Abstract
Study Objectives:
Sleep bruxism (SB) is characterized by tooth grinding and jaw clenching during sleep. Familial factors may contribute to the occurrence of SB. This study aims are: (1) revisit the prevalence and characteristics of SB in a large cross-sectional survey and assess familial aggregation of SB, (2) assess comorbidity such as insomnia and pain, (3) compare survey data in a subset of subjects diagnosed using polysomnography research criteria.
Methods:
A sample of 6,357 individuals from the general population in Quebec, Canada, undertook an online survey to assess the prevalence of SB, comorbidities, and familial aggregation. Data on familial aggregation were compared to 111 SB subjects diagnosed using polysomnography.
Results:
Regularly occurring SB was reported by 8.6% of the general population, decreases with age, without any gender difference. SB awareness is concomitant with complaints of difficulties maintaining sleep in 47.6% of the cases. A third of SB positive probands reported pain. A 2.5 risk ratio of having a first-degree family member with SB was found in SB positive probands. The risk of reporting SB in first-degree family ranges from 1.4 to 2.9 with increasing severity of reported SB. Polysomnographic data shows that 37% of SB subjects had at least one first-degree relative with reported SB with a relative risk ratio of 4.625.
Conclusions:
Our results support the heritability of SB-tooth grinding and that sleep quality and pain are concomitant in a significant number of SB subjects.
Citation:
Khoury S, Carra MC, Huynh N, Montplaisir J, Lavigne GJ. Sleep bruxism-tooth grinding prevalence, characteristics and familial aggregation: a large cross-sectional survey and polysomnographic validation. SLEEP 2016;39(11):2049–2056.
Keywords: sleep bruxism, prevalence, heritability, familial aggregation, pain
Significance.
This is the largest comprehensive cross-sectional study for the prevalence, characteristics, and familial aggregation of sleep bruxism (SB). Findings were further validated in polysomnography diagnosed SB subjects. Sleep bruxism is regularly reported by 8% of the general population and was shown to be concomitant with trouble maintaining sleep and the presence of chronic pain. Pre-menopausal woman present a high increase in the report of SB. SB was also shown to aggregate in families, but even more in the presence of chronic pain in a gender-specific way. This study sheds the light on the importance of chronic pain and gender in the report of first-degree relatives with SB.
INTRODUCTION
Sleep bruxism (SB) is a stereotyped sleep-related movement disorder characterized by teeth grinding and jaw clenching during sleep. SB mainly occurs in light sleep stages or during sleep transition periods.1 The major consequences of SB are headaches, tooth wear, and complaints from bed partner due to grinding noises.2 Its prevalence ranges from 5% to 8% in the general population and tends to decrease with age.3,4
The presence of orofacial pain is reported in a subgroup of subjects with SB, but the relationship between pain and SB is still unclear.5 These patients may report headaches, temporomandibular disorders (TMD), and pain in masticatory muscles upon awakening.1,6 A cross-sectional study showed that muscle activity during sleep was a good predictor for painful TMD in adolescents.7 However, other studies have found that low levels of SB are more correlated with TMD than higher levels of SB.8,9 It was also previously reported that as much as 70% of TMD patients with myofascial pain also showed evidences of rhythmic masticatory muscle activity (RMMA) upon clinical examination and ambulatory sleep recordings.10
SB may co-occur with other sleep disturbances including sleep apnea, insomnia, and restless legs syndrome.3 However the relationship between SB and sleep disturbances is weak and still under debate.11,12 As reported by questionnaires, SB is associated with obstructive sleep apnea in some children.13 In the case of sleep disorder breathing, SB is thought to be concomitant, but the cause to effect is still undetermined.14
Occurrence of SB is suspected to have a hereditary component. As early as 1966, it was established that the incidence of SB in blood relatives was high. In that study, SB was only diagnosed using questionnaires.15 In another study, 117 pairs of twins, 28 monozygotic (MZ) and 89 dizygotic (DZ) were diagnosed with SB from clinical examination using bruxo-facets as main criteria. No difference between twins and corresponding non-twins was found. However, the concordance rate for the pattern of mastication was 0.97 (MZ) and 0.61 (DZ).16 It was also observed that SB is a persistent trait between childhood and adulthood and that it can be attributed to genetic component.17 More recently, a study done in the Finnish twin cohort reported that genetic factors account for half of the variation in phenotypic liability to SB.18 The same results were also found in Japanese twins.19 In the studies cited above, with the exception of the last one, the diagnosis of SB used to assess heritability was based on self-report or clinical criteria; the absence of polysomnographic recording to confirm SB using validated research methodology restrains extrapolation of these findings.
In the present study, our aims are to: (1) revisit the prevalence of reported sleep bruxism-tooth grinding awareness using a cross-sectional survey from the general population; (2) determine the association between SB-tooth grinding awareness with other comorbidities including chronic pain, pain medication use, and sleep disturbances; (3) estimate the relative risk of reporting SB in first-degree relatives of SB pro-bands, using previously recorded sleep laboratory data from a cohort of young SB-tooth grinding adults using diagnosis validated research criteria.
METHODS
Cross-Sectional Survey
Using a representative sample of 6,357 adult individuals from the general population in Quebec, Canada, an online survey was undertaken in October 2015 to assess the prevalence of SB. The logistics of the survey were the responsibility of a private firm (CROP Inc. Montréal, Canada). The sampling was chosen to match as closely as possible the regional distribution in the province of Quebec with regards to age, gender, place of residency, spoken language, and years of education. The sample consisted of 3,087 males and 3,270 females, and the age distribution was as follows: 18–24 (11%); 25–34 (16%); 35–44 (16%); 45–54 (20%); 55–64 (17%), and 65+ (20%). The main language spoken at home was French for 85%, English for 12%, and other for 3%. All questions were provided in French and in English.
To determine the prevalence of SB-tooth grinding in the general population, we selected questions validated to use for screening of SB. Table S1 in the supplemental material lists the questions of the survey. For the question: “Do you grind your teeth during your sleep?” four possible answers could be chosen from “Regularly, on occasions, rarely, and never.” SB probands were grouped as positive probands when they answered “regularly” and “on occasions” and as negative pro-bands when they answered “rarely” and “never.” Another set of questions were designed to estimate the frequency of other symptoms pertaining to SB comorbidities, such as pain and insomnia. Finally, the prevalence of reporting SB-tooth grinding awareness by first-degree relatives was collected in order to determine the familial aggregation of SB.
Comparisons between groups of SB probands were made using a Pearson χ2 test (IBM SPSS Statistics version 22). Effect sizes were assessed by Cramer V coefficients, where values between 0 and 0.1 were considered weak, between 0.1 to 0.3 were considered small, between 0.3 to 0.5 medium, and ≥ 0.5 were considered to have a large effect. Odds ratio (OR) and their 95% confidence interval were calculated to determine the association between comorbidities and reported SB-tooth grinding in SB positive and negative probands.
The percentage of first-degree relatives with reported SB was estimated for each group of positive and negative pro-bands. The relative risk ration (λ) was calculated by dividing the prevalence of reported SB in first-degree relatives by the prevalence of reported SB in the general population. We calculated the crude recurrence risk as the ratio between the number of affected relatives and the total number of relatives.
The association between reported SB in first-degree relatives, frequency of SB, gender, and chronic pain were evaluated using binary logistic regression model. The outcome variable was report of SB in first-degree relatives. Predictors in the model included SB reported frequencies groups (dummy coded), no pain status, and male as reference values for chronic pain status and gender respectively. The predictors were introduced in the equation according to the Hosmer-Lemeshow goodness-of-fit of the model.
Polysomnographic Validation
For the second part of this study, we used polysomnographic data collected in a sleep laboratory from recruited subjects suffering from teeth grinding during sleep.8,20 Subjects were young and healthy adults recruited by advertising in our dental clinic as well as our campus at the Université de Montréal and were selected based on a history of jaw discomfort, dental tooth wear or sleep partner complaint of SB, and tooth-grinding history (> 3 nights/week). Subjects were invited to spend 2 nights of polysomnography in order to confirm and validate the presence of sleep bruxism, the first night for habituation and the second night for diagnosis. Absence of other sleep disorders was confirmed by the first night of polysomnographic recordings and the second night was used for SB diagnosis and data analysis. This study was approved by the ethics committee of our hospital. All subjects signed an informed consent.
Polysomnography using surface electrodes included electroencephalography (EEG), electromyography (EMG) on jaw muscles (masseters and temporalis), as well as video and audio recording described in details elsewhere.8,21 The following research criteria were used to validate the presence of SB: > 4 RMMA episode/h of sleep, > 25 EMG bursts/h of sleep and > 1 episode with grinding noise. The diagnosis of SB was confirmed in 111 subjects.
Diagnosed SB subjects were classified into 3 groups based on criteria established by Rompré et al.8 using SB-RMMA frequency. High (n = 27), moderate (n = 34), and low (n = 50) groups were defined using cluster analysis. The high frequency group had more than 50 EMG bursts/h of sleep; in the moderate group, they had between 25 and 50 EMG bursts/h of sleep; and the low group had less than 25 EMG bursts/h of sleep and the presence of noise.
Structured questionnaires were used to identify potential family members with SB. A total of 20.7% of subjects had missing answers on questionnaires on family members, and therefore were excluded from the percentage and relative risk calculations.
A one-way ANOVA was done to assess the difference among the 3 groups of SB-RMMA frequencies. Results were reported as mean with standard deviation. The relative risk ration (λ) was calculated by dividing the prevalence of reported SB in first-degree relatives by the prevalence of reported SB in the general population.
RESULTS
Cross-Sectional Survey
Prevalence of Self-Reported SB-Tooth Grinding
The self-reported prevalence of SB-tooth grinding is shown in Table 1. SB-tooth grinding was reported to occur regularly in 8.6% and on occasion in 13.7% of the general population. The SB positive probands account for 22.3% of the total population surveyed. Although there is a statistically significant difference in the frequency of the reported SB by males and females (Pearson χ2(3) = 10.95, P = 0.01), this difference is driven by those reporting regularly as opposed to rarely and the association between gender and frequency is weak (V = 0.04). When we combine the groups, results show that there is no difference in reported SB between genders; 46.5% of males belong to the SB positive probands group as opposed to 49.1% of males in the SB negative probands group (P = 0.09). The prevalence of SB tends to decrease with age whereas the report of SB positive probands is 30% in 18–34 y, 25% in 35–54 y, and 14% in 55+ y. As shown in Figure 1, females tend to have a peak of reported SB in the age group 45–54, which decreases at an older age.
Table 1.
Prevalence of demographic variables in reported SB-tooth grinding awareness frequencies.
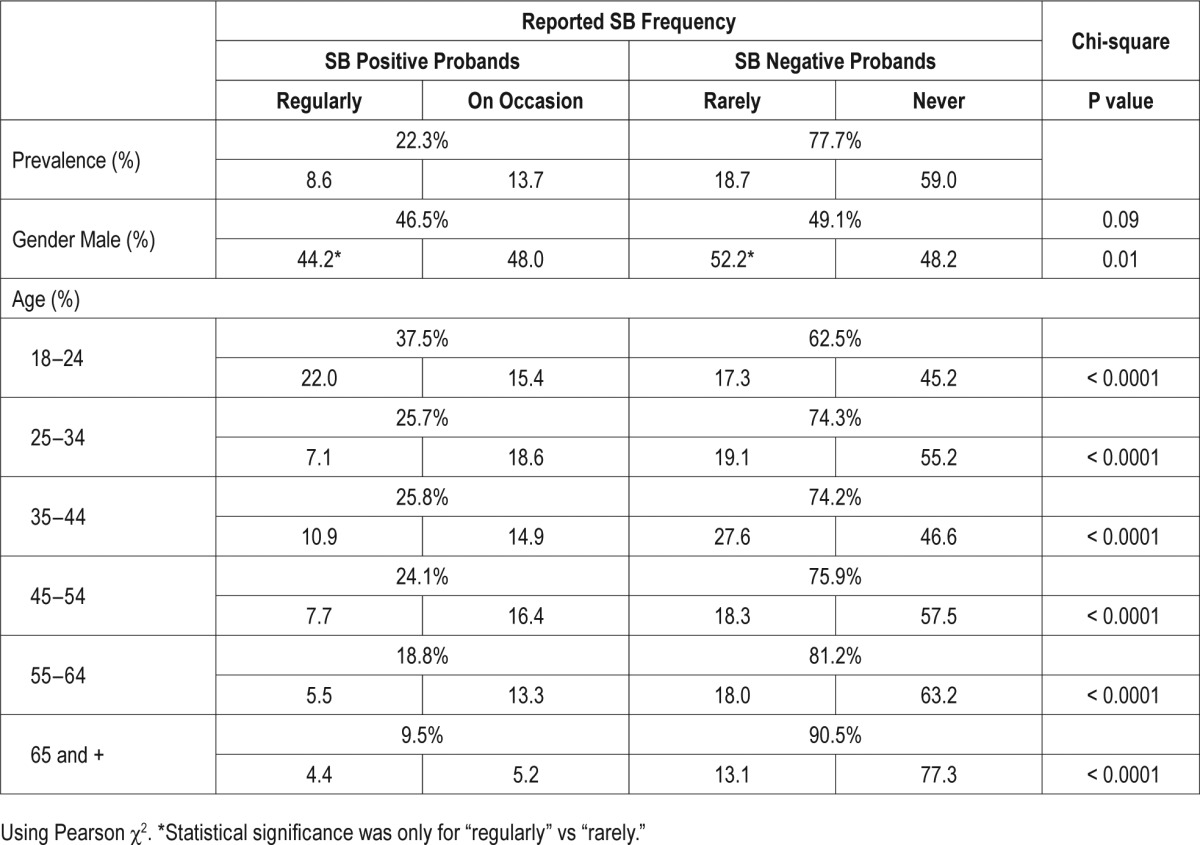
Figure 1.
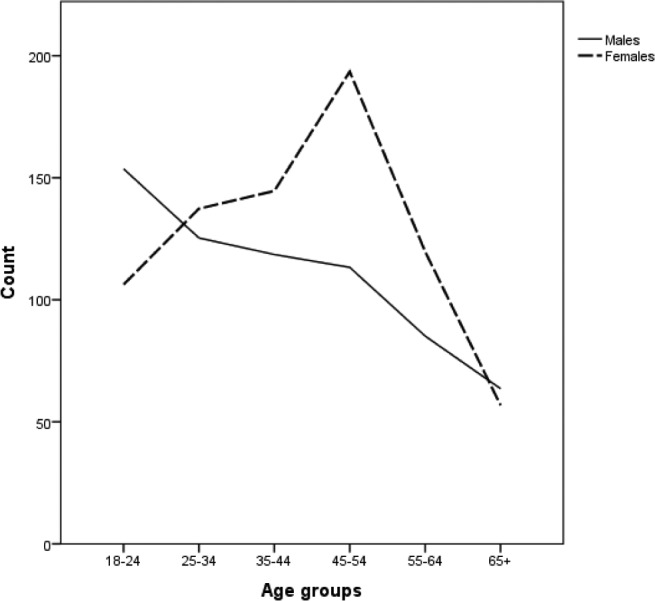
Age distribution of positive sleep bruxism probands by gender. The number of sleep bruxism positive probands in each age group, separated by gender is plotted.
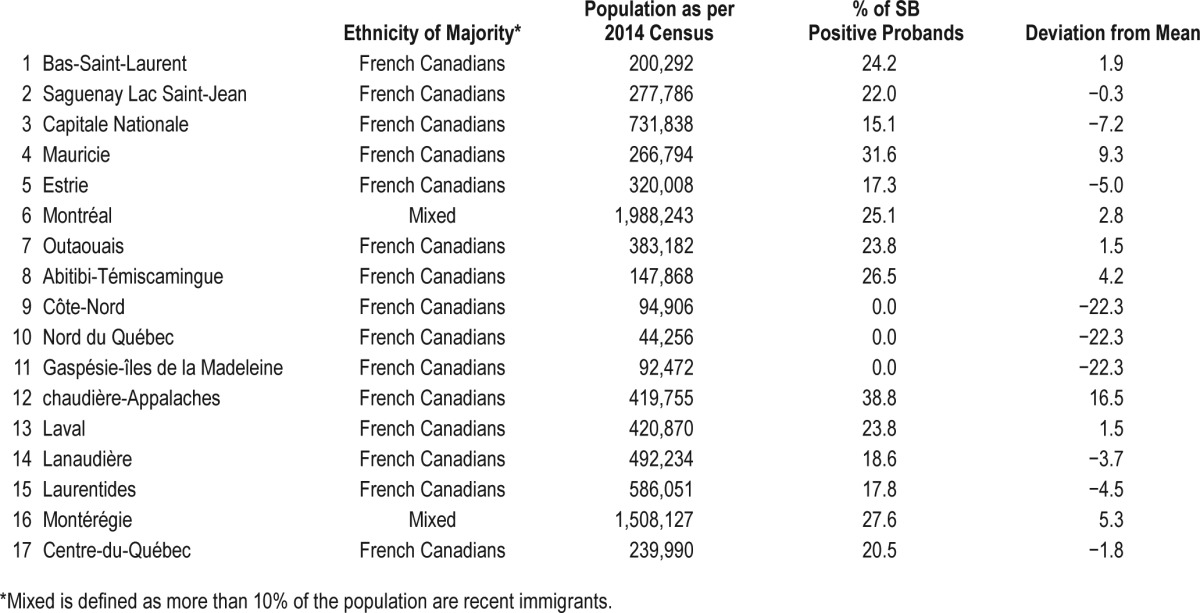
The highest prevalence of SB was reported in mid-sized cities (namely Chaudière-Appalaches, Mauricie, and Montérégie regions) (Table 2). These administrative regions are populated in majority by French Canadians issued from French ancestry descendants. Large metropolitan cities, where population is composed of diverse ethnicities, did not show more than average number of SB positive probands. Interestingly, regular and occasional SB were never reported (0%) in cities with a population of less than 100,000. There was no statistically significant difference in SB probands with regards to language spoken.
Table 2.
Distribution of SB positive probands in administrative regions in the province of Quebec.
Co-occurrence with Sleep Disturbances and Chronic Pain
In general, there is no difference in questionnaire report for sleep satisfaction between groups of different frequencies of reported SB. Among those reporting regular SB, most are satisfied with their sleep (30% are very satisfied, 33% are somewhat satisfied, 29% are little satisfied, and 8% are not satisfied).
Interestingly, SB positive probands show no difficulty falling asleep and in early awakening in comparison with SB negative probands. However, the prevalence of SB positive probands reporting more difficulties maintaining sleep was a little higher, at 47.6% in SB positive probands vs. 41.6% in negative SB proband subjects (χ2(1) = 16.2; P < 0.0001; V = 0.05; OR = 1.3; 95% CI (1.1–1.4)). Among the SB positive probands reporting difficulties maintaining sleep, 2.4% reported extremely severe difficulties, 20.3% very severe difficulties, 38.9% somewhat severe difficulties, 33.1% not very severe, and 5.3% not at all severe (χ2(4) = 74.6; P < 0.0001; V = 0.17).
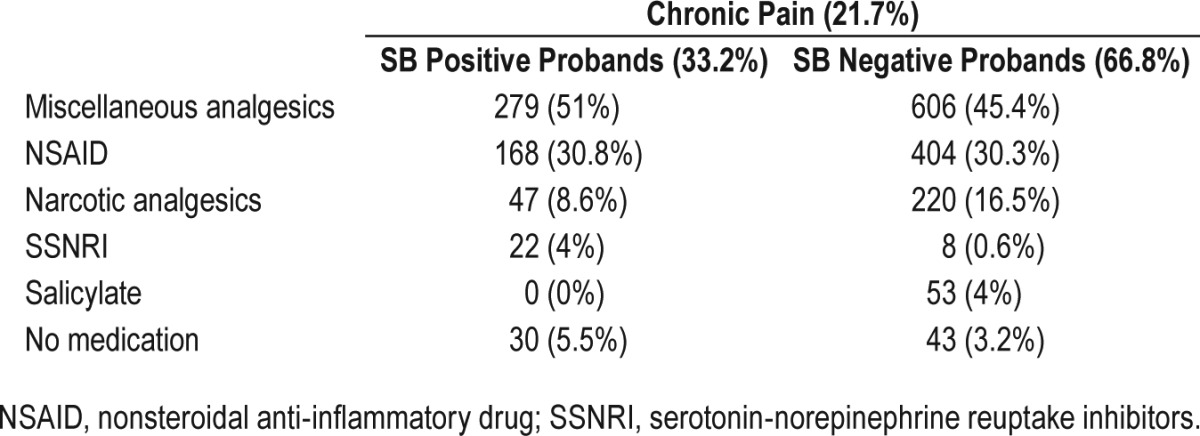
Thirty-three percent of SB positive probands reported pain in the last three months as compared with 19% for the SB negative probands (χ2(1) = 120.3; P < 0.0001; OR = 2.1 95% CI (1.8– 2.4)). The use of pain medication was assessed only in those who reported chronic pain, as those that did not suffer from chronic pain did not report taking any pain medication. From those who suffer from chronic pain, SB positive probands are much less medicated (n = 516) than SB negative probands (n = 1,291). SB positive probands use two times less narcotics (opioids) than SB negative probands, although this difference is not statistically significant (Table 3).
Table 3.
Medication classes use in individuals with chronic pain only.
Familial Aggregation of SB
The frequencies of reported SB by first-degree relatives are shown in Table 4. Overall, 21% of SB positive probands reported first-degree relatives with SB, as opposed to 10% of SB negative probands (χ2(1) = 141.2; P < 0.0001; V = 0.15). The proportion of family members with SB is increased with the severity of the frequency of reported SB. For instance, 25% of those reporting regular SB have first-degree relatives with SB, 18.9% of those with occasional SB have first-degree relatives with SB, 18.5% of those reporting SB rarely have first-degree relatives with SB, and finally, 6.9% of those never reporting SB have first-degree relatives with SB. These differences in proportions are statistically significant (χ2(3) = 266.3; P < 0.0001; V = 0.2).
Table 4.
Familial reports of SB-tooth grinding by first-degree relatives.
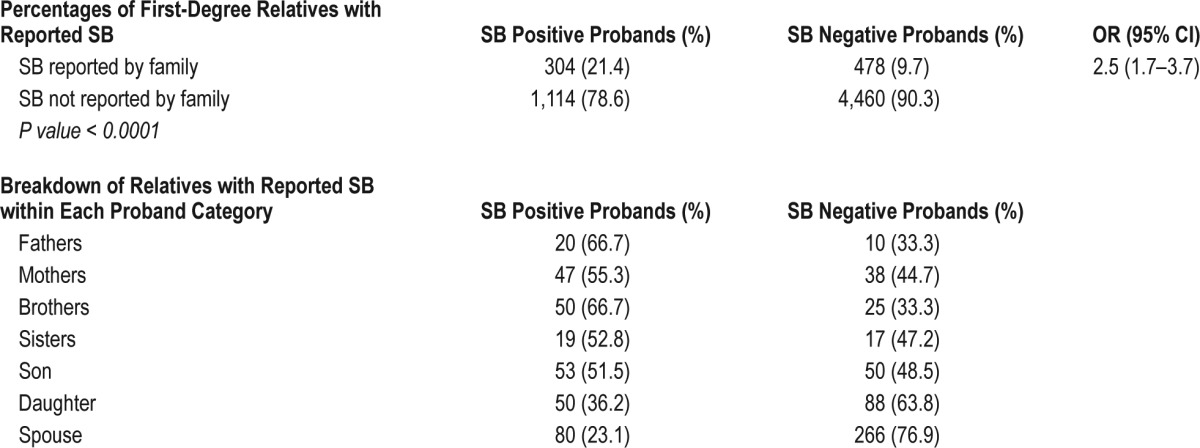
In any given sample, regardless of the SB proband's status, 12% of respondents claim that at least one family member grinds their teeth during sleep, with the majority being spouses and children.
Population sample data shows that there is a positive correlation between self-report of sleep bruxism and having a first-degree relative with sleep bruxism (Pearson χ2 = 23.12 (P < 0.0001)). The relative risk of a SB positive proband having a family member reporting SB is 2.5 (95% CI: 1.7–3.7). The crude recurrence risk of reporting SB in relatives is 0.22. The relative risk ration (λ) is 1 in SB positive probands but is increased with severity, for instance, the relative risk is 2.9 in the regularly reporting group, λ = 1.4 in the occasionally reporting group, λ = 1 in the rarely reporting group, and λ = 0.4 in the never reporting group.
In this section, we investigated whether the predisposition for SB positive probands to report first-degree relatives with SB can be modulated by gender and chronic pain by means of a 3-way interaction analysis. This analysis was conducted with the SB reported frequencies groups dummy coded in the model, and with no chronic pain status and male as the reference values for chronic pain status and gender respectively. Results are presented in Table 5.
Table 5.
Binary logistic regression for the report of first degree relatives with SB.
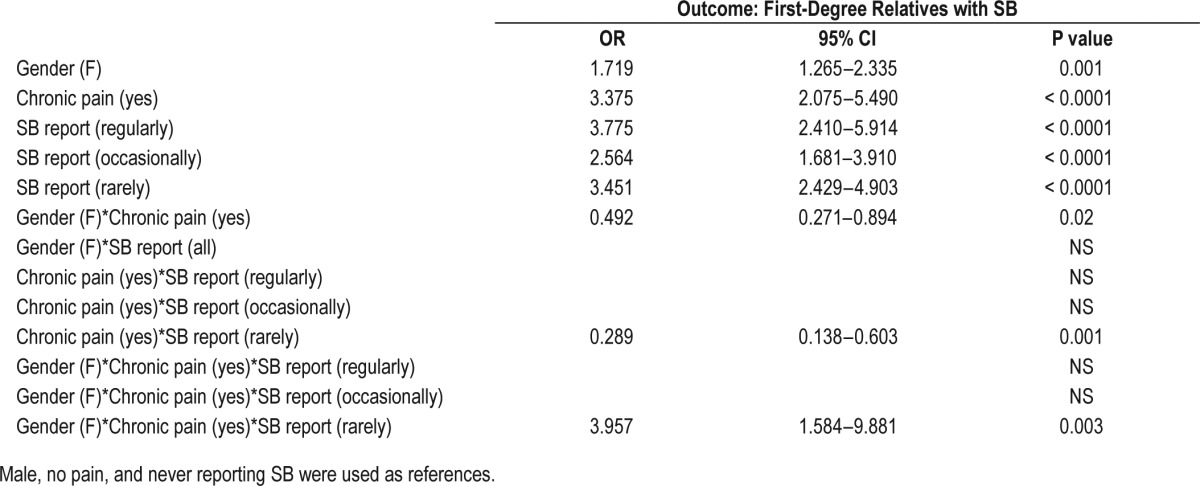
The regression model with introduced gender, chronic pain, and SB frequency explains 11.3% of the variation in having a first-degree relative with SB. Among subjects without pain and never reporting SB, females report more first-degree relatives with SB than males (OR = 1.72; 95% CI: 1.27–2.34; P = 0.001). Among males never reporting SB, chronic pain subjects also have more first-degree relatives with SB than those reporting no chronic pain (OR = 3.38; 95% CI: 2.08–5.49; P < 0.0001). Among males without pain, the odds of having a first-degree relative with SB are higher in subjects with self-reported SB. The group that reports SB frequently have an OR = 3.76; 95% CI: 2.41–5.91; P value < 0.0001 of having a family member with SB. Those reporting occasionally and rarely present an OR = 2.56; 95% CI: 1.68–3.91 and OR = 3.45; 95% CI: 2.43– 4.90, respectively (P < 0.0001).
A two-way interaction between gender and chronic pain shows that the effect of having chronic pain in females on the odds to report a first-degree family member with SB is lower than in males (OR = 0.49; 95% CI: 0.27–0.89; P value = 0.02). Also, another significant two-way interaction shows that the effect of having chronic pain in those reporting rarely SB on the odds to report first-degree family member with SB is lower than in those not reporting SB. Finally, the three-way interaction shows that the effect of reporting SB rarely with chronic pain in women increases the chance of having a first-degree relative with SB compared with men. (OR = 3.96; 95% CI: 1.58–9.85; P = 0.003).
Polysomnographic Validation
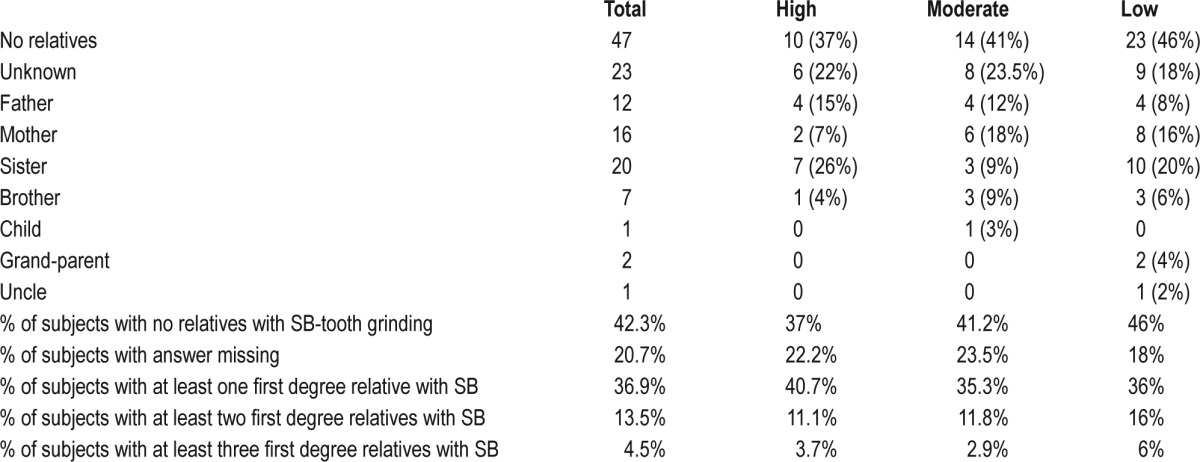
Polysomnography was used to diagnose SB in 111 subjects according to validated research criteria. Three groups were generated according to RMMA frequency. High, moderate. and low frequency SB-RMMA groups were homogeneous and comparable; they did not differ between gender or mean age of subjects (see Table 6). When asked about first-degree relatives, 37% of SB probands reported having first-degree relatives with SB (25.2% parents, 24.3% siblings, 2.7% extended family, 0.9% offspring). The percentage of reported relatives with SB was similar among high, moderate, and low frequency SB-RMMA groups (high: 40.7%, moderate: 35.3%, and low: 36%). Overall, the relative risk ratio (λ) of first-degree relative report of SB is 4.625 (see Table 7).
Table 6.
Characteristic of subjects within each SB-RMMA frequency groups.
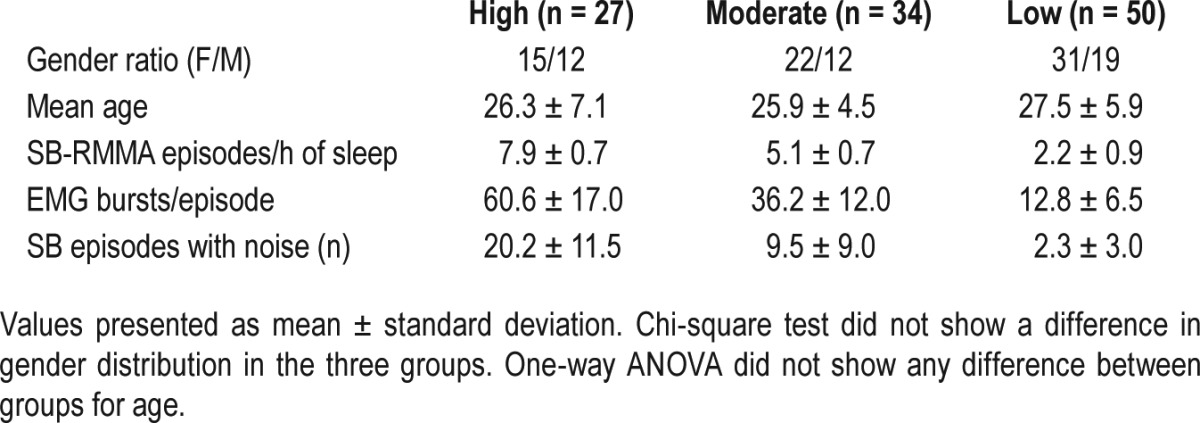
Table 7.
SB probands' report of first-degree relatives with SB.
DISCUSSION
With more than 6,000 individuals surveyed, this is the largest structured cross-sectional study undertaken to assess characteristics and heritability of self-reported SB. By using a large populational survey, this study was able to confirm previously reported characteristics of probable SB and provided novel information related to the subjective assessment of this parasomnia using questionnaires. First, the prevalence of regularly reported SB was established at 8.6% in the general population. This prevalence is in line with what was previously reported in smaller studies and in different sets of populations with similar age ranges.4 Our results also confirmed that there is no gender difference in the report of SB and that its prevalence decreases with age. An interesting augmentation in the prevalence of reported SB was noted in women aged 45–54 y. To our knowledge, this is the first peak in reported SB in women in this age group. This age range in women corresponds to hormonal changes, an increase in degenerative jaw bone disorders, and TMD.22,23 One can speculate that all these factors combined might exacerbate this increase in SB, or that some other factors, like pain and insomnia, are actually driving this increase. On the other hand, the three-way interaction analysis also shows that women with chronic pain have a higher chance of having first-degree relatives with SB, even if they do not report SB.
The geographic distribution of reported SB is in line with previous assumptions that there might be a founder's effect in the transmission of this trait. The regions where SB is most prevalent are constituted mainly of French Canadians from Quebec that derive from a founder's effect, and not recent immigrants and/or Native Americans.24 This finding supports the previously suggested hypothesis that SB might be attributed to a genetic component.17
Sleep satisfaction does not seem to be a complaint in the presence of SB. In fact, most SB probands report that they are very satisfied with their sleep quality. Interestingly, their complaint of insomnia is specifically related to complaints of maintaining sleep and not initiation or early awakening. This result could be explained by the higher sleep instability shown in SB as opposed to healthy controls during the phase A3 of their cyclic alternating pattern,25 as well as the related-arousal.26
The relationship between SB and report of chronic pain is difficult to assess. Chronic pain is reported more by SB positive probands; however, this group is less medicated than the SB negative probands. The major limitation in this association is that the nature of the pain is not specified and therefore a myriad of complaints could be included. The nature of the interaction between SB and chronic pain cannot be extrapolated to causality or consequence. Central pathophysiological mechanisms are considered to play a role in the initiation of SB,1,27 and many psychological factors like stress and anxiety seem to exacerbate SB, leading to a complex pattern of combined states. In this study, the gender difference also seems to play a major role in SB report, familial aggregation of SB, and chronic pain.
In the survey of medications taken by SB probands that suffer from chronic pain, it was striking to note that almost three times more SB positive probands were on SNRIs than those without SB. Many case studies have reported that SB can emerge following the administration SSRI and SNRI, namely duloxetine, paroxetine and fluoxetine but large controlled studies are lacking.28–32
Evidence has emerged showing that familial factors may contribute to the occurrence of SB, and this conclusion is important and opens the door for further genetic investigation. In fact, results show that relatives of SB positive probands have an increased risk of SB awareness compared to relatives of SB negative probands. The crude recurrence risk of reporting SB in positive probands is around 2, and is proportional to the severity of SB. Also, SB probands show a 2.9 relative risk of having a first-degree relative with reported SB. This relative risk is augmented to 4.6 when the diagnosis of SB is established using polysomnography.
We observed that one third of SB subjects diagnosed by polysomnography report at least one first-degree relative also suffering from SB. However, the severity of SB, measured by RMMA frequency, does not affect the percentage of affected family members with SB.
Recently, a group from Japan proposed a weak association of wake and sleep bruxism with a gene coding for serotonin receptor 2A (HTR2A),33 a nonspecific biomarker of several behavioural and cognitive functions. This study, along with many other heritability studies, justifies the investigation of genetic factors predisposing to SB alone as well as with the presence of comorbid sleep and pain complaints.
This study presents a methodological dichotomy that limits data extrapolation. In the cross-sectional survey, the assessment of chronic pain was not limited to the jaw or headaches. We therefore could not identify if there is an association between SB and TMD. In the second part using polysomnographic recording, as well as in the survey, we used self-reported awareness to assess familial co-occurrence of SB-tooth grinding. We recognize that self-reports have a limited capacity to detect true presence of SB in blood relatives.3,34
In conclusion, due to the large population surveyed, this study should represent a landmark in the field of sleep bruxism and should open the door for future studies on comorbidities between SB, sleep maintenance, gender, and chronic pain. This study should also present an argument in favor of future investigations of the genetics of SB. The high probability that heterogeneous environmental effects or other comorbidities like insomnia and pain are contributing to the genesis of SB suggests that powerful analysis models will be necessary to isolate the most relevant causes or mechanisms responsible for SB.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by the Canadian Institute for Health Research (CIHR). Dr. Lavigne holds a Canada Research Chair. Dr. Huynh is a FRQS Research Fellow. The authors have indicated no financial conflicts of interest. This cross-sectional survey was performed at the faculty of dental medicine; polysomnography was performed at the centre for advanced research in sleep medicine.
ACKNOWLEDGMENTS
The authors thank Christiane Manzini for data management, Diane Landry for data entry, Francine Guitard and Regis Schwab for sleep bruxism scoring, and Pierre Rompré for statistical advice.
REFERENCES
- 1.Carra MC, Huynh N, Lavigne GJ. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387–413. doi: 10.1016/j.cden.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Lobbezzo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40:2–4. doi: 10.1111/joor.12011. [DOI] [PubMed] [Google Scholar]
- 3.Maluly M, Andersen ML, Dal-Fabbro C, et al. Polysomnographic study of the prevalence of sleep bruxism in a population sample. J Dent Res. 2013;92:97S–103S. doi: 10.1177/0022034513484328. [DOI] [PubMed] [Google Scholar]
- 4.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–43. [PubMed] [Google Scholar]
- 5.Camparis CM, Siqueira JT. Sleep bruxism: clinical aspects and characteristics in patients with and without chronic orofacial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:188–93. doi: 10.1016/j.tripleo.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Mayer P, Heinzer R, Lavigne G. Sleep bruxism in respiratory medicine practice. Chest. 2016;149:262–71. doi: 10.1378/chest.15-0822. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes G, Franco-Micheloni AL, Siqueira JT, Goncalves DA, Camparis CM. Parafunctional habits are associated cumulatively to painful temporomandibular disorders in adolescents. Braz Oral Res. 2016;30 doi: 10.1590/1807-3107BOR-2016.vol30.0015. doi: 10.1590/1807-3107BOR-2016.vol30.0015. [DOI] [PubMed] [Google Scholar]
- 8.Rompré PH, Daigle-Landry D, Guitard F, Montplaisir JY, Lavigne GJ. Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res. 2007;86:837–42. doi: 10.1177/154405910708600906. [DOI] [PubMed] [Google Scholar]
- 9.Lavigne GJ, Rompré PH, Montplaisir JY, Lobbezoo F. Motor activity in sleep bruxism with concomitant jaw muscle pain. A retrospective pilot study. Eur J Oral Sci. 1997;105:92–5. doi: 10.1111/j.1600-0722.1997.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmitter M, Kares-Vrincianu A, Kares H, Bermejo JL, Schindler HJ. Sleep-associated aspects of myofascial pain in the orofacial area among temporomandibular disorder patients and controls. Sleep Med. 2015;16:1056–61. doi: 10.1016/j.sleep.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Saito M, Yamaguchi T, Mikami S, et al. Weak association between sleep bruxism and obstructive sleep apnea. A sleep laboratory study. Sleep Breath. 2016;20:703–9. doi: 10.1007/s11325-015-1284-x. [DOI] [PubMed] [Google Scholar]
- 12.Manfredini D, Guarda-Nardini L, Marchese-Ragona R, Lobbezoo F. Theories on possible temporal relationships between sleep bruxism and obstructive sleep apnea events. An expert opinion. Sleep Breath. 2015;19:1459–65. doi: 10.1007/s11325-015-1163-5. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira NM, Dos Santos JF, Dos Santos MB, Marchini L. Sleep bruxism associated with obstructive sleep apnea syndrome in children. Cranio. 2015;29:1–5. doi: 10.1080/08869634.2015.1097299. [DOI] [PubMed] [Google Scholar]
- 14.Balasubramaniam R, Klasser GD, Cistulli PA, Lavigne GJ. The link between sleep bruxism, sleep disordered breathing and temporomandibular disorders: an evidence-based review. J Dent Sleep Med. 2014;1:27–37. [Google Scholar]
- 15.Reding GR, Rubright WC, Zimmerman SO. Incidence of bruxism. J Dent Res. 1966;45:1198–204. doi: 10.1177/00220345660450042701. [DOI] [PubMed] [Google Scholar]
- 16.Lindqvist B. Bruxism in twins. Acta Odontol Scand. 1974;32:177–87. doi: 10.3109/00016357409002546. [DOI] [PubMed] [Google Scholar]
- 17.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Sleep bruxism based on self-report in a nationwide twin cohort. J Sleep Res. 1998;7:61–7. doi: 10.1046/j.1365-2869.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 18.Rintakoski K, Hublin C, Lobbezzo F, Rose RJ, Kaprio J. Genetic factors account for half of the phenotypic variance in liability to sleep-related bruxism in young adults: a nationwide Finnish twin cohort study. Twin Res Hum Genet. 2012;15:714–9. doi: 10.1017/thg.2012.54. [DOI] [PubMed] [Google Scholar]
- 19.Takaoka R, Ishigaki S, Yatani H, Ogata S, Hayakawa K. Evaluation of genetic factors involved in nocturnal electromyographic activity of masticatory muscles in twins. Clin Oral Investig. 2016 Mar 22; doi: 10.1007/s00784-016-1794-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Nashed A, Lanfranchi P, Rompré P, et al. Sleep bruxism is associated with a rise in arterial blood pressure. Sleep. 2012;35:529–36. doi: 10.5665/sleep.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavigne GJ, Rompré PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75:546–52. doi: 10.1177/00220345960750010601. [DOI] [PubMed] [Google Scholar]
- 22.Dias GM, Bonato LL, Guimaraes JP, et al. A Study of the association between sleep bruxism, low quality of sleep, and degenerative changes of the temporomandibular joint. J Craniofac Surg. 2015;26:2347–50. doi: 10.1097/SCS.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 23.Slade GD, Bair E, By K, et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain. 2011;12:T12–26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bherer C, Labuda D, Roy-Gagnon MH, Houde L, Tremblay M, Vézina H. Admixed ancestry and stratification of Quebec regional populations. Am J Phys Anthropol. 2011;144:432–41. doi: 10.1002/ajpa.21424. [DOI] [PubMed] [Google Scholar]
- 25.Carra MC, Rompré PH, Kato T, et al. Sleep bruxism and sleep arousal: an experimental challenge to assess the role of cyclic alternating pattern. J Oral Rehabil. 2011;38:635–42. doi: 10.1111/j.1365-2842.2011.02203.x. [DOI] [PubMed] [Google Scholar]
- 26.Kato T, Rompré P, Montplaisir JY, Sessle BJ, Lavigne GJ. Sleep bruxism: an oromotor activity secondary to micro-arousal. J Dent Res. 2001;80:1940–4. doi: 10.1177/00220345010800101501. [DOI] [PubMed] [Google Scholar]
- 27.Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil. 2008;35:476–94. doi: 10.1111/j.1365-2842.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 28.Sahin Onat S, Malas FU. Duloxetine-induced sleep bruxism in fibromyalgia successfully treated with amitriptyline. Acta Reumatol Port. 2015;40:391–2. [PubMed] [Google Scholar]
- 29.Albayrak Y, Ekinci O. Duloxetine-induced nocturnal bruxism resolved by buspirone: a case report. Clin Neuropharmacol. 2011;34:137–8. doi: 10.1097/WNF.0b013e3182227736. [DOI] [PubMed] [Google Scholar]
- 30.Uca AU, Uguz F, Kozak HH, et al. Antidepressant-induced sleep bruxism: prevalence, incidence, and related factors. Clin Neuropharmacol. 2015;38:227–30. doi: 10.1097/WNF.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 31.Ellison JM, Stanziani P. SSRI-associated nocturnal bruxism in four patients. J Clin Psychiatry. 1993;54:432–4. [PubMed] [Google Scholar]
- 32.Romanelli F, Adler DA, Bungay KM. Possible paroxetine-induced bruxism. Ann Pharmacother. 1996;30:1246–8. doi: 10.1177/106002809603001107. [DOI] [PubMed] [Google Scholar]
- 33.Abe Y, Suganuma T, Ishii M, et al. Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J Sleep Res. 2012;21:289–96. doi: 10.1111/j.1365-2869.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- 34.Raphael KG, Janal MN, Sirois DA, et al. Validity of self-reported sleep bruxism among myofascial temporomandibular disorder patients and controls. J Oral Rehabil. 2015;42:751–8. doi: 10.1111/joor.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


