Abstract
Study Objectives:
To describe parental reports of sleepiness and sleep duration in children with polysomnography (PSG)-confirmed obstructive sleep apnea (OSA) randomized to early adenotonsillectomy (eAT) or watchful waiting with supportive care (WWSC) in the ChildHood Adenotonsillectomy Trial (CHAT). We hypothesized children with OSA would have a larger improvement in sleepiness 6 mo following eAT compared to WWSC.
Methods:
Parents of children aged 5.0–9.9 y completed the Epworth Sleepiness Scale modified for children (mESS) and the Pediatric Sleep Questionnaire-Sleepiness Subscale (PSQ-SS). PSG was performed at baseline and at 7-mo endpoint. Children underwent early adenotonsillectomy or WWSC.
Results:
The mESS and PSQ-SS classified 24% and 53% of the sample as excessively sleepy, respectively. At baseline, mean mESS score was 7.4 ± 5.0 (SD) and mean PSQ-SS score was 0.44 ± 0.30. Sleepiness scores were higher in African American children; children with shorter sleep duration; older children; and overweight children. At endpoint, mean mESS score decreased by 2.0 ± 4.2 in the eAT group versus 0.3 ± 4.0 in the WWSC group (P < 0.0001); mean PSQ-SS score decreased 0.29 ± 0.40 in eAT versus 0.08 ± 0.40 in the WWSC group (P < 0.0001). Despite higher baseline sleepiness, African American children experienced similar improvement with adenotonsillectomy than other children. Improvement in sleepiness was weakly associated with improved apnea-hypopnea index or oxygen desaturation indices, but not with change in other polysomnographic measures.
Conclusions:
Sleepiness assessed by parent report was prevalent; improved more after eAT than after WWSC; and was not strongly predicted by sleep disturbances identified by PSG.
Clinical Trial Registration:
Childhood Adenotonsillectomy Study for Children with OSA (CHAT). ClinicalTrials.gov Identifier #NCT00560859.
Citation:
Paruthi S, Buchanan P, Weng J, Chervin RD, Mitchell RB, Dore-Stites D, Sadhwani A, Katz ES, Bent J, Rosen CL, Redline S, Marcus CL. Effect of adenotonsillectomy on parent-reported sleepiness in children with obstructive sleep apnea. SLEEP 2016;39(11):2005–2012.
Keywords: adenotonsillectomy, apnea-hypopnea index, Epworth Sleepiness Scale, OSA, OSAS, pediatric, Pediatric Sleep Questionnaire, polysomnogram, sleepiness
Significance.
Sleepiness in children with obstructive sleep apnea improved on two commonly used sleepiness questionnaires following adenotonsillectomy treatment. Improvement was greater in children who underwent adenotonsillectomy than watchful waiting with supportive care. Sleepiness was higher in children who were older, overweight, African American race, and who had shorter sleep duration. An improvement in sleepiness was weakly correlated with improvement in AHI and oxygen desaturation index on polysomnography. These findings highlight the need for more research to better understand the complexities and measurement of sleepiness in children. Clinically, sleepiness should be evaluated in patients with sleep-disordered breathing even if low AHI is observed on the polysomnogram to help guide treatment. The significant improvement in sleepiness in children receiving eAT compared to watchful waiting provides further support for interventions aimed at improving sleep-disordered breathing.
INTRODUCTION
Daytime sleepiness (DS) is a well-described component of obstructive sleep apnea (OSA) in adults. However, DS is often less obvious in pediatric OSA and not well understood. A few studies have reported on sleepiness in children with OSA, noting a variable prevalence of sleepiness.1,2 As a treatment for OSA, continuous positive airway pressure therapy and adenotonsillectomy both have been shown in nonrandomized studies to reduce parent-reported sleepiness.3–6 However, randomized studies have not been performed.
It is also unknown whether subjective DS as measured through parent report is correlated with the severity of OSA measured by polysomnography (PSG). Prior research has not identified consistent associations between DS and PSG variables,1,5,7 possibly due to differences in assessment of DS and study of children across broad age ranges and disease severity. Furthermore, DS among some children may manifest as hyper-activity rather than overt sleepiness.8
The Childhood Adenotonsillectomy Trial (CHAT) is a multicenter randomized controlled trial evaluating the effects of early adenotonsillectomy (eAT) compared to watchful waiting and supportive care (WWSC) on a range of health and behavioral outcomes in children with OSA.9,10 A prior publication reporting the study's primary outcomes briefly reported improvement of DS after eAT.9 The current study is an in-depth analysis of two commonly used parent-reported sleepiness questionnaires: the modified Epworth Sleepiness Scale (mESS)1,11 and the Sleepiness Subscale from the Pediatric Sleep Questionnaire, Sleep-Related Breathing Disorder Scale (PSQ-SS)12,13 in CHAT participants. This study also includes analysis of the relationship of sleepiness measures to PSG measures. Additionally, we report the sleep duration from parent-completed 5-day sleep journals and its relationship to subjective sleepiness. We hypothesized that children who underwent eAT would show a larger improvement in sleepiness scores on the mESS and the PSQ-SS than children in the WWSC group and that improvement in sleepiness would correlate with improvement in sleep measures.
METHODS
The study was approved by the Institutional Review Board of each participating institution. Informed consent was obtained from caregivers, and assent from children 7 y of age or older. The design of the CHAT study has been previously described in detail.10
Participants
In brief, children ages 5.0–9.9 y were recruited from pediatric sleep centers, general pediatric clinics, and otolaryngology clinics. Inclusion criteria included parental report of snoring, PSG showing an obstructive apnea-hypopnea index (AHI) ≥ 2 events per hour of sleep or an obstructive apnea index ≥ 1 per hour, and an otolaryngology evaluation showing that the child was a candidate for adenotonsillectomy. Exclusion criteria included AHI > 30, obstructive apnea index > 20, oxygen saturation < 90% for ≥ 2% total sleep time, significant health problems, medication use for psychiatric disorders or attention deficit hyperactivity disorder, developmental delays requiring school accommodations, recurrent tonsillitis, body mass index (BMI) z-score ≥ 3, and any known genetic, craniofacial, or neurologic disorders likely to affect the airway, cognition, or behavior.
Children were randomized into two groups: eAT and WWSC, with an intervention period of 7 mo. Children underwent the following at baseline and endpoint: standardized evaluations with cognitive and behavioral assessments, including sleepiness questionnaires, 5-day sleep journals, and PSG. Parents provided socioeconomic information including completion of high school education and income less than $30,000.
Sleepiness Questionnaires
See appendix A and B in the supplemental material for items on questionnaires.
Modified Epworth Sleepiness Scale
The mESS includes eight presented situations. Parents are asked to answer how likely their child is to fall asleep in each of the presented situations: no chance (0 points); slight chance (1 point); moderate chance (2 points); or high chance (3 points). A total score (maximum score of 24) is calculated as a sum of points from the 8 answers. The mESS was introduced in 2004 in a study on sleepiness in children with OSA.1 Another version of the mESS was introduced in 2009.14 The difference between the ESS for adults and the mESS for children includes word changes in two of the situations: situation 3 has different examples, “classroom or movie theater” in place of “theater or a meeting” and situation 7 removes the words “without alcohol.” Situation 8, related to driving, assumes that the child is a passenger in a car. However, this context is very similar to situation 4 (“As a passenger in a car for 1 h without a break”). The original 8-item mESS was the primary version used in this study. Because of the similarity of situations 4 and 8, two additional questions on likelihood of dozing were asked in the CHAT study (“doing homework or taking a test”; and “playing a videogame”) to introduce other daytime experiences typically encountered in childhood. These additional questions were used to create two additional versions of the mESS, with a different eighth item (mESS9 and mESS10) (see supplemental material for these analyses). A cutoff of > 10 on the original ESS is commonly used in adults to indicate abnormal sleepiness; no widely accepted cutoff exists in children.
Pediatric Sleep Questionnaire-Sleepiness Subscale
The PSQ-Sleep-Related Breathing Disorders Scale includes a total of 22 questions on snoring, excessive daytime sleepiness, and inattentive/hyperactive behaviors.12,13 This scale was previously validated in children without and with sleep-disordered breathing and correctly classified 86.4% of children, with a sensitivity of 0.85 and specificity of 0.87 in one group, and correctly classified 85% of children, with a sensitivity of 0.81 and specificity of 0.87 in a second group of children. The Sleepiness Subscale (PSQ-SS) consists of four questions. The items ask about feeling unrefreshed in the morning, being hard to wake up in the morning, a problem with daytime sleepiness, and sleepiness observed by a teacher. Answers are assigned a score of 1 for “yes,” and 0 for “no.” An overall score is calculated as the total score divided by the number of non-missing responses other than “Don't Know.” A score higher than 0.33 is considered significant for sleepiness.
Sleep Duration
Parents completed a sleep journal for their child for 5 nights at each time point overlapping with the baseline and endpoint visits. Parents were asked to record on a daily basis what time their child went to bed, woke up, the number of awakenings, and the number of naps. Average sleep duration was calculated over the 5-night period for each child.
Polysomnography
Each child underwent a standardized in-laboratory overnight PSG at baseline and endpoint, performed in an American Academy of Sleep Medicine (AASM)-accredited sleep center, certified for all CHAT study procedures; this has been described in detail in previous papers.9,10 The PSGs were scored at a central Sleep Reading Center (Case Western Reserve University; Brigham and Women's Hospital) following pediatric scoring recommendations of the AASM15 by registered polysomnologists who were blinded to all clinical data. Hypopneas were scored as a ≥ 50% reduction in airflow accompanied by a cortical arousal or ≥ 3% desaturation. Interscorer and intra-scorer reliability for key PSG parameters exceeded intraclass correlation coefficients of 0.90.
Statistical Analysis
Descriptive statistics were calculated for the participants and for subgroups defined by age, sex, race (Caucasian, African American vs. Other), ethnicity (non-Hispanic vs. Hispanic), and weight status (overweight/obese vs. other). Differences in mESS and PSQ-SS for each characteristic were analyzed using independent samples t-tests or analyses of variance with Bonferroni post hoc tests when appropriate. Bivariate associations were assessed with the Pearson rank correlation. Changes from baseline to endpoint sleepiness scores were analyzed separately for the eAT group and the WWSC group using paired t-tests. The effect of the interaction of time and group was assessed on mESS and PSQ-SS with a two-way analysis of variance. Multiple linear regression analysis was performed to determine associations of baseline and endpoint mESS with age, sex, race, BMI z-score, AHI, and sleep duration (by sleep diary) for the entire sample. Multiple linear regression analysis was performed to determine associations of socioeconomic status in relation to sleepiness. All analyses were done using SAS version 9.4 (Cary, NC) and significance levels were set at P < 0.05.
RESULTS
Participants
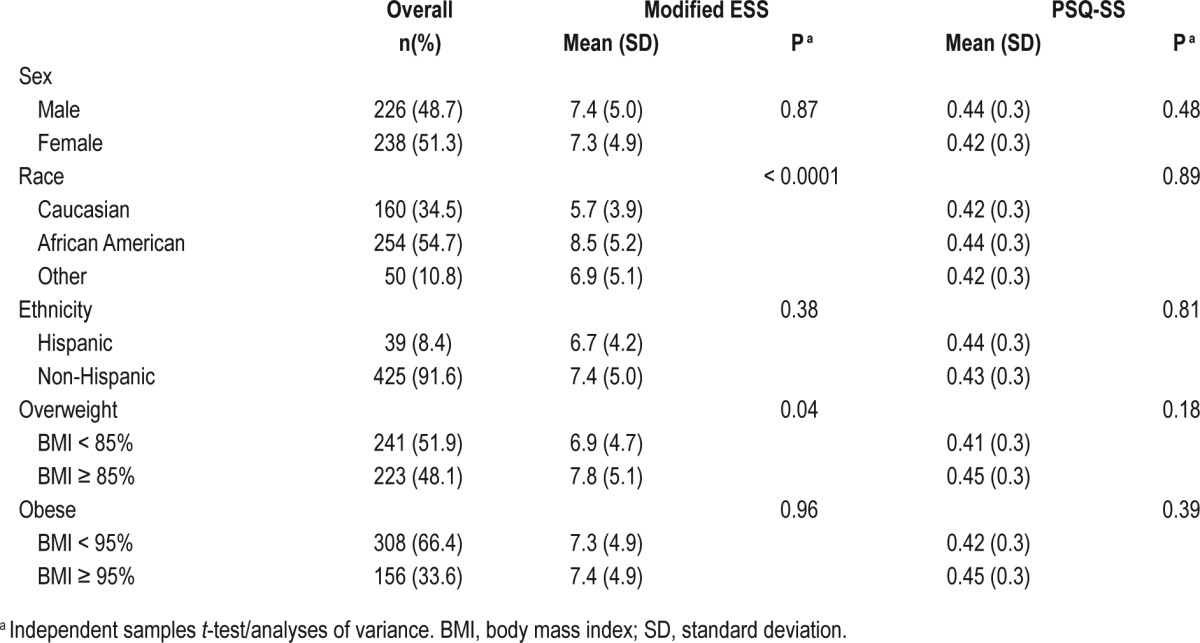
The mean ± standard deviation (SD) age of the group at baseline was 7.0 ± 1.4 y. At baseline, the mean AHI ± SD was 6.9 ± 5.7, with a median AHI (interquartile range) of 4.7 (2.7–8.8). African American children, in comparison to non-African American children, had an AHI of 7.6 ± 5.9 vs. 6.0 ± 5.3; P = 0.003). See Table 1 for a summary of baseline clinical characteristics and sleepiness questionnaires results.
Table 1.
Modified Epworth Sleepiness Scale and Pediatric Sleep Questionnaire-Sleepiness Scale scores by baseline characteristics.
Sleepiness Measures at Baseline
Modified Epworth Sleepiness Scale
At baseline, mean ± SD mESS score was 7.3 ± 4.9; 24.4% of children had a score of > 10 and 17 (3.2%) of children had a score of 0. African American children, in comparison to non-African American children, had a higher mean mESS score (8.5 ± 5.2 vs. 6.0 ± 4.2; P < 0.0001). No sex or ethnicity differences were observed. The mean mESS scores in under-weight/normal weight children were less than in overweight children (P < 0.05) (see Table 1). After adjusting for age, sex, race, ethnicity, and overweight status, the association of increased sleepiness with African American race remained a significant predictor of mESS (P < 0.0001 for African Americans compared to Caucasians). After adjusting for AHI, African American race remained a significant predictor of mESS (P < 0.001).
Pediatric Sleep Questionnaire-Sleepiness Subscale
At baseline, the mean ± SD baseline PSQ-SS composite score was 0.4 ± 0.3, with a range of 0–1, with higher values indicating increased sleepiness. Of 458 completed questionnaires, 246 (53%) had a composite score higher than 0.33. The PSQSS was statistically different by age (P = 0.01), with 5 y olds having a lower score than those 6 y and older. Five-y-old children, in comparison to children age 6 y or older, had a mean BMI z-score of 0.6 ± 1.3 vs. 1.0 ± 1.3 (P < 0.0001). There was no difference in AHI between the two groups, as 5-y-old children had a mean AHI of 6.9 ± 5.3 and children 6 y or older had a mean AHI of 6.9 ± 5.9 (P = 0.99). Sex, weight, race, and ethnicity were not significantly associated with the PSQ-SS score (see Table 1).
Change In Sleepiness Measures Over the Intervention Period
Modified Epworth Sleepiness Scale
Within the eAT group, the mean mESS improved from 7.2 ± 4.7 to 5.1 ± 4.4; P < 0.001. In contrast, within the WWSC group, the mean mESS did not significantly change (7.5 ± 5.2 to 7.1 ± 5.1; P = 0.36). Between the eAT and WWSC groups, the improvement in scores was significant (mean change in mESS was −2.0 ± 4.2 for the eAT group and −0.3 ± 4.0 for the WWSC group; P = 0.01 for the interaction of time and group) (see Figure 1). At endpoint, there were 76 of 464 (16.4%) children with mESS > 10. Of these, 22 of 210 (9.5%) had mESS > 10 in the eAT group, vs. 54 of 232 (23.3%) in the WWSC group (P < 0.0001).
Figure 1.
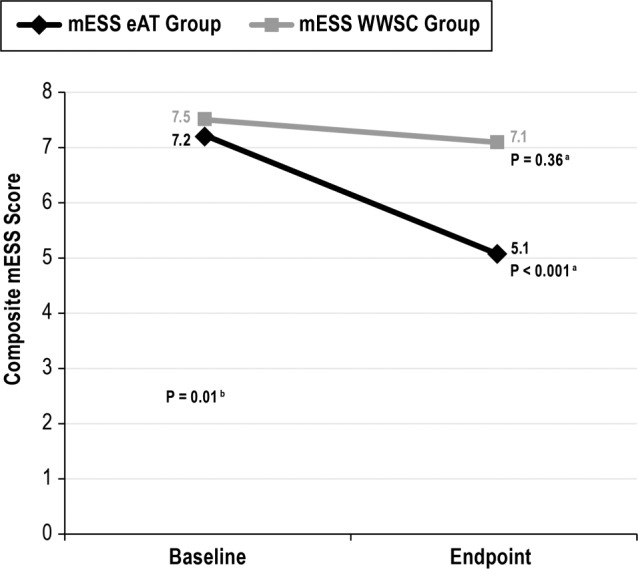
Change in modified Epworth Sleepiness Scale scores for early adenotonsillectomy and watchful waiting with supportive care groups. a Paired t-test. b Analysis of variance two-way interaction. There was a significant change in baseline and endpoint mESS scores in the eAT group. In addition, there was a significant difference in change between groups over time (P = 0.01; analysis of variance). eAT, early adenotonsillectomy group; mESS, modified Epworth Sleepiness Scale; WWSC, watchful waiting with supportive care group.
Pediatric Sleep Questionnaire-Sleepiness Subscale
Within the eAT group, the mean PSQ-SS improved from 0.42 ± 0.3 to 0.13 ± 0.2; P < 0.0001. Within the WWSC group, the mean PSQ-SS improved from 0.44 ± 0.3 to 0.36 ± 0.3; P = 0.002. Similar to mESS, a greater change was observed for the eAT group compared to the WWSC group (mean change in PSQ-SS was −0.3 ± 0.4 for the eAT group and −0.1 ± 0.4 for the WWSC group; P < 0.0001) (see Figure 2). At endpoint, there were 138 of 402 children (34.2%) with PSQ-SS > 0.33. Of those, 35 of 198 (17.7%) had PSQ > 0.33 in the eAT group, vs. 103 of 204 (50.4%) in the WWSC group (P = 0.0001).
Figure 2.
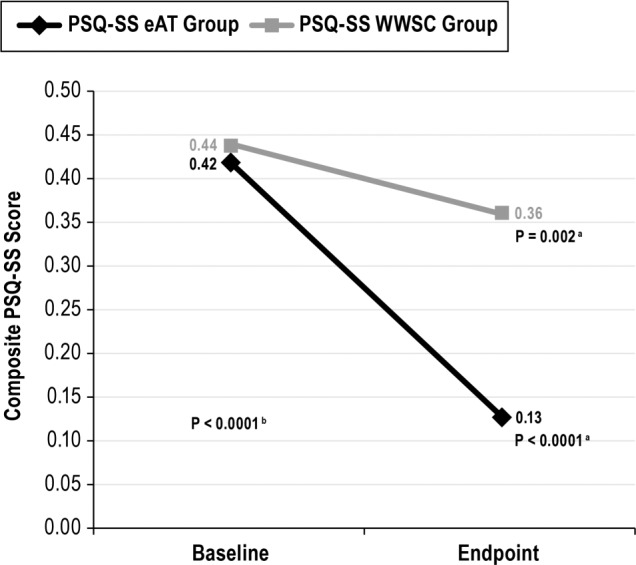
Change in Pediatric Sleep Questionnaire - Sleepiness Subscale scores for early adenotonsillectomy and watchful waiting with supportive care groups. a Paired t-test. b Analysis of variance two-way interaction. There was a significant change in baseline and endpoint modified Epworth Sleepiness Scale scores in the eAT group (n = 198) and in the WWSC group (n = 204). In addition, there was a significant difference in change between groups over time (P < 0.0001; analysis of variance). eAT, early adenotonsillectomy group; PSQ-SS, Pediatric Sleep Questionnaire - Sleepiness Subscale; WWSC, watchful waiting with supportive care group.
Associations between Sleepiness Measures and PSG Parameters
At baseline, a mESS score > 10 was found in 24.7% of children with an AHI of 2–4.9, 21% with an AHI of 5–10, and 27.0% with an AHI > 10. At baseline, a PSQ-SS score higher than 0.33 was found in 52% children with an AHI of 2–4.9, 52% with an AHI of 5–10, and 56% with an AHI > 10. These differences were not significant.
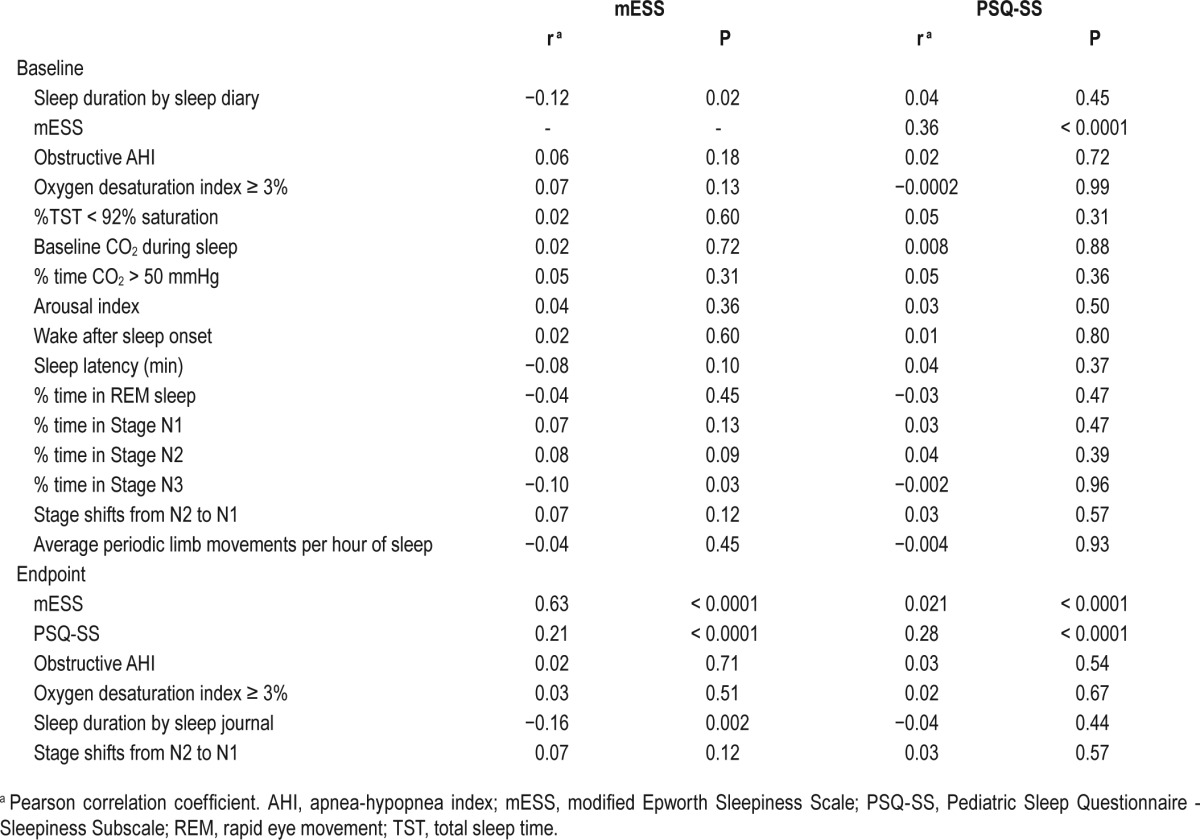
No significant associations were observed between baseline PSG parameters and baseline mESS or PSQ-SS scores other than a negative association between mESS and baseline stage N3 sleep (r = −0.10; P = 0.03) (see Table 2).
Table 2.
Correlations of selected sleep measures at baseline and endpoint with sleepiness scale scores at baseline.
A decrease in AHI over the intervention period was associated with improvement in sleepiness scores measured by both the mESS and PSQ-SS. Similar findings were observed for change in oxygen desaturation index ≥ 3% with change in mESS (see Table 3).
Table 3.
Correlation of changes in sleepiness scores with changes in selected polysomnography variables (over 6-mo intervention period).
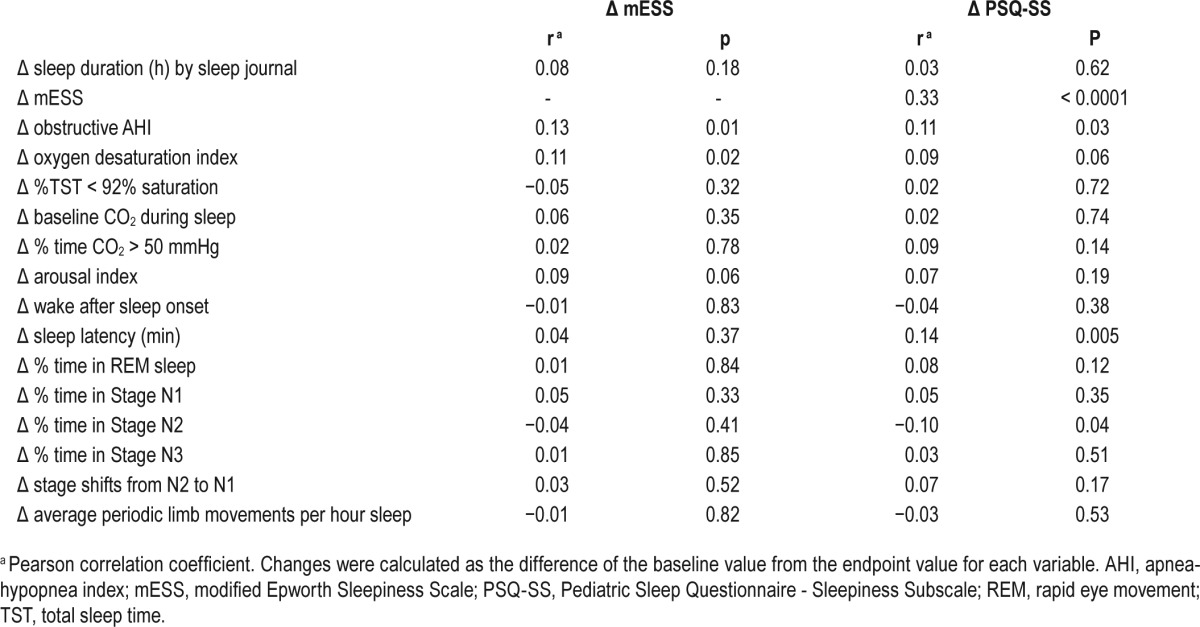
Among the 258 children whose AHI normalized to less than 2 at endpoint, regardless of study arm, a greater decrease in mean mESS score was observed as compared to the children whose PSG showed residual OSA (−1.5 ± 4.0 vs. −0.6 ± 4.6; P = 0.03, for combined group analysis). Similar results were observed for the mean PSQ-SS score (−0.18 ± 0.36 vs. −0.08 ± 0.4; P = 0.01).
Association between Sleepiness Measurements and Parent-Reported Sleep Duration by Sleep Journals
There were 376 sleep journals returned at baseline and 349 sleep journals returned at endpoint. To eliminate outliers, 8 sleep journals were excluded due to the average sleep duration being outside three SD of the mean. Children were reported to sleep an average duration of 9.48 ± 1.24 h at baseline and 9.46 ± 1.11 h at endpoint. Sleep duration at baseline was negatively and weakly correlated with a higher mESS score (r = −0.12, P = 0.02); however, it was not correlated with PSQSS (P = 0.45). Change in sleep duration over the intervention period was not associated with change in either sleepiness measurement.
Multivariable Modeling of Sleepiness
A linear regression model predicting mESS score at baseline from age, sex, race, BMI z-score, AHI, and sleep duration (by sleep diary) showed African American race was the only variable significantly associated with mESS (P < 0.0001) (see Table S1 in the supplemental material). Findings were similar for predicting mESS scores at endpoint (data not shown).
The role of socioeconomic status in relation to mESS showed higher caregiver income, African American race, and intervention arm of eAT predicted change in mESS scores; no interaction was observed between income and education (data not shown; all P ≤ 0.01).
DISCUSSION
This study extends findings of the CHAT study by further evaluating the prevalence of sleepiness and correlates of change in sleepiness to PSG parameters, sleep duration, and intervention effects at baseline and over the intervention period. In this sample of children with a range of OSA, who did not have prolonged oxyhemoglobin desaturation, and were otherwise healthy, we observed a high prevalence of sleepiness measured on two standardized questionnaires. Baseline sleepiness (measured by either the mESS or PSQ-SS) was higher in children who were older. Increased sleepiness, as measured by the mESS, also was associated with being overweight, short sleep duration, and African American race. However, after considering these factors, only African American race was significantly associated with sleepiness. Of the PSG measures, baseline mESS scores were negatively correlated with stage N3 sleep, but not with baseline AHI or other PSG parameters. Sleepiness, measured by either questionnaire, improved more with eAT than with WWSC. Improvement in sleepiness was associated weakly with improvement in AHI or oxygen desaturation index but not associated with change in other PSG measures or sleep duration. Furthermore, regardless of treatment arm, OSA resolution was associated with improved sleepiness.
Early literature has emphasized differences between childhood and adult OSA, including a rarity of complaints about sleepiness among children.2,16 However, inquiry about sleepiness more systematically, as in the current study and a previous report,5 suggests that the frequency of parental awareness about sleepiness in their children with OSA may be higher than is commonly realized.
The higher mESS scores among African American children (at both baseline and follow-up) are similar to previously reported findings in adults.17,18 It is unclear why African American children, compared to other children, had higher sleepiness scores on the mESS, but not on the PSQ-SS in our study, suggesting the questionnaires may assess sleepiness differently (PSQ-SS addresses morning sleepiness in addition to daytime sleepiness, and the mESS assesses daytime situational sleepiness) or that the questions may be interpreted differently. A prior study found that AA children nap more, but have less nocturnal sleep than non-African American children, with the same total amount (diurnal plus nocturnal) of sleep19; thus, it is possible the timing of sleep may affect parental interpretation of sleepiness. Alternatively, African American children may experience more evident sleepiness in reference to specific situations. Furthermore, as we reported before, African American children, compared to other children, have a higher baseline AHI, lower rates of normalization of PSG findings, and less relative improvement in caregiver-reported measures of behavior.9 When age, sex, race, BMI z-score, AHI, and sleep duration were jointly analyzed in a linear regression, only African American race predicted higher sleepiness scores. There are a number of possible reasons for increased sleepiness among African American children that may relate to OSA severity, usual patterns of diurnal vs. nocturnal sleep, and parents' expectations or perceptions of sleepiness and behavior. Given the importance of sleepiness on attention and cognition, further research is needed to elucidate the specific contributors to sleepiness in children.
Other correlates of baseline sleepiness included shorter sleep duration and overweight status. Sleep duration is a well-recognized determinant of daytime alertness and function in children.20 In comparison, the relationship between pediatric overweight/obesity and sleepiness is not as well understood.21 Research from adults suggests that obesity, independent from OSA, is associated with sleepiness,22 and that visceral fat and release of cytokines may contribute to sleepiness.23,24 While acknowledging the importance of treating OSA, our findings also highlight the importance of sleep duration and healthy weight.
The predictors of change in sleepiness with interventions in children are not well understood. The significant improvement in sleepiness in children receiving eAT compared to watchful waiting provides further support for interventions aimed at improving sleep-disordered breathing. Although African American children had higher mESS scores, the relative decrease in mESS scores following adenotonsillectomy was similar between African American and other children, thus supporting the use of adenotonsillectomy for treatment of OSA in both groups of children.
The generally poor correlation among objective measurements of sleep recorded by PSG and subjective sleepiness we observed parallel other research showing that subjective sleepiness is not strongly correlated with objective measurements of sleepiness in children5 or adults.25 These findings indicate the importance of evaluating for sleepiness during the clinical evaluation. If sleepiness is of concern for the child, treatment even in situations of low AHI may result in substantial improvement of daytime functioning of the child. Of interest, a weak association was observed at baseline between an increase in stage N3 sleep and lower score on the mESS, which is consistent with evidence that stage N3 sleep is “restorative.”26 Sleepiness as determined by multiple sleep latency tests, as opposed to subjective measures, may be more sensitive to pediatric OSA severity as reflected by standard polysomnographic measures2,5 or esophageal pressure monitoring.27 These results highlight the complexity of existing constructs for sleepiness, its measures, and its causes, all of which still remain poorly understood.
A challenge in the use of subjective measurements of sleepiness also is the absence of a widely agreed upon “cutoff” score on the mESS to indicate excessive daytime sleepiness in children. Using a value > 10, as is used in adults, 24.4% of the children in our sample were identified as having excessive daytime sleepiness. A lower pediatric cutoff may be appropriate,1 but further age-specific research is required to establish this. In contrast to the mESS, use of a PSQ-SS cutoff > 0.33 (answering two or more of four sleepiness questions) classified 53% of our sample with excessive daytime sleepiness. We also observed that the mESS and PSQ-SS were only modestly correlated with each other (rho = 0.36; P < 0.0001), and each correlated somewhat differently with other measures. The lack of closer correlation, however, is not surprising given previous observations that the manner in which subjective sleepiness is assessed can have strong influence on the answers. For example, among adults evaluated for OSA, scores on the ESS and answers to the simple question “How great a problem do you have with sleepiness (feeling sleepy, or struggling to stay awake) in the daytime?” also were only moderately associated (rho = 0.49).28 Further research is needed to better understand how to best assess sleepiness in children, considering issues of age, development, and parent-reporting.
Study strengths included a large sample, wide geographic and racial diversity, and use of standardized methods to assess baseline and follow-up measures. The study was limited by lack of a control group of children without OSA, and absence of objective measurements of sleepiness and daily sleep patterns.
CONCLUSIONS
In this randomized controlled trial of adenotonsillectomy for the treatment of pediatric OSA, we observed a significant improvement in parental reports of sleepiness on two easily administered sleepiness questionnaires, the mESS and the PSQ-SS, in children who underwent adenotonsillectomy compared to those who underwent watchful waiting with supportive care. Although African American children with OSA had higher sleepiness scores at baseline and endpoint on the mESS, their sleepiness scores improved with adenotonsillectomy intervention, similar to those of non-African American children. As sleepiness may have a profound effect on academic performance, sports performance, and personal relationships, these findings may be informative to parents of sleepy children with OSA who present for evaluation and treatment. Sleepiness was not well predicted by level of AHI or other clinical or physiological parameters and suggests the importance of evaluating sleepiness even in situations of a low AHI.
DISCLOSURE STATEMENT
Financial support for the study was provided by National Institutes of Health: UO1 HL83075, UL1RR024134, U54 RR023567. Respironics provided Novametrix capnographs for use in this study. There was no off-label or investigational use of products. Dr. Rosen has consulted for Jazz Pharmaceuticals, Advance-Medical, and Natus Medical. Dr. Redline reports that the study received equipment from Respironics for use in this trial. She also reports that Brigham and Women's Hospital received research grant support from ResMed Foundation and research equipment (unrelated to this study) from Philips-Respironics and ResMed Inc. The authors have indicated no financial conflicts of interest. The work was performed at the following 7 locations: University of Pennsylvania, Children's Hospital of Philadelphia, Philadelphia, PA; University of Cincinnati, Cincinnati Children's Hospital, Cincinnati, OH; Case Western Reserve University, University Hospitals, Cleveland, OH; University of Louisville, Kosair Children's Hospital, Louisville, KY; Saint Louis University, Cardinal Glennon Children's Hospital, St. Louis, MO; Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, NY; and Boston Children's Hospital, Harvard Medical School, Boston, MA.
ACKNOWLEDGMENTS
The CHAT gratefully acknowledges the superb support of the CHAT research staff: Jean Arnold, Mary Ellen Carroll, Mary Anne Cornaglia, Beth Ann Compton, Judith Emancipator, Melissa Fernando, Amanda Goodman, Xiaoling Hou, Elise Hodges, Laurie Karamessinis, Kim Lacy, Megan McDougall, Daniel Mobley, Michelle Nicholson, Deborah L. Ruzicka, Gauri Sathe, Nancy Scott, Susan Surovec, Omarya Vega, Xingmei Wang, Catherine Williams, Vikki Kociela, Theresa Friederich, Angela Orlando, Casey Critchlow, Ted Otto, Jennifer Griggs, Helen Moore, Charles Bright, Tim Soberg, Teresa Soberg, Michelle Perry, Amy Parker, Bonnie Bahr, Diane Roth, Bonnie Kallaos, Alicia McGlaughlin, Tara Albert, Alan Wild, John Stith, Tom Sanford, Anthony Mikulec, Jessica Luitjohan, Karen Snyder, and Laura Misuraca. We also appreciate the generous participation of the families enrolled in the study. We are grateful for the helpful guidance during the study of the CHAT Data and Safety Monitoring Board: Lynn Taussig, MD (Chair); Thomas Anders, MD; Julie Buring, ScD; Karina Davidson, PhD; Estelle Gauda, MD; Steven Piantadosi, MD, PhD; Bennett Shaywitz, MD; Benjamin Wilfond, MD; Tucker Woodson, MD; Robert Zeiger, MD.
REFERENCES
- 1.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 2.Gozal D, Wang M, Pope DW. Objective sleepiness measures in pediatric obstructive sleep apnoea. Pediatrics. 2001;108:693–7. doi: 10.1542/peds.108.3.693. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–51. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 4.Marcus CL, Radcliff J, Konstantinopoulou S, et al. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:998–1003. doi: 10.1164/rccm.201112-2167OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chervin RD, Weatherly RA, Ruzicka DL, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs other surgical care. Sleep. 2006;29:495–503. [PMC free article] [PubMed] [Google Scholar]
- 6.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:e769–78. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan EY, Ng DK, Chan C, et al. Modified Epworth Sleepiness Scale in Chinese children with obstructive sleep apnea: a retrospective study. Sleep Breath. 2009;13:59–63. doi: 10.1007/s11325-008-0205-7. [DOI] [PubMed] [Google Scholar]
- 8.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20:1185–92. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL, Moore RH, Rosen CL, et al. Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline S, Amin R, Beebe D, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34:1509–17. doi: 10.5665/sleep.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 12.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 13.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133:216–22. doi: 10.1001/archotol.133.3.216. [DOI] [PubMed] [Google Scholar]
- 14.Anderson B, Storfer-Isser A, Taylor HG, Rosen CL, Redline S. Association of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2009;123:e701–7. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and rechnical specifications, 1st ed. [Google Scholar]
- 16.Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108:610–8. doi: 10.1378/chest.108.3.610. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–84. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes AL, Spilsbury JC, Patel SR. The Epworth score in African American populations. J Clin Sleep Med. 2009;5:344–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8- year-old children. Pediatrics. 2005;115(1 Suppl):225–32. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber R, Cassoff J, Frenette S, Wiebe S, Carrier J. Impact of sleep extension and restriction on children's emotional lability and impulsivity. Pediatrics. 2012;130:e1155–61. doi: 10.1542/peds.2012-0564. [DOI] [PubMed] [Google Scholar]
- 21.Bonuck K, Chervin RD, Howe LD. Sleep-disordered breathing, sleep duration, and childhood overweight: a longitudinal cohort study. J Pediatr. 2015;166:632–9. doi: 10.1016/j.jpeds.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slater G, Pengo MF, Kosky C, Steier J. Obesity as an independent predictor of subjective excessive daytime sleepiness. Respir Med. 2013;107:305–9. doi: 10.1016/j.rmed.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Arnardottir ES, Maislin G, Schwab RJ, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep. 2012;35:921–32. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 25.Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology. 1999;52:125–31. doi: 10.1212/wnl.52.1.125. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel R, Köberle S, Allen SR. Significance of slow wave sleep: considerations from a clinical viewpoint. Sleep. 1986;9:66–79. doi: 10.1093/sleep/9.1.66. [DOI] [PubMed] [Google Scholar]
- 27.Chervin RD, Ruzicka DL, Hoban TF, et al. Esophageal pressures, polysomnography, and neurobehavioral outcomes of adenotonsillectomy in children. Chest. 2012;142:101–10. doi: 10.1378/chest.11-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chervin RD, Aldrich MS, Pickett R, Guilleminault C. Comparison of the results of the Epworth Sleepiness Scale and the Multiple Sleep Latency Test. J Psychosomatic Research. 1997;42:145–55. doi: 10.1016/s0022-3999(96)00239-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


