Abstract
Study Objectives:
Sleep is important for consolidation of hippocampus-dependent memories. It is hypothesized that the temporal sequence of nonrapid eye movement (NREM) sleep and rapid eye movement (REM) sleep is critical for the weakening of nonadaptive memories and the subsequent transfer of memories temporarily stored in the hippocampus to more permanent memories in the neocortex. A great body of evidence supporting this hypothesis relies on behavioral, pharmacological, neural, and/or genetic manipulations that induce sleep deprivation or stage-specific sleep deprivation.
Methods:
We exploit an experimental model of circadian desynchrony in which intact animals are not deprived of any sleep stage but show fragmentation of REM and NREM sleep within nonfragmented sleep bouts. We test the hypothesis that the shortening of NREM and REM sleep durations post-training will impair memory consolidation irrespective of total sleep duration.
Results:
When circadian-desynchronized animals are trained in a hippocampus-dependent contextual fear-conditioning task they show normal short-term memory but impaired long-term memory consolidation. This impairment in memory consolidation is positively associated with the post-training fragmentation of REM and NREM sleep but is not significantly associated with the fragmentation of total sleep or the total amount of delta activity. We also show that the sleep stage fragmentation resulting from circadian desynchrony has no effect on hippocampus-dependent spatial memory and no effect on hippocampus-independent cued fear-conditioning memory.
Conclusions:
Our findings in an intact animal model, in which sleep deprivation is not a confounding factor, support the hypothesis that the stereotypic sequence and duration of sleep stages play a specific role in long-term hippocampus-dependent fear memory consolidation.
Citation:
Lee ML, Katsuyama AM, Duge LS, Sriram C, Krushelnytskyy M, Kim JJ, de la Iglesia HO. Fragmentation of rapid eye movement and nonrapid eye movement sleep without total sleep loss impairs hippocampus-dependent fear memory consolidation. SLEEP 2016;39(11):2021–2031.
Keywords: circadian desynchrony, REM sleep fragmentation, NREM sleep fragmentation, memory consolidation
Significance.
The stereotypic sequence and duration of sleep stages—nonrapid eye movement (NREM) sleep and rapid eye movement (NREM) sleep—is believed to have a critical role in the consolidation of hippocampus-dependent memory. A large body of evidence supporting this hypothesis relies on experiments in which the duration of sleep stages is manipulated pharmacologically, genetically, through neural lesions, or by sleep deprivation. We exploit an animal model in which NREM and REM sleep are fragmented without fragmentation or loss of total sleep. Under these conditions, consolidation of long-term hippocampus-dependent fear memory is impaired and the impairment is associated with the fragmentation of NREM and REM sleep.
INTRODUCTION
Sleep is important for memory consolidation. Both total sleep and stage-specific sleep deprivation impair long-term memory consolidation, and recent evidence points toward a critical role of specific brain activity patterns during sleep in the consolidation of memory. Consolidation is believed to rely on the transfer of information from the initial encoding network to a new more stable memory network. The information transfer takes place when the network is not encoding new stimuli. Although this “transfer” of the memory trace could potentially take place during wakefulness, experimental evidence suggests that sleep represents a unique, ideal “off-line” state in which consolidation can occur.1–3
The temporal stereotypic sequence of the two basic sleep stages—nonrapid eye movement (NREM) sleep and rapid eye movement (REM) sleep—during the sleep cycle appears to be critical for proper memory consolidation.3 The sleep-stage sequential hypothesis proposes that during NREM sleep, particularly during slow wave sleep, nonadaptive memories are weakened and adaptive memories become stronger. Additionally, during REM sleep these adaptive memories are stored as more stable traces in the neocortex, where they can be integrated with preexisting memory networks. The sequential hypothesis has received further support by growing evidence of repeated reactivation or “replay” of neuronal activity patterns that represent newly encoded hippocampus-dependent memories during NREM and REM sleep.4 Together, this evidence has led to a new hypothesis for the role of sleep in memory consolidation, the “active system consolidation hypothesis.” According to this model, reactivations that occur during NREM sleep's slow waves mediate the distribution of memories temporarily stored in the hippocampus to more stable storage sites in the neocortex. Subsequently, during REM sleep, the reactivation in longer-term storage sites would lead to stable synaptic consolidation.
The sequential and active system consolidation hypotheses are supported by studies that have found a strong correlation between memory consolidation and the post-training duration of both NREM and REM sleep, as well as by studies that induce stage-specific sleep deprivation to impair consolidation. A key prediction of the active sleep consolidation hypothesis is that the experimental shortening of the duration of NREM and/or REM sleep bouts, independently of total sleep or stage-specific sleep duration, will impair memory consolidation. Shortening sleep-stage bouts without restricting total sleep duration is experimentally challenging, and this prediction has yet to be tested.
We use a rat model of circadian desynchrony that leads to predictable and stable disruption of temporal sleep-stage sequence without net losses in total sleep duration. The exposure of rats to an artificially short 22-h light-dark (LD) cycle (LD22) leads to internal desynchrony of circadian rhythms, including desynchrony of NREM and REM sleep timing. Here we show that despite of the desynchronization of sleep stages in LD22 animals, the amounts of wakefulness, total sleep, NREM sleep, and REM sleep are similar to those in animals under a 24-h LD cycle (LD24). When LD22 animals are trained in a hippocampus-dependent contextual-fear conditioning paradigm during days of normal sleep architecture, they show similar memory consolidation to LD24 animals. Instead, when LD22 animals are trained during days of disrupted sleep architecture, their memory consolidation is impaired. Analysis of sleep architecture in contextual fear-trained animals shows that although the post-training bout lengths of total sleep and wakefulness cannot predict memory consolidation, longer post-training NREM and REM sleep bout lengths are associated with stronger long-term memory consolidation. Our results suggest that the duration and timing of NREM and REM sleep—rather than that of total sleep per se—sustain a memory consolidation function, providing support for the sequential and active system consolidation hypotheses.
METHODS
Animals and Housing Conditions
Male Wistar rats, 2-mo old on arrival, were purchased from Charles River (Raleigh, NC) and individually housed in transparent polycarbonate cages (20 × 25 × 22 cm) fitted with infrared beam detectors. Light periods consisted of cool white light (50–150 lux) and dark periods of dim red light (1 lux). LD24 rats were housed in an LD cycle consisting of 12 h of light followed by a 12 h of darkness. LD22 rats were maintained under a LD cycle of 11 h of light and 11 h of darkness. After ∼15 days under LD22 conditions, desynchronization was confirmed by the appearance of two statistically significant rhythmic components of locomotor activity as determined by periodogram analysis (see next section).
Learning Paradigms
Contextual Fear Conditioning
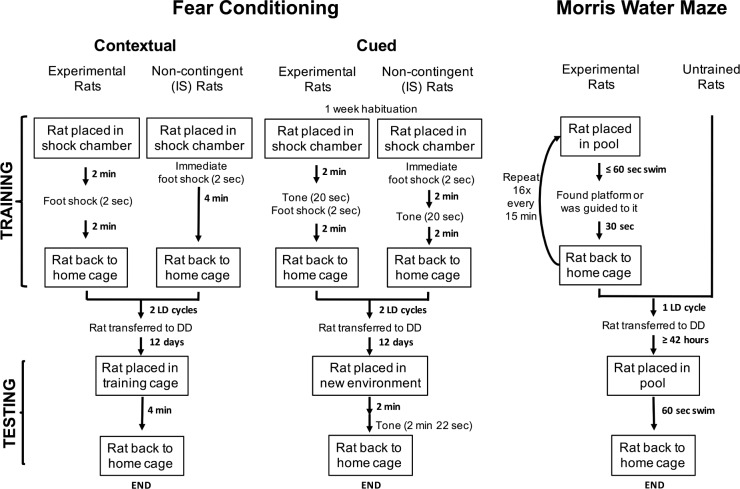
LD24 animals were handled daily during the dark phase and LD22 animals were handled daily close to lights onset or offset. Handling animals during or close to their subjective night is less likely to induce circadian phase shifts. Animals were trained and tested in dim red light conditions. Rats were trained on a contextual fear-conditioning task by placing them into a conditioning cage (Shock floor, Coulbourn Instruments, Allentown, PA) under dim red light in which they were allowed to explore (Figure 1). After 2 min, a 2-sec, 2.5-mA foot shock was delivered. Animals were then allowed to recover for 2 min and returned to home cages. Noncontingent (immediate-shock) control animals received a foot shock immediately after being placed into the conditioning cage and they were allowed to recover for 4 min before being returned to the home cage. These animals cannot associate the context with the aversive stimulus. Animals remained in their respective housing conditions (LD24 or LD22) for 2 days and then were transferred to constant dim red light for approximately 12 days; this period assures the resynchronization of circadian rhythms, which occurs within one to two cycles5 and allowed the test trial to be performed under the same circadian conditions and phase for all groups. To test memory consolidation, animals were placed in the conditioning chamber for 4 min under dim red light without delivering a shock. Each animal was tested only once for long-term memory to avoid the confounding effect of memory extinction. During training and testing, videos were recorded with an infrared Sony camcorder. Freezing behavior, defined as cessation of all but respiratory movement, was scored offline by at least two investigators blind to the training history of individual animals. Data were quantified and presented as percentage of time spent in freezing behavior.
Figure 1.
Schematic representation of the training and testing protocols used in the preset study. See Methods for a detailed description of each paradigm.
Cued Fear Conditioning
Animals were handled daily as for contextual fear conditioning. Habituation to the training chamber was done during the light phase three times over the course of 1 w prior to training (Figure 1). Training took place in the middle of the light phase and testing in the middle the subjective day. Rats were trained by placement in a conditioning cage pretreated with 5% ammonium 5% hydroxide under 50–75-lux light intensity. After 2 min a 20-sec tone was applied, followed by a 2-sec, 1-mA foot shock. Rats were allowed to recover for 2 min prior to being returned to the home cage. Noncontingent (immediate-shock) control animals were treated similarly, except that the foot shock was applied immediately upon their placement in the conditioning cage. These control animals were then allowed to explore the cage for 2 min, after which they were exposed to a 20 sec tone and allowed to explore the cage again for 2 min. These noncontingent animals cannot associate the cue (tone) with the aversive stimulus. After a single training trial, animals were kept in their respective housing conditions (LD24 or LD22) for 2 days and then released in constant dim red light conditions for 12 days prior to testing. Testing was done in a different environment from training to discard interference of the context on the results. A different chamber was kept under dim red light, with a conventional rat cage treated with 1% acetic acid. Rats were placed in testing cage and allowed to explore for 2 min. A constant tone was applied throughout the remaining of the test (for 2 min and 22 sec). The recording of videos and the freezing analysis were done similarly to the contextual fear-conditioning experiment.
Morris Water Maze
A 350-gallon circular pool (175-cm diameter, 43-cm depth), located in a separate room from the housing chamber, was surrounded by visual cues and under a light intensity of less than 50 lux. During training, an underwater hidden platform was placed in one of the quadrants in the pool. The training sessions lasted 5 h and were centered in the middle of the light phase, with a maximum of 8 animals per session (Figure 1). Each rat underwent 16 training trials with 15-min intertrial intervals, which has been shown to induce effective spatial orientation learning.6 Training trials consisted of placing the rat in the water facing the wall of the pool at semirandom positions. If the animal did not find the platform after 60 sec, the experimenter guided the animal to it. The rat was allowed to be in the platform for 30 sec to look at visual cues. Swimming patterns were recorded with a Panasonic camera mounted in the ceiling using the Pinnacle Studio Movie Box video capture system. After training, animals were kept in one extra LD cycle (either 22 h or 24 h long depending on the group) and then released in constant dim red light for at least 42 h. Untrained animals were kept in LD24 cycle during approximately same amount of time and also released in constant dim red light for at least 42 h prior to the probe test. Data collection for untrained animals took place in two different batches, 2 animals for the first set and 10 animals for the second set, for a total of 12 animals combined. The probe test sessions were centered in the middle of the subjective day and took place under the same light intensity as the training. During testing, the platform was removed and the rat's swimming patterns were recorded for 60 sec. The analysis of trajectories, crossings at the platform location, swim distances and latency to reach the platform location were performed using the MatLab (Mathworks, Natick, MA) image processing toolbox and the MouseMove (Charles Kopec, Carlos Brody laboratory, http://brodywiki.princeton.edu//wiki/index.php/MouseMove) tracking software package.
Electrocorticographic Recordings
Sleep in rats was recorded by implanting electrocorticographic (ECoG) electrodes, which enable the recording of electrical activity from the cerebral cortex. Briefly, rats were anesthetized using isoflurane. ECoG electrodes were placed over the frontal and parietal cortices and the leads from the ECoG electrodes were routed to a Teflon pedestal attached to the skull with dental cement. After 5 days of recovery, the ECoG electrodes were connected to an amplifier through a wire attached to a swivel. ECoG signals (128-Hz sampling rate) were amplified, passed through filters, digitized, and recorded in 10-sec bins. Electromyographic (EMG) recording electrodes were implanted into the dorsal neck muscles to enhance the accuracy of sleep stage scoring. Low EMG activity is characteristic of sleep periods.
Sleep Scoring
The vigilance states of wakefulness, NREM sleep, and REM sleep were determined offline in 10-sec epochs by the same operator, masked to the light/dark cycle and circadian phase at which the recording was taken. Reliability of the operator in sleep scoring was determined by scoring several hours of sleep and comparing this scores to those of researchers with experience in sleep scoring, with a ≥ 90% agreement. Wakefulness was characterized by fast low-amplitude ECoG waves in coincidence with high EMG-monitored muscle activity. NREM sleep was associated with slow high-amplitude ECoG waves and low muscle activity. In contrast, REM sleep is characterized by fast low-amplitude ECoG waves, appearance of theta ECoG (visualized through a fast Fourier transform), and muscle paralysis. The percentage of time spent in each stage was calculated for every 10 min. Bins were categorized as REM sleep if more than 20% of the bin was occupied by REM sleep. If the bin contained less than 20% REM sleep, it was defined as the stage that occupied the most time. Delta activity was calculated as the product of the percent of delta power (0.5–4 Hz) by the duration of NREM sleep within a 10-min bin.
Data Analysis and Statistics
Locomotor activity was recorded at a frequency of once per minute, and then binned at 10-min intervals. The Sokolvove and Bushell χ2 periodogram7 was used to estimate the period of statistically significant oscillations in the circadian range. LD22 animals that did not exhibit two significant periods were excluded from the study. Statistical analysis was performed by either one- or two-way analysis of variance (ANOVA) followed by post hoc comparisons when appropriate. Dunnett comparisons were used to compare against immediate-shock animals (nonlearning controls). Tukey comparisons were used to compare groups against each other, and Sidak contrasts for planned comparisons. In every case, results are considered significant with an α of 0.05. When there was no homogeneity of variance, data were transformed to Log (X + 1), because in the only case this was necessary the data contained zeroes. Correlation analysis was executed using Spearman correlation analysis. All statistical analysis was performed using the Prism 6.0d software (GraphPad Software, La Jolla, CA).
Exponential coefficients for individual survival curves of each sleep stage (1-min bins) were calculated using JMP 11.0.0 (SAS Institute, Cary, NC), from the least-squares best fitting curve according to the function:
where X(t) is the estimated survival for each sleep stage duration (in 10-sec steps), X0 is the survival for the shortest duration (10 sec, with a survival of 100%), t is the step number (1 for an interval of 10 sec, 2 for an interval of 20 sec, and so on) and a is the exponential coefficient for the best fit curve. This exponential coefficient was used as a direct measure of how transient (or fragmented) a sleep stage was.
RESULTS
Forced Desynchronized Rats Show Abnormal Timing of REM and NREM Sleep without Sleep Deprivation
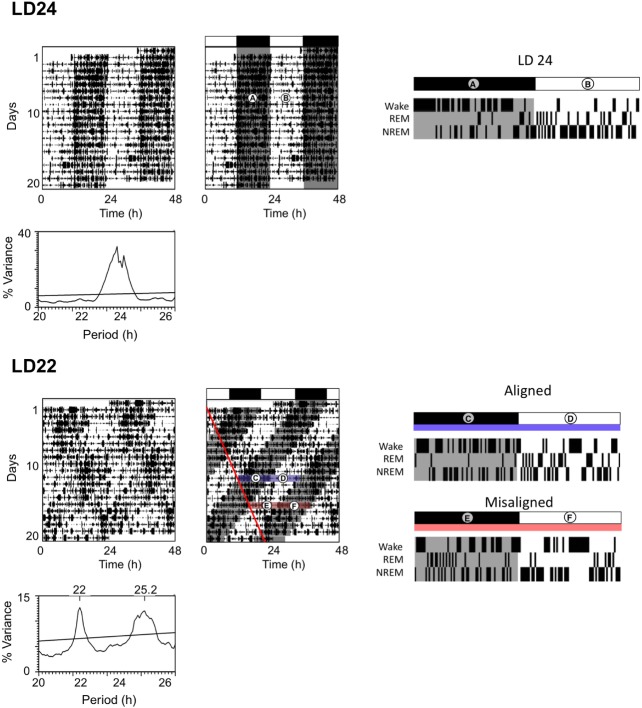
When rats are housed under LD24 loco-motor activity occurs primarily in the dark phase, as expected for a nocturnal animal (Figure 2A). Similarly, ECoG scoring of vigilance states shows wakefulness primarily during the dark phase (Figure 2A), and REM and NREM sleep stages primarily during the light phase (Figure 2B). Under LD22 forced desynchrony, some rhythms within the same animal are en-trained to the 22-h LD cycle and others are dissociated from the 22-h LD cycle, oscillating with a ∼25-h period; locomotor activity exhibits both periodicities (Figure 2). During the LD22 “aligned” phases (Figure 2C and 2D), the activity bouts from each rhythm overlap and wakefulness and sleep bouts have a similar temporal distribution as in an LD24 animal. In contrast, during LD22 “misaligned” phases (Figure 2E and 2F), the activity bout of the 22-h rhythm overlaps with the rest bout of the ∼25-h rhythm and vice versa. Analysis of ECoG sleep recordings in these animals reveals that whereas NREM sleep is temporally organized according to both periodicities, REMS only exhibits a ∼25-h rhythm.8,9 The different rhythmicity of each sleep stage leads to a cycle that alternates between days in which sleep architecture (the temporal distribution of NREM and REM sleep) is similar to that of LD24 rats (aligned days, Figure 2C and 2D), and days in which wakefulness does not occur primarily during the dark phase and REM predominates in the dark phase (misaligned days, Figure 2E and 2F).
Figure 2.
Sleep under LD22 forced desynchrony. Left panel, Representative locomotor activity records of rats housed under LD24 and LD22 conditions. Locomotor activity (infrared beam interruptions) is plotted as black marks for each 48-h period (double-plotted actogram), with days inserted vertically. The same actogram is shown twice. On the right actogram, white and black horizontal bars indicate light and dark phases of the LD cycle, respectively. Gray shading indicates the times of darkness on successive days. Red line in right LD22 actogram marks the onset of the locomotor activity rhythm that is not entrained by the LD cycle. Periodogram analysis (below each actogram) reveals that the only statistically significant periodicity in the LD24 animal corresponds to a period of 24 h, whereas two periodicities are statistically significant in the LD22 animal (periods indicated on top of periodogram). Y-axes on periodogram represent the % variance explained by oscillations with each specific period. Right panel, Hypnograms indicate vigilance states in 10-min bins for a 24-h period in LD24 animals and for a 22-h period in LD22 animals. Bars above hypnograms represent the LD schedule and gray shaded areas within the plots represent dark phases. For the LD24 hypnogram, (A) represents the light phase and the (B) the dark phase. For the LD22 hymnograms, blue horizontal overlay bar represents aligned phase—(C) dark aligned phase and (D) light aligned phase—and red horizontal overlay bar represents misaligned phase—(E) dark misaligned phase and (F) light misaligned phase. LD, light-dark.
Forced Desynchronized Rats Show No Deficits in Total Sleep, NREM Sleep, or REM Sleep, but Show NREM and REM Sleep Fragmentation
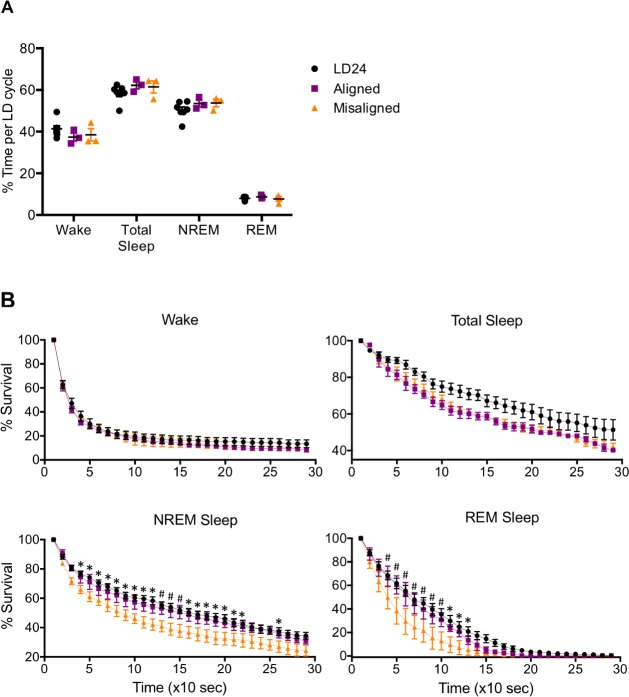
Desynchrony between sleep stages observed in LD22 rats is the result of the differential regulation of NREM and REM sleep by the homeostatic and circadian regulatory processes, respectively.8–10 We tested whether this abnormal timing of sleep stages leads to deficits in total sleep or in specific sleep stages. We calculated the percent of sleep throughout: (1) a 24-h period in LD24-housed animals, (2) a 22-h period in LD22-housed animals during aligned phases, or (3) a 22-h period in LD22-housed animals during misaligned phases. Figure 3A shows that LD22 animals display no deficits in either total sleep or specific sleep stages when compared to LD24 animals (one-way ANOVA: wake [F(2,10) = 1.17, P = 0.35]; total sleep [F(2,10) = 1.25, P = 0.33]; NREM sleep [F(2,10) = 1.34, P = 0.31]; REM sleep [F (2,10) = 0.63, P = 0.55]), indicating that the force-desynchronized protocol does not induce deprivation of total sleep or specific sleep stages.
Figure 3.
LD22 forced desynchrony induces nonrapid eye movement (NREM) and rapid eye movement (REM) sleep fragmentation without fragmentation of total sleep or deprivation of any specific sleep stage. (A) Percentage of time spent in each sleep stage in LD24 control animals, LD22 aligned animals or LD22 misaligned animals. One-way analysis of variance: Wake (F(2,10) = 1.17, P = 0.35); total sleep (F(2,10) = 1.25, P = 0.33); NREM sleep (F(2,10) = 1.34, P = 0.31); REM sleep (F(2,10) = 0.63, P = 0.55). (B) Survival analysis of wake, total sleep, NREM sleep, and REM sleep. See Table 1 for two-way analysis of variance results. When the effect of group or the interaction was significant, Tukey multiple comparison tests were done (P = 0.05): *LD22 misaligned differs from LD24; #LD22 misaligned differs from both LD22 aligned and LD24. n = 7 (LD24); n = 3 (LD22 aligned and misaligned). LD, light-dark.
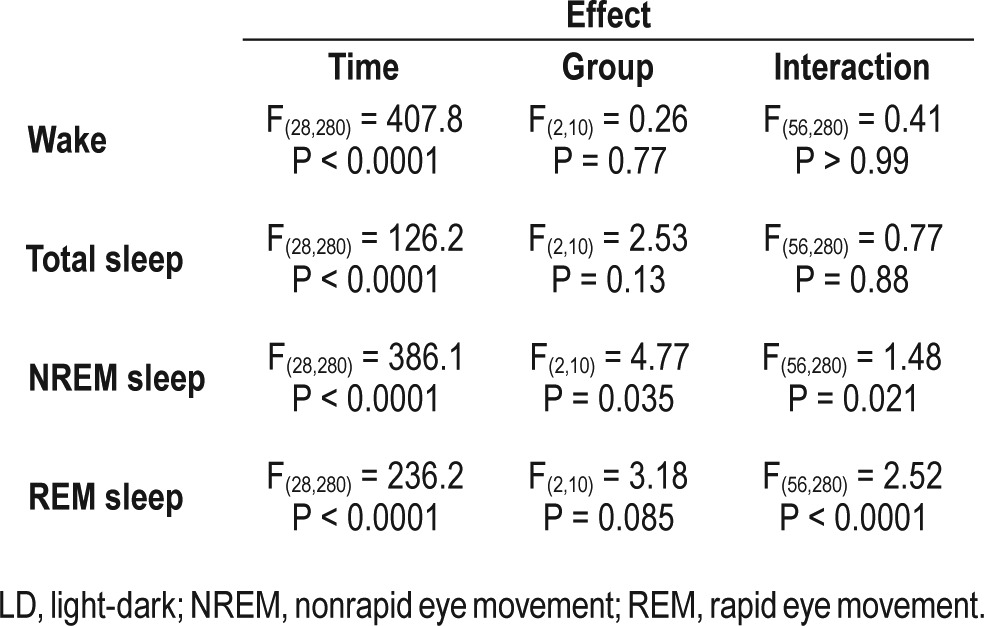
A similar percentage of time spent in each sleep stage over 1 day in all groups does not necessarily mean that the duration of each sleep stage event is conserved in all groups. The specific duration of a specific sleep stage within the day can result from a few long bouts or of many short bouts. To determine whether the bout duration of each sleep stage was different between the three groups, we performed survival analysis of each sleep stage for each animal. Figure 3B shows that survival curves of sleep and wakefulness bouts were similar in LD24, LD22 aligned, and LD22 misaligned animals. In contrast, the survival for NREM and REM sleep were overall significantly shorter for LD22 misaligned animals than in the other groups (see Table 1 for statistical results). These results indicate that although the cumulative duration of sleep stages does not differ between the three experimental groups, NREM and REM sleep bouts are shorter and therefore more fragmented in LD22 misaligned animals. Furthermore, because the survival of total sleep does not differ between groups, the fragmentation of REM and NREM sleep can only be explained by more frequent switches between REM and NREM sleep within each sleep bout.
Table 1.
Two-way analysis of variance results for the survival of each sleep stage for the three experimental groups: LD24, LD22 aligned, and LD22 misaligned animals.
Long-Term Consolidation of Hippocampus-Dependent Contextual Fear Memory is Impaired by Circadian Misalignment
The fragmentation of NREM and REM sleep in the absence of total sleep deprivation or total sleep fragmentation offers a unique opportunity to test the prediction that the shortening of NREM and REM sleep bouts should impair the consolidation of long-term hippocampus-dependent memory, even when sleep bouts have a normal duration. To test this prediction, we trained rats on a hippocampus-dependent contextual fear-conditioning task, which only requires a single training trial and leaves a memory trace that can persist up to several months.11,12 The single training trial was timed to either the aligned or misaligned phases of desynchrony, allowing us to precisely assess deficits in acquisition or consolidation. LD24 and LD22 rats were trained in the middle of the light phase. LD22 rats were subdivided into two groups: (1) trained during the aligned phase and (2) trained during the misaligned phase. Following training, rats remained in their respective LD schedules for 2 days, and then were placed under constant dim red light for ∼12 days before they were tested at the same circadian time for all groups. A single test trial was performed during each animal's subjective day.
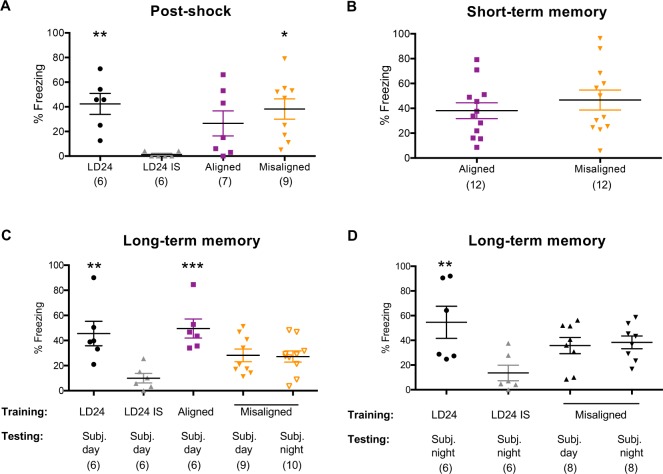
Figure 4A shows that most rats in all groups except immediate-shock control animals displayed freezing during the 2-min post-shock period, a normal response to an aversive stimulus (one-way ANOVA: F(3,24) = 4.51, P = 0.012 and Tukey comparisons). Furthermore, a 4-min test trial conducted 15 min after training, in which the animal was placed back in the shock chamber but no shock was delivered, showed that rats in the LD22 aligned and misaligned groups displayed normal short-term memory (Figure 4B, two-tailed Student t test: t(22) = 0.83, P = 0.41). When long-term memory was as -sessed, animals trained under LD24 conditions or under LD22 aligned phases showed significantly higher freezing scores than immediate-shock animals (Figure 4C, one-way ANOVA: F(4,32) = 5.75, P = 0.0013 and Dunnett comparisons). However, LD22 animals trained during misaligned phases showed freezing scores that were not statistically different from immediate-shock controls (Dunnett comparisons). These results suggest that the misalignment of circadian rhythms does not impair short-term memory but is associated with impairment in the consolidation of long-term contextual fear conditioning memory. The incongruence of circadian times between training and testing sessions can impair memory retrieval.13,14 An alternative explanation for the lower test freezing scores of animals trained during LD22 misaligned days could be that memory was indeed normally consolidated but because the circadian phase during the light phase of the misalignment corresponds to the subjective night of the ∼25-h rhythm, the animals are unable to retrieve this memory during the subjective day under DD. To test for this possibility, a second group of LD22 misaligned animals was trained during the light phase but the test 12 days after releasing them into DD took place during the subjective night. These animals also displayed freezing levels that were not significantly different from immediate-shock controls (Figure 4C, Dunnett comparison). Planned contrasts revealed that LD24-trained animals and LD22 animals trained during aligned phases did not differ from each other (P > 0.05). Similarly, mis-aligned groups in Figure 4C did not differ from each other (P > 0.05). Whereas each misaligned group was not statistically different from LD24-trained animals or LD22 animals trained during aligned phases, both misaligned groups pooled together had significantly lower freezing scores than either LD24-trained animals (P = 0.0481) or LD22 animals (P = 0.0135).
Figure 4.
Training during LD22 misaligned phases leads to impaired long-term memory consolidation of hippocampus-dependent contextual fear conditioning. (A) Post-shock freezing during training. Whereas immediate-shocked control animals (LD24 IS) do not freeze during training, LD24, LD22 aligned, and LD22 misaligned animals show normal freezing responses. One-way analysis of variance: F(3,24) = 4.51, P = 0.012; *P = 0.05, **P = 0.01 different from LD24 IS, Dunnett multiple comparisons test. (B) Freezing during short-term memory test. LD22 aligned and misaligned animals show similar short-term (15 min post-training) memory consolidation. Two-tailed Student t test: t(22) = 0.83, P = 0.41. (C) Training during the light phase of LD24 and LD22 aligned days, but not during the light phase of LD22 misaligned days, leads to higher test freezing responses than training with an immediate-shock. Bottom of x-axis indicates training and testing conditions; all animals were trained during the light phase; one-way analysis of variance: F(4,32) = 5.75, P = 0.0013. (D) Training during the dark phase of LD24 but not during the dark phase of LD22 misaligned days, leads to higher test freezing responses than training with an immediate shock. Bottom of x-axis indicates training and testing conditions; all animals were trained during the dark phase; one-way analysis of variance: F(3,24) = 4.05, P = 0.0184. *P = 0.05, **P = 0.01, ***P = 0.001 different from LD24 immediate shock, Dunnett multiple comparisons test. Number of animals is indicated in brackets.
The possibility remains that circadian misalignment only impairs memory consolidation when animals are trained at specific phases of the LD cycle. Nocturnal rodents typically display low activity during the light phase, and this phase—in combination with the internal misalignment of circadian rhythms—could be particularly detrimental to memory consolidation. We tested this possibility by training LD22 misaligned animals during the dark phase and testing them either during the subjective day or during the subjective night after their release into DD. Although LD24 control animals trained during the dark phase and tested during the subjective night showed significantly higher freezing scores than immediate-shock controls, neither of the two groups trained in the dark phase during misalignment show significantly higher freezing than controls (Figure 4D, one-way ANOVA: F (3,24) = 4.05, P = 0.0184 and Dunnett comparisons). Planned contrasts revealed that misaligned groups in Figure 4D did not differ from each other (P > 0.05). Although a planned comparison showed that both misaligned groups pooled together did not differ from LD24-trained animals (P = 0.0810), this difference was significant in a one-tailed Student t-test (t(20) = 1.73, P = 0.0496). Thus, the lower long-term learning performance of animals trained during a state of circadian misalignment is most likely accounted for by reduced—although not abolished—long-term memory consolidation that is independent of the phases of training or testing.
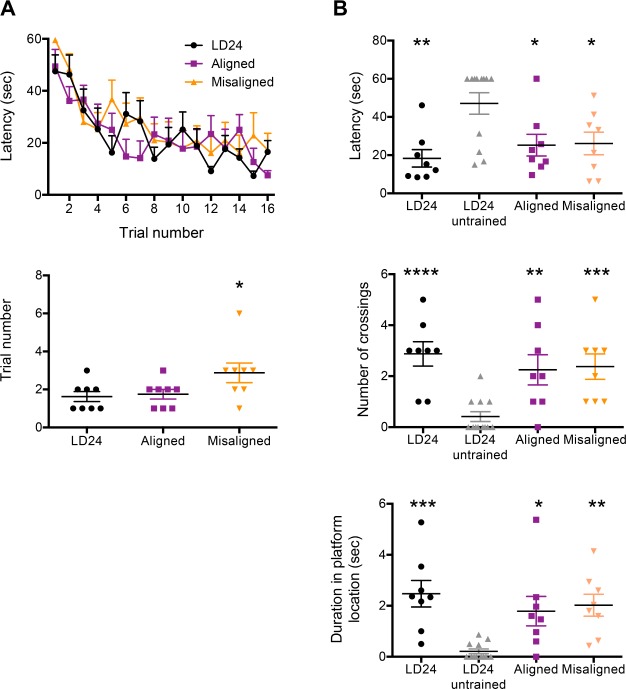
The hippocampus is also involved in spatial navigation memory, and lesion studies have indicated that different hippocampus regions may be involved in fear vs. spatial memories.11 To assess the effect of circadian misalignment on long-term hippocampal spatial memory, we trained LD24, LD22 aligned, or LD22 misaligned animals in a modified Morris water maze paradigm.6 During successive training trials, all three groups showed a significant and similar reduction in the latency to reach the hidden platform (Figure 5A, top panel), indicating a similar pattern of memory acquisition (two-way ANOVA: Trial number [F(15,315) = 9.08, P < 0.0001], Group [F(2,21) = 0.56, P = 0.58], Interaction [F(30,315) = 0.94, P = 0.56]). Overall, the trajectories used to reach the platform during the first 6 training trials did not differ between groups (two-way ANOVA: Trajectory [F(4,84) = 4.01, P = 0.0051], Group [F(2,21) = 0.56, P = 0.58] and Interaction [F (8,84) = 0.35, P = 0.95; data not shown]), indicating that there was no bias in the swimming pattern during memory acquisition. However, LD22 misaligned animals required significantly more trials to reach the platform on their own during training than LD24 or LD22 aligned animals (Figure 5A, bottom panel; one-way ANOVA: F(2,21) = 3.58, P = 0.046). Animals in all three groups displayed normal long-term spatial memory in a probe test. Compared to nontrained control animals, animals in all three groups showed a shorter latency to reach the platform location (one-way ANOVA: F(3,32) = 5.67, P = 0.0031), higher number of crossings over the original platform location (one-way ANOVA: F (3,32) = 10.99, P < 0.0001) and longer time spent on this location (one-way ANOVA: F (3,32) = 7.15, P = 0.0008; Figure 5B). The average speed to reach the platform during testing was similar amongst all groups (oneway ANOVA, F(3,32) = 0.70, P = 0.56), indicating normal motor swimming abilities for animals in all groups (data not shown).
Figure 5.
Training under LD22 forced desynchrony conditions has no effect on hippocampus-dependent spatial memory consolidation. (A) Responses during the Morris water maze training trials. Top panel: Latency to reach the hidden platform during successive training trials for LD24, LD22 aligned, and LD22 misaligned animals. Two-way analysis of variance (ANOVA): Trial (F(15,315) = 9.08, P < 0.0001); group (F(2,21) = 0.56, P = 0.58); interaction (F(30,315) = 0.94, P = 0.56). Bottom panel: Training trial number on which rats reached the hidden platform by their own. One-way ANOVA: F(2,21) = 3.58, P = 0.046. *P = 0.05 different from LD24, Dunnett multiple comparisons test. (B) Responses during the probe test. Top panel: Latency for the first crossing in the original platform location. One-way ANOVA: F(3,32) = 5.67, P = 0.0031. Center panel: Number of crossings over the original platform location. One-way ANOVA: F(3,32) = 10.99, P < 0.0001. Bottom panel: Total duration on the original location of the platform. One-way ANOVA: F(3,32) = 7.15, P = 0.0008. *P = 0.05, **P = 0.01, ***P = 0.001, ****P = 0.0001 different from LD24 untrained, Dunnett multiple comparisons test. n = 8 for LD24, aligned and misaligned; n = 12 for LD24 untrained. LD, light-dark.
Long-Term Consolidation of Hippocampus-Independent Cued Fear Memory is Not Impaired by Circadian Misalignment
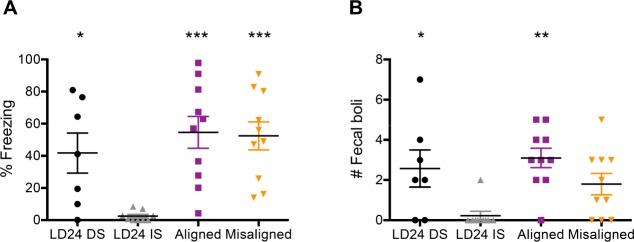
Our results indicate that the misalignment of circadian rhythms impairs memory consolidation in a hippocampus-dependent contextual fear-conditioning task. The active system consolidation hypothesis for the role of sleep on memory consolidation has been specifically proposed for hippocampus-related memories. We investigated whether long-term memory consolidation in a paradigm that involves a fear conditioning but is independent of the hippocampus, is affected by the misalignment of circadian rhythms. A new set of animals from each of the same three experimental groups was exposed to a single training trial in a cued fear-conditioning task. A single 1-mA foot shock was used as an aversive stimulus but in this case the shock was preceded by a 20-sec tone. Figure 6A shows that all three experimental groups showed a significantly higher freezing response during the test trial than immediate-shock animals in which the tone and the foot shock were presented in a noncontingent manner (one-way ANOVA: F(3,32) = 7.80, P = 0.0005). The number of fecal boli after the presentation of the tone, an estimate of the animals' distress, showed similar results (Figure 6B; one-way ANOVA: F(3,32) = 5.35, P = 0.0042). Therefore, cue-dependent, hippocampus-independent memory consolidation is not impaired by misalignment of sleep stages.
Figure 6.
Training under LD22 forced desynchrony conditions has no effect on long-term memory consolidation of hippocampus-independent cued fear conditioning. (A) Post-tone freezing scores during long-term memory test from animals trained under LD24, LD22 aligned, and LD22 misaligned conditions. One-way ANOVA: F(3,32) = 7.80, P = 0.0005. (B) Number of fecal boli during test trial. One-way ANOVA: F(3,32) = 5.35, P = 0.0042. *P = 0.05, ***P = 0.001 different from LD24 immediate shock, Dunnett multiple comparisons test. n = 7 (LD24); n = 9 (LD24 IS); n = 10 (aligned); n = 10 (misaligned). LD, light-dark.
NREM and REM Sleep Fragmentation, but Not Total Sleep Fragmentation, is Associated with Impaired Hippocampus-Dependent Fear Memory Consolidation
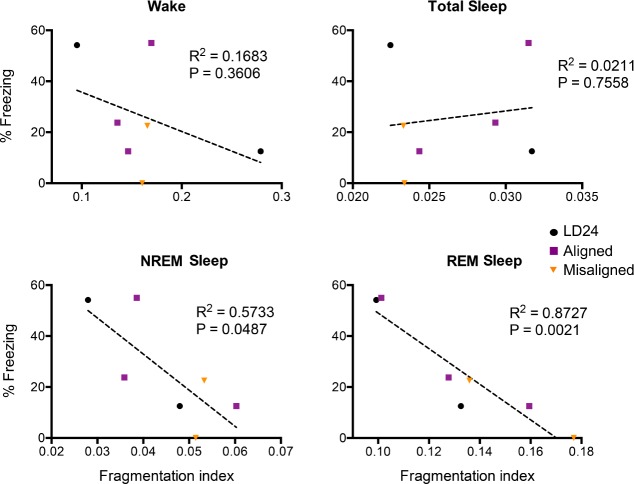
Our data show that hippocampus-dependent fear memory consolidation is impaired in animals with fragmented sleep stages. However, forced desynchronized rats show desynchrony in other circadian rhythms that could be responsible for memory deficits.5,9,15,16 To determine whether memory deficits in LD22 misaligned animals are associated specifically with our observed fragmentation of NREM and REM sleep, we repeated our contextual fear conditioning experiments in all three experimental groups while simultaneously recording the vigilance states with ECoG electrodes after training for each animal. We plotted a survival curve and fitted an exponential decay curve for each animal and sleep stage during the light phase one day after training. The exponential coefficient of this curve is inversely proportional to the duration of the specific stage before transitioning to another, and represents a fragmentation index for each sleep stage. Figure 7 shows that fragmentation of neither wakefulness (P = 0.3606) nor total sleep (P = 0.7558) was associated with long-term memory— freezing during the test trial. In contrast, the fragmentation index for both NREM sleep (P = 0.0487) and REM sleep (P = 0.0021) had a negative linear relationship with freezing scores. In other words, shorter bout durations of NREM and REM sleep after training were correlated with poor memory consolidation, regardless of the group to which the animals belonged. Delta activity during the same post-training period was not associated with memory consolidation (R2 = 0.0491, P = 0.63). These results, although based in a small number of animals (n = 7), indicate that the post-training fragmentation of REM and NREM sleep is associated with poor long-term hippocampus-dependent fear memory.
Figure 7.
Rapid eye movement (REM) and nonrapid eye movement (NREM) sleep fragmentation, but not total sleep fragmentation, are associated with impaired memory consolidation in hippocampus-depended contextual fear conditioning. For each sleep stage, the fragmentation index is drawn from the exponential decay coefficient of the survival curve for that stage in each animal. Correlation coefficient (R2) and associated P value for the regression analysis are shown in each case (n = 7).
DISCUSSION
We show that the experimental fragmentation of REM and NREM sleep without fragmentation of total sleep per se is associated with impairment of consolidation of contextual fear memory. This finding is consistent with the interpretation that sleep can only sustain memory consolidation when its architecture allows for bouts of REM and NREM sleep that are sufficiently long. This interpretation is further supported by the fact that long-term memory performance is positively associated with the length of post-training REM and NREM sleep bouts but not associated with the length of post-training bouts of total sleep. Our results in a genetically, neurologically, and pharmacologically intact animal model without sleep deprivation as a confounding factor are in line with the proposed function of the stereotypic temporal sequence of REM and NREM sleep throughout the sleep cycle in memory consolidation. The active system consolidation hypothesis for memory consolidation during sleep predicts that disruption of the temporal structure of REM and NREM sleep should impair memory consolidation even in the absence of the stressful effects of sleep deprivation. Our findings support this prediction.
The disruption of sleep architecture induced in the forced desynchronized rat does not impair spatial memory consolidation. Both contextual fear conditioning and spatial orientation memories rely on an intact hippocampus. However, lesion studies targeting subregions of the hippocampus first suggested that the dorsal hippocampus in rodents has cognitive functions without emotional content, such as spatial navigation in the Morris water maze,17 whereas the ventral hippo-campus appears to be involved in both the coding of emotional stimuli and their association with environmental stimuli such as context in a contextual fear-conditioning task. This segregation of functions between the dorsal and ventral hippo-campus, corresponding to the posterior and anterior regions in primates, respectively, has not only support in lesion and pharmacological studies, but also in tract-tracing, functional magnetic resonance imaging studies in humans and, lately, in gene-expression patterns that match the anatomical subregions and their functions.11 Our results suggest that hippocampus-dependent spatial memory consolidation is more resilient to disruptions in post-training sleep architecture than contextual fear conditioning. Interestingly, consolidation of cued fear memory was unaffected by the sleep disruption induced by circadian desynchrony, suggesting that emotional memories that are less dependent on hippocampal processing may not rely on the sleep consolidation mechanisms that contextual fear memories rely on.
Previous studies have shown that sleep fragmentation without extended periods of sleep deprivation impair memory consolidation. In fact, fragmentation of sleep and specific sleep stages associated with aging or obstructive sleep apnea appears to be a critical contributing factor to reduced cognitive performance.18,19 Accordingly, allowing REM sleep interruption in patients with obstructive sleep apnea by acutely withdrawing their treatment through positive airway pressure reduces performance in a spatial memory task.20 In rats and mice, experimentally induced sleep fragmentation through different methods also induces deficits in spatial learning, and this is associated with reduced long-term potentiation in the hippocampus.21–23 Together, this experimental evidence supports a critical role for a minimum REM or NREM sleep duration for proper memory consolidation. In contrast to our study, however, all these protocols to induce sleep fragmentation result in shorter total sleep, REM sleep, or NREM sleep durations. Even in the case in which a specific stage is not reduced in overall duration, total sleep is always fragmented in these protocols. Our results are unique in demonstrating that memory consolidation deficits are associated with fragmented REM and NREM sleep, even in the absence of fragmented sleep. Interestingly, despite the fact that the post-training fragmentation of NREM sleep was predictive of poor memory consolidation, the total delta activity during the same post-training period was not. This result suggests that for slow wave sleep to facilitate memory consolidation after training it must manifest itself in bouts that are above a minimum duration.
Internal misalignment of circadian rhythms, including de-synchrony between the homeostatic and circadian regulation of sleep, is an outcome of challenges to the circadian system such as jetlag and nocturnal shift work.24,25 Prior studies showed that circadian desynchrony disrupts learning and memory.26 Circadian disruption through constant light exposure impairs hippocampus-dependent memory.27,28 In Siberian hamsters, a one-time photic treatment that results in circadian arrhythmicity impairs novel-object recognition.29 Circadian desynchrony by simulated jet lag or forced desynchrony impairs fear-conditioning recall.30–34 Under circadian desynchrony induced by scheduled feeding, this memory impairment is associated with a desynchronized phase of the hippocampus circadian oscillator and reduced long-term potentiation.34 In humans, international airline cabin crews with years of chronic jetlag had reduced temporal lobe volume and memory deficits.35 Long-term (> 10 y) shift work is associated with impaired cognitive function.36 Internal misalignment of circadian rhythms, including sleep stages, is also present in mood disorders such as major depression, winter depression, and bipolar disorder,37,38 and learning is typically impaired in these mood disorders. Our results suggest that cognitive deficits associated with environmental challenges to the circadian system or mood disorders could result in part from impaired memory consolidation due to the misalignment of sleep stages.
Importantly, internal desynchrony of circadian rhythms is not restricted to the misalignment of sleep stages but it involves desynchrony of other circadian rhythms. Among these rhythms, the release of corticosterone is disrupted during mis-aligned days in the forced desynchronized rat.15 Post-training increases in corticosterone levels have been associated with increased consolidation in cued and contextual fear memory.39,40 Thus, impaired contextual fear memory consolidation during LD22 misaligned phases could reflect an inability to increase glucocorticoid release. We do not think this is the case. Mis-aligned LD22 rats have intermediate levels of corticosterone throughout the LD cycle, and compared to aligned LD22 rats, they have higher levels during the light phase and lower levels during the dark phase.15 Despite these contrasting differences in each phase, misaligned animals trained either during the light or dark phases showed impaired memory consolidation relative to aligned animals. Nevertheless, the possibility remains that circadian misaligned animals are unable to mount a glucocorticoid increase that favors memory consolidation. Future studies in the desynchronized rat or other models should determine to what extent the misalignment of other behavioral and physiological circadian rhythms contribute to deficits in memory consolidation.
The circadian system of mammals is under regulation by a master circadian clock within the suprachiasmatic nucleus (SCN) of the hypothalamus. In mice, lesions of the SCN or genetic disruption of the molecular circadian clock result in memory deficits.41,42 However, SCN lesions rescue learning deficits in arrhythmic Siberian hamsters, suggesting that an arrhythmic master circadian clock in these animals actively inhibits memory consolidation.43 Together, these results suggest that the circadian integrity of the neuronal network within the SCN is important for normal memory consolidation. In support of this view, we have previously shown that desynchrony of sleep stages in the forced desynchronized rat emerges from the desynchrony of specific neuronal sub-populations within the SCN.8,9 Our current findings indicate that the local circadian misalignment of neurons within the SCN circuitry, through the desynchrony of sleep stages, may impair higher cognitive functions such as long-term hippo-campus memory consolidation.
DISCLOSURE STATEMENT
This was not an industry supported study. Support was provided by NSF Award IOS0909716 to Dr. de la Iglesia. Mykhaylo Krushelnytskyy was supported by a Mary Gates Fellowship for Undergraduate Research. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Dan Storm and Trongha Phan for suggestions on learning experimental design. We thank Vicky Herrera for analysis of behavioral tests. We thank Drs. Yonathan Munk, Frederick Reitz, Andres Barrias and Waldo Cerpa for technical assistance.
REFERENCES
- 1.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 2.Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci. 2010;11:218. doi: 10.1038/nrn2762-c1. author reply. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci. 2011;15:343–51. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.de la Iglesia HO, Cambras T, Schwartz WJ, Diez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ, Lee HJ, Welday AC, et al. Stress-induced alterations in hippocampal plasticity, place cells, and spatial memory. Proc Natl Acad Sci U S A. 2007;104:18297–302. doi: 10.1073/pnas.0708644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–60. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 8.Lee ML, Swanson BE, de la Iglesia HO. Circadian timing of REM sleep is coupled to an oscillator within the dorsomedial suprachiasmatic nucleus. Curr Biol. 2009;19:848–52. doi: 10.1016/j.cub.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cambras T, Weller JR, Angles-Pujoras M, et al. Circadian desynchronization of core body temperature and sleep stages in the rat. Proc Natl Acad Sci U S A. 2007;104:7634–9. doi: 10.1073/pnas.0702424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Iglesia HO, Lee ML. A time to wake, a time to sleep. In: Aguilar-Roblero R, Fanjul-Moles ML, Díaz-Muñoz M, editors. Mechanisms of Circadian Systems in Animals and Their Clinical Relevance. New York, NY: Springer International; 2014. p. 380. [Google Scholar]
- 11.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goosens KA. Hippocampal regulation of aversive memories. Curr Opin Neurobiol. 2011;21:460–6. doi: 10.1016/j.conb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralph MR, Sam K, Rawashdeh OA, Cain SW, Ko CH. Memory for time of say (time temory) Is encoded by a circadian oscillator and Is distinct from other context memories. Chronobiol Int. 2013;30:540–7. doi: 10.3109/07420528.2012.754449. [DOI] [PubMed] [Google Scholar]
- 14.Cain SW, McDonald RJ, Ralph MR. Time stamp in conditioned place avoidance can be set to different circadian phases. Neurobiol Learn Mem. 2008;89:591–4. doi: 10.1016/j.nlm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Wotus C, Lilley TR, Neal AS, et al. Forced desynchrony reveals independent contributions of suprachiasmatic oscillators to the daily plasma corticosterone rhythm in male rats. PLoS One. 2013;8:e68793. doi: 10.1371/journal.pone.0068793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz MD, Wotus C, Liu T, et al. Dissociation of circadian and light inhibition of melatonin release through forced desynchronization in the rat. Proc Natl Acad Sci U S A. 2009;106:17540–5. doi: 10.1073/pnas.0906382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015;10:97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathol Biol. 2014;62:233–40. doi: 10.1016/j.patbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Varga AW, Kishi A, Mantua J, et al. Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. J Neurosci. 2014;34:14571–7. doi: 10.1523/JNEUROSCI.3220-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace E, Kim do Y, Kim KM, et al. Differential effects of duration of sleep fragmentation on spatial learning and synaptic plasticity in pubertal mice. Brain Res. 2015;1615:116–28. doi: 10.1016/j.brainres.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Tartar JL, Ward CP, McKenna JT, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–48. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward CP, McCoy JG, McKenna JT, Connolly NP, McCarley RW, Strecker RE. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res. 2009;1294:128–37. doi: 10.1016/j.brainres.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17:489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- 25.Sack RL, Blood ML, Lewy AJ. Melatonin rhythms in night shift workers. Sleep. 1992;15:434–41. doi: 10.1093/sleep/15.5.434. [DOI] [PubMed] [Google Scholar]
- 26.Smarr BL, Jennings KJ, Driscoll JR, Kriegsfeld LJ. A time to remember: the role of circadian clocks in learning and memory. Behav Neurosci. 2014;128:283–303. doi: 10.1037/a0035963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckel-Mahan KL, Phan T, Han S, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11:1074–82. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujioka A, Fujioka T, Tsuruta R, Izumi T, Kasaoka S, Maekawa T. Effects of a constant light environment on hippocampal neurogenesis and memory in mice. Neurosci Lett. 2011;488:41–4. doi: 10.1016/j.neulet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Ruby NF, Hwang CE, Wessells C, et al. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105:15593–8. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tapp WN, Holloway FA. Phase shifting circadian rhythms produces retrograde amnesia. Science. 1981;211:1056–8. doi: 10.1126/science.7193351. [DOI] [PubMed] [Google Scholar]
- 31.Fekete M, van Ree JM, Niesink RJ, de Wied D. Disrupting circadian rhythms in rats induces retrograde amnesia. Physiol Behav. 1985;34:883–7. doi: 10.1016/0031-9384(85)90008-3. [DOI] [PubMed] [Google Scholar]
- 32.Loh DH, Navarro J, Hagopian A, Wang LM, Deboer T, Colwell CS. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS One. 2010;5:e12546. doi: 10.1371/journal.pone.0012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neto SP, Carneiro BT, Valentinuzzi VS, Araujo JF. Dissociation of the circadian rhythm of locomotor activity in a 22 h light-dark cycle impairs passive avoidance but not object recognition memory in rats. Physiol Behav. 2008;94:523–7. doi: 10.1016/j.physbeh.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Loh DH, Jami SA, Flores RE, et al. Misaligned feeding impairs memories. eLife. 2015;4:e09460. doi: 10.7554/eLife.09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–8. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 36.Marquie JC, Tucker P, Folkard S, Gentil C, Ansiau D. Chronic effects of shift work on cognition: findings from the VISAT longitudinal study. Occup Environ Med. 2015;72:258–64. doi: 10.1136/oemed-2013-101993. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–52. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- 38.Lewy AJ. Circadian misalignment in mood disturbances. Curr Psychiatry Rep. 2009;11:459–65. doi: 10.1007/s11920-009-0070-5. [DOI] [PubMed] [Google Scholar]
- 39.Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–11. [PubMed] [Google Scholar]
- 40.Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Phan TX, Chan GC, Sindreu CB, Eckel-Mahan KL, Storm DR. The diurnal oscillation of MAP (mitogen-activated protein) kinase and adenylyl cyclase activities in the hippocampus depends on the suprachiasmatic nucleus. J Neurosci. 2011;31:10640–7. doi: 10.1523/JNEUROSCI.6535-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wardlaw SM, Phan TX, Saraf A, Chen X, Storm DR. Genetic disruption of the core circadian clock impairs hippocampus-dependent memory. Learn Mem. 2014;21:417–23. doi: 10.1101/lm.035451.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez F, Lu D, Ha P, et al. Circadian rhythm. Dysrhythmia in the suprachiasmatic nucleus inhibits memory processing. Science. 2014;346:854–7. doi: 10.1126/science.1259652. [DOI] [PMC free article] [PubMed] [Google Scholar]