Summary
Long Interspersed Element-1 (LINE-1 or L1) retrotransposons encode two proteins (ORF1p and ORF2p) that are required for retrotransposition. The L1 Element Amplification Protocol (LEAP) assays the ability of L1 ORF2p to reverse transcribe L1 RNA in vitro. Ultracentrifugation or immunoprecipitation is used to isolate L1 ribonucleoprotein particle (RNP) complexes from cultured human cells transfected with an engineered L1 expression construct. The isolated RNPs are incubated with an oligonucleotide that contains a unique sequence at its 5′ end and a thymidine-rich sequence at its 3′ end. The addition of dNTPs to the reaction allows L1 ORF2p bound to L1 RNA to generate L1 cDNA. The resultant L1 cDNAs then are amplified using polymerase chain reaction (PCR) and the products are visualized by gel electrophoresis. Sequencing the resultant PCR products then allows product verification. The LEAP assay has been instrumental in determining how mutations in L1 ORF1p and ORF2p affect L1 reverse transcriptase (RT) activity. Furthermore, the LEAP assay has revealed that the L1 ORF2p RT can extend a DNA primer with mismatched 3′ terminal bases when it is annealed to an L1 RNA template. As the LINE-1 biology field gravitates toward studying cellular proteins that regulate LINE-1, molecular genetic and biochemical approaches such as LEAP, in conjunction with the LINE-1 cultured cell retrotransposition assay, are essential to dissect the molecular mechanism of L1 retrotransposition.
Keywords: LINE-1, reverse transcriptase, ribonucleoprotein particle (RNP), L1 Element Amplification Protocol (LEAP)
1. Introduction
Long Interspersed Element-1s (LINE-1s or L1s) are the only active, autonomous transposable elements in the human genome (1, 2). L1s mobilize through an RNA intermediate by a “copy and paste” process termed retrotransposition (reviewed in (3)). An average individual human genome contains ~80–100 active (i.e., retrotransposition-competent L1s (RC-L1s) (4, 5). RC-L1s are ~6 kb in length and encode two proteins (ORF1p and ORF2p) that are required for their retrotransposition (1, 6–8). L1 ORF1p is an approximately 40 kDa RNA binding protein (9–15) with nucleic acid chaperone activity (16, 17). L1 ORF2p is an approximately 150 kDa protein (18–21) with both endonuclease (EN) (6) and reverse transcriptase (RT) activities (22).
Although L1 retrotransposition has had a dramatic impact on the structure of the human genome, the detailed molecular mechanism of L1 retrotransposition requires elucidation (reviewed in (3)). Assays using Ty1/L1 ORF2p expression constructs (22, 23), as well as L1 ORF2p produced in recombinant expression systems (24–26), have revealed that L1 ORF2p contains reverse transcriptase activity. Similarly, a recombinant L1 ORF2p EN domain produced in E. coli contains DNA endonuclease activity that can generate single-strand DNA breaks at thymidine-rich sequences in double-stranded DNA, leading to nicks that contain a 5′ phosphate and 3′ hydroxyl group (6).
The above experiments, in conjunction with the LINE-1 cultured cell retrotransposition assay (7) and molecular genetic approaches (reviewed in (3)), have demonstrated that L1 ORF1p and ORF2p preferentially associate with their encoding L1 RNA in cis (27–29), leading to the formation of a ribonucleoprotein particle (RNP) that is a necessary intermediate for retrotransposition (18, 21, 30). Components of the L1 RNP are thought to gain access to the nucleus, where the L1 ORF2p EN activity generates a single-strand endonucleolytic nick in genomic DNA at a thymidine-rich consensus sequence (e.g., 5′-TTTT/A-3′, 5′-TTTC/A-3′, 5′-TTTA/A-3′ etc., where “/” denotes the scissile bond) (31–35). This endonucleolytic activity liberates a 3′-hydroxyl group, which can serve as a primer for the L1 ORF2p RT activity to initiate the reverse transcription of L1 RNA by a process termed target site-primed reverse transcription (TPRT) (Figure 1) (6, 24, 36). However, the difficulty in purifying high levels of recombinant L1 ORF2p in vitro, and the fact that L1 ORF2p is made in low quantities in vivo (as few as one molecule of L1 ORF2p is made per L1 RNA (37)), has hampered efforts to study L1 TPRT at the molecular level.
Figure 1. Target site-primed reverse transcription (TPRT).
The L1 RNA (grey-yellow-blue-grey line), ORF1p (yellow circles), and ORF2p (blue circle) minimally constitute the L1 RNP. The L1 RNP targets genomic DNA (black bold lines) and nicks one T-rich strand exposing a free 3′OH. L1 ORF2p RT uses the T-rich DNA strand as a primer for reverse transcription of the L1 RNA template. After initial priming, L1 ORF2p RT synthesizes a complementary L1 cDNA strand (green arrow) using L1 RNA as a template. Second strand synthesis and completion of integration remain to be elucidated from L1 retrotransposition but may follow the Bombyx mori R2 retrotransposon model of integration (52).
Epitope and RNA tagging strategies have been used to detect L1 ORF1p, L1 ORF2p, and L1 RNA from engineered L1 expression constructs (18, 20, 21, 28, 30). Importantly, these technologies also have allowed the discrimination of the L1 proteins and RNA produced from transfected L1 expression constructs from the proteins and RNAs expressed from endogenous L1s. In 2006, we developed an assay, termed the L1 Element Amplification Protocol (LEAP), that allows the detection of ORF2p reverse transcriptase activity in RNP preparations derived from cultured human cells transfected with engineered L1 expression constructs (28). The LEAP assay uses a similar rationale that has been used to detect RT activity from mitochondrial plasmids of Neurospora crassa (38) and is similar to the strategy employed to detect telomerase activity using the telomere repeat amplification protocol (TRAP) assay (39).
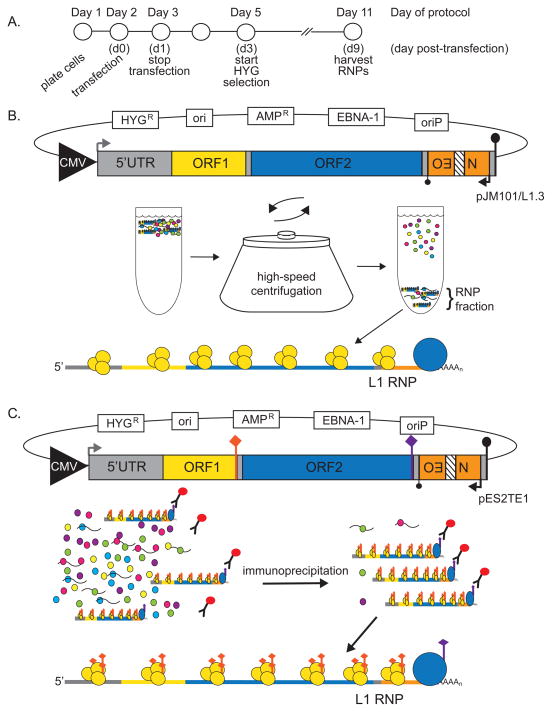
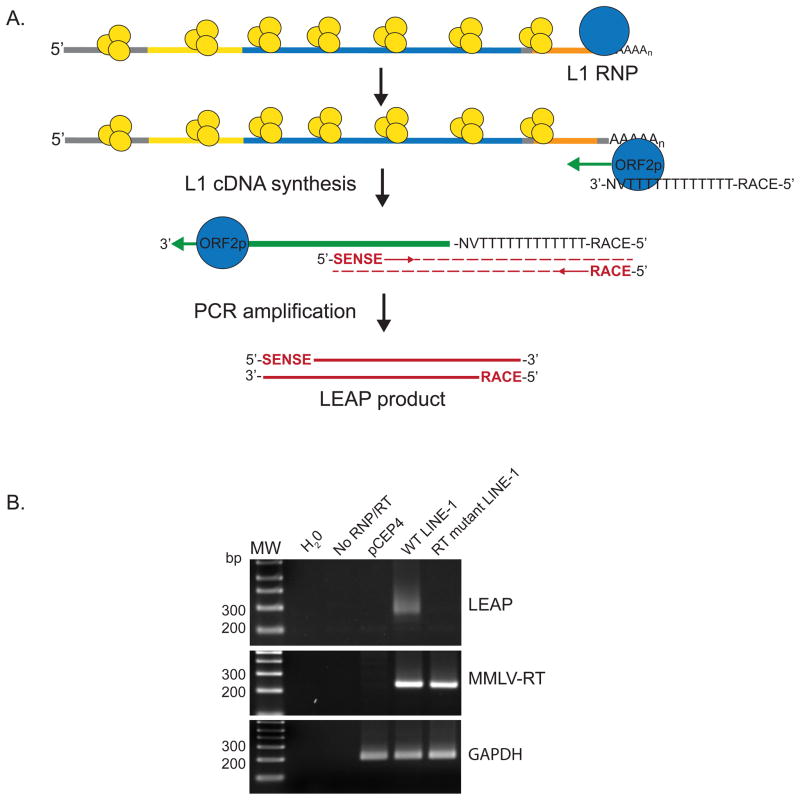
For the LEAP assay, HeLa-JVM cells are transfected with engineered L1 constructs that express versions of ORF1p and ORF2p that contain different epitope tags at their respective carboxyl termini (Figure 2) (18, 28, 30). Hygromycin B is used to select for HeLa-JVM cells containing the engineered L1 constructs. Cellular RNP complexes then are isolated from hygromycin B-resistant HeLa-JVM cells using differential centrifugation through a sucrose cushion (Figure 2B) (28, 30). Alternatively, L1 RNPs can be isolated from hygromycin B-resistant HeLa-JVM cells by immunoprecipitation, using an antibody directed against the L1 ORF2p carboxyl terminal epitope tag (Figure 2C) (18). The resultant RNP then is incubated with an oligonucleotide (i.e., a LEAP adapter) to prime cDNA synthesis (Figure 3A) (28). The LEAP adapter contains a unique sequence at the 5′ end (RACE) followed by 12 thymidines (dT12) and ends with VN nucleotides (where V represents adenosine (A), guanosine (G), or cytidine (C), and N represents any nucleotide). The L1 cDNAs are PCR-amplified using oligonucleotide primers to the RACE sequence and the engineered L1 construct. The LEAP PCR products then are visualized by gel electrophoresis and can be subsequently cloned and sequenced to characterize the products (Figure 3B).
Figure 2. Enrichment of the L1 RNP.
(A) A timeline of the LEAP assay is depicted and described in the Methods. Days of the protocol are noted above and the corresponding days post-transfection (d0-14) are noted below. (B) The pJM101/L1.3 L1 construct contains the L1.3 element (accession no. L19088). The pCEP4 plasmid (Life Technologies) backbone encodes for the EBNA-1 (EBNA-1) viral protein and contains an origin of viral replication (oriP), a hygromycin B-resistance gene (HYGR), the cytomegalovirus (CMV) promoter (large black triangle) and an SV40 polyadenylation signal (large black lollipop) for plasmid replication, hygromycin-selection, and transcription, respectively, in mammalian cultured cells. The pCEP4 backbone also has a bacterial origin of replication (ori) and an ampicillin-resistance gene (AMPR) for replication and ampicillin-selection (respectively) in E.coli. (For details of the mneoI reporter cassette, please see the “LINE-1 Cultured Cell Retrotransposition Assay” chapter in this volume.) After transfection and hygromycin B-selection in HeLa-JVM cells, the cells are lysed and subjected to high-speed centrifugation through a sucrose cushion. After centrifugation, cellular RNPs are enriched in the pellet fraction. This fraction contains the L1 RNA bound by L1 ORF1p and ORF2p, which minimally constitutes the L1 RNP (see Figure 1). (C) The pES2TE1 L1 construct (18), like pJM101/L1.3, contains the L1.3 element in a pCEP4 (Life Technologies) backbone. Unlike pJM101/L1.3, the pES2TE1 construct encodes a T7-tagged (orange diamond) ORF1p and a FLAG-HA-tagged (purple diamond) ORF2p (18). After transfection and hygromycin B selection, HeLa cells are lysed and subjected to immunoprecipitation using an anti-FLAG antibody conjugated to beads (red circle with black “Y”). Immunoprecipitated complexes contain L1 ORF1p and ORF2p bound to its encoding RNA, which minimally constitutes the L1 RNP.
Figure 3. The LEAP Assay.
(A) The L1 RNP minimally consists of L1 ORF1p (yellow circles) and ORF2p (blue circle) bound to the L1 RNA (multicolored line). The L1 RNP is incubated with a 5′-RACE-T12VN-3′ primer and dNTPs. The L1 RT activity (green arrow) of ORF2p initiates L1 cDNA (green line) synthesis. Subsequently, the L1 cDNA is amplified using an engineered LINE-1 construct-specific primer (SENSE) and a RACE primer (red arrows), resulting in the LEAP product (double red line). (B) LEAP products can be resolved by electrophoresis and visualized by staining. LEAP products (top panel), L1 RNA present in RNPs (MMLV-RT; middle panel), and GAPDH RNA levels (GAPDH; lower panel) are shown. A standard LEAP assay includes a water control PCR reaction (H20); a control reaction without any RT (No RNP/RT); a reaction using RNPs from empty vector-transfected cells (pCEP4); a reaction with wild type L1 RNPs (WT LINE-1); and a reaction with RT-mutant L1 RNPs (RT mutant LINE-1). The molecular weight (MW) ladder sizes are shown in base pairs (bp). L1 ORF1p and ORF2p protein levels can also be detected by western blot analyses (not shown) (18, 28).
Data obtained from the LEAP assay have revealed that, unlike retroviral reverse transcriptases (e.g., Moloney murine leukemia virus (MMLV)-RT), the L1 RT can initiate reverse transcription from a DNA primer with mismatched 3′ terminal bases when it is annealed to an L1 RNA template (28). Indeed, the ability of L1 RT to extend mismatched DNA primer/RNA template duplexes explains how certain genomic DNA sequences (e.g., 5′-TTTA/A or 5′-TTTC/A, where ‘/’ corresponds to the L1 EN nick) can serve as primers to initiate TPRT in vivo (31, 35, 40). That being stated, increasing the length of DNA primer 3′ terminal mismatches to 4 mismatched nucleotides decreases the efficiency of reverse transcription (28, 41). Notably, the presence of different VN dinucleotide pairs and poly (A) tail lengths in the resultant LEAP products allows independent products to be distinguished from one another in a single LEAP reaction (28). Finally, the use of gene specific primers has allowed the identification of cellular mRNAs that can be reverse transcribed at low levels by L1 RT in trans (28, 42–44).
LEAP adapters also have been designed to mimic genomic integration sites observed in cultured cell experiments. For example, the LEAP assay was used to examine how endonuclease-deficient L1s are able to integrate at dysfunctional telomeres in Chinese hamster ovary (CHO) cell lines that are defective in components required for the non-homologous end-joining (NHEJ) pathway of DNA repair (45, 46). Here, the LEAP adapter was modified to mimic potential telomere ends (e.g., 5′-RACE-(TTAGGG)3-3′, 5′-RACE-(TTAGGG)3TT-3′, 5′-RACE-(TTAGGG)3TTAG-3′, etc.). A LEAP adapter with a telomeric repeat ending in 5′-(TTAGGG)3TT-3′ was more efficient to prime first strand L1 cDNA synthesis than an adapter ending in 5′-(TTAGGG)3-3′ (45). Characterization of the LINE-1 cDNA/primer junctions of these LEAP products revealed that they generally contained a perfect telomere repeat followed by a poly (T) sequence that resulted from the reverse transcription of the L1 poly (A) mRNA; hence, the LEAP products recapitulated the structure of endonuclease-deficient LINE-1 retrotransposition events observed in NHEJ-deficient CHO cells (33, 45, 46). Indeed, these data further demonstrated that L1 RNP preparations are associated with a nuclease activity that can process the oligonucleotide adapter prior to its use as a primer in the LEAP reaction (45).
The LEAP assay also has been used to determine whether missense mutations in L1 ORF1p or ORF2p affect L1 RT activity (18, 28, 45). For example, LEAP reactions revealed that missense mutations in ORF1p that adversely affect L1 retrotransposition by decreasing the ability of ORF1p to bind L1 RNA retain LEAP activity (18, 28). Similarly, missense mutations in the EN or cysteine-rich domain of L1 ORF2p adversely affect L1 retrotransposition but retain LEAP activity (18, 28, 45).
A number of cellular proteins that interact with L1 RNPs recently have been identified and the overexpression of a subset of these proteins can adversely affect L1 retrotransposition in cultured human cells (21, 47, 48). The LEAP assay can be used to determine if these cellular proteins affect L1 RT activity. For example, the LEAP assay revealed that the L1 mRNA template remains annealed to the L1 cDNA in an RNA/DNA hybrid after reverse transcription, protecting the L1 cDNA from APOBEC3A-mediated cytidine deamination (49). However, the addition of RNase H (an enzyme that degrades RNA in an RNA/DNA hybrid) to the LEAP reaction renders the L1 cDNA susceptible to cytidine deamination (49). These data, in conjunction with cultured cell based experiments, revealed that APOBEC3A inhibits L1 retrotransposition, in part, by deaminating the transiently exposed single-strand L1 cDNA generated during TPRT (49).
In sum, the LEAP assay has been used to elucidate mechanistic details of TPRT. The following protocol has been adapted from previously published studies and is optimized for HeLa cells (18, 28). Important experimental controls and technical tips are highlighted in the Notes section of this protocol.
2. Materials
Special care should be taken when preparing materials for L1 RNP isolation and RT-PCR reactions. Prepare all solutions in DNase/RNase-free water. All lab instruments, consumables (e.g., pipettor, pipets, conical tubes, and microcentrifuge tubes) and solutions should be RNase-free and used only for RNA work. Work areas should be cleaned regularly with RNaseZap® (Life Technologies #AM9786) or other similar cleaning reagent.
2.1 Cell Culture Medium and Transfection Reagents
HeLa cells: we typically use HeLa-JVM cells for our assays (7, 28, 50).
Dulbecco’s Modified Eagle Medium (DMEM) (with 4.5 g/L D-glucose, Life Technologies #11960-051) containing 10% fetal bovine serum (FBS) (Sigma #F2442 or Life Technologies #26140-079), and 1X Pen Strep Glutamine (100 U/mL penicillin, 100 μg/mL streptomycin, and 292 μg/mL glutamine, Life Technologies #10378-016). This is called HeLa-JVM DMEM growth medium in the protocol below.
1X phosphate-buffered saline (PBS), pH 7.4, sterilized
L1 expression plasmid constructs (e.g., pJM101/L1.3 (Figure 2B; (5)) or pES2TE1 (Figure 2C; (18))
A cell counter (e.g., Countess® Automated Cell Counter, Life Technologies #C10227) or hemocytometer
Tissue culture T-25, T-75, or T-175 flasks (Corning #353108, #353136, or #353112, respectively)
FuGENE® 6 (Promega #E2691)
Opti-MEM® I (Life Technologies #31985-062)
Hygromycin B (Life Technologies #10687-010)
2.2 Lysis Buffer
1.5 mM KCl (Sigma #P3911)
2.5 mM MgCl2 (Sigma #M2670)
5 mM Tris-HCl, pH 7.5 (Life Technologies #15567-027)
1% deoxycholic acid (Calbiochem #264103)
1% Triton X-100 (Sigma #T8787)
1X Complete Protease Inhibitor Cocktail, EDTA-free (Roche #11873580001, add fresh)
UltraPure™ DNase/RNase free water (Life Technologies #10977-015)
2.3 Sucrose Stock Solution, 47%
80 mM NaCl (Sigma #S9888)
5 mM MgCl2
20 mM Tris-HCl, pH 7.5
UltraPure™ sucrose (Life Technologies #15503-022), 47% weight to volume (w/vol) in UltraPure™ DNase/RNase free water
1 mM dithiothreitol (DTT) (Fisherbrand™ #BP172, add fresh)
1X Complete Protease Inhibitor Cocktail, EDTA-free (add fresh)
UltraPure™ DNase/RNase free water
Sterile Filter System, 0.22 μm CA 500 mL bottle (Corning #430769)
2.4 Sucrose dilution buffer
80 mM NaCl
5 mM MgCl2
20 mM Tris-Cl, pH7.5
1 mM DTT (add fresh)
1X Complete Protease Inhibitor Cocktail, EDTA-free (add fresh)
UltraPure™ DNase/RNase free water
2.5 IP Flag buffer
100 mM KCl
20 mM Tris-HCl pH 8.0 (Life Technologies #15568-025)
1 mM DTT (add fresh)
10% UltraPure™ Glycerol (Life Technologies #15514-011)
0.1% IGEPAL CA-630 (Sigma #I8896)
1X Complete Protease Inhibitor Cocktail, EDTA-free (add fresh)
UltraPure™ DNase/RNase free water
2.6 Flag elution buffer
IP FLAG buffer (see 2.5 above)
200 μg/mL 3X FLAG peptide (Sigma #F4799)
2.7 LEAP reaction buffer (L1 reverse transcriptase reaction)
50 mM Tris-HCl, pH 7.5
50 mM KCl
5 mM MgCl2
10 mM DTT (add fresh)
0.2 μM 3′ RACE Adapter (see Note 1)
20 U recombinant RNasin® ribonuclease inhibitor (Promega #N2511)
0.2 mM dNTPs (Life Technologies #18427-013)
0.05% Tween®-20 (Fisherbrand™ #BP337)
UltraPure™ DNase/RNase free water
2.8 LEAP PCR reaction mix
2.9 Other
RNaseZap® RNase decontamination solution (Life Technologies #AM9786)
Cell scrapers, small or large (e.g., Fisherbrand™ #08-100-241 or #08-100-242, respectively)
Conical tubes, 15 mL (e.g., Nunc™ #362694)
Barrier pipette tips (e.g., Neptune #BT1250.N, #BT200, #BT20, and #BT10XL)
M-MLV Reverse Transcriptase (MMLV-RT) (Promega #M1701)
RNeasy Mini Kit (Qiagen #74104)
Agarose and low-melt agarose (Life Technologies #16500-500 and #16520-100, respectively) with 1X TAE
Ethidium bromide (Sigma #E8751), GelRed™ Nucleic Acid Stain (Biotium #41003-1) or SYBER® Safe DNA gel stain (Life Technologies #S33102)
EZview™ Red ANTI-FLAG® M2 Affinity Gel (Sigma #F2426) (see Note 3)
Bradford reagent (Bio-Rad Protein Assay, #500-0006)
PCR machine (e.g., Applied Biosystems 2720 Thermal Cycler, Bio-Rad iCycler, or Eppendorf Mastercycler proS)
Thin-walled PCR 96-well plate (Fisherbrand™ #14230232) and adhesive PCR film (Thermo Scientific™ #AB-0558) or 0.2 mL tubes (Corning #3745)
Refrigerated centrifuge that can accommodate conical tubes (e.g., Eppendorf 5702R)
Thermo Scientific Sorvall MTX 150 Micro-Ultracentrifuge
Micro-ultracentrifuge tubes (Thermo Scientific #45237)
Nucleic acid electrophoresis system (e.g., Bio-Rad Sub-Cell GT cell #170-4401)
QIAquick Gel Extraction Kit (Qiagen #28706)
PCR product cloning kit (e.g., Zero Blunt® PCR cloning kit, Life Technologies #K2700-20)
3. Methods
Detailed below is a scaled-down version of the original LEAP assay (28) as well as an alternative protocol using immunoprecipitation (18) instead of ultracentrifugation to isolate L1 RNPs. This protocol is optimized for HeLa cells but has also been used for other human cell lines (21, 42–44). Hygromycin B concentrations and transfection protocols should be optimized for any different cell lines used for this assay.
3.1 LINE-1 RNP isolation using ultracentrifugation (Figure 2A and 2B)
Day 1 – Plate cells: Seed 2×106 HeLa-JVM cells in HeLa-JVM growth medium in a T-75 tissue culture flask. Cells are grown in a humidified incubator at 37°C with 7% CO2 (see Note 4).
Day 2 – Transfect cells: Cells typically are transfected 14 to 16 hours post-plating, day zero (d0) (Figure 2A), using the FuGENE® 6 transfection reagent following the manufacturer’s instructions. The LEAP assay should include the following transfection conditions: 1) an empty vector (e.g., pCEP4, Life Technologies); 2) a wild type LINE-1 expression plasmid (e.g., pJM101/L1.3 (5)); and 3) an RT mutant LINE-1 plasmid (e.g., pJM105/L1.3, which has a D702A mutation in the ORF2 RT domain (29)) (see Note 5). Prepare a transfection mix in a 1.5 mL microcentrifuge tube containing 8 μg of the pCEP4 or LINE-1 expression plasmid and 32 μL FuGENE® 6 and 500 μL Opti-MEM® I. Incubate the solution at room temperature for 20 minutes. Add the transfection mix to the growth medium of one flask of cells.
Day 3 – Stop the transfection: Approximately 16 to 24 hours post-transfection, one day post-transfection (d1) (Figure 2A), aspirate medium from the cells and add fresh DMEM growth medium to the cells.
Days 5–11 – Select for transfected cells: Begin drug selection 3 days post-transfection (d3) and continue until 9 days post-transfection (d9). Grow cells in DMEM growth medium containing 200 μg/mL hygromycin B. Change the hygromycin B-containing media every day until 9 days post-transfection (d9) (Figure 2A).
Day 11 – Harvest the cells for ultracentrifugation: Rinse the cells once with cold 1X PBS. Aspirate the 1X PBS. Add 5 mL cold 1X PBS and scrape the cells from the flask using a cell scraper. Pellet the cells by centrifugation at 3,000xg at 4°C for 5 minutes. Transfected cells from one T-75 flask can be divided into 2 aliquots and can be stored at −80°C for at least one month without affecting reproducibility (see Note 6).
Lyse cells: Add 250 μL Lysis Buffer (Materials 2.2) to the cell pellets. Pipette up and down to resuspend the pellets. Let the cells sit on ice for 15 minutes. Centrifuge the cell lysates at 3,000xg at 4°C for 10 minutes. Transfer the supernatants to new clean tubes and keep on ice. A small aliquot of the whole cell lysates should be flash frozen in an ethanol/dry ice bath and stored at −80°C (see Note 7).
Prepare the sucrose cushion: Using the Sucrose Dilution buffer (Materials 2.4), dilute the 47% sucrose stock solution (Materials 2.3) to 8.5% and 17% sucrose solutions. Prepare 500 μL of the 17% sucrose solution and 250 μL of the 8% sucrose solution per sample. Carefully layer the sucrose solutions such that 500 μL of the 17% sucrose solution is at the bottom and 250 μL of the 8% sucrose solution is on top in a 1 mL micro-ultracentrifuge tube. On top of the 8% sucrose, gently layer 150 to 200 μL of the cell lysate. If using a fixed angle rotor, leave an empty space between samples or use sealable tubes to minimize possible cross-contamination during the spin.
Run the micro-ultracentrifuge: Spin the cell lysates at 168,000xg at 4°C for 2 hours.
Resuspend the RNP: After centrifugation, a pellet should be visible at the bottom of the tube. Aspirate off the sucrose solution, being careful not to disturb the pellet. Resuspend the pellet in 50 to 100 μL 1X Complete Protease Inhibitor Cocktail, EDTA-free (in DNase/RNase-free water) by gently pipetting up and down. Quantitate protein concentrations of RNPs using the Bradford protein assay reagent. If needed, dilute RNPs with 1X Complete Protease Inhibitor Cocktail, EDTA-free to a concentration of 1.5 to 2 μg/μL of total protein. To preserve the enzymatic activity upon storage, dilute the RNPs to a concentration of 0.75 to 1 μg/μL of total protein with glycerol (final 50% volume/volume glycerol concentration). Divide the RNPs into 25 to 50 μL aliquots, flash freeze in an ethanol/dry ice bath, and store the RNPs at −80°C (see Note 7).
3.2 LINE-1 RNP isolation using immunoprecipitation (Figures 2A and 2C)
Day 1 – Plate cells: Seed 6×106 HeLa-JVM cells in HeLa-JVM DMEM growth medium in a T-175 tissue culture flask.
Day 2 – Transfect cells: Cells typically are transfected 14 to 16 hours post-plating, day zero (d0) (Figure 2A) using the FuGENE® 6 transfection reagent following the manufacturer’s instructions. The LEAP assay using RNPs isolated by immunoprecipitation should include the following transfection conditions: 1) an empty vector (e.g., pCEP4, Life Technologies); 2) an epitope-tagged wild type LINE-1 plasmid (e.g., pES2TE1 (18)); and 3) a non-tagged LINE-1 plasmid (e.g., pJM101/L1.3 (5), which does not have any epitope tag on ORF1p or ORF2p) (see Note 5). Prepare a transfection mix in a 1.5 mL microcentrifuge tube containing 20 to 30 μg of the pCEP4 or LINE-1 expression plasmid, 80 to 120 μL FuGENE® 6, and 1 mL Opti-MEM® I. Incubate the solution at room temperature for 20 minutes. Add the transfection mix to the growth medium of one flask of cells.
Day 3 – Stop the transfection: Approximately 16 to 24 hours post-transfection, one day post-transfection (d1) (Figure 2A), aspirate the medium from the cells and add fresh DMEM growth medium to the cells.
Days 5–11 – Select for transfected cells: Begin drug selection 3 days post-transfection (d3) and continue until 9 days post-transfection (d9). Grow cells in DMEM growth medium containing 200 μg/mL hygromycin B. Change the hygromycin B-containing media every day until 9 days post-transfection (d9) (Figure 2A).
Day 11 – Harvest the cells for immunoprecipitation: Rinse the cells once with cold 1X PBS. Aspirate the 1X PBS. Add 10 mL cold 1X PBS and scrape the cells from the flask using a cell scraper. Pellet the cells by centrifugation at 3,000xg at 4°C for 5 minutes. Transfected cells from one T-175 flask can be divided into 2 aliquots and can be stored at −80°C for at least one month without affecting reproducibility.
Lyse the cells: Add IP Flag buffer (Materials 2.5) three times the volume of the cell pellet. For example, if the volume of the cell pellet is approximately 100 μL, add 300 μL of the buffer. Pipette up and down to resuspend the pellets. Let the cells sit on ice for 15 minutes. Centrifuge the cell lysates at 3,000xg at 4°C for 10 minutes. Transfer the lysates to new clean tubes and keep on ice. A small aliquot of the whole cell lysates should be flash frozen in an ethanol/dry ice bath and stored at −80°C (see Note 7).
Determine the cell lysate protein concentration using the Bradford protein assay reagent.
Prepare immunoprecipitation: For each immunoprecipitation, incubate 3 mg of protein from the cell lysate with 20 μL of equilibrated EZview™ Red ANTI-FLAG® M2 Affinity Gel (see Note 3) at 4°C overnight on a rotating wheel or nutator (see Note 8). Collect the beads by centrifugation at 3,000xg at 4°C for 10 minutes. Resuspend the beads in 1 mL cold IP Flag buffer. Wash the beads 4 more times with 1 mL cold IP Flag buffer.
Elute the RNPs: Incubate the beads with 50 μL IP Flag buffer containing 200 μg/μL of 3XFLAG peptide at 4°C for 1 hour on a rotating wheel or nutator. Spin down the beads at 6,000xg at 4°C for 5 minutes and transfer the eluates to new tubes (see Note 7).
3.3 LEAP Reactions and PCR
The following reactions are performed the same for RNPs prepared by ultracentrifugation or immunoprecipitation. Reactions are prepared in a PCR workstation equipped with HEPA-filtered air circulation and UV-sterilization.
L1 reverse transcription reaction: Incubate 1 μL L1 RNP [0.75 to 1.0 μg] or IP sample with the LEAP reaction buffer (Materials 2.7) in a total volume of 50 μL at 37°C for 1 hour. LEAP cDNAs can be stored at −20°C.
PCR amplification of L1 RT-synthesized cDNA: Add 1 μL of LEAP cDNA to the LEAP PCR reaction mix (Materials 2.8) in a total volume of 50 μL (see Note 9).
-
The LEAP PCR program is as follows:
1 cycle 94°C for 3 minutes; 35 cycles 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 30 seconds; 1 cycle 72°C for 7 minutes; 4°C hold LEAP products are visualized by electrophoresis on a 2% agarose gel (equal parts agarose to low-melt agarose) in 1X TAE stained with ethidium bromide, GelRed™, or SYBER® Safe nucleic acid stains (Figure 3B). LEAP bands can be excised, extracted using the QIAquick Gel Extraction Kit, and cloned into a commercial sequencing vector (e.g., Zero Blunt® PCR Cloning Kit). These plasmids can then be sequenced for characterization of individual LEAP products within one reaction.
Acknowledgments
The authors would like to thank Nancy Leff for helpful comments during the preparation of this manuscript. This work was supported in part by NIH grant GM060518 to J.V.M. Authors were supported in part by fellowships from the American Cancer Society #PF-07-059-01GMC (H.C.K.), the NHGRI #T32-HG00040 (D.A.F.), the Japan Society for the Promotion of Science, the Uehara Memorial Foundation and the Kanae Foundation (T.M.), and an International postdoctoral fellowship from the Fondation pour la Recherche Medicale (A.J.D.). J.V.M. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
. The LEAP adapters for the RT reaction work best if they are HPLC-purified (Integrated DNA Technologies (IDT, Iowa, USA)). LEAP Adapter sequence: 5′-GCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTTTTTTTVN-3′, where V is A, G, or C and N is any nucleotide (28).
- SENSE: 5′-GGGTCCGAAATCGATAAGCTTGGATCCAGAC-3′. This “SENSE” primer is specific to the transfected engineered L1 construct and does not amplify endogenous L1s (28).
. EZview™ Red ANTI-FLAG® M2 Affinity Gel is the anti-FLAG M2 antibody covalently linked to agarose beads. Anti-FLAG beads should be equilibrated according to the manufacturer’s instructions. To equilibrate the anti-FLAG beads, spin down the affinity gel solution at 8200xg at 4°C for 30 seconds and aspirate the supernatant being careful not to disturb the beads. Resuspend the beads in at least 10 times the packed bead volume in IP Flag buffer. Repeat this wash 4 more times. Spin the beads a final time and resuspend the beads in an equal volume of the packed bead volume in IP Flag buffer. For example, if your packed bead volume is 200 μL, resuspend the beads in 200 μL IP Flag buffer. This 50% slurry of anti-FLAG beads is the working stock of equilibrated EZview™ Red ANTI-FLAG® M2 Affinity Gel.
. HeLa-JVM cells can also be seeded at 2×105 cells per T-25 cell culture flask or 6×106 cells per T-175 cell culture flask. For cells in a T-25 flask, prepare a transfection mix in a 1.5 mL microcentrifuge tube containing 1 μg of the pCEP4 or LINE-1 expression plasmid, 4 μL FuGENE® 6, and 100 μL Opti-MEM® I. For cells in a T-175 flask, prepare a transfection mix in a 1.5 mL microcentrifuge tube containing 20 to 30 μg of the pCEP4 or LINE-1 expression plasmid, 80 to 120 μL FuGENE® 6 and 1mL Opti-MEM® I. Incubate the solution at room temperature for 20 minutes. Add the transfection mix to the growth medium of one flask of cells.
. Transcription of L1 elements being tested in the LEAP assay should be driven by the CMV promoter and have the pCEP4 backbone, which allows for episomal replication of the plasmid and hygromycin B-selection of cells containing the L1 expression construct. It is difficult to detect LEAP activity from L1 expression constructs where L1 transcription is driven by the promoter activity in the L1 5′UTR.
. For ultracentrifugation, the entire cell pellet from a T-25 flask should be used for ultracentrifugation. The cell pellet from one T-175 flask can be divided into 4 equal aliquots and stored at −80°C.
. MMLV-RT and western blot analyses should be done to show comparable RNA and protein levels, respectively, of the isolated L1 RNP. Similarly, RNA and L1 proteins can be analyzed from whole cell lysates and compared to the isolated L1 RNP fractions. RNA is purified from RNPs using the Qiagen RNeasy kit. The purified, DNA-free RNA is reverse transcribed and PCR amplified using the same adapters in the LEAP reactions but with MMLV-RT instead of L1 RNPs. The L1 cDNAs are PCR amplified using the same primers and conditions as listed for the LEAP PCR but for 25–30 cycles. Control cellular RNAs (e.g., GAPDH) are PCR amplified using gene-specific primers (28). Western blots of RNPs expressed from pES2TE1 are performed using anti-T7 antibody (Millipore #69522-3) to detect L1 ORF1p or anti-HA (3F10 clone, Roche #11867423001) to detect L1 ORF2p (18, 30). Untagged L1 ORF1p can be detected from L1 RNPs and cell lysates using an anti-ORF1p antibody (30, 48, 51).
. Cell lysates should be pre-cleared with agarose beads if immunoprecipitation is done with anti-FLAG-coupled agarose beads. Other resins, such as Dynabeads (Life Technologies), may have less non-specific protein binding and may not require pre-clearing.
. LEAP cDNAs may also be subjected to qPCR and quantified as detailed in Kulpa and Moran, 2006 and Doucet et al., 2010 (18, 28).
Conflict of Interest
J.V.M. is an inventor on the patent: “Kazazian, H.H., Boeke, J.D., Moran, J.V., and Dombrowski, B.A. Compositions and methods of use of mammalian retrotransposons. Application No. 60/006,831; Patent No. 6,150,160; Issued November 21, 2000.” J.V.M. has not made any money from this patent and voluntarily discloses this information.
References
- 1.Dombroski BA, Mathias SL, Nanthakumar E, Scott AF, Kazazian HH., Jr Isolation of an active human transposable element. Science. 1991;254:1805–1808. doi: 10.1126/science.1662412. [DOI] [PubMed] [Google Scholar]
- 2.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 3.Beck CR, Garcia-Perez JL, Badge RM, Moran JV. LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet. 2011;12:187–215. doi: 10.1146/annurev-genom-082509-141802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH., Jr Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci U S A. 2003;100:5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sassaman DM, Dombroski BA, Moran JV, Kimberland ML, Naas TP, DeBerardinis RJ, Gabriel A, Swergold GD, Kazazian HH., Jr Many human L1 elements are capable of retrotransposition. Nat Genet. 1997;16:37–43. doi: 10.1038/ng0597-37. [DOI] [PubMed] [Google Scholar]
- 6.Feng Q, Moran JV, Kazazian HH, Jr, Boeke JD. Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell. 1996;87:905–916. doi: 10.1016/s0092-8674(00)81997-2. [DOI] [PubMed] [Google Scholar]
- 7.Moran JV, Holmes SE, Naas TP, DeBerardinis RJ, Boeke JD, Kazazian HH., Jr High frequency retrotransposition in cultured mammalian cells. Cell. 1996;87:917–927. doi: 10.1016/s0092-8674(00)81998-4. [DOI] [PubMed] [Google Scholar]
- 8.Scott AF, Schmeckpeper BJ, Abdelrazik M, Comey CT, O’Hara B, Rossiter JP, Cooley T, Heath P, Smith KD, Margolet L. Origin of the human L1 elements: proposed progenitor genes deduced from a consensus DNA sequence. Genomics. 1987;1:113–125. doi: 10.1016/0888-7543(87)90003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohjoh H, Singer MF. Cytoplasmic ribonucleoprotein complexes containing human LINE-1 protein and RNA. EMBO J. 1996;15:630–639. [PMC free article] [PubMed] [Google Scholar]
- 10.Hohjoh H, Singer MF. Ribonuclease and high salt sensitivity of the ribonucleoprotein complex formed by the human LINE-1 retrotransposon. J Mol Biol. 1997;271:7–12. doi: 10.1006/jmbi.1997.1159. [DOI] [PubMed] [Google Scholar]
- 11.Holmes SE, Singer MF, Swergold GD. Studies on p40, the leucine zipper motif-containing protein encoded by the first open reading frame of an active human LINE-1 transposable element. J Biol Chem. 1992;267:19765–19768. [PubMed] [Google Scholar]
- 12.Khazina E, Weichenrieder O. Non-LTR retrotransposons encode noncanonical RRM domains in their first open reading frame. Proc Natl Acad Sci U S A. 2009;106:731–736. doi: 10.1073/pnas.0809964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolosha VO, Martin SL. In vitro properties of the first ORF protein from mouse LINE-1 support its role in ribonucleoprotein particle formation during retrotransposition. Proc Natl Acad Sci U S A. 1997;94:10155–10160. doi: 10.1073/pnas.94.19.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolosha VO, Martin SL. High-affinity, non-sequence-specific RNA binding by the open reading frame 1 (ORF1) protein from long interspersed nuclear element 1 (LINE-1) J Biol Chem. 2003;278:8112–8117. doi: 10.1074/jbc.M210487200. [DOI] [PubMed] [Google Scholar]
- 15.Martin SL. Ribonucleoprotein particles with LINE-1 RNA in mouse embryonal carcinoma cells. Mol Cell Biol. 1991;11:4804–4807. doi: 10.1128/mcb.11.9.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan KE, Hickman AB, Jones CE, Ghirlando R, Furano AV. Polymerization and nucleic acid-binding properties of human L1 ORF1 protein. Nucleic Acids Res. 2012;40:813–827. doi: 10.1093/nar/gkr728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin SL, Bushman FD. Nucleic acid chaperone activity of the ORF1 protein from the mouse LINE-1 retrotransposon. Mol Cell Biol. 2001;21:467–475. doi: 10.1128/MCB.21.2.467-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doucet AJ, Hulme AE, Sahinovic E, Kulpa DA, Moldovan JB, Kopera HC, Athanikar JN, Hasnaoui M, Bucheton A, Moran JV, Gilbert N. Characterization of LINE-1 ribonucleoprotein particles. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ergun S, Buschmann C, Heukeshoven J, Dammann K, Schnieders F, Lauke H, Chalajour F, Kilic N, Stratling WH, Schumann GG. Cell type-specific expression of LINE-1 open reading frames 1 and 2 in fetal and adult human tissues. J Biol Chem. 2004;279:27753–27763. doi: 10.1074/jbc.M312985200. [DOI] [PubMed] [Google Scholar]
- 20.Goodier JL, Ostertag EM, Engleka KA, Seleme MC, Kazazian HH., Jr A potential role for the nucleolus in L1 retrotransposition. Hum Mol Genet. 2004;13:1041–1048. doi: 10.1093/hmg/ddh118. [DOI] [PubMed] [Google Scholar]
- 21.Taylor MS, Lacava J, Mita P, Molloy KR, Huang CR, Li D, Adney EM, Jiang H, Burns KH, Chait BT, Rout MP, Boeke JD, Dai L. Affinity proteomics reveals human host factors implicated in discrete stages of LINE-1 retrotransposition. Cell. 2013;155:1034–1048. doi: 10.1016/j.cell.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathias SL, Scott AF, Kazazian HH, Jr, Boeke JD, Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991;254:1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- 23.Dombroski BA, Feng Q, Mathias SL, Sassaman DM, Scott AF, Kazazian HH, Jr, Boeke JD. An in vivo assay for the reverse transcriptase of human retrotransposon L1 in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:4485–4492. doi: 10.1128/mcb.14.7.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cost GJ, Feng Q, Jacquier A, Boeke JD. Human L1 element target-primed reverse transcription in vitro. EMBO J. 2002;21:5899–5910. doi: 10.1093/emboj/cdf592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piskareva O, Denmukhametova S, Schmatchenko V. Functional reverse transcriptase encoded by the human LINE-1 from baculovirus-infected insect cells. Protein Expr Purif. 2003;28:125–130. doi: 10.1016/s1046-5928(02)00655-1. [DOI] [PubMed] [Google Scholar]
- 26.Piskareva O, Schmatchenko V. DNA polymerization by the reverse transcriptase of the human L1 retrotransposon on its own template in vitro. FEBS Lett. 2006;580:661–668. doi: 10.1016/j.febslet.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 27.Esnault C, Maestre J, Heidmann T. Human LINE retrotransposons generate processed pseudogenes. Nat Genet. 2000;24:363–367. doi: 10.1038/74184. [DOI] [PubMed] [Google Scholar]
- 28.Kulpa DA, Moran JV. Cis-preferential LINE-1 reverse transcriptase activity in ribonucleoprotein particles. Nat Struct Mol Biol. 2006;13:655–660. doi: 10.1038/nsmb1107. [DOI] [PubMed] [Google Scholar]
- 29.Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, Boeke JD, Moran JV. Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol. 2001;21:1429–1439. doi: 10.1128/MCB.21.4.1429-1439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulpa DA, Moran JV. Ribonucleoprotein particle formation is necessary but not sufficient for LINE-1 retrotransposition. Hum Mol Genet. 2005;14:3237–3248. doi: 10.1093/hmg/ddi354. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert N, Lutz-Prigge S, Moran JV. Genomic deletions created upon LINE-1 retrotransposition. Cell. 2002;110:315–325. doi: 10.1016/s0092-8674(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 32.Jurka J. Sequence patterns indicate an enzymatic involvement in integration of mammalian retroposons. Proc Natl Acad Sci U S A. 1997;94:1872–1877. doi: 10.1073/pnas.94.5.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrish TA, Gilbert N, Myers JS, Vincent BJ, Stamato TD, Taccioli GE, Batzer MA, Moran JV. DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat Genet. 2002;31:159–165. doi: 10.1038/ng898. [DOI] [PubMed] [Google Scholar]
- 34.Myers JS, Vincent BJ, Udall H, Watkins WS, Morrish TA, Kilroy GE, Swergold GD, Henke J, Henke L, Moran JV, Jorde LB, Batzer MA. A comprehensive analysis of recently integrated human Ta L1 elements. Am J Hum Genet. 2002;71:312–326. doi: 10.1086/341718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Symer DE, Connelly C, Szak ST, Caputo EM, Cost GJ, Parmigiani G, Boeke JD. Human l1 retrotransposition is associated with genetic instability in vivo. Cell. 2002;110:327–338. doi: 10.1016/s0092-8674(02)00839-5. [DOI] [PubMed] [Google Scholar]
- 36.Luan DD, Korman MH, Jakubczak JL, Eickbush TH. Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non-LTR retrotransposition. Cell. 1993;72:595–605. doi: 10.1016/0092-8674(93)90078-5. [DOI] [PubMed] [Google Scholar]
- 37.Alisch RS, Garcia-Perez JL, Muotri AR, Gage FH, Moran JV. Unconventional translation of mammalian LINE-1 retrotransposons. Genes Dev. 2006;20:210–224. doi: 10.1101/gad.1380406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuiper MT, Akins RA, Holtrop M, de Vries H, Lambowitz AM. Isolation and analysis of the Neurospora crassa Cyt-21 gene. A nuclear gene encoding a mitochondrial ribosomal protein. J Biol Chem. 1988;263:2840–2847. [PubMed] [Google Scholar]
- 39.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert N, Lutz S, Morrish TA, Moran JV. Multiple fates of L1 retrotransposition intermediates in cultured human cells. Mol Cell Biol. 2005;25:7780–7795. doi: 10.1128/MCB.25.17.7780-7795.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monot C, Kuciak M, Viollet S, Mir AA, Gabus C, Darlix JL, Cristofari G. The specificity and flexibility of l1 reverse transcription priming at imperfect T-tracts. PLoS Genet. 2013;9:e1003499. doi: 10.1371/journal.pgen.1003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An W, Dai L, Niewiadomska AM, Yetil A, O’Donnell KA, Han JS, Boeke JD. Characterization of a synthetic human LINE-1 retrotransposon ORFeus-Hs. Mob DNA. 2011;2:2. doi: 10.1186/1759-8753-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai L, Taylor MS, O’Donnell KA, Boeke JD. Poly(A) binding protein C1 is essential for efficient L1 retrotransposition and affects L1 RNP formation. Mol Cell Biol. 2012;32:4323–4336. doi: 10.1128/MCB.06785-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandal PK, Ewing AD, Hancks DC, Kazazian HH., Jr Enrichment of processed pseudogene transcripts in L1-ribonucleoprotein particles. Hum Mol Genet. 2013;22:3730–3748. doi: 10.1093/hmg/ddt225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopera HC, Moldovan JB, Morrish TA, Garcia-Perez JL, Moran JV. Similarities between long interspersed element-1 (LINE-1) reverse transcriptase and telomerase. Proc Natl Acad Sci U S A. 2011;108:20345–20350. doi: 10.1073/pnas.1100275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrish TA, Garcia-Perez JL, Stamato TD, Taccioli GE, Sekiguchi J, Moran JV. Endonuclease-independent LINE-1 retrotransposition at mammalian telomeres. Nature. 2007;446:208–212. doi: 10.1038/nature05560. [DOI] [PubMed] [Google Scholar]
- 47.Goodier JL, Cheung LE, Kazazian HH., Jr Mapping the LINE1 ORF1 protein interactome reveals associated inhibitors of human retrotransposition. Nucleic Acids Res. 2013;41:7401–7419. doi: 10.1093/nar/gkt512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moldovan JB, Moran JV. The zinc-finger antiviral protein ZAP inhibits LINE and Alu retrotransposition. PLoS Genet. 2015 doi: 10.1371/journal.pgen.1005121. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson SR, Narvaiza I, Planegger RA, Weitzman MD, Moran JV. APOBEC3A deaminates transiently exposed single-strand DNA during LINE-1 retrotransposition. Elife. 2014;3:e02008. doi: 10.7554/eLife.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hulme AE, Bogerd HP, Cullen BR, Moran JV. Selective inhibition of Alu retrotransposition by APOBEC3G. Gene. 2007;390:199–205. doi: 10.1016/j.gene.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leibold DM, Swergold GD, Singer MF, Thayer RE, Dombroski BA, Fanning TG. Translation of LINE-1 DNA elements in vitro and in human cells. Proc Natl Acad Sci U S A. 1990;87:6990–6994. doi: 10.1073/pnas.87.18.6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen SM, Eickbush TH. R2 target-primed reverse transcription: ordered cleavage and polymerization steps by protein subunits asymmetrically bound to the target DNA. Mol Cell Biol. 2005;25:6617–6628. doi: 10.1128/MCB.25.15.6617-6628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]