Abstract
Study Objectives:
Episodes of brief limb ischemia (remote preconditioning) in mice induce tolerance to modeled ischemic stroke (focal brain ischemia). Since stroke outcomes are in part dependent on sleep-wake history, we sought to determine if sleep is critical for the neuroprotective effect of limb ischemia.
Methods:
EEG/EMG recording electrodes were implanted in mice. After a 24 h baseline recording, limb ischemia was induced by tightening an elastic band around the left quadriceps for 10 minutes followed by 10 minutes of release for two cycles. Two days following remote preconditioning, a second 24 h EEG/EMG recording was completed and was immediately followed by a 60-minute suture occlusion of the middle cerebral artery (modeled ischemic stroke). This experiment was then repeated in a model of circadian and sleep abnormalities (Bmal1 knockout [KO] mice sleep 2 h more than wild-type littermates). Brain infarction was determined by vital dye staining, and sleep was assessed by trained identification of EEG/EMG recordings.
Results:
Two days after limb ischemia, wild-type mice slept an additional 2.4 h. This additional sleep was primarily comprised of non-rapid eye movement (NREM) sleep during the middle of the light-phase (i.e., naps). Repeating the experiment but preventing increases in sleep after limb ischemia abolished tolerance to ischemic stroke. In Bmal1 knockout mice, remote preconditioning did not increase daily sleep nor provide tolerance to subsequent focal ischemia.
Conclusions:
These results suggest that sleep induced by remote preconditioning is both sufficient and necessary for its neuroprotective effects on stroke outcome.
Citation:
Brager AJ, Yang T, Ehlen JC, Simon RP, Meller R, Paul KN. Sleep is critical for remote preconditioning-induced neuroprotection. SLEEP 2016;39(11):2033–2040.
Keywords: mouse, ischemia, homeostatic, circadian, neuroprotection, tolerance
Significance.
The daily gain in sleep after limb ischemia (i.e., remote preconditioning) appears to be sufficient and necessary for improving tolerance to subsequent focal ischemia. Remote preconditioning (rPC) has been investigated in a number of clinical trials for stroke, but none of these trials have considered sleep gains or changes in sleep following rPC. Sleep disturbances are common in clinical settings due to patient monitoring. If sleep were to be interrupted, then any benefit of rPC would hypothetically be lost.
INTRODUCTION
Brief (non-injurious) ischemia in a hind limb vascular bed protects the brain from subsequent ischemic injury,1,2 a phenomenon known as remote preconditioning. The mediators of remote preconditioning may include small molecules such as humoral factors, and/or neurogenic mechanisms.1 While the exact mechanisms are still not clear, clinical trials of remote preconditioning suggest this may be a powerful approach to ameliorate ischemic brain injury following stroke.2
Multiple lines of evidence suggest a complex, reciprocal interaction between modeled ischemic stroke outcomes and sleep-wake processes. For instance: (1) ischemic brain injury enhances non-rapid eye movement (NREM) sleep in rodents,3,4 (2) sleep deprivation prior to brain ischemia reduces injury (neuroprotective),3,5,6 (3) sleep deprivation after brain ischemia increases injury,4,7 and (4) functional recovery from ischemic brain injury is improved through pharmacological promotion of sleep.8 In lieu of existing literature in rodent models, the present study investigated a causal interaction between ischemic stroke outcome and sleep amount after limb ischemia.
In this study, we investigated the effect of remote preconditioning on sleep in C57 wild-type mice and C57 mice with a knockout of the Bmal1 gene (ARNTL Aryl hydrocarbon receptor nuclear translocator-like: Bmal1 KO). Bmal1 KO mice do not exhibit circadian rhythms,9 and exhibit a number of sleep phenotypes including hypersomnia and an impaired ability to recover from sleep loss (> 2h additional spontaneous sleep/day vs. wild-type C57 mice).10 The Bmal1 KO allowed us to examine if the neuroprotective effect of remote preconditioning, induced by transient limb ischemia, is sensitive to sleep and circadian mechanisms. The present study is the first-ever to show that remote preconditioning can alter daily amounts of sleep, and that sleep is critical for the ability of remote preconditioning to protect against ischemic brain injury.
METHODS
Animals
Animal procedures were performed at Morehouse School of Medicine, Atlanta, GA, at facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and in accordance with protocols approved by the Morehouse School of Medicine IACUC, as well as the principles outlined in the National Institute of Health Guide for the Care and Use of Laboratory Animals. Adult (10–12 weeks), male C57BL/6 were obtained from Charles River Laboratories (Charles River, MO), and were used to develop the model of remote preconditioning. Bmal1 KO mice and wild-type littermates were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in a temperature-controlled vivarium (23°C) under an entrained 12 h-12 h light-dark cycle with food and water provided ad libitum.
EEG/EMG Implants
Electroencephalograph (EEG) and electromyography (EMG) electrodes were implanted in mice anesthetized with isoflurane (induced at 2%, maintained at 1.5%). Four stainless steel epidural screw electrodes stabilized a prefabricated head mount (Pinnacle Technologies, KS). The first 2 electrodes (frontal and ground) were located 1.5 mm anterior to bregma and 1.5 mm on either side of the central suture. The second 2 electrodes (parietal and common reference) were located 2.5 mm posterior to bregma and 1.5 mm on either side of the central suture. Electrical continuity between the screw electrode and head mount was achieved with silver epoxy. EMG activity was monitored using stainless-steel Teflon coated wires inserted bilaterally into the nuchal muscles. The head mount (integrated 2x3 pin grid array) was secured to the skull with dental acrylic. Mice were recovered for at least 2 weeks prior to baseline sleep recording.
EEG/EMG Recordings
One week prior to the EEG/EMG recordings, mice were moved to the recording chamber and connected to a lightweight tether attached to a low-resistance commutator mounted over the cage (Pinnacle Technologies, KS). This enabled complete freedom of movement throughout the cage. With the exception of the tether, the home cage environment was maintained. Recording of EEG/EMG waveforms began at lights-on on day 8. Data acquisition was performed on a PC running polysomnographic software (Sirenia Acquisition, Pinnacle Technologies, KS). EEG signals were low-pass filtered with a 60 Hz cutoff and collected continuously at a sampling rate of 400 Hz. After collection, EEG/EMG waveforms were classified in ten-second epochs as: (1) wake (low-voltage, high-frequency EEG; high-amplitude EMG); (2) non-rapid eye movement (NREM sleep; high-voltage, mixed-frequency EEG; low- amplitude EMG); or rapid-eye movement (REM) sleep (low-voltage EEG with a predominance of theta activity [6–10 Hz]; very low amplitude EMG) by a trained observer. Classification of wake, NREM sleep, and REM sleep was primarily determined from the frontal electrode located 1.5 mm anterior to bregma and on the left side of the central suture. EEG epochs determined to have artifact (interference caused by scratching, movement, eating, or drinking) were excluded from analysis. Artifact comprised less than 5% of all recordings used for analysis.
Remote Preconditioning
Under anesthesia with isoflurane (induced at 2%, maintained at 1.5%) the upper left hind limb was ligated with an elastic band for 10 min, released for 10 min, and re-ligated for another 10 min (2 cycles of 10 min hind limb ischemia). Sham controls were anesthetized with isoflurane, but did not undergo ligation. Animals were recovered for 72 h prior to subsequent middle cerebral artery occlusion.
Middle Cerebral Artery Occlusion (MCAO)
Mice were anesthetized with isoflurane and subjected to modeled ischemic stroke of the right hemisphere induced by MCAO using the monofilament suture method (adapted from Bederson et al.11). The stroke surgeon was blinded with respect to the preconditioning status of the animals. Briefly, a silicone-coated 7-0 monofilament nylon surgical suture was threaded through the external carotid artery into the internal carotid artery to block the middle cerebral artery blood flow at its origin and maintained for 60 min producing focal ischemia. Attenuation of cerebral blood flow (CBF) was monitored continually with laser Doppler imaging (Perimed; Suffern, NY), during stroke to ensure that it dropped below 80% of control values. No animals were removed from the experiment. The suture was then removed to restore blood flow (confirmed by CBF).
Histology
To designate the area of infarcted tissue, the brain was serially sectioned into 1 mm-thick coronal sections and immersed in the vital dye (2%) 2,3,5-triphenyltetrazolium hydrochloride (TTC). The technique is quantitatively equivalent to conventional staining of brain sections with hematoxylin and eosin.11 The volume of infarcted tissue in comparison to surviving tissue was calculated from images of each section imported into Adobe Photoshop (Adobe, CA) using the histogram function. Areas of infarction were summated and multiplied by the section thickness. The percent infarct volume was calculated by dividing the volume of infarcted tissue by total volume of the contralateral hemisphere multiplied by 100 (adapted from Pignataro et al.12). Infarct volume was not examined in mice that died 0–48 h after stroke.
Experimental Design of Sleep-Wake Recordings
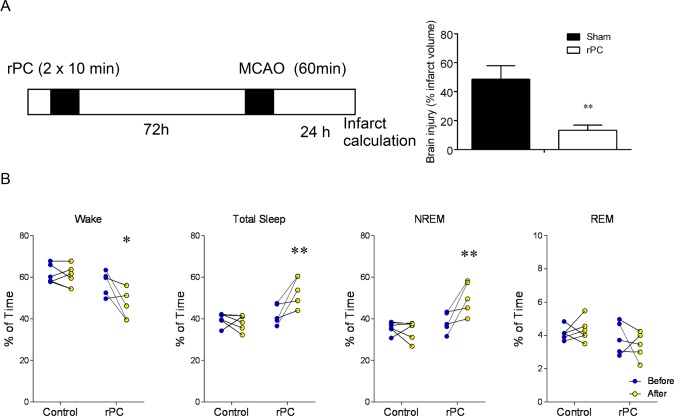
A schematic of the experimental design is provided in Figure 1A. Immediately after a 24 h baseline EEG/EMG recording, mice were removed from the tethered recording system to undergo remote preconditioning or no remote preconditioning (sham) under isoflurane anesthesia. Mice were immediately returned to the tethered recording system, and a second 24-h EEG/EMG recording was undertaken 48–72 h later. This time window aimed to control for any residual postoperative effects of isoflurane on EEG waveforms. Immediately following the second EEG/EMG recording, mice were subjected to MCAO during the middle of the light-phase. Mice were allowed to recover for 12 h in their postoperative surgical cages before being re-attached to the tethered recording system. A third 24-h EEG/ EMG recording was undertaken 48–72 h later in preconditioned mice. Each treatment group (remote preconditioning vs. sham) had 12 mice with 6 mice for each genotype (WT vs. Bmal1 KOs).
Figure 1.
Two days after remote preconditioning, total sleep increases by over 2 h. (A) Left panel: Experimental timeline for validating our paradigm of remote preconditioning (rPC) and modeled ischemic stroke via middle cerebral artery occlusion (MCAO). Mice were not outfitted with EEG/EMG electrodes. Brain infarct volume is expressed as a percentage of total contralateral hemisphere volume (adapted from ref. 12). (A) Right panel: Means ± SEM percent infarcted tissue in preconditioned mice and sham (anesthetized) mice. (B) Percent time mice spent in wake, total sleep (NREM+REM), and NREM and REM sleep prior to (blue) and two days after remote preconditioning (yellow) or no remote preconditioning (control) **P < 0.05, two-way ANOVA with Dunn-Sidak multiple comparison. n = 5 per treatment.
Statistics
Statistical analysis of the data was performed using Graphpad prism (v 6.0) and levels of significance were set at P < 0.05. Main effects for treatment and genotype were examined with one- or two-way ANOVAs where appropriate with post hoc Dunn-Sidak multiple comparisons test. An unpaired t-test compared extent of infarcted tissue in experiments with two groups.
RESULTS
Remote Preconditioning Protects Against Focal Brain Ischemia
In agreement with previously published studies,13,14 we first determined that animals subjected to remote preconditioning prior to middle cerebral artery occlusion had significant reductions in brain infarct volume: 13.3 ± 3.6% of brain was infarcted in previously preconditioned mice compared to 48.6 ± 9.4% of brain for sham controls subjected to MCAO (n = 6/treatment; P = 0.005; Figure 1A). Under this circumstance, brains of mice were collected for analyses of brain infarct volume 24 h post-MCAO (Figure 1A). Mortality rates were modest for preconditioned mice (15%; one of six mice died within 72 h post-MCAO) but high for sham controls (50%; three of six mice died within 72 h post-MCAO).
Remote Preconditioning Increases Daily Amounts of NREM Sleep
Once we determined that our modality of remote preconditioning protected against focal brain ischemia, C57 mice were outfitted with EEG/EMG electrodes and baseline recordings made; there were no significant differences in wake and sleep in the baseline recordings of the 2 groups; Figure 1B. Animals were subjected to either remote preconditioning or sham procedure. In agreement with the first experiment in mice not outfitted with EEG/EMG electrodes, there was a significant reduction in infarct volume in EEG fitted mice subjected to remote preconditioning: 11.4 ± 3.1% for preconditioned mice compared to 44.2 ± 6.8% for sham controls (n = 6/treatment; P = 0.002; unpaired t-test). Animals subjected to remote preconditioning spent significantly more time asleep compared to sham-treated animals: 53.6 ± 2.9% vs. 39.7 ± 1.8% of the 24 h recording, respectively (P = 0.002; F1,9 = 16.4; two-way ANOVA [treatment]; Figure 1B). Mice slept 2.4 h more after remote preconditioning compared to baseline levels (P = 0.03; F 1,9 = 6.1; two-way ANOVA [time]). Consistent with this observation, remote preconditioned animals spend less time awake compared with sham-treated controls: 46.4 ± 3.2% vs. 60.3 ± 2.8% of the 24 h recording, respectively (P = 0.009; F 1,9 = 10.9; two-way ANOVA [treatment]; Figure 1B).
NREM and REM sleep amounts were analyzed, and revealed that NREM sleep increased following remote preconditioning by 30.8 ± 12.6% compared to baseline (P = 0.004; F 1,9 = 14.9; two-way ANOVA [time]; Figure 1B). In contrast, there was no significant change in durations of REM sleep following remote preconditioning (P = 0.07; F1,9 = 4.2; two-way ANOVA [time]; Figure 1B). In sham controls, neither NREM nor REM sleep changed from baseline levels (P > 0.05, both [time]; Figure 1B).
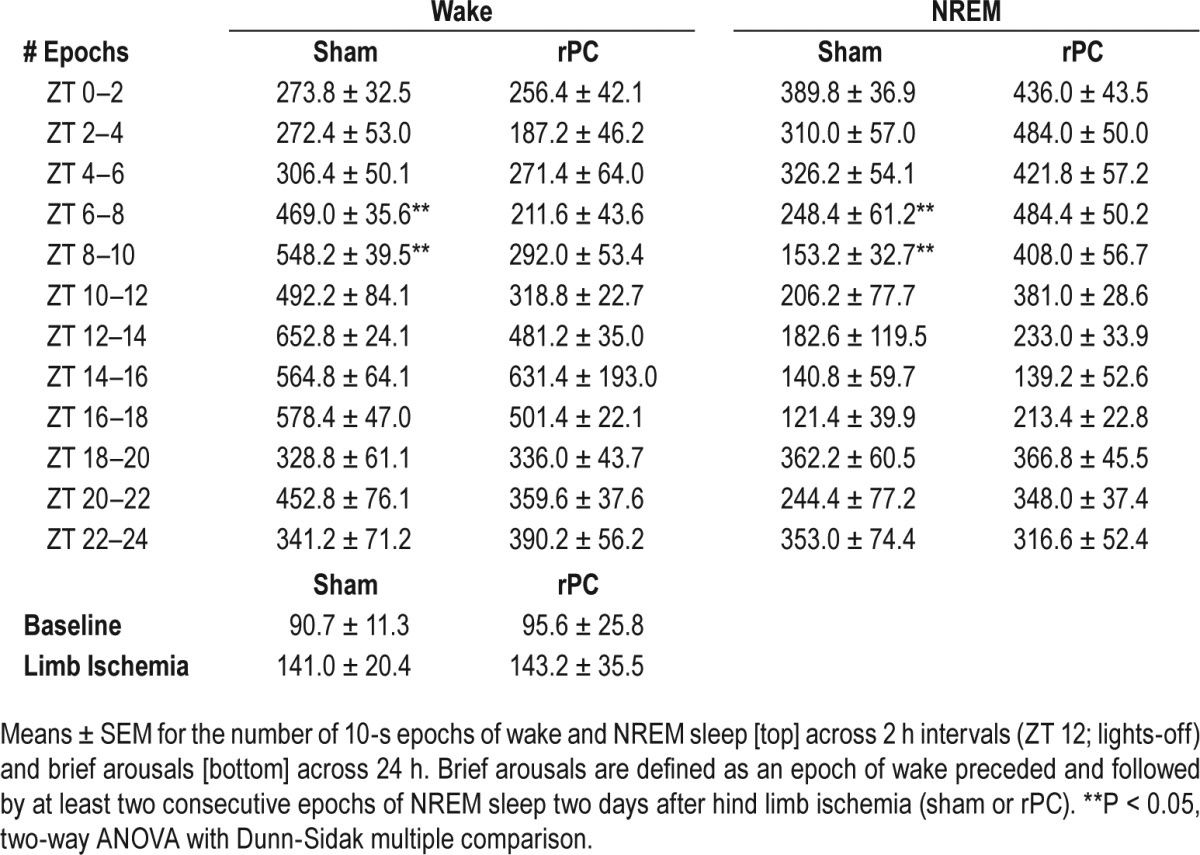
Finally, we quantified: (1) 24-h bout duration and bout counts for wake; (2) the temporal distribution of wake in 2-h intervals; and (3) the number of brief arousals (relative to baseline levels). A brief arousal was defined as a recording epoch of wake preceded and followed by at least two consecutive recording epochs of NREM sleep. These analyses would lend credence to the idea that additional sleep induced by remote conditioning was not a homeostatic rebound response to poor wake efficiency and is in fact sleep-dependent. Wake bout duration and count represented the average length and number, respectively, of consecutive (10-s) epochs of wake across the 24-h recording period. There was no difference for bout duration (P = 0.27; unpaired t-test) or bout count (P = 0.80; unpaired t-test) of wake (P = 0.63; unpaired t-test) in preconditioned mice compared to sham controls. Despite no treatment-dependent difference in the 24-h average, preconditioned mice slept through the mid-active period of the light-phase; a time of day when inbred (C57) mice entrained to a 12 h-12 h light cycle are typically active (ZT 6: P = 0.002; F1,9 = 20.8; two-way ANOVA [time] (wake); P = 0.002; F1,9 = 8.9; two-way ANOVA [time] (NREM); ZT 8; P = 0.005; F1,9 = 14.8; two-way ANOVA [time] (wake); P = 0.005; F1,9 = 15.1; two-way ANOVA [time] (NREM); Table 1). Thus, these additional “siestas” in preconditioned mice compared to sham controls likely underlie the neuroprotective effects of limb ischemia. Further, neither preconditioning nor sham procedure altered the number of brief arousals from baseline levels (P > 0.05, all; two-way ANOVA [treatment and time]; Table 1),
Table 1.
Preconditioned mice have additional “siestas” without a compromise in wake efficiency.
Sleep Gain Following Remote Preconditioning Occurs during the Light-Phase
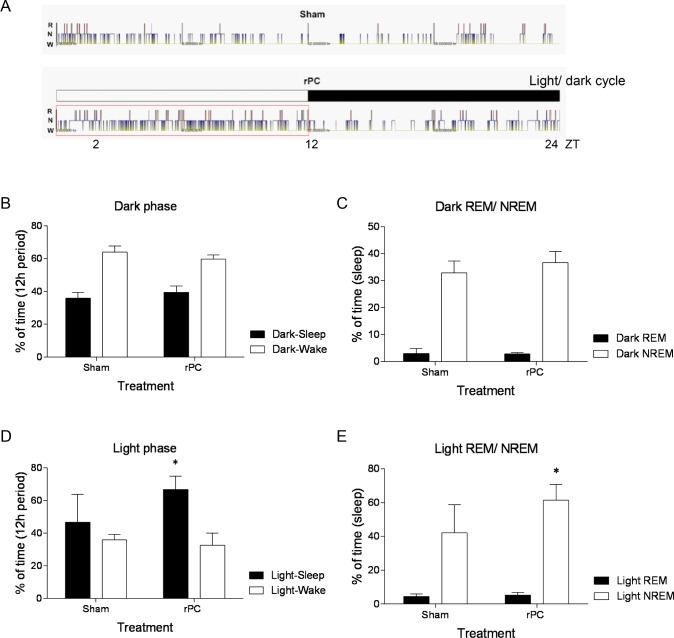
We examined differences in sleep-wake states across the 12-h dark (active)-phase and 12-h light (rest)-phase (Figure 2A). During the dark-phase, preconditioned mice and sham controls were awake for comparable amounts (Figure 2B; P > 0.05, both). When the mice did sleep, preconditioned mice and sham controls spent comparable amounts of time in either NREM or REM sleep (Figure 2C; P > 0.05). During the light-phase, preconditioned mice slept more than sham controls (P = 0.03; F1,16 = 5.4; two-way ANOVA [treatment]; Figure 2D). This additional sleep two days after remote preconditioning was primarily comprised of NREM sleep (P = 0.049; F 1,16 = 4.5; two-way ANOVA [treatment]; Figure 2E). Thus, remote preconditioning that results in neuroprotection also increases sleep.
Figure 2.
NREM sleep increases across the light-phase two days after remote preconditioning. (A) Representative hypnograms of wake (W), NREM sleep (N), and REM sleep (R) across the 12 h light-phase/12 h dark-phase in preconditioned mice (remote preconditioning) and sham (anesthetized) mice. ZT 12, lights-off; ZT 24, lights-on. Means ± SEM percent time spent awake or asleep across the 12 h dark-phase (B) and 12 h light-phase (D) and time spent in either REM or NREM sleep across the 12 h dark-phase (C) and 12 h light-phase (E). **P < 0.05, two-way ANOVA with Dunn-Sidak multiple comparison. n = 5 per treatment.
Sleep Gain Following Remote Preconditioning is Necessary for Neuroprotection
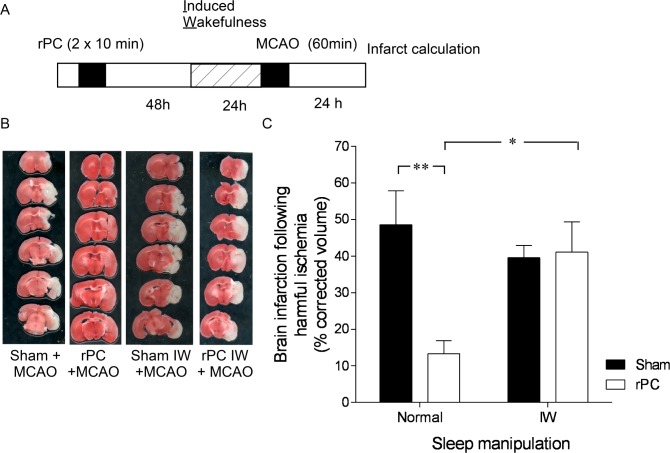
Next, we sought to identify if the additional sleep was necessary for the ability of remote preconditioning to protect the brain against modeled ischemic stroke. Two days after remote preconditioning, we prevented increases in sleep using an induced wakefulness procedure (Figure 3A). Induced wakefulness consisted of mild cage rattling and poking for 5 h during the light-phase. A dose-response curve from a previous study found that 5 h of induced wakefulness is necessary to deprive mice of 2.4 h of sleep.15
Figure 3.
Neuroprotection from stroke is lost with deprivation of induced sleep two days after preconditioning. (A) Experimental timeline for induction of remote preconditioning, induced wakefulness (IW), and modeled ischemic stroke via middle cerebral artery occlusion (MCAO). (B) Representative coronal sections showing the absence of 2,3,5-triphenyltetrazolium hydrochloride staining in infarcted tissue (white area) 24 h after modeled ischemic stroke in preconditioned and sham (anesthetized) mice subjected to IW or left undisturbed. (C) Means ± SEM infarcted tissue in preconditioned and sham (anesthetized) mice subjected to IW or left undisturbed. **P < 0.05, two-way ANOVA with Dunn-Sidak multiple comparison. n = 6 per treatment.
Remarkably, the brain was less tolerant to modeled ischemic stroke in mice that underwent induced wakefulness. Brain infarct volume in preconditioned mice subjected to induced wakefulness was 40.2 ± 9.2% compared to 12.1 ± 1.6% in preconditioned mice left undisturbed (P = 0.02; F1,20 = 6.3; two-way ANOVA; Figure 3B, 3C). Brain infarct volume did not change in sham controls subjected to induced wakefulness; infarcted tissue averaged 40.2 ± 9.8% in sham-treated animals subjected to the induced wakefulness procedure and 48.6 ± 15.5% in sham-treated animals not subjected to the induced wakefulness procedure (P > 0.05, post hoc; Figure 3C).
Remote Preconditioning Does Not Affect Sleep or Promote Neuroprotection in Bmal1 Knockout Mice
In the next set of experiments, we sought to determine whether Bmal1, a direct regulator of both circadian rhythms and sleep, affected both ischemic tolerance and sleep gain following remote preconditioning. To approach this, we measured sleep and brain infarct volume in Bmal1 KO mice. Baseline levels of sleep and wakefulness in Bmal1 KOs in the present study were comparable to baseline levels reported by Laposky et al.10 Bmal1 KOs slept more than wild-type littermates (WTs; P = 0.0001; F1,23 = 39.4; two-way ANOVA). Total sleep represented 52.5 ± 1.4% of the 24-h recording for Bmal1 KOs compared to 41.1 ± 1.1% of the 24 h recording for WTs.
Two days after remote preconditioning, total sleep did not change from baseline levels in Bmal1 KOs; total sleep averaged 51.8 ± 1.7% of the 24 h recording period (P > 0.05; twoway ANOVA [time]; Figure 4C). There was no change in total sleep and wake from baseline levels in Bmal1 KOs subjected to sham procedure (P > 0.05; two-way ANOVA [time] Figure 4C). There was also no change in the temporal distribution of wake in 2 h intervals or the number of brief arousals from baseline levels in Bmal1 KOs subjected to remote preconditioning or sham procedure (P > 0.05; two-way ANOVA [time]).
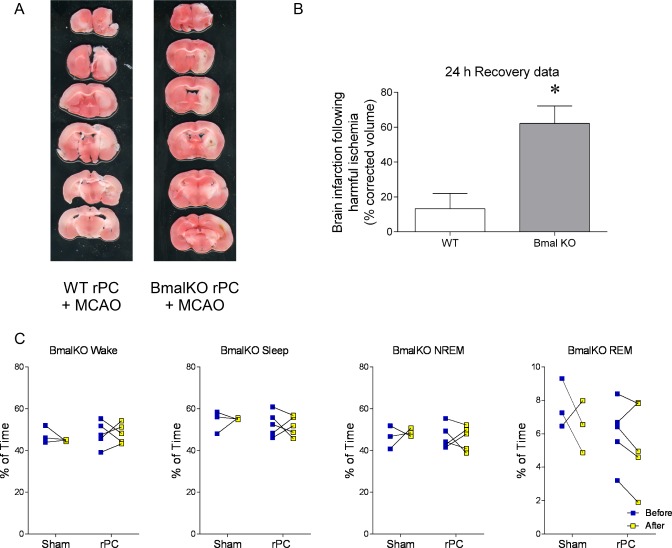
Figure 4.
Bmal1 knock-outs (KO) are not responsive to the sleep-promoting and neuroprotective actions of remote preconditioning. (A) Representative coronal sections showing the absence of 2,3,5-triphenyltetrazolium hydrochloride staining in infarcted tissue (white area) 24 h after modeled ischemic stroke in preconditioned mice bearing whole-body Bmal1 knock-out (KO) and wild-type (WT) littermates. These mice were not outfitted with EEG/EMG electrodes. (B) Means ± SEM infarcted tissue in preconditioned WTs (left) and Bmal1 KOs (right). (C) Percent time that Bmal1 KOs spent in wake, total sleep (NREM+REM), and NREM and REM sleep prior to and two days after remote preconditioning, or no remote preconditioning (sham) **P < 0.05, twoway ANOVA with Dunn-Sidak multiple comparison. n = 3 for shams, 4 for preconditioned.
Although Bmal1 KOs slept more than WTs prior to remote conditioning, the two strains had similar amounts of daily sleep after remote preconditioning; there were no differences in 24 h bout durations for wake (P = 0.32; unpaired t-tests) or NREM sleep (P = 0.18; unpaired t-tests) or bout counts for wake (P = 0.35; unpaired t-tests) or NREM sleep (P = 0.34; unpaired t-tests) between preconditioned WTs and Bmal1 KOs. However, preconditioned WTs did have one additional “siesta” compared to preconditioned Bmal1 KOs during the mid-active period of the light-phase (P = 0.048; F1,9 = 5.7; two-way ANOVA [time] (wake); P = 0.04; F1,9 = 5.1; two-way ANOVA [time] (NREM)).
Following the remote preconditioning procedure, Bmal1 KOs were less tolerant to modeled ischemic stroke and had significantly more infarcted tissue compared to WTs measured 24 h following MCAO (13.3 ± 3.6%, WTs; 62.2 ± 6.2%, KOs; P = 0.0001; unpaired t-test; Figure 4A, 4B). In addition, a similar effect of Bmal1 knockout preventing tolerance was observed when brain infarct volume was determined 72 h following MCAO: 11.4 ± 2.9% vs. 32.7 ± 3.8%; P = 0.0047; n = 6 and 3, respectively). Mortality after stroke was ∼50% for Bmal1 KOs regardless if the mice were preconditioned.
Despite Sleep Gain, Focal Brain Ischemia Increases Brief Arousals in Pre-Conditioned Mice
Previous studies in rodents not subjected to remote or focal preconditioning have shown that focal brain ischemia increases daily sleep but also increases sleep fragmentation.3–5,7 We report similar results as these previous studies following focal brain ischemia in preconditioned WTs; daily amounts of total sleep (NREM+REM) increased (33.6 ± 13.9% increase) from baseline levels: (P = 0.04; two-way ANOVA [time]) with a preference for NREM sleep (40.9 ± 17.0% increase from baseline levels; P = 0.04; two-way ANOVA [time]), but not REM sleep (P = 0.05; two-way ANOVA [time]). Despite a sleep gain, the number of brief arousals increased by ∼3.0-fold from baseline levels following focal brain ischemia (P = 0.01; twoway ANOVA [time]; 95.6 ± 25.8 vs. 256.6 ± 26.0). In preconditioned Bmal1 KOs, focal brain ischemia did not change wake or sleep from baseline levels (P > 0.05, all; two-way ANOVA).
DISCUSSION
In the present study, we report a 2.4 h increase in sleep in mice subjected to a remote preconditioning paradigm. We show tolerance to modeled ischemic stroke induced by remote preconditioning is lost: (1) when mice were prevented from getting 2.4 h of additional sleep in the form of more naps after remote preconditioning; and (2) in mice with a mutation that abolishes circadian rhythms and induces hypersomnia (∼ 2 h more spontaneous sleep compared to wild-type littermates).9,10 Overall, these results demonstrate that sleep is critical for the ability of remote preconditioning to protect against modeled ischemic stroke in mice.
Disruptive sleep can have both positive and negative effects on stroke outcome. Total sleep deprivation of a short duration (3–9 h) prior to ischemic brain injury has been reported as neuroprotective, reducing the extent of tissue injury.3,5 Conversely, sleep deprivation and sleep-wake fragmentation after ischemic brain injury has been shown to exacerbate brain injury and impede neurological recovery.4,6 In the present study, we found that increases in sleep after remote preconditioning improved tolerance to ischemic brain injury. We also found that the brain was less tolerant to modeled ischemic stroke if mice were prevented from gaining this additional sleep after remote preconditioning. These findings demonstrate that sleep contributes to the neuroprotective actions of remote preconditioning in promoting tolerance to modeled ischemic stroke and improving stroke outcome. But, the mechanisms of action are unclear.
We found that remote preconditioning selectively increases daily of amounts of non-rapid eye movement (NREM) sleep: a state of sleep characterized by tissue recovery, repair, and removal of metabolic waste. Similar to controls, daily amounts of rapid eye movement (REM) sleep changed ∼12 min from baseline levels (2%) in a few preconditioned mice, but remained stable following preconditioning in other mice. Thus, there was no overall main effect of preconditioning on REM sleep. There were also minimal changes in the consolidation of wake after remote preconditioning. These lack of effects suggest that additional sleep induced by remote conditioning was not a homeostatic rebound response. Since validating our model of remote preconditioning and its induction of sleep, the next step is to determine a mechanism of action1.
We also studied sleep induction in response to remote preconditioning and ischemic stroke in mice bearing whole-body Bmal1 knockout (KO). Previous studies suggest hippocampal Bmal1 does not change in mice subjected to ischemic brain injury.15 However, systemic Bmal1 ablation in smooth muscle cells impaired daily rhythms of vasoconstriction in mesenteric arteries, blood pressure and promotes vascular injury,17,18 which can affect the risk of hemorrhaging after modeled ischemic stroke. Beyond these studies, there is essentially no direct evidence for Bmal1 regulation of stroke outcome either in the absence or presence of any modality of preconditioning. Therefore, this study is the first to examine this relationship.
In the present study, we found that Bmal1 KO mice: (1) show no significant change in wake and total sleep following remote preconditioning; and (2) show no significant neuroprotection against modeled ischemic stroke following remote preconditioning. Collectively, these findings in Bmal1 KOs suggest that core clock genes may modulate the benefits of sleep during the neuroprotective phase of preconditioning. Since this mouse model sleeps more than WT mice (∼2 h/daily), this also suggests additional sleep induced by a mutation is not sufficient to protect the brain against modeled ischemic stroke.
Sleep following focal brain ischemia in rodents has been examined previously.3–5,7 In these studies, rodents were not subjected to preconditioning. The present study in preconditioned mice found similar changes in sleep parameters post-MCAO compared to these previous studies.3–5,7 This includes: (1) increases in non-rapid eye movement (NREM) sleep; and (2) increased sleep fragmentation identified in the present study through measures of brief arousals. In summary, this suggests that a sleep gain post-MCAO is likely not affected by remote preconditioning.
To summarize, we found that remote preconditioning induces sleep, and neuroprotection against focal brain ischemia is lost if a remote preconditioning- induced sleep gain is impeded. Remote preconditioning (rPC) has been investigated in a number of clinical trials for stroke,2 exercise performance,19 and myocardial infarction. While some studies suggest positive findings others show relatively poor efficacy.20,21 However, none of these trials of rPC have considered sleep gains or changes in sleep following rPC. Sleep disturbances are common in clinical settings due to patient monitoring. If sleep were to be interrupted, then any benefit of rPC would hypothetically be lost. The clear implication of our study is that sleep may need to be factored into the analysis of remote preconditioning studies.
DISCLOSURE STATEMENT
This was not an industry supported study. Federal financial support was provided by: F32HL116077 to Dr. Brager, R01NS078410 to Dr. Paul, U54NS060659 to Dr. Paul, G12MD007602 to Dr. Meller and Dr. Paul, P50HL117929 to Dr. Paul, and R01NS59588 to Dr. Meller. The authors have indicated no financial conflicts of interest. The work was performed at Morehouse School of Medicine, Atlanta, GA.
REFERENCES
- 1.Meller R, Simon R. A critical review of mechanisms regulating remote preconditioning-induced brain protection. J App Physiol. 2015;119:1135–42. doi: 10.1152/japplphysiol.00169.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–61. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 3.Moldovan M, Constantinescu AO, Balseanu A, Oprescu N, Zagrean L, Popa-Wagner A. Sleep deprivation attenuates experimental stroke severity in rats. Exp Neurol. 2010;222:135–41. doi: 10.1016/j.expneurol.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Gao B, Cam E, Jaeger H, Zunzunegui C, Sarnthein J, Bassetti CL. Sleep disruption aggravates focal cerebral ischemia in the rat. Sleep. 2010;33:879–87. doi: 10.1093/sleep/33.7.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cam E, Gao B, Imbach L, Hodor A, Bassetti CL. Sleep deprivation before stroke is neuroprotective: a pre-ischemic conditioning related to sleep rebound. Exp Neurol. 2013;247:673–9. doi: 10.1016/j.expneurol.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Weil ZM, Norman GJ, Karelina K, et al. Sleep deprivation attenuates inflammatory responses and ischemic cell death. Exp Neurol. 2009;218:129–36. doi: 10.1016/j.expneurol.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zunzunegui C, Gao B, Cam E, Hodor A, Bassetti CL. Sleep disturbance impairs stroke recovery in the rat. Sleep. 2011;34:1261–9. doi: 10.5665/SLEEP.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodor A, Palchykova S, Gao B, Bassetti CL. Baclofen and gamma-hydroxybutyrate differentially altered behavior, EEG activity and sleep in rats. Neuroscience. 2014;284:18–28. doi: 10.1016/j.neuroscience.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 9.Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 11.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 12.Pignataro G, Meller R, Inoue K, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:1040–7. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- 13.Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006;404:170–5. doi: 10.1016/j.neulet.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151:1099–103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brager AB, Ehlen JC, Castanon-Cervantes O, et al. Sleep loss and the inflammatory response under chronic environmental circadian disruption. PLoS One. 2013;8:e67532. doi: 10.1371/journal.pone.0063752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tischkau SA, Cohen JA, Stark JT, Gross DR, Bottum KM. Time-ofday affects expression of hippocampal markers for ischemic damage induced by global ischemia. Exp Neurol. 2007;208:314–22. doi: 10.1016/j.expneurol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anea CB, Zhang M, Stepp DW, et al. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–7. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch S, Della-Morte D, Dave KR, Sacco RL, Perez-Pinzon MA. Biomarkers for ischemic preconditioning: finding the responders. J Cereb Blood Flow Metab. 2014;34:933–41. doi: 10.1038/jcbfm.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tie HT, Luo MZ, Li ZH, et al. Remote ischemic preconditioning fails to benefit pediatric patients undergoing congenital cardiac surgery: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e1895–912. doi: 10.1097/MD.0000000000001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretti C, Cavallero E, D'Ascenzo F, et al. The European and Chines cardiac and renal remote ischemic preconditioning study (EUROCRIPS); study design and methods. J Cardiovasc Med (Hagerstown) 2015;16:246–52. doi: 10.2459/JCM.0000000000000098. [DOI] [PubMed] [Google Scholar]