-TTGCTACAGG GTTTCATCCA-3', Reverse: 5'-CATATTGCTGTCCAGTTCATC TC-3'), Noxa (Forward: 5'-ACTGTGGTTCTGGCGCAGAT-3', Reverse: 5'-TTGAGCACACTCGTCCTTCAA-3'), Puma (Forward: 5'-ATGCCTGCCTCACCTTCATCT-3', Reverse: 5'-AGC ACAGGATTCACAGTCTGGA-3'), Bcl2 (Forward: 5'-TTATAA GCTGTCACAGAGGGG-3', Reverse: 5'-GAACTCAAAGAAG GCCACAATCCTC-3'), Mcl1 (Forward: 5'-GAGGAGGAAGA GGACCTATACC-3', Reverse: 5'-AGTTTCTGCTAATGGTTC GATGAAG-3'), and GAPDH (Forward: 5'-TGGCAAAGTG GAGATTGTTGCC-3', Reverse: 5'-AA GATGGTGATGGGCT TCCCG-3').
Statistical analysis
Comparisons between two groups were performed using Student’s t-test. In cases where more than two groups were compared to each other, one way analysis of variance (ANOVA) was conducted followed by Tukey’s HSD test. All statistical analyses were performed using SPSS statistical software. A P value of less than 0.05 was considered statistically significant.
Acknowledgments
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2013).
References
- 1.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. (2008);73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 2.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. (2003);22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 3.Pabla N, Dong G, Jiang M, et al. Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest. (2011);121:2709–2722. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia Z, Wang N, Aoyagi T, Wang H, Liu H, Yang T. Amelioration of cisplatin nephrotoxicity by genetic or pharmacologic blockade of prostaglandin synthesis. Kidney Int. (2011);79:77–88. doi: 10.1038/ki.2010.331. [DOI] [PubMed] [Google Scholar]
- 5.Oh GS, Kim HJ, Choi JH, et al. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. (2014);85:547–560. doi: 10.1038/ki.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sah R, Geracioti TD. Neuropeptide Y and posttraumatic stress disorder. Mol Psychiatry. (2013);18:646–655. doi: 10.1038/mp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos-Carvalho A, Elvas F, Alvaro AR, Ambrósio AF, Cavadas C. Neuropeptide Y receptors activation protects rat retinal neural cells against necrotic and apoptotic cell death induced by glutamate. Cell Death Dis. (2013);4:e636. doi: 10.1038/cddis.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Körner M, Waser B, Reubi JC. Neuropeptide Y receptor expression in human primary ovarian neoplasms. Lab Invest. (2004);84:71–80. doi: 10.1038/labinvest.3700009. [DOI] [PubMed] [Google Scholar]
- 9.Gonçalves J, Ribeiro CF, Malva JO, Silva AP. Protective role of neuropeptide Y Y2 receptors in cell death and microglial response following methamphetamine injury. Eur J Neurosci. (2012);36:3173–3183. doi: 10.1111/j.1460-9568.2012.08232.x. [DOI] [PubMed] [Google Scholar]
- 10.Park MH, Jin HK, Min WK, et al. Neuropeptide Y regulates the hematopoietic stem cell microenvironment and prevents nerve injury in the bone marrow. EMBO J. (2015);34:1648–1660. doi: 10.15252/embj.201490174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humanes B, Lazaro A, Camano S, et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int. (2012);82:652–663. doi: 10.1038/ki.2012.199. [DOI] [PubMed] [Google Scholar]
- 12.Panesso MC, Shi M, Cho HJ, et al. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int. (2014);85:855–870. doi: 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linkermann A, Himmerkus N, Rölver L, et al. Renal tubular Fas ligand mediates fratricide in cisplatin-induced acute kidney failure. Kidney Int. (2011);79:169–178. doi: 10.1038/ki.2010.317. [DOI] [PubMed] [Google Scholar]
- 14.Jiang M, Yi X, Hsu S, Wang CY, Dong Z. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol. (2004);287:F1140–1147. doi: 10.1152/ajprenal.00262.2004. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Ma H, Shao J, et al. A Role for Tubular Necroptosis in Cisplatin-Induced AKI. J Am Soc Nephrol. (2015);26:2647–2658. doi: 10.1681/ASN.2014080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SW, Jin L, Piao SG, Chung BH, et al. Inhibition of dipeptidyl peptidase IV protects tacrolimus-induced kidney injury. Lab Invest. (2015);95:1174–1185. doi: 10.1038/labinvest.2015.93. [DOI] [PubMed] [Google Scholar]
- 17.Katagiri D, Hamasaki Y, Doi K, et al. Protection of glucagon-like peptide-1 in cisplatin-induced renal injury elucidates gut-kidney connection. J Am Soc Nephrol. (2013);24:2034–2043. doi: 10.1681/ASN.2013020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smiałowska M, Domin H, Zieba B, et al. Neuroprotective effects of neuropeptide Y-Y2 and Y5 receptor agonists in vitro and in vivo. Neuropeptides. (2009);43:235–249. doi: 10.1016/j.npep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. (2007);72:53–62. doi: 10.1038/sj.ki.5002256. [DOI] [PubMed] [Google Scholar]
- 20.Hwang HS, Park IY, Kim DW, et al. PEP-1-FK506BP12 inhibits matrix metalloproteinase expression in human articular chondrocytes and in a mouse carrageenan-induced arthritis model. BMB Rep. (2015);48:407–412. doi: 10.5483/BMBRep.2015.48.7.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L, Su P, Li R, et al. Knockdown of microtubule actin crosslinking factor 1 inhibits cell proliferation in MC3T3-E1 osteoblastic cells. BMB Rep. (2015);48:583–588. doi: 10.5483/BMBRep.2015.48.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]