Abstract
Study Objectives:
Obstructive sleep apnea (OSA) results from the interaction of several physiological traits; specifically a compromised upper airway anatomy and muscle function, and two key non-anatomical deficits: elevated loop gain and a low arousal threshold. Although continuous positive airway pressure (CPAP) is an efficacious treatment, it is often poorly tolerated. An alternative approach could involve administering therapies targeting the non-anatomic causes. However, therapies (oxygen or hypnotics) targeting these traits in isolation typically improve, but rarely resolve OSA. Therefore, our aim was to determine how the combination of oxygen and eszopiclone alters the phenotypic traits and OSA severity and to assess the baseline phenotypic characteristics of responders/nonresponders to combination therapy.
Methods:
In a single-blinded randomized crossover study, 20 OSA patients received combination therapy (3 mg eszopiclone and 40% oxygen) versus placebo/sham air, with 1 w between conditions. Under each condition, we assessed the effects on OSA severity (clinical polysomnography) and the phenotypic traits causing OSA using CPAP manipulations (research polysomnography).
Results:
Combination therapy reduced the apnea-hypopnea index (51.9 ± 6.2 vs. 29.5 ± 5.3 events/h; P < 0.001), lowered both the ventilation associated with arousal (5.7 ± 0.3 vs. 5.2 ± 0.3 L/min; P = 0.05) and loop gain (3.3 ± 0.5 vs. 2.2 ± 0.3; P = 0.025). Responders to therapy (apnea-hypopnea index reduced by > 50% to below 15 events/h; n = 9/20) had less severe OSA (P = 0.001), a less collapsible upper airway (P = 0.01) and greater upper airway muscle effectiveness (P = 0.002).
Conclusions:
The combination of lowering loop gain and raising the arousal threshold is an effective therapy in patients whose anatomy is not severely compromised. Our work demonstrates that combining therapies that target multiple traits can resolve OSA in selected individuals.
Clinical Trial Registration:
ClinicalTrials.gov, ID: NCT01633827
Citation:
Edwards BA, Sands SA, Owens RL, Eckert DJ, Landry S, White DP, Malhotra A, Wellman A. The combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibility. SLEEP 2016;39(11):1973–1983.
Keywords: arousal threshold, loop gain, phenotyping, sleep apnea
Significance.
Given that obstructive sleep apnea (OSA) has both anatomical and non-anatomical causes (i.e., inadequate upper airway muscle function, a high loop gain, and a low arousal threshold), we assessed whether the combination of oxygen and eszopiclone 1) simultaneously lowers loop gain and raises the arousal threshold and 2) whether these changes in OSA physiology markedly reduce OSA severity in those patients with a mild to moderate upper airway collapsibility. Our study demonstrates that the combination of drugs/agents targeting loop gain and the arousal threshold is a beneficial treatment option in patients whose anatomy is not severely compromised. The major clinical implication of this study is that combining therapies directed to an OSA patient's underlying pathophysiology is an effective strategy and takes us one step closer to individualized therapy for patients with OSA.
INTRODUCTION
Obstructive sleep apnea (OSA) is a highly prevalent disorder with serious cardiovascular1–8 and neurocognitive consequences,9 yet a considerable number of patients do not tolerate the most commonly prescribed treatment, continuous positive airway pressure (CPAP).10–15 Therefore, novel approaches to OSA treatments are needed.
The key to offering new treatments is to recognize that OSA is a multifactorial disorder,16–20 and is not simply due to compromised upper airway physiology (i.e., increased collapsibility, poor dilator muscle responsiveness). Accumulating evidence demonstrates that two key non-anatomical traits contribute substantially to the pathogenesis of OSA: (1) an over-sensitive ventilatory control system (i.e., high loop gain), and (2) a low respiratory arousal threshold.
We and others have used therapies to manipulate these non-anatomical traits individually in an attempt to treat OSA without CPAP. For instance, the non-anatomical traits can be targeted with oxygen or acetazolamide to reduce loop gain21,22 or hypnotics to raise the arousal threshold.23,24 Such monotherapies typically result in an improvement but rarely a complete resolution of OSA, because (1) the anatomical deficiency is too severe (i.e., a highly collapsible airway), (2) the effects of these therapies on individual traits are not large enough, or (3) more likely, manipulation of a single trait is not adequate to overcome the multifactorial nature of this disorder.
Surprisingly, few attempts have been made to treat OSA with a combination of therapies. Accordingly, we sought to examine how the combination of oxygen (to reduce loop gain) and eszopiclone (to raise the arousal threshold) alters the phenotypic traits and OSA severity. We hypothesized that combination therapy substantially improves sleep apnea severity, and that those individuals with a mild to moderate upper airway collapsibility will obtain a greater benefit versus those with more severe upper airway collapsibility. Preliminary results of this analysis have been published in abstract form.25
METHODS
Participants
Twenty-two patients with a previous diagnosis of OSA (defined as an apnea-hypopnea index [AHI] > 10 events/h) were recruited from an existing database of patients (i.e., sample of convenience) that consisted of patients from our sleep clinic and the general community (Figure 1). The sample of OSA patients was a heterogeneous population (i.e., unselected), which was necessary in order to determine the physiological characteristics of those who would gain the greatest benefit from combination therapy. The first patient was recruited in September 2012 and the last patient follow-up was completed in June 2014. Six patients were currently compliant CPAP users, defined as more than 4 h CPAP/night for more than 2 mo; in the remaining patients, the diagnosis was recent and treatment had not begun. Subjects were excluded if they had a history of renal failure, neuromuscular disease, or other major neurological disorders; uncontrolled diabetes; heart failure; uncontrolled hypertension; thyroid disease; or any other unstable medical condition. Subjects were also excluded if they had any sleep disorder other than OSA (e.g., periodic leg movement syndrome, restless legs syndrome, insomnia, central sleep apnea/Cheyne-Stokes respiration), or if they were taking any medications known to affect sleep/arousal, breathing, or upper airway muscle physiology. Written informed consent was obtained before participation in the study, which was approved by the Partners' Human Research Committee. The study was registered with clinicaltrials.gov (NCT01633827).
Figure 1.
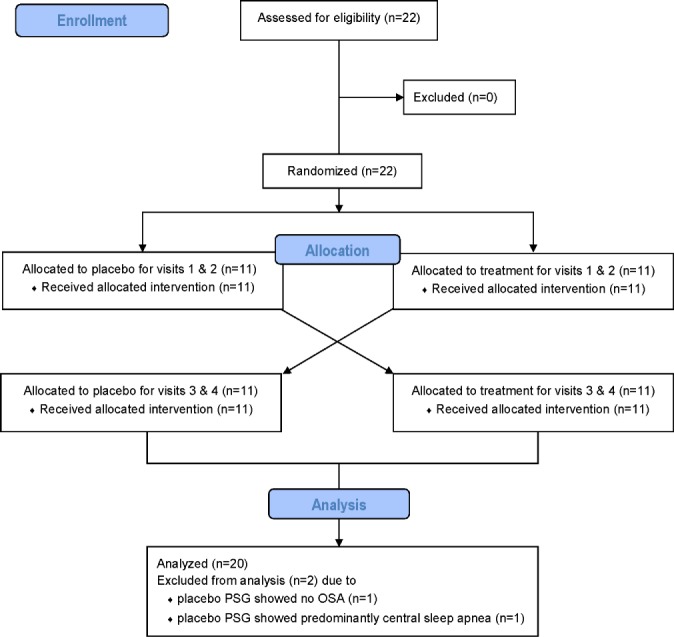
Flow diagram of the enrollment, randomization, and analysis procedures.
Experimental Design
We employed a single-blinded placebo-controlled crossover study design in which participants were randomized to the allocation order (placebo first or treatment first). A member of the team not involved in patient enrollment and outcome assessment generated the allocation sequence. Subjects remained blinded to allocation. Each participant performed the following procedures under active treatment and placebo conditions. Participants completed both a clinical and research polysomnogram (PSG) separated by at least 2 days. During the treatment arm, participants took eszopiclone (3 mg by mouth) at bedtime and slept with supplemental oxygen (FIO2 = 0.4) throughout the night. Doses were chosen based on our previously published data examining the effect of these individual therapies.22,23,26 During the placebo arm, subjects took a placebo at bedtime and were placed on room air while they slept. Participants then had approximately a 1-w washout period prior to crossover to the other arm of the study, whereby the clinical and research PSGs were repeated.
Study Measurements
Clinical PSG
To determine the severity of OSA (i.e., AHI) we performed full in-laboratory PSG. Upon arrival, subjects completed the Epworth Sleepiness Scale and Pittsburgh Sleep Quality Index questionnaires and instrumentation performed with a standard clinical montage that included electroencephalogram, electrooculogram, submentalis and anterior tibialis electromyogram (EMG), electrocardiogram, nasal pressure and thermistor, respiratory effort (piezoelectric bands placed around the chest and abdomen), body position, and arterial oxygen saturation monitored at the finger. End-tidal O2 and CO2 were continuously recorded from a catheter placed inside the nostril and measured with an O 2/CO2 analyzer (Vacumed, Ventura, CA). Last, subjects wore a Venturi mask (Teleflex Medical, Research Triangle Park, NC) for the delivery of oxygen/room air. All signals were sampled at 125 Hz and displayed using Spike 2 software (Cambridge Electronic Design Ltd, Cambridge, UK). Sleep state and respiratory events and arousals were scored according to standard clinical criteria27 and an O2-independent alternative criteria (see supplemental material) by a single experienced sleep technician blinded to treatment condition. In addition to the standard clinically reported PSG variables, we also calculated the percentage of the night spent in stable breathing (defined as no events or arousals for more than 3 min). To control for sleeping position, OSA severity was assessed during at least 4 h of supine sleep.
Research PSG
Subjects slept with the same monitoring equipment as the clinical PSG. Additionally they were fitted with a sealed nasal mask (Gel Mask; Philips Respironics, Murrysville, PA) attached to a pneumotachometer (model 3700A; Hans-Rudolph, Kansas City, MO) for measuring airflow. The mask was connected to a positive/negative pressure source (Pcrit 3000, Philips Respironics) to enable rapid switching between CPAP levels. The pressure in the mask was measured by a pressure transducer (Validyne, Northridge, CA) connected to a port in the mask. CO2 was continuously recorded from a catheter placed inside the nostril and measured with a capnograph (Vacumed). Epiglottic pressure (Pepi) was determined by a pressure catheter (model MCP-500; Millar, Houston, TX) advanced through the nostril and placed 1 to 2 cm caudal to the base of the tongue under direct visualization and securely taped in place. To insert the catheter, both nostrils were initially decongested (0.05% oxymetazoline HCl), and the more patent nostril was anesthetized (4% lidocaine HCl). Genioglossal muscle EMG (EMGGG) was recorded by two Teflon-coated stainless steel finewire intramuscular electrodes (Cooner Wire Company, Chatsworth, CA) with 2 mm of Teflon removed from the tip were inserted periorally into the genioglossus using a 25-gauge needle 3 to 4 mm on either side of the frenulum to a depth of approximately 1.5 cm after surface anesthesia (4% lidocaine HCl) to create a bipolar EMG recording.
Measuring the Traits Responsible for OSA and Modeling Their Interaction
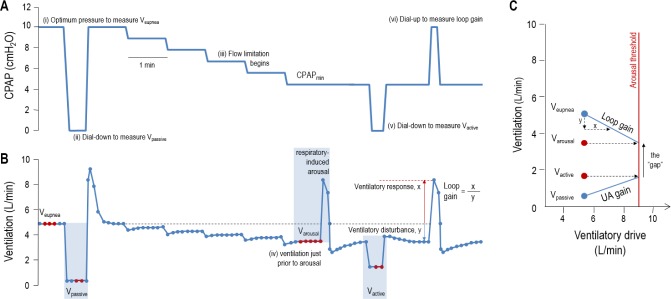
The traits were assessed in units of ventilation during supine nonrapid eye movement (NREM) (stages N2-3) sleep using our previously published method,28 which manipulates CPAP and assesses the consequent changes in ventilation (Figure 2; also see the supplemental material for further detail) described as follows:
Eupneic ventilation (Veupnea): ventilation on therapeutic CPAP, which represents the subject's asleep ventilatory requirement.
Passive collapsibility (Vpassive): ventilation through a passive airway at CPAP = 0 cmH2O and at eupneic ventilatory drive.
Ventilation that causes an arousal (Varousal): during the slow CPAP reduction, Varousal is the minimum ventilation that can be tolerated before an arousal.
Active collapsibility (Vactive): ventilation (at CPAP = 0 cmH2O) that can be achieved through a maximally active airway without arousal.
Ventilatory control sensitivity (i.e., loop gain): increase in ventilatory drive (measured as the ventilatory overshoot following a switch to optimal CPAP) in response to a steady-state reduction in ventilation.
Multiple measurements of each trait were made throughout the night, which were then averaged to produce a single value for each individual. These traits are then graphically depicted (Figure 2C) in order to determine the arousal threshold and the effectiveness of the upper airway muscles (i.e., upper airway gain). The difference between Varousal and Vactive, which we refer to as the physiologic “gap,” quantifies the ventilatory insufficiency and thereby predicts whether stable breathing or OSA will emerge. For example, a positive “gap” predicts the presence of OSA because the ventilation needed to avoid arousal cannot be achieved through the activated airway (Vactive < Varousal). A negative “gap” predicts that stable breathing is pos sible without arousal (Vactive > Varousal).
Figure 2.
Technique for determining the physiological traits using continuous positive airway pressure (CPAP) dial-downs. The obstructive sleep apnea (OSA) traits are measured by manipulating CPAP during supine nonrapid eye movement (NREM) sleep (A) and measuring the changes in ventilation (B). After determining ventilation on optimum CPAP (Veupnea; i), the pressure was dialed down to measure passive V0 (ii), the ventilation at CPAP = 0 when pharyngeal muscles are inactive. CPAP was then lowered until flow limitation started (iii) and arousals occurred intermittently. Ventilation just prior to arousal (iv) was defined as the “ventilation causing arousal” (Varousal). During stable breathing between arousals, CPAP was dialed down or up to obtain active V0 (v) and loop gain (vi), respectively. Active V0 is the ventilation at CPAP = 0 when pharyngeal muscles are maximally activated. Loop gain is the ventilatory response (overshoot in ventilation above Veupnea) divided by the ventilatory disturbance (reduction in ventilation below Veupnea). (C) These four ventilations can be used to calculate the arousal threshold and upper airway response and illustrate how all four traits interact to manifest the absence or presence of OSA. After the patient's loop gain is known, the ventilation that causes arousal was translated into the ventilatory drive that causes the arousal, which is referred to as the arousal threshold (i.e., 9 L/min). When the arousal threshold is known, starting at the passive V0 we can say that if the ventilatory drive is increased to a level near the arousal threshold, then the pharyngeal muscles will activate and increase V0 to the measured level active V0. The slope of this line is called the upper airway gain (or response) and is a measure of the upper airway muscle function. The steeper this slope, the better the upper airway response to an increase in ventilatory drive, and the larger the distance between the active and passive V0. In order to avoid having OSA (i.e., achieve stable breathing), Vactive must be above Varousal.
In addition to measuring the traits in units of ventilation or ventilatory drive, we also assessed (1) the upper airway anatomy/collapsibility by measuring the passive critical closing pressure (Pcrit), (2) the arousal threshold as the nadir Pepi prior to arousal, and (3) the upper airway muscle responsiveness as the increase in peak EMGGG (%maximum) per change in Pepi across flow-limited breaths during the CPAP dial-downs (see supplemental material).
Responder and Statistical Analysis
In order to determine the physiological characteristics of the patients who gained the greatest benefit from this combination of therapies, similar to previously published criteria,29 patients were categorized as “responders” to therapy if their treatment AHI was less than 15 events/h and reduced by more than 50% of the baseline value. These criteria were chosen to allow the widest applicability for clinical practice.
Either paired t-tests or Wilcoxon signed-rank tests were used to assess the effects of combination therapy as appropriate (SigmaPlot, Systat Software, San Jose, CA). A value of P ≤ 0.05 was considered significant. Values are presented as means ± standard error of the mean or medians [interquartile range] as appropriate.
RESULTS
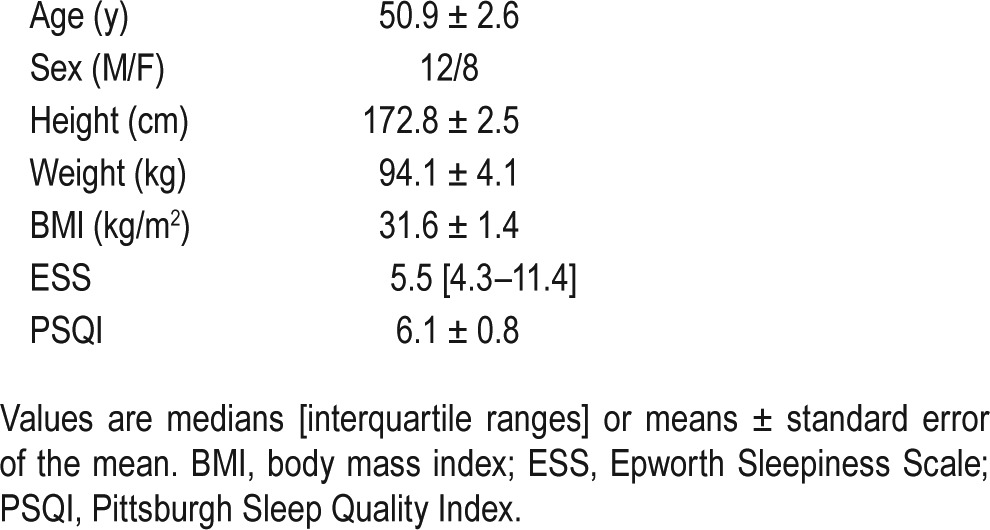
During the placebo arm, one participant did not have OSA and another exhibited predominantly central sleep apnea; both were excluded from the analysis (see Figure 1). Subject demographics for the 20 remaining unselected patients are shown in Table 1.
Table 1.
Patient characteristics.
Effect of Combination Therapy on OSA Severity
The effect of combination therapy on both sleep architecture and sleep-disordered breathing (clinical PSG) is summarized in Table 2. Briefly, combination therapy significantly increased total sleep time by 10% (P = 0.04), driven by an increase in N2 sleep. Combination therapy lowered the supine AHI (in all except one patient) by 47% (see Figure 3) in our unselected patients via a reduction in the frequency of both obstructive apneas and hypopneas. Furthermore, improvements in the supine AHINREM remained significant, and of a similar magnitude when the PSGs were scored using an alternative criteria that characterized events using only a 30% reduction in nasal airflow without the need for arousal or desaturation (see supplemental material). Combination therapy also improved the arousal index, and percentage of the night spent in stable breathing as well as all oxygenation indices (i.e., mean overnight saturation, nadir saturation, and time spent less than 90%). Despite the improvement in OSA severity, the duration of the residual obstructive apneas and hypopneas were slightly but significantly increased on therapy.
Table 2.
Effect of combination therapy on sleep architecture and sleep-disordered breathing.
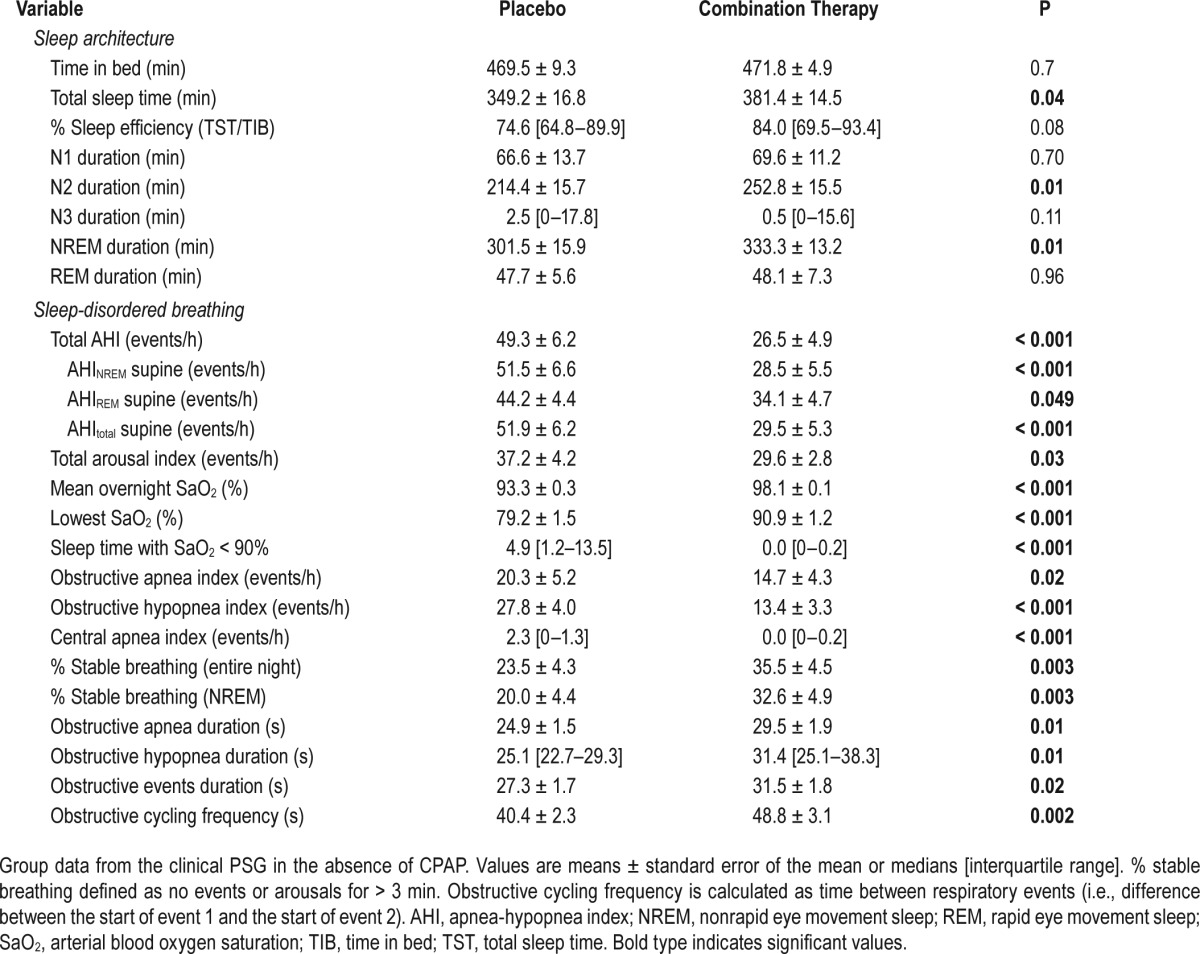
Figure 3.
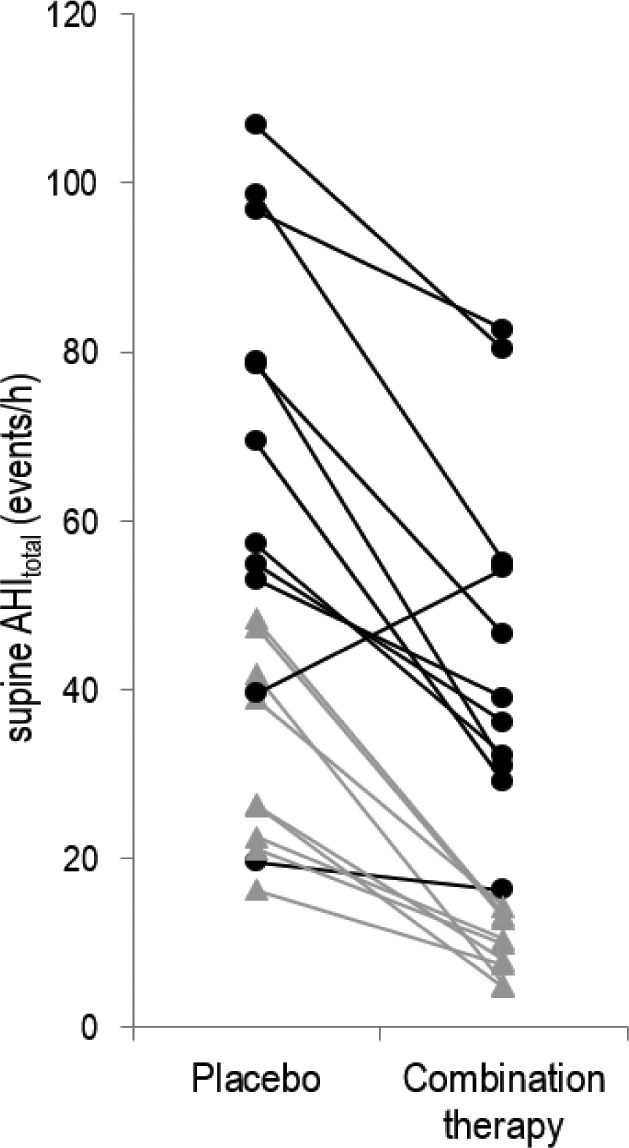
Individual effects of combination therapy on obstructive sleep apnea (OSA) severity. Combination therapy significantly reduced the overall supine apnea-hypopnea index (AHI) in all but one individual. Notably this individual had a low loop gain at baseline and a robust upper airway muscle response. However, with therapy, for reasons that are unclear, combination therapy unexpectedly increased loop gain (by 150%) and attenuated their upper airway muscle function (by 84%), which consequently widened the gap between Varousal and Vactive: such a widening is consistent with the increase in OSA severity observed. Black circles represent those patients considered nonresponders to therapy, whereas gray triangles represent responders (see text for definitions of responders/nonresponders). Of note, in six patients (approximately one-third of all unselected patients) OSA was completely resolved (AHI < 10 events/h) with combination therapy.
Effect of Combination Therapy on the OSA Traits
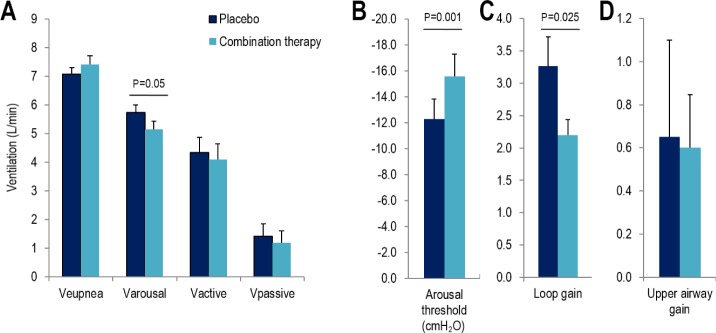
The effect of combination therapy on the OSA traits is depicted in Figure 4. Combination therapy significantly reduced the ventilation causing arousal (5.7 ± 0.3 vs. 5.2 ± 0.3 L/min; P = 0.05) and loop gain (3.3 ± 0.5 vs. 2.2 ± 0.3; P = 0.025). When the arousal threshold was estimated in units of ventilatory drive, it also tended to increase with therapy but the difference was not statistically significant (P = 0.16). There was a significant increase in the arousal threshold when assessed as the nadir epiglottic pressure prior to arousal (P = 0.001). In contrast, compared to placebo, combination therapy did not alter the eupneic ventilation (Veupnea), the passive upper airway collapsibility assessed using either Vpassive or Pcrit (see supplemental material), the maximum ventilation achievable without arousal (Vactive) or the upper airway gain/effectiveness.
Figure 4.
The effect of combination therapy on the traits. Combination therapy significantly reduced Varousal (A) and loop gain (C) while also increasing the arousal threshold (B) (measured as nadir epiglottic pressure). Combination therapy did not alter any of the remaining traits: Veupnea, Vactive & Vpassive (A), upper airway gain (D).
Responders to Therapy
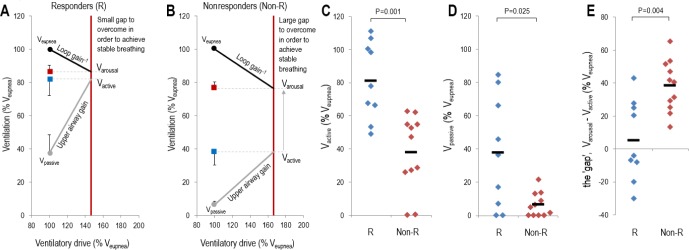
Nine of 20 patients (45%) were classified as “responders” to therapy (8 patients or 40% were responders using the alternative oxygen independent scoring criteria: see supplemental material). The baseline physiological characteristics (i.e., traits) of “responders” are summarized in Table 3 and Figure 5. In terms of their clinical and PSG characteristics, responders (n = 9) had less severe OSA (P = 0.001) and longer hypopnea durations (P = 0.02) compared to nonresponders. There was no difference in age, sex, and body mass index between the groups. When examining the underlying phenotypical traits, responders had a less collapsible upper airway (assessed using either Vpassive; P = 0.01, or Pcrit; P = 0.025) and could achieve a higher ventilation without arousal (Vactive; P = 0.002); responders also tended to have a lower arousal threshold but this finding failed to reach statistical significance (P = 0.16). This observation means that responders to therapy had a smaller “gap” (P = 0.002) [i.e., the distance between Vactive and the minimum level of ventilation that could be tolerated without arousal (Varousal)] that needed to be overcome by alterations in the phenotypic traits. The differences in the traits observed between responders and nonresponders remained significant even after the values were normalized as a percentage of Veupnea (see Figure 5).
Table 3.
Baseline characteristics of patients who were responsive and nonresponsive to combination therapy.
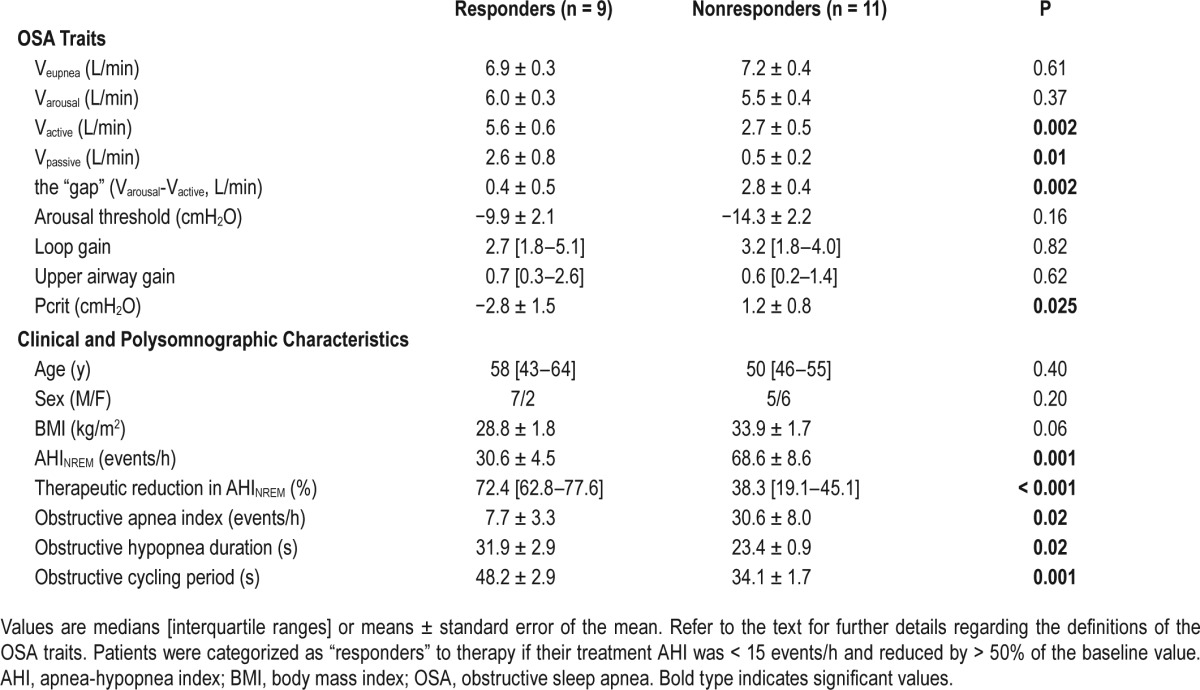
Figure 5.
Baseline physiological characteristics of responders to therapy. (A,B) The baseline group model diagrams for responders (R) and nonresponders (Non-R), respectively. Each model diagram illustrates how the four physiological traits interact to produce obstructive sleep apnea in each group. Data are expressed as a percentage of the eupneic ventilation (Veupnea). Responders to therapy had a higher maximum ventilation achievable without arousal (Vactive) (C) and a less collapsible airway under passive conditions (Vpassive) (D), which ultimately results in a smaller difference between Varousal and Vactive, called the physiologic “gap.” This small difference in responders means that only reductions in loop gain and increases in the arousal threshold are necessary for the intersection of the loop gain and upper airway gain lines to occur to the left of the arousal threshold line, thus yielding stable breathing.
DISCUSSION
The major findings of the current study are that combination therapy improved OSA severity (i.e., AHI) in 19 of 20 unselected patients, with 9 patients being considered “responders” to therapy when the clinical PSGs were scored using conventional methods. Furthermore, the improvements in OSA severity remained similar (although the number of responders was reduced to 8 patients) when the PSGs were scored using an alternative O2 independent criteria. Nonetheless, the improvement in OSA severity (regardless of criteria used) was due to the therapies' ability to close the “physiological gap” by reducing loop gain and increasing the arousal threshold (i.e., the remaining traits were not altered), allowing a lower ventilation to be achieved without arousal. Importantly, combination therapy was most effective in patients with minimal collapsibility and a smaller physiological “gap.” Therefore, the combination of drugs/agents targeting two key non-anatomical traits (loop gain and the arousal threshold) responsible for OSA may be an effective treatment option in patients whose anatomy is not severely compromised. Although the current study examined the acute effects of combination therapy on OSA severity in unselected patients, the major implication of our work is that it now provides proof of concept that a combination of treatments directed at an individual's underlying pathophysiology might be an effective alternative treatment strategy for certain patients. Importantly, such findings take us one step closer to individualized therapy for patients with OSA.
Effects of Combination Therapy on the Traits and Severity of OSA
Altering Loop Gain
The current study demonstrated that in unselected patients, supplemental oxygen lowered loop gain by approximately one third of its baseline value, a similar magnitude to that reported in previous studies.22,26 Furthermore, we observed a significant correlation between the reduction in loop gain and the reduction in OSA severity, suggesting that those who had the greatest decrease in loop gain benefit substantially from combination therapy. This finding supports a causal role for loop gain in OSA pathogenesis and suggests that maximally lowering loop gain will likely resolve OSA in patients with relatively “good” passive anatomy/collapsibility and a small physiological “gap.” Given the observed relationship between the reduction in loop gain and reduction in OSA severity, the development of new therapies with a more consistent and potent effect on lowering loop gain in order to reduce OSA severity more markedly, will likely be of great benefit to the field.
In contrast to the findings of the current study and others,30 Xie et al.31 reported that hyperoxia had no effect on mean AHI (room air vs. hyperoxia: 39 ± 6 vs. 34 ± 6 events/h, P = 0.25) and found there was no relationship between anatomy/collapsibility and the changes in AHI across participants. Apparent discrepancies are possibly explained by their greater average age (+10 y), more severe average collapsibility (Pcrit in the current study [see supplemental material]: −2.8 ± 1.5 cmH2O vs. Xie et al.: −0.9 ± 1.8 cmH2O) or a lower loop gain to start with in the study by Xie et al. and were thus less responsive to manipulation of this trait (as loop gain decreases with aging).32
Altering the Arousal Threshold
To date, the effect of hypnotics on both the arousal threshold and OSA severity has been minimally studied. Recent work by our group23 has suggested that compared to placebo, eszopiclone significantly increases the arousal threshold by 4 cmH2O, a finding comparable to the 3.3 cmH2O increase observed in the current study. Furthermore, both the current and previous studies have demonstrated that eszopiclone increased the total sleep time (driven by an increase in stage N2), and reduced the number of arousals (spontaneous and respiratory-induced) throughout the night. The study conducted by Eckert et al.23 highlighted the fact that individuals with a low arousal threshold achieved a greater improvement in their OSA severity. Notably there was a similar trend in the current study for responders to have a lower arousal threshold compared to the nonresponders (note that this trend became significant when we conducted our responder analysis using the alternative oxygen independent criteria; see supplemental material).
Combining Oxygen and a Hypnotic Has an Additive Effect in Reducing OSA Severity
A limitation of the current study design was the failure to assess how oxygen or eszopiclone altered the AHI independently. However, it would appear, based on published effect sizes of the individual therapies, that the combination of oxygen and a hypnotic improves OSA more than either treatment alone. In a recent meta-analysis of the effects of oxygen therapy in over 300 OSA patients, Mehta et al.30 demonstrated that oxygen reduced the AHI by 20% (18% to 28% interquartile range). Similarly, our previous work22 demonstrated that oxygen therapy reduced the AHI by 33%, with 2 of 12 patients (17%) being considered responders using the definition used in the current study. Furthermore, we have shown23 that the hypnotic eszopiclone reduced the AHI by a similar magnitude as oxygen (22%), with 2 of 17 patients (12%) considered responders. It is noteworthy that all of these studies were conducted in unselected patients. The current study, which was similarly conducted in unselected patients with a broad range of apnea severity, reduced the overall AHI by 47%, with 45% of our patients being considered responders. Although speculative, the evidence does lend support to the concept that combining oxygen and a hypnotic has an additive effect on reducing OSA severity.
Comparison With the PALM Scale
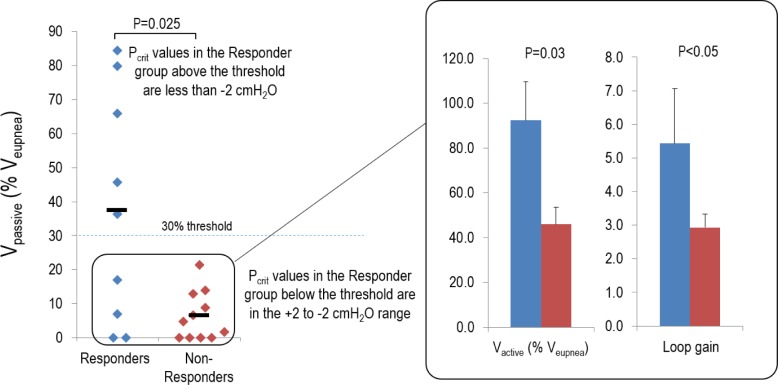
Our group recently proposed the three-point (Passive critical closing pressure of the upper airway, Arousal threshold, Loop gain, and Muscle responsiveness [PALM]) scale designed to categorize individual patients with OSA based on several key pathophysiologic traits.20 The objective of the PALM scale was to provide a conceptual framework in which to stimulate future hypothesis-driven testing of therapies that target specific underlying mechanisms in specific OSA patients. According to PALM criteria 3, 19% of unselected OSA patients (compared to the 20% of patients enrolled in the our study) were identified as having a modest vulnerability to upper airway collapse (defined as a Pcrit ≤ 2 cmH2O) and were expected to be candidates for one or a combination of targeted therapies with an increased likelihood that non-anatomic interventions would be beneficial in these patients. In support of this concept, all four patients in this category in our study (i.e., equivalent measurement is a Vpassive [expressed as a percentage of Veupnea] > 30%) were responders to therapy; importantly 3 of 4 had an AHI less than 10 events/h on therapy.
Most of our patients (70%) would be classified as PALM criteria 2, defined as those individuals with a Pcrit between −2 to +2 cmH2O (which equates to a Vpassive [expressed as a percentage of Veupnea] < 30%). Although Eckert et al.20 proposed that these patients will likely require a combination of anatomic and non-anatomical therapies to treat OSA, the current work suggests that approximately 25% of these patients can be effectively treated without an anatomical intervention (see Figure 5; four individuals were classified as responders despite having a poor airway [Vpassive < 30%]). When comparing the differences in the baseline characteristics of the four responders versus the nonresponders in this subgroup (see Figure 6), we found that responders have both a higher Vactive (92.7 ± 2.3 vs. 76.4 ± 3.7 %Veupnea; P = 0.03) and a higher loop gain (5.4 ± 1.6 vs. 2.9 ± 0.4; P < 0.05). Such findings highlight the importance of understanding the individual causes of OSA as well as the interaction of the traits is critical in predicting the effect of non-anatomical therapies.
Figure 6.
Physiological characteristics of responders with a poor anatomy/collapsibility. Data are expressed as a percentage of the eupneic ventilation (Veupnea). Individuals with a Vpassive below 30% of their eupneic ventilation (Veupnea) were classified as having a poor anatomy. Note that four of nine responders fit this category, whereas all of the nonresponders had a poor anatomy. When comparing the physiological differences between the responders (shown in blue) and nonresponders (shown in red) in this subset (see inset), responders had a higher maximum ventilation achievable without arousal (Vactive) and a higher loop gain.
Does Combination Therapy Have a Future in Sleep Medicine?
After a patient has received a diagnosis of OSA, the first line of therapy is usually a trial of CPAP. Because only approximately 50% of these patients will continue to use CPAP beyond 3 mo,33,34 a substantial number of patients must look for alternative treatment options. Although there are now a number of alternative treatment options, including behavioral approaches (i.e., weight loss, exercise, or avoiding sleep in the supine position), surgical remedies (i.e., uvulopalatopharyngoplasty or the placement of a hypoglossal nerve stimulator29) and medical interventions (i.e., oral appliances and drugs/ agents such as oxygen therapy,26 respiratory stimulants such as acetazolamide,21,35 or hypnotics23,36), they each have variable success rates. The key, we believe, to understanding better this variable efficacy of non-CPAP therapies lies in the knowledge that OSA is caused by a combination of physiological factors and these alternative treatments only target/manipulate one of these traits. Importantly, few attempts have been made to date to treat OSA with combination therapies, often either in very small numbers of patients or when the physiology causing OSA was not determined.
Inoue and colleagues37 assessed the combined effect of acetazolamide and uvulopalatopharyngoplasty on OSA severity and showed that this combination led to a greater reduction in AHI than either of the individual treatments alone. Although this study was only conducted in five patients with severe OSA, it provided initial support for the concept that combination therapy may be viable in treating OSA. Similarly, Dieltjens et al.38 demonstrated the additional reduction in OSA severity patients experienced when sleeping in the lateral position was done in conjunction with wearing an oral appliance. Last, Oliven et al.39 assessed the effect of a combination of electrical stimulation of the genioglossus with mandibular advancement (targeting muscle responsiveness and anatomy/collapsibility respectively) on the Pcrit in 14 OSA patients under propofol-induced anesthesia. Although the authors did not assess the effect of these interventions on OSA severity, their findings indicate that there was an additive improvement in P crit compared with individual manipulations, with a number of patients in the combination group achieving a Pcrit ≤ 5 cmH2O, which should lead to a resolution of OSA.20,40 The current work, which assesses both physiological mechanisms and OSA severity, extends the findings from the prior smaller studies to provide support for an innovative approach to OSA management: combining multiple therapies directed at one or more of the interacting physiological traits that underlie the disorder. Combination therapy is used in multiple medical conditions, including diabetes, hypertension, heart failure and cancer, to maximize therapeutic effectiveness (as well as to minimize toxicity and improve patient acceptance). The current study suggests that OSA could potentially be treated in a similar way.
Translating Physiological Research Into Clinical Practice and Methodological Considerations
Our findings suggest that if decreases in CPAP were performed to zero pressure as part of a routine clinical CPAP titration night, we might be able to use this information to stream patients into alternative therapies as part of standard clinical practice, without the need for formal physiological phenotyping. As highlighted by the results of this study, patients who can achieve more than 30% of the resting ventilation during a decrease to zero CPAP are likely to be good candidates for non-anatomical therapies. Furthermore, an individual's ventilatory control sensitivity41 or arousal threshold42 can be determined from a standard PSG which may help provide key information as to which of the non-anatomical traits needs targeting and is likely to be important in identifying patients that may have a poor anatomy (Vpassive < 30%), yet still respond to these therapies, thus making personalized treatment a reality. For those patients with a vulnerable anatomy, recent data suggest that lateral positioning43 can dramatically improve Vpassive (compare 2.8 ± 1.8 vs. 50.8 ± 9.5 % Veupnea; P < 0.001) which raises the interesting prospect that the nonresponders in our study could be converted into responders by placing them in the lateral position in combination with oxygen and a hypnotic.
Although this personalized approach to OSA treatment is enticing, future research needs to demonstrate that not only do such therapies improve OSA (i.e., AHI), but that they also abolish snoring as well as improve the adverse health consequences associated with the disorder (i.e., reduce daytime sleepiness, lower blood pressure, improve diabetes, etc). Furthermore, given that the improvement in overall AHI observed in the current study was mainly driven by a large reduction in the NREM AHI (as opposed to rapid eye movement), the combination of oxygen and a hypnotic may not be suitable for the treatment of rapid eye movement-predominant OSA. Based on physiological principles rather than our current data, we recommend that —outside the setting of supervision within clinical trials—hypnotics should not be used in OSA patients with severe desaturation because there is the potential to prolong the time to arousal and worsen the duration and severity of the hypoxemia/hypercapnia experienced. A consideration that must also be taken into account when interpreting our findings is that the patients recruited into this study came largely from an existing database of patients, which may be less representative of a clinical population sample. However, given that the clinical (i.e., anthropometric and polysomnographic variables) and physiological (i.e., OSA traits) characteristics of our patients are similar to those reported in the literature, we think that our findings remain generalizable to the general OSA population. An additional limitation of the current work is that we have only assessed the effect of combination therapy on 1 night. Thus, longitudinal studies will need to be conducted to determine if the treatments remain effective, or whether patients develop a tolerance to the drugs/agents that reduces their efficacy over time.
CONCLUSIONS
The current study demonstrates that the combination of oxygen and eszopiclone therapy closes the physiological gap causing OSA by reducing loop gain and allowing a lower ventilation to be achieved without arousal. This combination of nonanatomical therapies improved the AHI in 95% of patients studied, and was most effective in patients with a less collapsible airway and when loop gain was greatly reduced. Combining nonanatomical interventions can therefore be effective for OSA treatment in a recognizable subset of selected patients. Given the availability of techniques to measure these traits, and the emergence of simplified approaches using routine clinical information, this work has the potential to provide individualized treatment for OSA patients.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the National Institutes of Health: 5R01HL102321-02 and P01HL095491 as well as the Harvard Catalyst Clinical Research Center: UL1 RR 025758-01. Dr. Edwards is supported by the National Health and Medical Research Council (NHMRC) of Australia's CJ Martin Overseas Biomedical Fellowship (1035115). Dr. Eckert was supported by the American Heart Association (10SDG3510018), an NHMRC of Australia Overseas Biomedical Fellowship (510392), and is currently supported by a NHMRC R.D. Wright Fellowship (1049814). Dr. Sands was supported by the National Health and Medical Research Council of Australia and R.G. Menzies Foundation (1053201, 1035115) and is currently supported by the American Heart Association (15SDG25890059). Dr. Malhotra is supported by NIH RO1 HL085188 and K24 HL132105. Dr Landry is supported by a NHMRC ‘NeuroSleep’ Center for Research Excellence (CRE) post-doctoral fellowship. Dr. Owens consults for Apnex Medical, Apnicure and Philips Respironics. Dr. Malhotra was a consultant for Philips Respironics, SHC, SGS, Apnex Medical, Pfizer, Apnicure, but has relinquished all outside personal income since May 2012. Dr. White was the chief medical officer for Philips Respironics until 12/31/12 but is now a consultant. Dr White is also the chief scientific officer for Apnicure Inc. as of January 2013. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Lauren Hess, Erik Smales, Pam DeYoung, and Alison Foster for their laboratory assistance. Author contributions: Conception and design - BAE, DJE, DPW, AM, AW; Acquisition, analysis and interpretation - all authors; Drafting the manuscript for important intellectual content and final approval - all authors.
REFERENCES
- 1.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieto F, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model [see comments] J Clin Invest. 1997;99:106–9. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men [see comments] Lancet. 1990;336:261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 6.Wessendorf TE, Thilmann AF, Wang YM, Schriber A, Konietzko N, Teschler H. Fibrinogen levels and obstructive sleep apnea in ischemic stroke. Am J Respir Crit Care Med. 2000;162:2039–42. doi: 10.1164/ajrccm.162.6.2001048. [DOI] [PubMed] [Google Scholar]
- 7.Hoffstein V, Mateika S. Cardiac arrhythmias, snoring, and sleep apnea. Chest. 1994;106:466–71. doi: 10.1378/chest.106.2.466. [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 9.Redline S, Strauss ME, Adams N, et al. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20:160–7. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- 10.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea [see comments] Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 11.Engelman H, Wild MR. Improving CPAP use by patients with the sleep apnea/hypopnea syndrome (SAHS) Sleep Med Rev. 2003;71:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 12.Campos-Rodriguez F, Martinez-Alonso M, Sanchez-de-la-Torre M, Barbe F Spanish Sleep Network. Long-term adherence to continuous positive airway pressure therapy in non-sleepy sleep apnea patients. Sleep Med. 2016;17:1–6. doi: 10.1016/j.sleep.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 13.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 14.Pieters T, Collard P, Aubert G, Dury M, Delguste P, Rodenstein DO. Acceptance and long-term compliance with nCPAP in patients with obstructive sleep apnoea syndrome. Eur Respir J. 1996;9:939–44. doi: 10.1183/09031936.96.09050939. [DOI] [PubMed] [Google Scholar]
- 15.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 16.Wellman A, Eckert DJ, Jordan AS, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol. 2011;110:1627–37. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–41. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 18.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–70. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 20.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. Journal Physiol. 2012;590:1199–211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–51. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci. 2011;120:505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–12. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards BA, Sands SA, Owens RL, et al. Combination therapy for the treatment of obstructive sleep apnea. Am J Respir Crit Care Med. 2013:A3759. [Google Scholar]
- 26.Edwards BA, Sands SA, Owens RL, et al. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J Physiol. 2014;592:4523–35. doi: 10.1113/jphysiol.2014.277210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 28.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114:911–22. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strollo PJ, Jr., Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–49. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 30.Mehta V, Vasu TS, Phillips B, Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2013;9:271–9. doi: 10.5664/jcsm.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie A, Teodorescu M, Pegelow DF, et al. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J Appl Physiol. 2013;115:22–33. doi: 10.1152/japplphysiol.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards BA, Wellman A, Sands SA, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37:1227–36. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 34.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 35.Edwards BA, Connolly JG, Campana LM, et al. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep. 2013;36:281–5. doi: 10.5665/sleep.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in obstructive sleep apnea patients with a low arousal threshold. Sleep. 2014;37:811–9. doi: 10.5665/sleep.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue Y, Takata K, Sakamoto I, Hazama H, Kawahara R. Clinical efficacy and indication of acetazolamide treatment on sleep apnea syndrome. Psychiatry Clin Neurosci. 1999;53:321–2. doi: 10.1046/j.1440-1819.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 38.Dieltjens M, Vroegop AV, Verbruggen AE, et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 2015;19:637–44. doi: 10.1007/s11325-014-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliven R, Tov N, Odeh M, et al. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol. 2009;106:1668–73. doi: 10.1152/japplphysiol.91501.2008. [DOI] [PubMed] [Google Scholar]
- 40.Kirkness JP, Schwartz AR, Schneider H, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol. 2008;104:1618–24. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–18. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190:1293–300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joosten SA, Edwards BA, Wellman A, et al. The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep. 2015;38:1469–78. doi: 10.5665/sleep.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.