Abstract
Introduction
Although anaplastic thyroid carcinoma (ATC) is rare, it is one of the most aggressive human cancers. The optimal multimodal therapy policy of ATC is still debated, and a standardized treatment strategy remains to be established. This study aimed to evaluate the management aspect and prognosis of ATC.
Materials and Methods
The data were analyzed retrospectively for 50 patients with ATC to evaluate the clinical characters, management and factors influencing survival. Survival analysis was performed by Kaplan-Merier method and log-rank test, and multivariate analysis was performed using Cox proportional hazard model.
Results
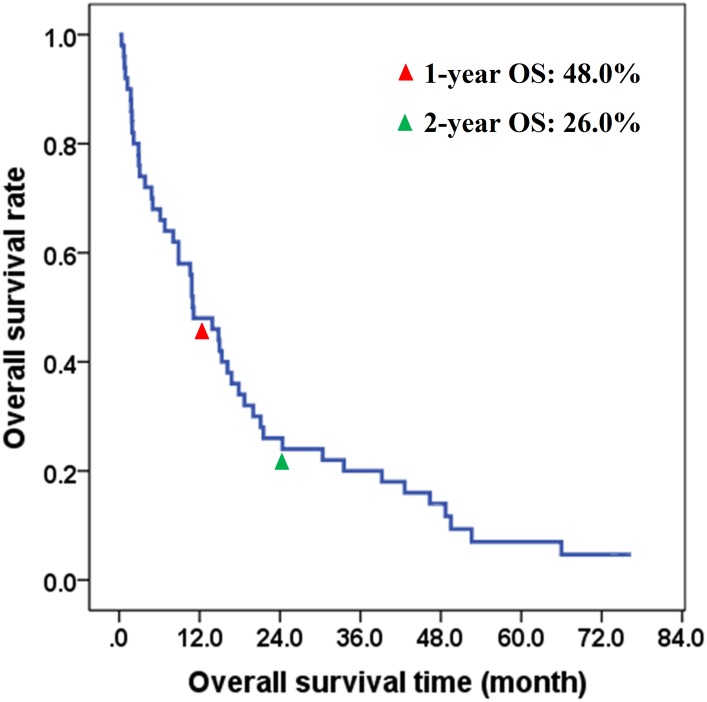
The 1-year and 2-year overall survival rates (OS) were 48.0% and 26.0% respectively in all patients, with the 2-year OS of 40.0% and 31.0% and 6.3% for stage IVA, IVB and IVC respectively (P <0.05). In stage IVA and IVB patients, combined surgery with radiotherapy improved overall survival, and the 2-year OS were 50.0% and 35.7% respectively in the group with combined surgery with radiotherapy and the group with surgery with only (P <0.05). Postoperative radiotherapy improved local control rate in stage IVA and IVB patients (P <0.05). However, surgery, radiotherapy or chemotherapy could not improve the survival of stage IVC patients. Multivariate analysis showed that distant metastases, surgery, radiotherapy and tumor residue could predict the prognosis.
Conclusion
Combined surgery and radiotherapy could improve overall survival in stage IVA and IVB patients. Patients with ATC have a bad prognosis. Distant metastases, surgery, radiotherapy and tumor residue are the most important factors affecting the prognosis.
Introduction
Anaplastic thyroid carcinoma (ATC) accounts for 1–2% of all thyroid malignancies with an annual incidence of about one to two cases per million [1, 2]. Although ATC is rare, it is one of the most aggressive human cancers, and it causes up to 40% of deaths from thyroid cancer. The average survival time of ATC is only 6 to 8 months and the 5-year survival rate of ATC is only 0 ~ 10% [1, 3].
Despite different treatment approaches, ATC grows rapidly, invades adjacent tissues, and most patients die due to uncontrolled local tumor invasion or distant metastases. The treatment options for ATC include surgery, chemotherapy and radiotherapy. Some studies showed that multimodal therapy combining surgery, chemotherapy and radiotherapy, might achieve better results in avoiding death from local invasion and improving survival in some patients; however, ATC has an extremely low cure rate even with the very best treatments, and treatment of ATC is mostly palliative [4–6]. Surgical resection with adjuvant radiation therapy and chemotherapy may prolong survival or improve quality of life, however strong evidence is needed to support this conclusion [7, 8]. The optimal multimodal therapy policy is still debated and a standardized treatment strategy remains to be established. Furthermore, the rare incidence of ATC and its aggressive nature make it difficult to compare the outcomes of different treatments, especially in studies with small cohorts [4, 5].
In this study, we retrospectively reported the clinical and pathologic outcome of a scale of 50 ATC patients treated in our institution, in order to evaluate the best treatment strategy and the factors affecting prognosis.
Patients and Methods
Patients
Fifty ATC patients were treated in our hospital between January 1990 and December 2006, accounting for 2.55% of the patients with thyroid malignant tumor (50/1963) during the same period, including 26 males and 24 females with a male to female ratio of 1.08:1. The age ranged from 36 to 85 years old, with a median age of 60 years. The medical histories ranged from 10 days to 24 months.
The main clinical manifestations included: neck mass in 38 cases, hoarseness in 9 cases, difficulty breathing in 9 cases, difficulty swallowing in 8 cases and neck pain in 13 cases. In all the patients, 35 cases were involved in unilateral thyroid, 15 cases in bilateral thyroid and 21 cases had enlarged cervical lymph nodes (21/50, 42.0%) with the most common area was ipsilateral Level IV. Sixteen cases had distant metastases on their initial hospitalization (16/50, 32.0%), including lung metastasis in 12 cases, ipsilateral radius metastasis in 1 case and multiple bilateral rib metastasis in 3 cases. According to the standard of TNM staging (UICC, 2010), 15 cases were Stage IVA, 19 cases were Stage IVa and 16 cases were Stage IVC. Written Ethics Approval and Patient Consent from the Hospital Research Ethics Committee of Sun Yat-sen University Cancer Center and Sixth Hospital were obtained, and participants provided their written informed content to participate this study.
Treatment
Treatment of the tumor and thyroid: for patients with tumor limited in unilateral thyroid lobe, total thyroidectomy was performed; while thyroidectomy with extensive resection of the surrounding tissues was performed for patients with tumor involved the surrounding tissues. Treatment of cervical lymph nodes: for patients with clinical or pathological cervical lymph nodes, level II to VI neck dissection was performed; while for patients with clinical negative cervical lymph nodes, level VI neck dissection was performed. Patients just underwent tracheotomy and tumor biopsy when they suffered from a wide range of tumor, severely invaded trachea (narrow diameter < 0.5 cm), or poor health status. Surgical margins are classified by the pathologist as R0 and R1 others; R0 indicates that no cancerous cells seen microscopically, while R1 means that cancerous cells can be seen microscopically; however the others includes tracheotomy, biopsy or non-surgical treatment.
Postoperative radiotherapy with a dose of more than 40 Gy is recommended in stage IVA and IVB patients and palliative doses should also be used to improve quality of life in some patients with widespread disease.
Follow-up
Follow-up information was obtained by the telephone and outpatient review. Follow-up time started from the date of the first treatment and ended by December 15, 2015.
Statistical Methods
SPSS 19.0 software was used for statistical analysis, counting data by chi-square test. Survival analysis was performed by Kaplan-Merier method, comparison among/between groups was performed using log-rank test, and multivariate analysis was carried out using Cox proportional hazard model. P<0.05 was considered statistically significant.
Results
Treatment results
In all these cases, 38 cases accepted total or extensive thyroidectomy with neck dissection in 16 cases (17 sides), and 3 cases accepted palliative resection of cervical lymph node, and 3 cases just underwent tracheotomy and tumor biopsy (Tables 1 and 2). The primary tumors were confined in the thyroid gland in 10 cases (10/50, 25.64%), while the tumor invaded out of the thyroid capsule in 29 cases (29/50, 74.36%). Microscopic positive surgical margin was found in 21 cases (R1) after radical surgery. The invaded surrounding structures included anterior cervical muscle and/or sternocleidomastoid in 21 cases, recurrent laryngeal nerve in 12 cases, trachea in 14 cases, esophagus in 10 cases, larynx in 3 cases, internal jugular vein or common carotid artery in 17 cases, jugular vein angle and clavicle in 2 cases, anterior superior mediastinum in 3 cases. Cervical lymph node metastasis was found in 21 cases (21/50, 42.0%), with ipsilateral Level IV as the most common involved area (11/19, 57.9%).
Table 1. Clinical characteristics of the 50 ATC patients.
| Factor | Cases | Overall survival | P value | |
|---|---|---|---|---|
| 1-year | 2-year | |||
| Gender | ||||
| Female | 24 | 45.8 | 16.7 | 0.254 |
| Male | 26 | 50.0 | 34.6 | |
| Age | ||||
| ≤ 60 years | 26 | 53.8 | 30.8 | 0.261 |
| > 60 years | 24 | 41.7 | 20.8 | |
| Diameter of primary tumor | ||||
| ≤ 4 cm | 17 | 47.1 | 29.4 | 0.808 |
| > 4 cm | 33 | 48.5 | 24.2 | |
| T stage | ||||
| T4a | 19 | 63.2 | 36.8 | 0.125 |
| T4b | 31 | 38.7 | 19.4 | |
| Strap muscle invasion | ||||
| No | 28 | 50.0 | 35.7 | 0.462 |
| Yes | 22 | 45.5 | 13.6 | |
| Recurrent laryngeal nerve invasion | ||||
| No | 41 | 46.3 | 29.3 | 0.488 |
| Yes | 9 | 55.6 | 11.1 | |
| Trachea invasion | ||||
| No | 30 | 56.7 | 33.3 | 0.192 |
| Yes | 20 | 35.0 | 15.0 | |
| Esophageal invasion | ||||
| No | 40 | 50.0 | 27.5 | 0.255 |
| Yes | 10 | 40.0 | 20.0 | |
| Lymph node metastasis | ||||
| No | 29 | 48.3 | 34.5 | 0.187 |
| Yes | 21 | 47.6 | 14.3 | |
| Distant metastasis | ||||
| No | 34 | 58.8 | 35.3 | 0.006 |
| Yes | 16 | 25.0 | 6.3 | |
| Stage | ||||
| VIA | 15 | 60.0 | 40.0 | 0.021 |
| VIB | 19 | 57.9 | 31.6 | |
| VIC | 16 | 25.0 | 6.3 | |
| Surgery | ||||
| R0 | 17 | 76.5 | 47.1 | 0.000 |
| R1 | 21 | 47.6 | 23.8 | |
| Other | 12 | 8.3 | 0.0 | |
| Radiotherapy | ||||
| No | 34 | 35.3 | 17.6 | 0.004 |
| Yes | 16 | 75.0 | 43.8 | |
| Chemotherapy | ||||
| No | 42 | 47.6 | 26.2 | 0.270 |
| Yes | 8 | 50.0 | 25.0 | |
| Tumor residue | ||||
| No | 21 | 76.2 | 52.4 | 0.000 |
| Yes | 29 | 27.6 | 6.9 | |
Table 2. Treatment methods of the 50 ATC patients.
| Treatment group | Case(s) |
|---|---|
| Surgery only | 21 |
| Surgery plus radiotherapy or chemotherapy | 17 |
| Chemoradiotherapy | 1 |
| Chemotherapy only | 2 |
| Tracheotomy, biopsy or other | 9 |
Sixteen patients underwent radiotherapy including postoperative (external beam) radiotherapy in 15 cases, with the dose varying from 30 to 70 Gy, including more than 40 Gy in 15 cases. And 8 cases received chemotherapy, including postoperative adjuvant chemotherapy in 5 cases and palliative chemotherapy in 3 cases (Tables 1 and 2). The chemotherapeutic agents were mainly MAID (ifosfamide, adriamycin, and dacarbazine) for 2 ~ 5 courses.
Twenty-nine cases had tumor residual after their first treatments, including 6 cases just underwent tracheotomy and tumor biopsy without further treatments.
Survival conditions
By the latest follow-up on December 15, 2015, 3 cases were still alive and 47 cases died from cancer-related diseases, including 35 cases died of primary tumor, and 12 cases died from distant metastases. The 1-year, 2-year and 5-year overall survival rates / cancer-specific survival rates were 48.0%, 26.0% and 4.7% respectively in all patients, with the 2-year overall survival / cancer-specific survival rates of 40.0%, 31.0% and 6.3% for stage IVA, IVB and IVC respectively (P <0.05). The survival curve was demonstrated in Fig 1.
Fig 1. Overall survival of 50 ATC Patients.
Local control rate
After the first treatment, 29 patients survived with tumor (29/50, 56.0%), and the other 21 patients survived without tumor (21/50, 42.0%). The 1-year and 2-year local control rates were 26.0% (13/50) and 16.0% (8/50), respectively.
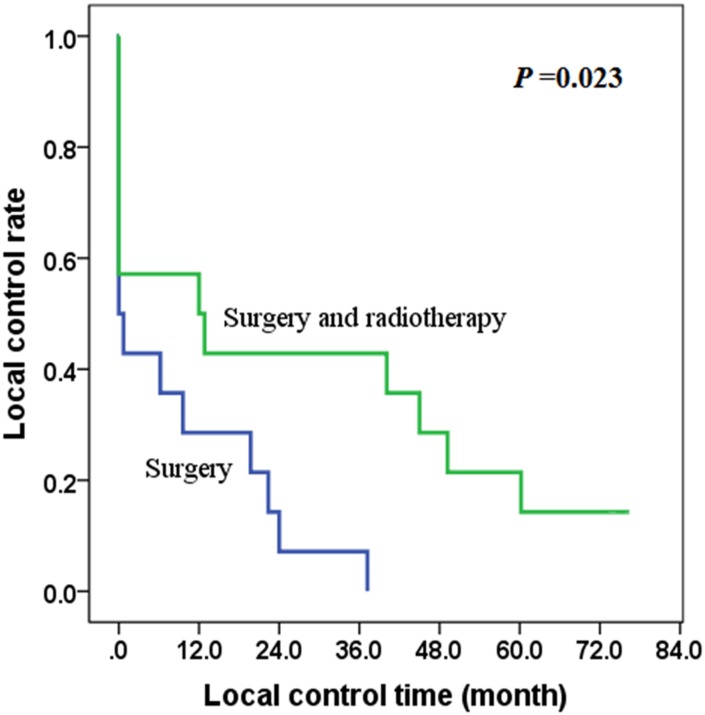
In patients without distant metastasis (IVA and IVB), most cases were treated mainly with surgery (S) or combined surgery with postoperative radiotherapy (SR), and there was no significant difference in the R0/R1 ratio between the S group (7/7, 50.0%) and the SR group (5/9, 55.6%) (χ2 = 0.583, P = 0.704). The local control rate of the SR group was better than that of the S group (χ2 = 5.139, P = 0.023), with the 2-year local control rates of 42.9% and 7.1% respectively in the SR group and S group (Fig 2).
Fig 2. The effect of radiotherapy on local control of stage IVA and IVB patients.
The role of radical surgery on the survival of patients with ATC
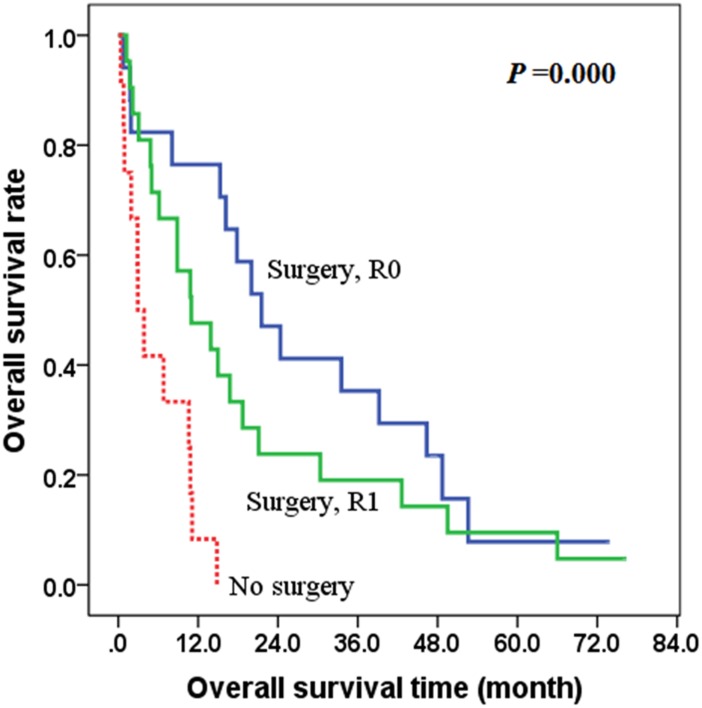
In all patients, radical (extensive) surgical treatment was performed in 38 cases. For surgical margin, 17 cases were R0 and 21 cases were R1. The overall survival of the R0 group or R1 group was better than that of the group without surgery (χ2 = 17.364, P = 0.000), with the 2-year overall survival rates of 58.3%, 31.3% and 0.0% respectively in the R0 group, R1 group and the group without radical surgery (Fig 3).
Fig 3. The effect of radical surgery on overall survival of ATC patients.
The role of postoperative radiotherapy on the survival of stage IVA and IVB patients
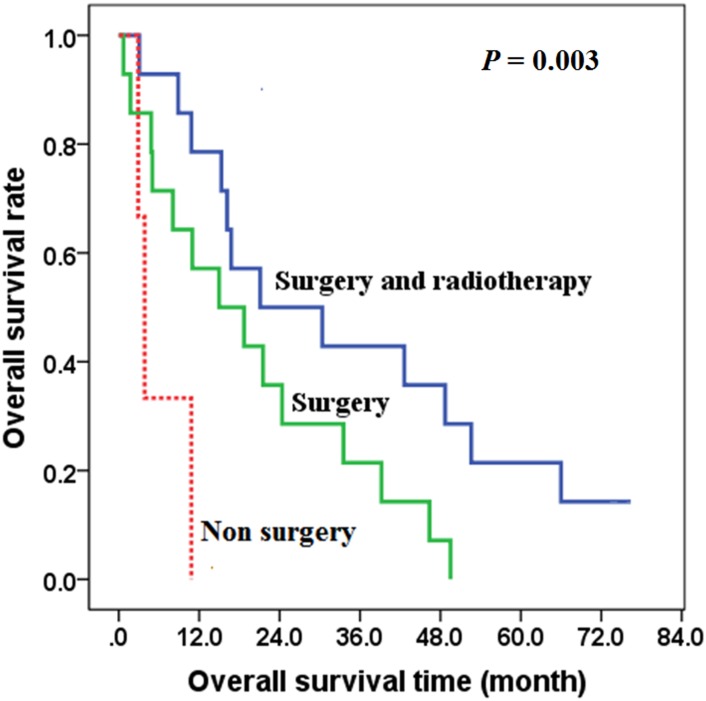
In patients without distant metastasis (IVA and IVB), the overall survival of the group treated with combined surgery and postoperative radiotherapy was better than the group treated with only surgery (χ2 = 4.584, P = 0.032), with the 2-year overall survival rates of 50.0% and 35.7% respectively in the postoperative radiotherapy group and surgery only group (Fig 4). There was no significant difference in the R0/R1 ratio between the group treated with surgery only (7/7, 50.0%) and the group with postoperative radiotherapy (5/9, 55.6%) (χ2 = 0.583, P = 0.704).
Fig 4. The effects of postoperative radiotherapy on overall survival of stage IVA and IVB patients.
The survival of patients with non-surgical treatment was worse than the postoperative radiotherapy group or surgery group (χ2 = 11.493, P = 0.003) (Table 3).
Table 3. The overall survival of different treatment groups of stage IVA and IVB patients.
| Factor | Cases | Overall survival | P value | |
|---|---|---|---|---|
| 1-year | 2-year | |||
| Surgery+ radiotherapy | 14 | 78.6 | 50.0 | |
| Surgery only | 14 | 57.1 | 35.7 | 0.003* |
| Radiotherapy or chemotherapy | 3 | 0.0 | 0.0 | |
* Surgery with radiotherapy vs Surgery only: P = 0.032.
The role of different treatment methods on the survival of stage IVC patients
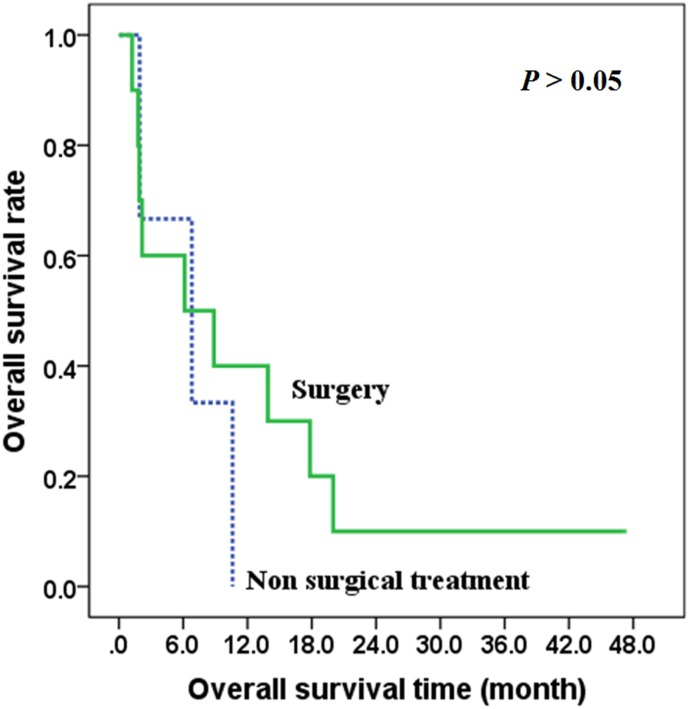
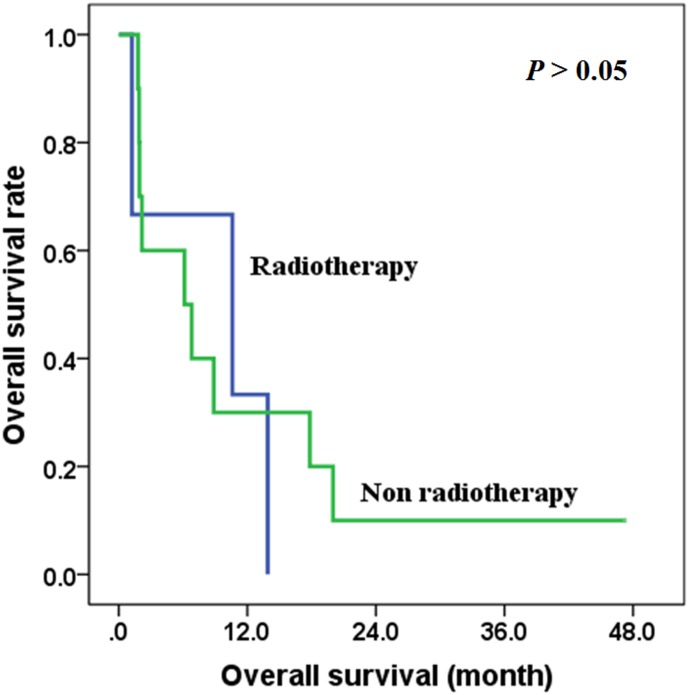
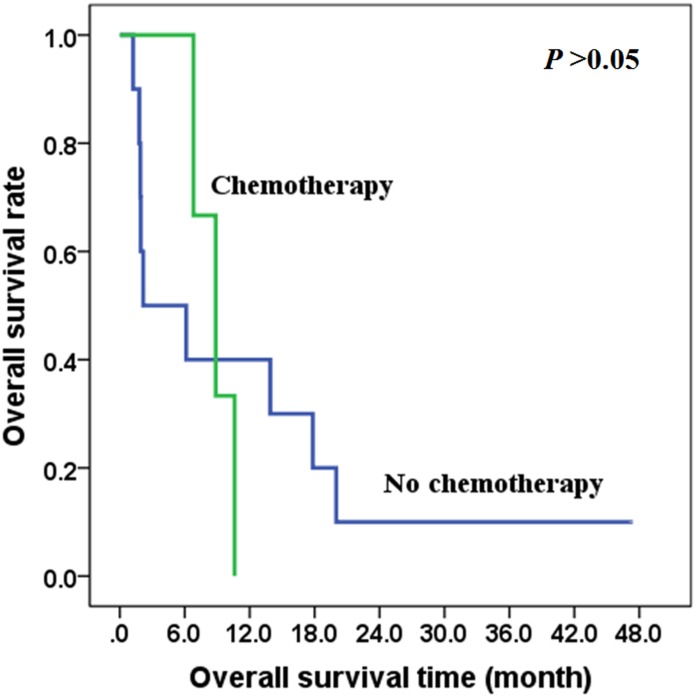
In patients with distant metastasis (IVC), there was no difference between the overall survival time of those treated with surgery and those with non-surgical treatment (χ2 = 0.413, P = 0.521), with the 2-year overall survival rates of 50.0% and 35.7% respectively in the two groups (Fig 5). Also, there was no difference between the overall survival time of those treated with radiotherapy and those treated without radiotherapy (χ2 = 0.125, P = 0.724) (Fig 6), and there was no difference between the overall survival time of those treated with chemotherapy and those treated without chemotherapy (χ2 = 0.041, P = 0.839) (Fig 7).
Fig 5. The effects of surgery on overall survival of stage IVC patients.
Fig 6. The effects of radiotherapy on overall survival of stage IVC patients.
Fig 7. The effects of chemotherapy on overall survival of stage IVC patients.
Multivariate analyses of the prognostic factors on overall survival
In order to evaluate the important factors affecting survival of patients, the possible factors that may affect prognosis, such as gender, age (> 60 years old, ≤60 years old), diameter of primary tumor, tumor confined to the thyroid gland or not, lymph node metastasis, trachea invasion, esophageal invasion, distant metastasis, surgical treatment, radiotherapy, chemotherapy and tumor residue were analyzed by multivariate Cox regression analysis. The results showed that the independent factors affecting the prognosis of patients underwent treatment were primary tumor size (diameter) less than 4 cm, distant metastases, surgery, radiotherapy, chemotherapy and tumor residue (Table 4). Multivariate analysis showed that distant metastases, surgery, radiotherapy and tumor residue could predict the prognosis.
Table 4. Multivariate analysis results of factors affecting the prognosis.
| Factors | B | SE | Wald | P | Exp(B) |
|---|---|---|---|---|---|
| Age | -.282 | .404 | .486 | .486 | .754 |
| Diameter of primary tumor ≤ 4 cm | -1.333 | .407 | 10.718 | .001 | .264 |
| Limited to gland | -.485 | .466 | 1.081 | .299 | .616 |
| Strap muscle invasion | .350 | .419 | .699 | .403 | 1.419 |
| Trachea invasion | .254 | .432 | .345 | .557 | 1.289 |
| Esophageal invasion | .181 | .448 | .162 | .687 | 1.198 |
| Lymph node metastasis | -.231 | .362 | .406 | .524 | .794 |
| Distant metastasis | 1.235 | .397 | 9.678 | .002 | 3.438 |
| Surgery | -1.104 | .532 | 4.306 | .038 | .331 |
| Radiotherapy | -1.215 | .448 | 7.350 | .007 | .297 |
| Chemotherapy | -1.757 | .587 | 8.972 | .003 | .173 |
| Tumor residue | 2.575 | .590 | 19.022 | .000 | 13.131 |
Discussion
ATC accounts for 1% ~ 2% of all thyroid tumors, with the characteristics of fast progression, strong local invasion, high distant metastasis rate and so on. The tumor has a very poor prognosis, and the average survival time is approximately 6 to 8 months [1, 3, 7, 9]. Multimodal therapy combining surgery, chemotherapy and radiation therapy, might achieve better results in improving survival in some patients; however, ATC has a very low cure rate even with the extremely radical treatments [3, 4].
Compared with differentiated thyroid cancer, one of the notable characteristics of ATC is the aggressive invasion of the thyroid capsule and the adjacent structures, and early neck lymph nodes metastasis and distant metastasis [10]. In this study, 31 of the 50 cases (62.0%) with tumor invasion through the thyroid capsule, and the most common invaded surrounding structures included the strap muscle, recurrent laryngeal nerve, trachea and esophagus. The tumor was hardly removed completely in patients with extensive tumor invasion. Thirty-eight patients underwent extensive thyroidectomy, but local microscopic tumor residue was found in 21 cases. Tumor residue was a main factor affecting prognosis; therefore early screen of cancer and taking active measures in initial treatment, such as multimodal treatment, may help to improve prognosis [5]. ATC patients have a high regional lymph node metastasis rate [11], such as 42.0% in this group, with level IV as the most common area. Additionally, ATC patients have a high distant metastasis rate, such as 32.0% in this group with the lung as the most common metastatic site.
The main treatment strategies of ATC are surgery, radiotherapy chemotherapy and biotherapy [4–7, 12], and radical surgical treatment is still a key therapeutic method affecting the prognosis. This study showed that there was significant difference in survival rates among the radical surgical treatment group and non-surgical treatment group (P = 0.000). Some scholars found that survival outcomes were significantly higher in patients with resectable tumors than in those with unresectable tumors [2, 7, 10, 13]. Sugitani’s study showed that the 1-year survival rate was found to be significantly higher in resectable tumors than that in the unresectable tumors (39.0% and 10.0%, respectively; P < 0.0001), which showed that the important role of surgical treatment in ATC [13]. R0 (negative surgical margin), and R1 (gross resection, positive microscopic margin) might result in substantial improvement in local control and survival; however, most of these studies are biased because they are retrospective and not randomized to control for bias factors such as extent of disease or adjuvant treatments [14, 15].
This study also showed that postoperative radiotherapy can improve OS in stage IVA and IVB patients (P = 0.032), and the 2-year overall survival rates of 50.0% and 35.7% respectively in the postoperative radiotherapy group and the surgery only group. Also, our study indicated that postoperative radiotherapy (more than 40 Gy) improved local control rate in stage IVA and IVB patients (P = 0.023). Furthermore, multivariable analysis showed that radiotherapy is an independent factor affecting prognosis. Some scholars also suggested that postoperative radiotherapy might be an effective way in the treatment of ATC [5, 16]. A study from Glaser SM showed that high-dose radiotherapy (>59.4 Gy) resulted in improved survival in ATC, and radiotherapy is a prognostic factor [17]. Therefore, we recommend postoperative radiotherapy with a dose of more than 40 Gy in stage IVA and IVB patients and palliative doses should also be used to improve quality of life in some patients with widespread disease.
Chemotherapy drugs, including cisplatin, doxorubicin, vincristine, were used to treat ATC patients commonly; however, it is still controversial whether chemotherapy can prolong the survival time and improve prognosis [18, 19]. Theoretically, chemotherapy can control the small metastatic sites around the main primary tumor, reduce the tumor dissemination or increase the tumor resection rate by shrinking the tumor, or improve the effect of radiotherapy and improve the long-term curative effect. Nevertheless, it is lacked of strong evidence to prove the above opinions [18, 19]. Our study also showed that chemotherapy might be an independent factor affecting prognosis in multivariate analysis; however, this study is limited in chemotherapy cases by sample size, and this opinion still needs to be confirmed by extending the sample. A study from Haigh confirmed postoperative chemoradiotherapy can improve the survival rate [2]. Busnard B thought preoperative chemoradiotherapy can improve tumor resection rate and improve prognosis [20], however, Mclve found it didn’t significantly prolong survival time in patients who accepted a comprehensive treatment based on surgery and chemoradiotherapy [21]. Prospective studies with enlarged sample size are needed to confirm whether comprehensive treatment is better than simply surgical treatment.
The prognosis of ATC patient is very poor, and this study showed that the 1-year and 2-year overall survival rates were 48.0% and 26.0%, respectively in all patients. This study also indicated that the independent factors affecting the prognosis of patients underwent treatment were primary tumor size (diameter) less than 4 cm, distant metastases, surgical treatment, radiotherapy and tumor residue. Some studies showed that white blood cell count and whether to accept surgery plus postoperative radiotherapy were the independent factors influencing the prognosis of ATC [22]. Lo C evaluated the factors influencing the prognosis of ATC and found that age may be associated with prognosis, limited lesion means a better prognosis, while with or without differentiated thyroid cancer may have nothing to do with the prognosis; the size of primary tumor was associated with prognosis, and the tumor resection rate was higher when the tumor diameter was less than 5 to 6 cm, which leads to a good prognosis [23]. Therefore, age and tumor size may also be prognostic factors of ATC patient [17, 24]. However, most of these studies are retrospective and biased and prospective randomized studies are needed in order to confirm such opinions.
Data Availability
All relevant data are within the paper and there is no Supporting Information.
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 81272955 and 81302368) and Guangdong Province Natural Science Foundation (2015A030313058).
References
- 1.Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Annals of surgical oncology. 2006;13(4):453–464. 10.1245/ASO.2006.05.042 [DOI] [PubMed] [Google Scholar]
- 2.Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001;91(12):2335–2342. [PubMed] [Google Scholar]
- 3.Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103(7):1330–1335. 10.1002/cncr.20936 [DOI] [PubMed] [Google Scholar]
- 4.Ito K, Hanamura T, Murayama K, Okada T, Watanabe T, Harada M, et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head & neck. 2012;34(2):230–237. 10.1002/hed.21721 [DOI] [PubMed] [Google Scholar]
- 5.Yau T, Lo CY, Epstein RJ, Lam AK, Wan KY, Lang BH. Treatment outcomes in anaplastic thyroid carcinoma: survival improvement in young patients with localized disease treated by combination of surgery and radiotherapy. Annals of surgical oncology. 2008;15(9):2500–2505. 10.1245/s10434-008-0005-0 [DOI] [PubMed] [Google Scholar]
- 6.McLarnon A. Thyroid cancer: Pazopanib alone is not effective against anaplastic thyroid cancer. Nature reviews Endocrinology. 2012;8(10):565 10.1038/nrendo.2012.136 [DOI] [PubMed] [Google Scholar]
- 7.Polistena A, Monacelli M, Lucchini R, Triola R, Conti C, Avenia S, et al. The role of surgery in the treatment of thyroid anaplastic carcinoma in the elderly. International journal of surgery. 2014;12 Suppl 2:S170–176. 10.1016/j.ijsu.2014.08.347 [DOI] [PubMed] [Google Scholar]
- 8.Sun XS, Sun SR, Guevara N, Marcy PY, Peyrottes I, Lassalle S, et al. Indications of external beam radiation therapy in non-anaplastic thyroid cancer and impact of innovative radiation techniques. Critical reviews in oncology/hematology. 2013;86(1):52–68. 10.1016/j.critrevonc.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 9.O'Neill JP, O'Neill B, Condron C, Walsh M, Bouchier-Hayes D. Anaplastic (undifferentiated) thyroid cancer: improved insight and therapeutic strategy into a highly aggressive disease. The Journal of laryngology and otology. 2005;119(8):585–591. 10.1258/0022215054516197 [DOI] [PubMed] [Google Scholar]
- 10.Lee DY, Won JK, Lee SH, Park do J, Jung KC, Sung MW, et al. Changes of Clinicopathologic Characteristics and Survival Outcomes of Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid: official journal of the American Thyroid Association. 2016;26(3):404–413. 10.1089/thy.2015.0316 [DOI] [PubMed] [Google Scholar]
- 11.Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid: official journal of the American Thyroid Association. 2013;23(6):709–713. 10.1089/thy.2012.0252 [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Shen Z, Xiao J, Yang Y, Huang W, Zhou Z, et al. Acetylcholinesterase overexpression mediated by oncolytic adenovirus exhibited potent anti-tumor effect. BMC cancer. 2014;14:668 10.1186/1471-2407-14-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugitani I, Miyauchi A, Sugino K, Okamoto T, Yoshida A, Suzuki S. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World journal of surgery. 2012;36(6):1247–1254. 10.1007/s00268-012-1437-z [DOI] [PubMed] [Google Scholar]
- 14.Brown RF, Ducic Y. Aggressive surgical resection of anaplastic thyroid carcinoma may provide long-term survival in selected patients. Otolaryngology—head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013;148(4):564–571. 10.1177/0194599813477364 [DOI] [PubMed] [Google Scholar]
- 15.Smallridge RC. Approach to the patient with anaplastic thyroid carcinoma. The Journal of clinical endocrinology and metabolism. 2012;97(8):2566–2572. 10.1210/jc.2012-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tennvall J, Lundell G, Wahlberg P, Bergenfelz A, Grimelius L, Akerman M, et al. Anaplastic thyroid carcinoma: three protocols combining doxorubicin, hyperfractionated radiotherapy and surgery. British journal of cancer. 2002;86(12):1848–1853. 10.1038/sj.bjc.6600361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glaser SM, Mandish SF, Gill BS, Balasubramani GK, Clump DA, Beriwal S. Anaplastic thyroid cancer: Prognostic factors, patterns of care, and overall survival. Head & neck. 2016;38 Suppl 1:E2083–2090. 10.1002/hed.24384 [DOI] [PubMed] [Google Scholar]
- 18.Pushkarev VM, Starenki DV, Saenko VA, Pushkarev VV, Kovzun OI, Tronko MD, et al. Differential effects of low and high doses of Taxol in anaplastic thyroid cancer cells: possible implication of the Pin1 prolyl isomerase. Experimental oncology. 2008;30(3):190–194. [PubMed] [Google Scholar]
- 19.Heron DE, Karimpour S, Grigsby PW. Anaplastic thyroid carcinoma: comparison of conventional radiotherapy and hyperfractionation chemoradiotherapy in two groups. American journal of clinical oncology. 2002;25(5):442–446. [DOI] [PubMed] [Google Scholar]
- 20.Busnardo B, Daniele O, Pelizzo MR, Mazzarotto R, Nacamulli D, Devido D, et al. A multimodality therapeutic approach in anaplastic thyroid carcinoma: study on 39 patients. Journal of endocrinological investigation. 2000;23(11):755–761. 10.1007/BF03345066 [DOI] [PubMed] [Google Scholar]
- 21.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130(6):1028–1034. 10.1067/msy.2001.118266 [DOI] [PubMed] [Google Scholar]
- 22.Sun C, Li Q, Hu Z, He J, Li C, Li G, et al. Treatment and prognosis of anaplastic thyroid carcinoma: experience from a single institution in China. PloS one. 2013;8(11):e80011 10.1371/journal.pone.0080011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo CY, Lam KY, Wan KY. Anaplastic carcinoma of the thyroid. American journal of surgery. 1999;177(4):337–339. [DOI] [PubMed] [Google Scholar]
- 24.Kim TY, Kim KW, Jung TS, Kim JM, Kim SW, Chung KW, et al. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head & neck. 2007;29(8):765–772. 10.1002/hed.20578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and there is no Supporting Information.