Abstract
Study Objectives:
Previous laboratory studies in narcolepsy patients showed altered core body and skin temperatures, which are hypothesised to be related to a disturbed sleep wake regulation. In this ambulatory study we assessed temperature profiles in normal daily life, and whether sleep attacks are heralded by changes in skin temperature. Furthermore, the effects of three months of treatment with sodium oxybate (SXB) were investigated.
Methods:
Twenty-five narcolepsy patients and 15 healthy controls were included. Core body, proximal and distal skin temperatures, and sleep-wake state were measured simultaneously for 24 hours in ambulatory patients. This procedure was repeated in 16 narcolepsy patients after at least 3 months of stable treatment with SXB.
Results:
Increases in distal skin temperature and distal-to-proximal temperature gradient (DPG) strongly predicted daytime sleep attacks (P < 0.001). As compared to controls, patients had a higher proximal and distal skin temperature in the morning, and a lower distal skin temperature during the night (all P < 0.05). Furthermore, they had a higher core body temperature during the first part of the night (P < 0.05), which SXB decreased (F = 4.99, df = 1, P = 0.03) to a level similar to controls. SXB did not affect skin temperature.
Conclusions:
This ambulatory study demonstrates that daytime sleep attacks were preceded by clear changes in distal skin temperature and DPG. Furthermore, changes in core body and skin temperature in narcolepsy, previously only studied in laboratory settings, were partially confirmed. Treatment with SXB resulted in a normalisation of the core body temperature profile. Future studies should explore whether predictive temperature changes can be used to signal or even prevent sleep attacks.
Citation:
van der Heide A, Werth E, Donjacour CE, Reijntjes RH, Lammers GJ, Van Someren EJ, Baumann CR, Fronczek R. Core body and skin temperature in type 1 narcolepsy in daily life; effects of sodium oxybate and prediction of sleep attacks. SLEEP 2016;39(11):1941–1949.
Keywords: narcolepsy, sleep, core body temperature, skin temperature
Significance.
Narcolepsy type 1 (narcolepsy with cataplexy) is a disorder of the regulation of sleep and wakefulness. Core body and skin temperature are known to play an important role in sleep and wake regulation. This is the first study that explores the interaction between temperature regulation and sleep in narcolepsy in normal daily life. The differences in temperature between narcolepsy patients and controls might be associated with the known excessive daytime sleepiness and disturbed nocturnal sleep in narcolepsy. Furthermore, daytime sleep attacks were preceded by clear changes in skin temperature. In the future these findings could lead to methods to warn narcolepsy patients or prevent falling asleep when not appropriate. However, future studies have to be performed.
INTRODUCTION
Core body and skin temperature are closely linked to sleep and alertness.1–3 In healthy controls, wake is associated with a relatively high core body temperature and a relatively low skin temperature.4,5 The opposite pattern, i.e., a decreased core body temperature and an increased skin temperature, is seen during sleep. Transitions from wake to sleep and vice versa are characterized by changes in these temperatures. Sleep onset is preceded by a decline in core body temperature and an increase in skin temperature. The decrease in core body temperature is mediated through increased skin perfusion, which consequently leads to the increase in skin temperature, facilitating cooling of the body.5,6 A relatively high distal skin temperature compared to proximal skin temperature, i.e., a high distal-to-proximal temperature gradient (DPG), was demonstrated to promote sleep onset.1 When a “sleepy state of core body and skin temperature” is seen during wake, this is typically associated with lowered vigilance.7 This may probably be called “sleep promoting,” since it is also seen immediately before sleep onset.
Narcolepsy type 1 (narcolepsy with cataplexy) is a disorder of the regulation of sleep and wakefulness. Besides the pathognomonic symptom cataplexy, narcolepsy patients suffer from excessive daytime sleepiness (EDS), hypnagogic hallucinations, sleep paralysis and disturbed nocturnal sleep.8,9 In studies concerning temperature measurements in narcolepsy patients, an altered diurnal temperature profile has been demonstrated in both core body and skin temperature. Narcolepsy patients are reported to have a relatively high daytime distal skin temperature, a lowered proximal skin temperature, and subsequently a higher DPG during daytime.4,10,11 Furthermore, a lower nocturnal distal skin temperature was seen in narcolepsy.11 Studies regarding core body temperature in narcolepsy were not conclusive. Results vary from an elevated core body temperature to a lowered core body temperature.4,12–15
The hypothesized relationship between an altered temperature pattern and disturbed sleep wake regulation in narcolepsy was supported by temperature manipulation studies. These studies demonstrated that cooling the distal skin resulted in a better ability to maintain wakefulness, and increasing core body temperature improved vigilance during the day.4 Furthermore, counteracting the altered nocturnal skin temperature pattern by subtle skin temperature manipulations during the night improved nocturnal sleep.16
All these previous studies regarding skin temperature and most of the studies concerning core body temperature were performed in a laboratory setting, but whether laboratory findings are representative for actual daily life has not been assessed. The current study was performed to overcome this limitation and to further explore the interaction between temperature regulation and sleep in narcolepsy in normal daily life. We aimed first to assess whether in an ambulatory setting, sleep attacks are heralded by changes in skin temperature. Our second aim was to further explore core body and skin temperature profiles and sleep in narcolepsy type 1 in daily life, before and after stable monotherapy with sodium oxybate (SXB) a widely used drug to improve nocturnal sleep, cataplexy, and EDS in narcolepsy.17 We expected to confirm the previously found daytime increased distal and lowered proximal skin temperature and consequently an increased DPG. Furthermore, we measured 24-h core body temperature patterns during day and night; these patterns have not been studied in detail before.
Patients and controls underwent 24-h ambulatory core body and skin temperature measurement together with ambulatory polysomnography. Additionally, the patients who were treated successfully with SXB monotherapy underwent the same measurements after three months of stable treatment with SXB.
METHODS
Subjects
Twenty-five narcolepsy patients (16 male; mean BMI 27.6) were recruited from the narcolepsy outpatient clinics of Leiden University Medical Centre, The Netherlands (n = 14) and University Hospital Zurich, Switzerland (n = 11) between 2007 and 2012. They all fulfilled the criteria for narcolepsy type 1 according to the International Classification of Sleep Disorders, Third Edition (ICSD-3).18 Of 25 patients, 23 underwent a lumbar puncture, which revealed an undetectable hypocretin-1 in all these patients.
Patients were all naïve to SXB treatment, and were scheduled to start with SXB. The decision for treatment with SXB was part of their therapeutic plan; i.e., no patients were put on SXB treatment for the purpose of participation in this study. Six patients used narcoleptic medication other than SXB, which was stopped at least 14 days prior to the start of the study.
Fifteen healthy controls (9 male; The Netherlands [n = 9]; Switzerland [n = 6]; mean BMI 25.2), free of any neurologic or psychiatric disease were recruited using notices in local newspapers. Controls were matched for age and gender.
Exclusion criteria for both patients and controls were cognitive impairment due to neurological disorders other than sleep-wake disorders, the use of hypnotics or sleep-wake active drugs other than SXB, and age below 18 or above 70 years.
The protocol was approved by the medical ethical committees of both institutions, and written informed consent was obtained from all subjects prior to the study.
Study Design
At baseline, all subjects underwent an ambulatory baseline 24-h temperature measurement and polysomnography simultaneously. Following the baseline study, 23 patients were treated with SXB by their treating physician. They received the usual therapeutic dose of SXB (4.5–9.0 g/day). Due to side effects and/or environmental factors, 7 patients discontinued SXB within 3 months. Subsequently, after three months of stable single-drug treatment a second 24-h temperature measurement and polysomnography was performed in 16 patients. Controls followed the procedure only at baseline.
Temperature Measurement
The temperature measurement methods have previously been described.11 A wireless monitoring system was used for core body temperature measurement: an ingestible and biocompatible capsule with Vitalsense monitor (Mini Mitter Company Inc., Respironics, Bend, OR, USA).19
Thermochron iButtons (type DS1921H-F50; Maxim Integrated products, Inc., Sunnyvale, CA, USA) were used for wireless skin temperature measurement.20 Skin temperature was measured at 7 locations: left infraclavicular area, both hands, abdomen (1 cm above the umbilicus), left midthigh (musculus rectus femoris), and both feet. Distal skin temperature contained the temperatures at medial metatarsal area at the plantar sides of both feet and the thenar area at the palmar side of both hands.21 Proximal skin temperature was obtained from the infraclavicular, the thigh, and abdominal temperature. To calculate the DPG, proximal skin temperature was subtracted from the distal skin temperature.
Both core body temperature and skin temperature were sampled once per minute with a resolution of 0.125°C.
Sleep Recording and Analysis
Polysomnographic sleep recording was performed in all subjects during day and night for 24 h with a portable Embletta X100 (Leiden) or a portable Embla A10 (Zürich) recorder (Embla Broomfield, CO, USA). Sleep recordings were scored according to the American Academy of Sleep Medicine criteria by an experienced sleep technician.22
Additionally, daytime sleep attacks were classified based on the following criteria: (1) a period of any sleep stage (1, 2, 3, or REM) during daytime, (2) for at least 2 consecutive min, (3) no sleep was registered at least 10 min prior to the sleep attack.
Data Analysis and Statistics
Because of skewed distribution, the Mann Whitney U test was used to compare sleep characteristics and number of daytime naps between patients and controls at baseline, and the related-samples Wilcoxon signed rank test was used to analyze sleep characteristics and daytime naps before and during SXB treatment in patients.
For subsequent analyses on the 24-h profiles, the mean temperature of each episode of 30 min was calculated. Group differences, group by time of day differences, and treatment by time of day effects on temperature were analysed with Generalized Linear Model for repeated measures with HuynhFeldt corrections (IBM SPSS 20, Inc., Chicago, IL, USA) with between factor narcolepsy, within factors SXB, and time of day and covariate geographical site (Leiden or Zürich). This analysis was separately performed with the real clock time data and with the data anchored for nocturnal bedtimes (with the actual bedtime coded as time-point 0). Analysis was run on the 24-h data, and separately for daytime and nighttime. Where narcolepsy or SXB related differences reached significance, post hoc t-tests were used to evaluate differences in the time of day.
Mixed effects logistic regression analysis (R version 3.1.1, R Foundation for Statistical Computing, Vienna, Austria) was performed to evaluate the effect of temperature on the onset of spontaneous daytime sleep attacks in patients at baseline. Since these temperatures were measured once per minute, and sleep scoring was performed in 30-s epochs, the temperatures were interpolated into 30-s values. Nocturnal temperatures were excluded. For all analysis the outcome variable was sleep onset, which was binomially coded for every 30-s epoch as wake = 0 and sleep onset = 1 (further sleep epochs were excluded from analysis. The different temperatures (proximal, distal, and DPG) and time of day were entered into the model as fixed effects. Intercepts for subjects were defined as random effects. To evaluate different epoch durations prior to sleep onset, 3 analyses were performed, each with a different regressor representing the temperature profile in an epoch prior to sleep onset. The first analysis evaluated the last temperature value during the epoch prior to sleep onset. The second and third regressor evaluated the predictive value of monotonic changes in temperature prior to sleep onset. To this end, the second regressor evaluated changes immediately preceding a sleep bout, quantified as the difference between the last temperature readout immediately prior to the 30-s epoch and the temperature 5 min before. The third regressor evaluated slower changes, which were quantified as the difference between the last temperature readout immediately prior to the 30-s epoch and the temperature 15 min before. These 3 analyses were repeated for each of the temperatures (proximal, distal and DPG). P values were obtained by likelihood ratio tests of the full model with the effect in question against the model without the effect in question.
RESULTS
Subjects
Twenty-five patients (mean age 34.8 ± 3.4 years) and 15 controls (mean age 33.9 ± 14.0 years) were included. Sixteen patients were available for the second study part. The average of their scheduled treatment dosages was 5.8 ± 2.2 g SXB/night.
Sleep
Sleep characteristics are given in Table 1. Due to technical problems, polysomnographic registration failed in one patient, and two controls did not give permission for polysomno-graphic registration.
Table 1.
Sleep variables before and after SXB administration.
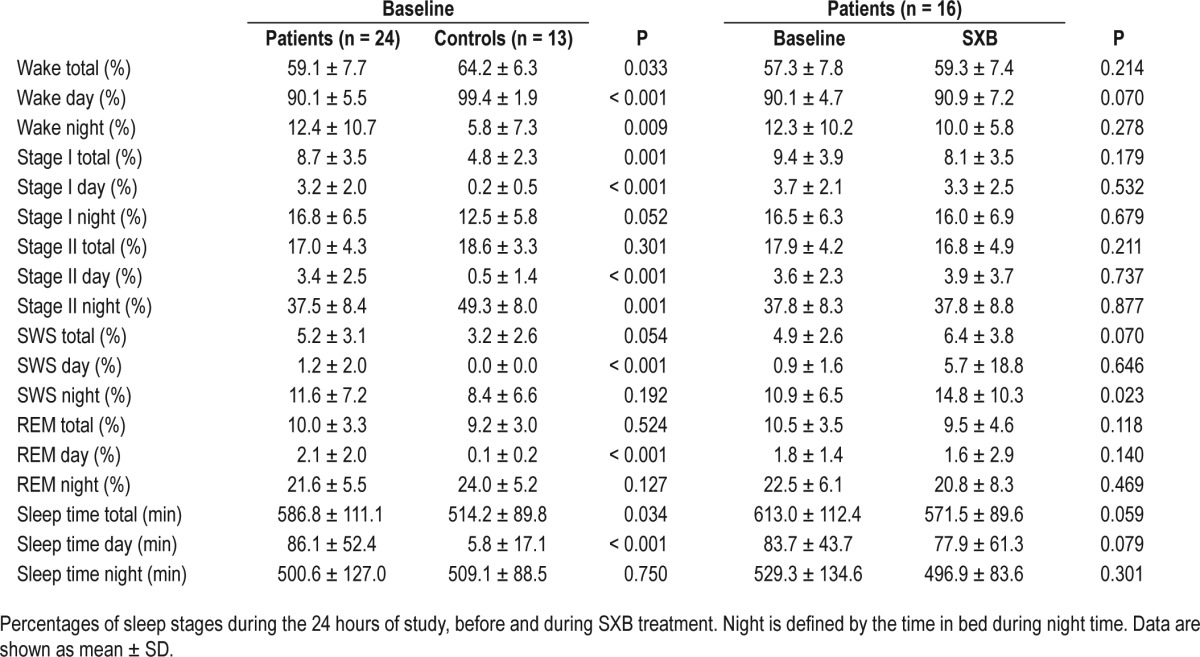
During the day, patients were significantly less awake than controls (P < 0.001). Subsequently, all sleep stages were more frequently seen during the day in patients compared to controls. Patients were more awake during the night, but spent overall more time asleep during 24 hours. The distribution of sleep stages during the night was similar in patients and controls. In patients, treatment with SXB resulted in an increase of slow wave sleep during the night (P = 0.023), and in a trend toward more time spent awake during the day.
Daytime napping occurred in all patients at baseline and in 13 of 16 patients during SXB treatment, varying from 1 to 7 naps per patient at baseline and from 0 to 9 naps per patient during treatment. Three controls had 1 daytime nap each; the other controls did not show any daytime sleep. At baseline, patients had significantly more daytime naps than controls (baseline mean number of naps for patients and controls, respectively 2.79 ± 0.318 and 0.23 ± 0.122, P < 0.001). Significant improvement in the number of daytime naps was seen in patients during SXB treatment (baseline mean number of naps for patients at baseline and during treatment with SXB, respectively 2.79 ± 0.318 and 1.88 ± 0.539, P = 0.037).
Temperature in Narcolepsy: Patients vs. Controls
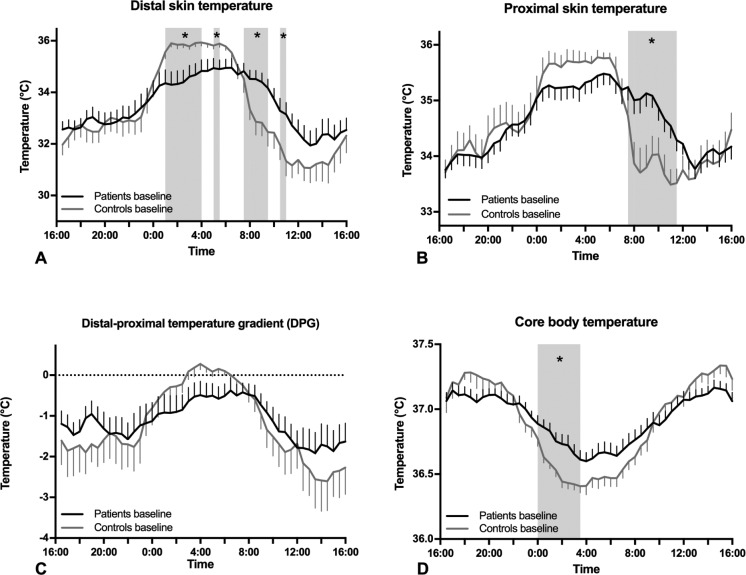
Temperature profiles are shown in Figure 1 and the results of statistical analysis in Table 2. No differences were seen between patients and controls by analysis over the full 24 hours. Analysis of the effect of group by time of day demonstrated a significant effect of narcolepsy by time of day for proximal skin temperature and a trend for core body temperature (respectively, F = 2.93, df = 10.23, P < 0.01, and F = 1.92, df = 7.32, P = 0.07).
Figure 1.
Mean ± SEM temperature profiles of patients vs. controls. (A) distal skin temperature in patients (black) and controls (gray) at baseline, (B) proximal skin temperature in patients and controls at baseline, (C) distal-proximal temperature gradient (DPG) in patients and controls at baseline (D) core body temperature in narcolepsy patients and controls at baseline. The gray area indicates the period during which the temperature significantly differed according the post-hoc tests (*P < 0.05).
Table 2.
Results of analysis of temperatures of controls vs. patients at baseline.
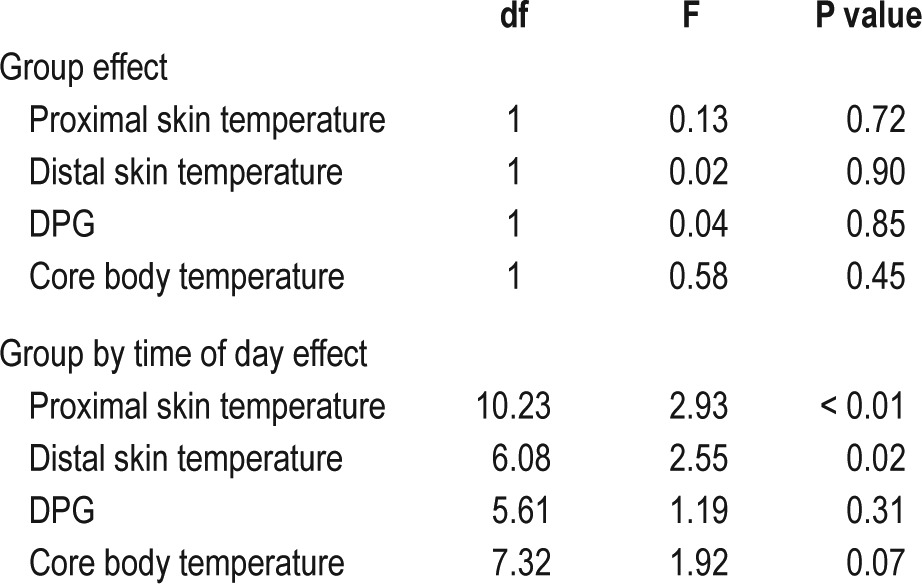
Separate analysis of daytime and nighttime temperatures demonstrated a trend toward a higher core body temperature in patients during the entire night (F = 3.50, df = 1, P = 0.07). Furthermore, a significant effect of group by time of day was seen for proximal skin temperature during the day and for both proximal and distal skin temperature during the night (respectively, F = 3.25, df = 6.86, P = 0.03; F = 2.69, df = 4.35, P = 0.03; and F = 3.14, df = 2.83, P = 0.03).
Post hoc tests indicated a significantly (P < 0.05) higher proximal skin temperature in patients between 07:30 and 11:30, a significantly higher core body temperature in patients between 00:00 and 03:30, a significantly lower distal skin temperature in patients between 01:00–04:00 and 05:00–05:30, and a significantly higher distal skin temperature in patients between 07:30–09:30 and 10:30–11:00.
A similar analysis was performed anchored to the bedtimes instead of actual clock times. This did not change the findings. This analysis is not presented here but can be found in Figures S1, S2 and Tables S1, S2 in the supplemental material.
In conclusion, as compared to controls, patients had a higher proximal and distal skin temperature in the morning, and a lower distal skin and a higher core body temperature during the night.
Temperature in Narcolepsy Patients: Baseline vs. SXB (Table 3, Figure 2)
Table 3.
Results of analysis of temperatures at baseline vs during SXB treatment in patients.
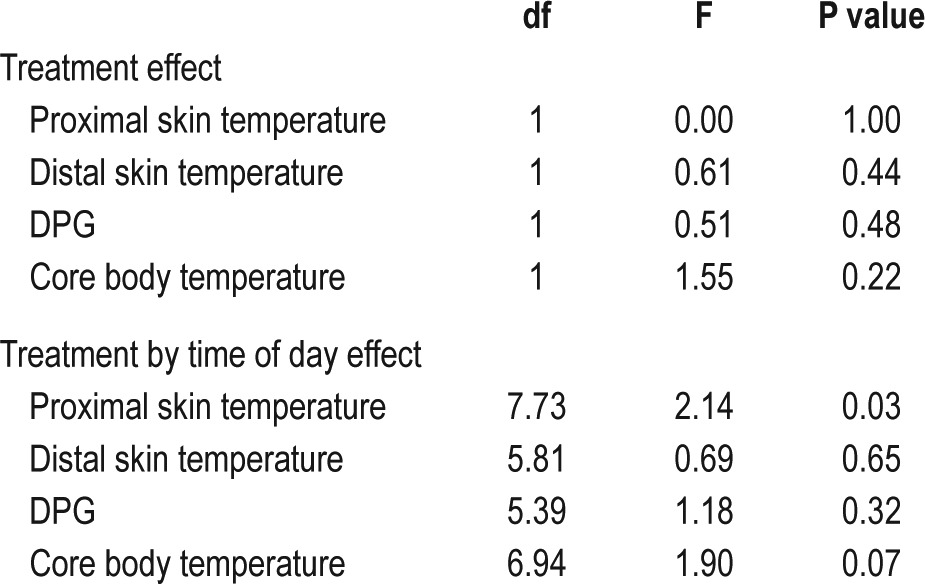
Figure 2.
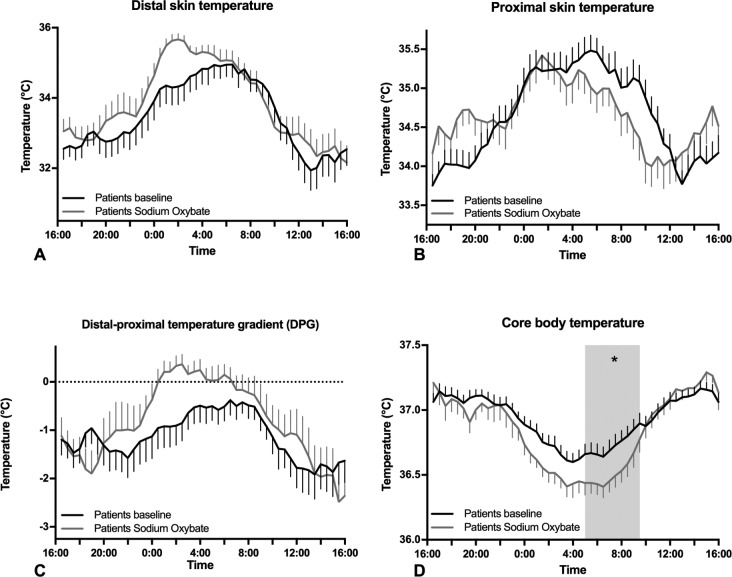
Mean ± SEM temperature profiles of patients at baseline and during SXB administration (A) distal skin temperature in patients at baseline (black) and during SXB administration (gray), (B) proximal skin temperature in patients at baseline and during SXB administration, (C) distal-proximal temperature gradient (DPG) in patients at baseline and during SXB administration (D) core body temperature in narcolepsy patients at baseline and during SXB administration. The gray area indicates the period during which the temperature significantly differed according the post-hoc tests (*P < 0.05).
No significant differences in temperature were seen at baseline compared to during treatment with SXB). Analysis of treatment by time of day revealed a significant effect on proximal skin temperature and nearly on core body temperature (respectively F = 2.14, df = 7.73, P = 0.03; F = 1.90, df = 6.94, P = 0.07). Furthermore, in post hoc tests, core body temperature was lower after SXB treatment from 05:00 to 09:30.
Additional separate daytime and night time analysis demonstrated that core body temperature was lower during the night during SXB treatment (F = 4.99, df = 1, P = 0.03), and analysis of the effect of treatment by time of day demonstrated a significant effect for proximal skin temperature during daytime (F = 3.45, df = 7.71, P < 0.01).
Summarizing, treatment with SXB in narcolepsy lowered nocturnal core body temperature to a level seen in controls. Indeed, no significant difference in nocturnal core body temperature was found for patients during treatment with SXB compared to controls (F = 0.0, df = 1, P = 0.94).
The Predictive Value of Temperature Changes on the Onset of Daytime Naps (Table 4, Figure 3)
Table 4.
Effect of temperature on daytime nap probability.
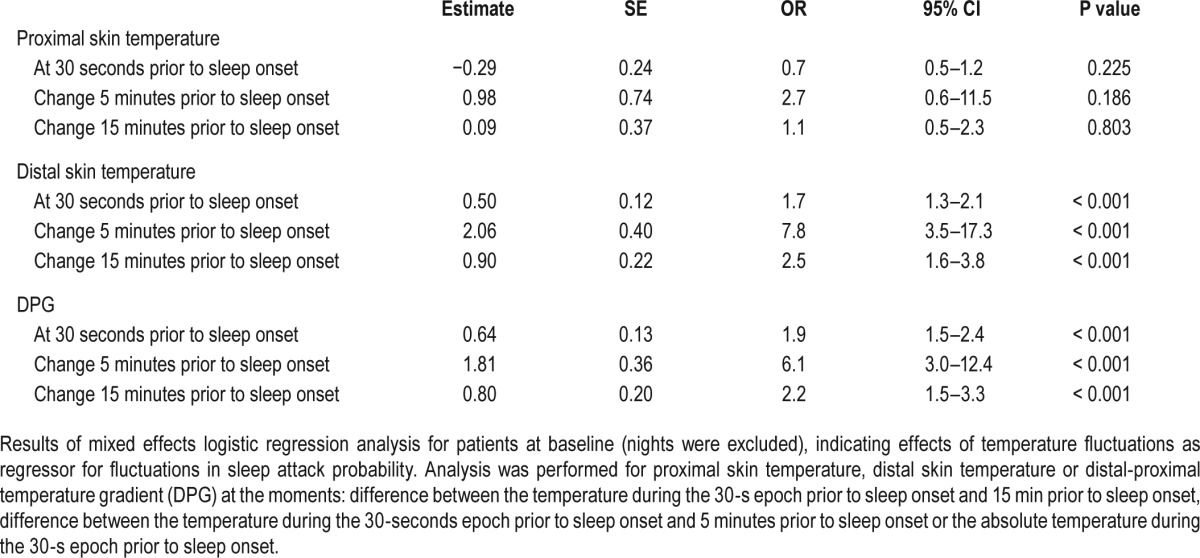
Figure 3.
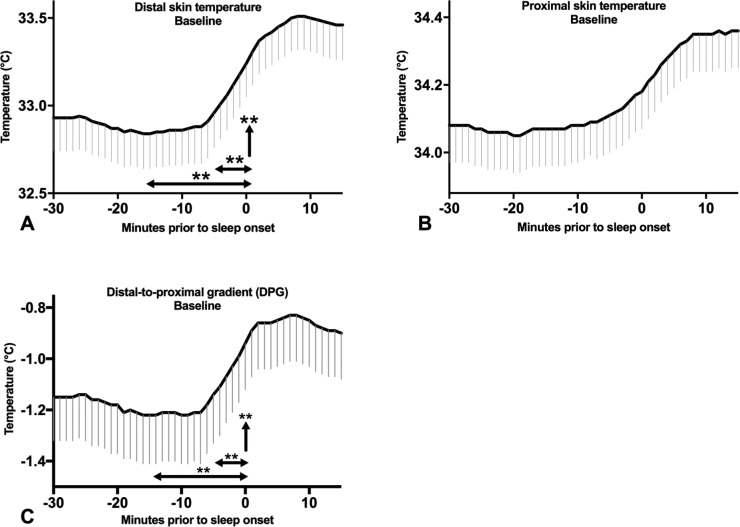
Temperature prior to daytime sleep onset in patients at baseline. Mean ± SEM temperatures in patients at baseline from 30 minutes prior to sleep onset until 15 minutes after sleep onset. (A) Distal skin temperature (B) Proximal skin temperature (C) Distal-proximal temperature gradient (DPG). The horizontal arrows indicate the temperature change 15 and 5 minutes prior to sleep onset, the vertical arrow indicates the temperature 30 seconds prior to sleep onset. **P < 0.01.
Since daytime napping was rare in controls, only daytime naps in patients were analyzed. Mixed effects logistic regression analysis in patients at baseline revealed that the probability of sleep onset increased with a higher distal skin temperature (Odds ratio [OR] and 95% confidence interval [CI] 1.7 [1.3– 2.1]/°C/30 seconds, P < 0.001) and DPG (OR1.9 [CI 1.5–2.4], P < 0.001) during the previous epoch. Furthermore, the probability of sleep onset increased the steeper the slope of increasing distal skin temperature and DPG over the previous 5 minutes (respectively OR 7.8 [CI 3.5–17.3], P < 0.001, and OR 6.1 [CI 3.0–12.4], P < 0.001) and 15 minutes (respectively OR 2.5 [CI 1.6–3.8], P < 0.001, and OR 2.2 [CI 1.5–3.3], P < 0.001) prior to sleep onset prior to falling asleep was found. No predictive value of proximal skin temperature in daytime sleep onset was seen. During treatment with SXB, similar effects were found, although less strong (Table S3 in the supplemental material).
Core body temperature was excluded from this analysis; due to technical problems, too many core body temperature measurements were missing to perform this analysis reliably. In patients at baseline 11% of the measurements were missing, during treatment with SXB 3% and in controls 21%.
DISCUSSION
We aimed to assess whether in everyday life, sleep attacks are heralded by changes in skin temperature, and to further explore temperature regulation and sleep in narcolepsy type 1.
Interestingly, changes in distal skin temperature and DPG strongly predicted the onset of daytime sleep attacks. Compared to controls, patients had a higher core body temperature during the first part of the night and a different time course of distal skin, proximal skin and core body temperature. Treatment with SXB resulted in normalization (i.e., lowering) of core body temperature during the night, an increased amount of slow wave sleep during the night and a reduced number of daytime naps.
Distal Skin Temperature and DPG Changes Are Predictive for Sleep Onset
An exciting finding in the present study is the predictive value of distal skin temperature and DPG in daily life daytime sleep attacks, 30 seconds, 5 minutes, 15 minutes and before sleep onset. In particular the increase of distal skin temperature and DPG in the five minutes prior to sleep onset are strongly related to the onset of sleep attacks. These findings may be evaluated for possible use as a warning of sleep attack risk in narcolepsy patients. It is tempting to imagine the development of a temperature-driven alarm or even cooling devices, to warn narcolepsy patients or even prevent them from falling asleep when not appropriate.
Previous temperature manipulation studies in healthy young and elderly subjects, insomniacs and narcolepsy patients showed that manipulation of skin temperature can influence the ability to maintain wakefulness, daytime vigilance, sleep onset latency, and sleep depth. In all subjects, distal skin warming decreased sleep onset latency and enhanced sleep depth.16,23,24 In narcolepsy patients, distal skin cooling increased the ability to maintain wakefulness.4 In elderly and insomniacs proximal skin warming markedly slowed the response speed in vigilance tasks.25
Altered Thermoregulatory Profile in Narcolepsy
Patients had a higher core body temperature during the first part of the night, which is in line with the only other ambulatory core body temperature study that has been performed in narcolepsy.14 All other previous studies concerning core body temperature were performed in a laboratory setting, and showed a large variation in methods and results.11–13,15 As high core body temperature is associated with higher vigilance or wakefulness,4,6 a nightly higher core body temperature might be related to the disturbed nocturnal sleep in narcolepsy.
In previous laboratory studies,10,11 daytime proximal skin temperature was repeatedly described to be lower in patients than in controls, a finding that could not be replicated in the current study. Conversely, we even found a higher proximal skin temperature in the morning in patients. However, none of these previous studies included ambulatory patients. These previous studies were respectively performed when subjects stayed (semi) supine during the measurements, and during a Multiple Sleep Latency Test (MSLT).10,11 Since body position directly influences skin and core body temperature,26 this might be an explanation for the differences between previous findings and ours. As a consequence of the absence of a lowered proximal skin temperature, no higher distal-proximal temperature gradient (DPG) was found in patients. Furthermore, the time course of both distal and proximal skin temperature is similar (Figure 1), so significant differences in DPG are not expected.
The different time course of distal skin temperature in patients, being higher in the morning and lower during night time, does correspond with the findings in the previous studies.10,11 As described in the introduction section, a high distal skin temperature during the day is associated with lowered vigilance and increased sleep propensity,7 and low distal skin temperature is expected to correspond with an increased maintenance of wakefulness.4 Subsequently, a lowered nocturnal distal skin temperature might be related to a disturbed nocturnal sleep.
The Effects of SXB on Temperature Profiles in Narcolepsy
Patients had an increased nocturnal core body temperature compared to controls prior to treatment, while during treatment with SXB, core body temperature reached levels similar to the levels seen in controls. This normalisation of nocturnal core body temperature might be associated with the known improvement of nocturnal sleep in patients during SXB treatment.27 However, in our previous study no treatment effects on core body temperature were seen at all.11 In that study, patients were only treated with SXB for 5 consecutive days, probably a too short period to detect these changes in core body temperature. Furthermore, methodology differed in that patients stayed (semi) supine throughout the whole study. This difference in methodology might as well be an explanation for the absence of an increase, i.e. normalization, of daytime proximal skin temperature in patients after treatment with SXB described in our previous study.11 Nevertheless, based on manipulation studies,16,25,28 an increase of proximal skin temperature lowers vigilance and increases sleep onset. Therefore, this previously found increased daytime proximal skin temperature would be in contradiction with the known effects of SXB, i.e. a reduction of daytime sleep attacks.
Limitations
Since previous studies were performed in laboratory settings, the results are not representative for daily life. For this reason, we performed our study ambulatory, which is a benefit in measuring and predicting daytime sleep attacks, however, due to being ambulatory, all circumstances differed between the subjects. Subjects were allowed to follow their usual habits; therefore all bedtimes differed, food and drinks varied, as well as their temperatures. Furthermore, subjects went outside whenever they wanted while the climate differed season by season, and due to technical problems core body temperature measurements were partially incomplete in a few subjects. These differences make the comparison of temperature profiles more difficult, however, we already performed these measurements in a laboratory setting.11 Moreover, no information about body position, which influences temperature, was available. All these differences probably influenced core body and skin temperatures and its analysis. Moreover, due to several clinical and/or personal reasons, not all included patients started or continued treatment with SXB for a period of at least three months, making the posttreatment group smaller.
CONCLUSIONS
Daytime sleep attacks were preceded by clear changes in skin temperature. An increase in distal skin temperature and DPG during the fifteen minutes, 30 seconds, and even more during the five minutes prior to daytime sleep onset, was highly significantly associated with the occurrence of sleep attacks. It is intriguing to speculate whether these findings in the future might possibly lead to methods to warn narcolepsy patients or prevent falling asleep when not appropriate.
In an ambulatory setting, core body temperature was higher and distal skin temperature lower during the night in narcolepsy patients compared to controls. These changes might be associated with the known disturbed nocturnal sleep in narcolepsy. Furthermore, the previously described higher daytime distal skin temperature was also seen in the present study, although only in the morning. This finding might be related to excessive daytime sleepiness in narcolepsy. Treatment with SXB improved nocturnal sleep and normalised the core body temperature profile.
DISCLOSURE STATEMENT
This study was supported by UCB pharma. Dr. Lammers has served as a paid member of the UCB advisory board and received lecture fees and conference travel support from UCB pharma. Dr. Lammers also did consultancy for UCB pharma and provided expert testimony for UCB pharma, Jazz pharmaceuticals, and Bioprojet. Dr. Donjacour received lecture fees from UCB pharma. Funding for participation of Dr. Van Someren was provided by Project NeuroSIPE 10738 of the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO) and partly funded by the Ministry of Economic Affairs, Agriculture and Innovation; both in The Hague, the Netherlands. Dr. Baumann received lecture fees from Roche and did consultancy for Roche, Dr. Baumann also received funding from UCB pharma and Abbvie. The other authors report no financial conflict of interest. For this study we used SXB (Xyrem) from UCB Pharma.
ACKNOWLEDGMENTS
The authors thank J.G. van Dijk, S. Overeem, C. Bassetti and R. Khatami for their involvement in study design; and M. Bach, J. Meier, S. Weber and J.G. van Vliet – de Regt for their help in acquiring the data.
REFERENCES
- 1.Krauchi K, Cajochen C, Werth E, et al. Warm feet promote the rapid onset of sleep. Nature. 1999;401:36–7. doi: 10.1038/43366. [DOI] [PubMed] [Google Scholar]
- 2.Krauchi K, Cajochen C, Werth E, et al. Functional link between distal vasodilatation and sleep-onset latency? Am J Physiol Regul Integr Comp Physiol. 2000;278:R741–8. doi: 10.1152/ajpregu.2000.278.3.R741. [DOI] [PubMed] [Google Scholar]
- 3.van Someren EJ. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol Int. 2000;17:313–54. doi: 10.1081/cbi-100101050. [DOI] [PubMed] [Google Scholar]
- 4.Fronczek R, Raymann RJEM, Romeijn N, et al. Manipulation of core body and skin temperature improves vigilance and maintenance of wakefulness in narcolepsy. Sleep. 2008;31:233–40. doi: 10.1093/sleep/31.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Someren EJ. Mechanisms and functions of coupling between sleep and temperature rhythms. Prog Brain Res. 2006;153:309–24. doi: 10.1016/S0079-6123(06)53018-3. [DOI] [PubMed] [Google Scholar]
- 6.Raymann RJ, Swaab DF, van Someren EJ. Skin temperature and sleep-onset latency: changes with age and insomnia. Physiol Behav. 2007;90:257–66. doi: 10.1016/j.physbeh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Romeijn N, van Someren EJ. Correlated fluctuations of daytime skin temperature and vigilance. J Biol Rhythms. 2011;26:68–77. doi: 10.1177/0748730410391894. [DOI] [PubMed] [Google Scholar]
- 8.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 9.Luca G, Haba-Rubio J, Dauvilliers Y, et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res. 2013;22:482–95. doi: 10.1111/jsr.12044. [DOI] [PubMed] [Google Scholar]
- 10.Fronczek R, Overeem S, Lammers G-J, et al. Altered skin-temperature regulation in narcolepsy relates to sleep propensity. Sleep. 2006;29:1444–9. doi: 10.1093/sleep/29.11.1444. [DOI] [PubMed] [Google Scholar]
- 11.van der Heide A, Donjacour CEHM, Pijl H, et al. The effects of sodium oxybate on core body and skin temperature regulation in narcolepsy. J Sleep Res. 2015;24:566–75. doi: 10.1111/jsr.12303. [DOI] [PubMed] [Google Scholar]
- 12.Dantz B, Edgar DM, Dement WC. Circadian rhythms in narcolepsy: studies on a 90 minute day. Electroencephalogr Clin Neurophysiol. 1994;90:24–35. doi: 10.1016/0013-4694(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 13.Grimaldi D, Agati P, Pierangeli G, et al. Hypocretin deficiency in narcolepsy with cataplexy is associated with a normal body core temperature modulation. Chronobiol Int. 2010;27:1596–608. doi: 10.3109/07420528.2010.504907. [DOI] [PubMed] [Google Scholar]
- 14.Mosko SS, Holowach JB, Sassin JF. The 24-hour rhythm core temperature in narcolepsy. Sleep. 1983;6:137–46. doi: 10.1093/sleep/6.2.137. [DOI] [PubMed] [Google Scholar]
- 15.Pollak CP, Wagner DR. Core body temperature in narcoleptic and normal subjects living in temporal isolation. Pharmacol Biochem Behav. 1994;47:65–71. doi: 10.1016/0091-3057(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 16.Fronczek R, Raymann RJEM, Overeem S, et al. Manipulation of skin temperature improves nocturnal sleep in narcolepsy. J Neurol Neurosurg Psychiatry. 2008;79:1354–7. doi: 10.1136/jnnp.2008.143610. [DOI] [PubMed] [Google Scholar]
- 17.Billiard M, Bassetti C, Dauvilliers Y, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13:1035–48. doi: 10.1111/j.1468-1331.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2014. International Classification of Sleep Disorders, 3rd edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne C, Lim CL. The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br J Sports Med. 2007;41:126–33. doi: 10.1136/bjsm.2006.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Marken Lichtenbelt WD, Daanen HA, Wouters L, et al. Evaluation of wireless determination of skin temperature using iButtons. Physiol Behav. 2006;88:489–97. doi: 10.1016/j.physbeh.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Raymann RJ, Swaab DF, van Someren EJ. Cutaneous warming promotes sleep onset. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1589–97. doi: 10.1152/ajpregu.00492.2004. [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SQ. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 23.Ebben MR, Spielman AJ. The effects of distal limb warming on sleep latency. Int J Behav Med. 2006;13:221–8. doi: 10.1207/s15327558ijbm1303_5. [DOI] [PubMed] [Google Scholar]
- 24.Raymann RJ, Swaab DF, van Someren EJ. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131:500–13. doi: 10.1093/brain/awm315. [DOI] [PubMed] [Google Scholar]
- 25.Raymann RJ, van Someren EJ. Time-on-task impairment of psychomotor vigilance is affected by mild skin warming and changes with aging and insomnia. Sleep. 2007;30:96–103. doi: 10.1093/sleep/30.1.96. [DOI] [PubMed] [Google Scholar]
- 26.Tikuisis P, Ducharme MB. The effect of postural changes on body temperatures and heat balance. Eur J Appl Physiol Occup Physiol. 1996;72:451–9. doi: 10.1007/BF00242275. [DOI] [PubMed] [Google Scholar]
- 27.Lammers GJ, Arends J, Declerck AC, et al. Gamma hydroxybutyrate and narcolepsy: a double-blind placebo-controlled study. Sleep. 1993;16:216–20. doi: 10.1093/sleep/16.3.216. [DOI] [PubMed] [Google Scholar]
- 28.Raymann RJEM, Van Someren EJW. Diminished capability to recognize the optimal temperature for sleep initiation may contribute to poor sleep in elderly people. Sleep. 2008;31:1301–9. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.