Abstract
Background
The features related to the prognosis of patients with mucinous breast cancer (MBC) remain controversial. We aimed to explore the prognostic factors of MBC and develop a nomogram for predicting survival outcomes.
Methods
The Surveillance, Epidemiology, and End Results (SEER) database was searched to identify 139611 women with resectable breast cancer from 1990 to 2007. Survival curves were generated using Kaplan-Meier methods. The 5-year and 10-year cancer-specific survival (CSS) rates were calculated using the Life-Table method. Based on Cox models, a nomogram was constructed to predict the probabilities of CSS for an individual patient. The competing risk regression model was used to analyse the specific survival of patients with MBC.
Results
There were 136569 (97.82%) infiltrative ductal cancer (IDC) patients and 3042 (2.18%) MBC patients. Patients with MBC had less lymph node involvement, a higher frequency of well-differentiated lesions, and more estrogen receptor (ER)-positive tumors. Patients with MBC had significantly higher 5 and10-year CSS rates (98.23 and 96.03%, respectively) than patients with IDC (91.44 and 85.48%, respectively). Univariate and multivariate analyses showed that MBC was an independent factor for better prognosis. As for patients with MBC, the event of death caused by another disease exceeded the event of death caused by breast cancer. A competing risk regression model further showed that lymph node involvement, poorly differentiated grade and advanced T-classification were independent factors of poor prognosis in patients with MBC. The Nomogram can accurately predict CSS with a high C-index (0.816). Risk scores developed from the nomogram can more accurately predict the prognosis of patients with MBC (C-index = 0.789) than the traditional TNM system (C-index = 0.704, P< 0.001).
Conclusions
Patients with MBC have a better prognosis than patients with IDC. Nomograms could help clinicians make more informed decisions in clinical practice. The competing risk regression model, as a more rational model, is recommended for use in the survival analysis of patients with MBC in the future.
Introduction
Breast cancer is the most common cancer in women worldwide. Mucinous breast cancer (MBC) is a rare and special type, presenting with substantial extracellular mucin, and its incidence was reported to range from 1% to 6% for all primary breast cancers [1–4]. MBC is distinct from breast cancer, and its uniqueness should be considered in clinical practice. MBC is commonly seen in elderly, postmenopausal patients and is generally considered to have a favorable prognosis. Previous studies have found that MBC tumors have specific characteristics, such as a high expression of hormone receptors and low expression of human epidermal growth factor receptor 2 (EGFR2/HER2) [3–7]. In previous studies, MBC has been categorized into type A tumor, type B tumor or type AB tumor [1,8,9]. Type B tumor and neuroendocrine tumor seemed to constitute a spectrum of lesions [10]. Micro-papillary MBC is also considered a special type B subgroup with poor prognosis [11]. Additionally, the microsatellite instability (MSI) phenotype is remarkably rare in MBC compared with mucinous carcinomas present at other anatomical sites [12,13].
Previous clinical studies reported that patients with MBC had bias that resulted from a small sample size or limited time of follow-up due to its relative rarity. The treatment guidelines for optimal local and systemic control of MBC are mostly extrapolated from the treatment experience with IDC and have not undergone rigorous validation in MBC patients. The lack of a particular prognosis evaluation system for MBC has resulted in uniform treatment for MBC.
Currently, nomograms have been developed in the majority of cancer types [14–16]. Nomograms have been accepted as a reliable and alternative tool, or even as a new standard[17], to assist clinicians with making convenient individual predictions. In this study, using a large, nationwide, population-based data, we retrospectively investigated the clinicopathological characteristics of MBC. Furthermore, we attempt to establish a nomogram for patients with MBC based on the clinicopathological data.
Materials and Methods
Patient selection and data processing
The Surveillance, Epidemiology, and End Results (SEER) database (http://seer.cancer.gov/) is sponsored by the National Cancer Institution with the aim of collecting information about the cancer incidence and outcome. The current SEER database collects and publishes cancer data from 18 population-based cancer registries among 14 states across the United States, representing approximately 30% of the United States population. The SEER database is collected and released annually, reflecting the most updated information. The SEER data do not capture information about surgery or radiation provided in the past four months of diagnosis nor is there information about recurrence or metastasis that is detected subsequent to the initial diagnosis. We received permission to access the research data (Reference Number: 10263-Nov2015). The study was approved by the review board of Zhejiang University Jinhua hospital. SEER.Stat software was utilized to identify patients with breast cancer in 1990–2007. The specific inclusion criteria were as follows: (1) Years of diagnosis were from 1990 to 2007. (2) Patients without distant metastases. (3) Histological type ICD-O-3 was limited to 8500/3 (IDC) and 8480/3 (MBC). The exclusion criteria were as follows: (1) Patients lacking documentation of race, age at diagnosis and marital status. (2) Patients younger than 20 years old or older than 80 years old. (3) Patients with multiple primary tumors (excluded to make the analyses of cancer-specific survival (CSS) more consistent). (4) Patients who survived less than one month (for the detailed inclusion and exclusion criteria, see S1 Fig).
Statistical analyses
All of the cases were regrouped according to the 7th American Joint Committee on Cancer (AJCC) TNM staging system. Race was divided into white, black and other. The hormone-receptor status of the tumor was stratified to hormone receptor (HoR) -positive [estrogen receptor (ER)-positive/progesterone receptor (PR)-positive, ER-negative/PR-positive and ER-positive/PR-negative] and HoR-negative (ER-negative/PR-negative). The cutoff age of 70 was achieved through the X-tile program [18] (S1 Fig). Age was classified into young (< or = 70 years old) and old (> 70 years old) groups. Marital status was regrouped as married, single (never married or having a domestic partner) or divorce (separated, divorced and widowed).
The distribution of the histological type in different subgroups was analyzed using Chi-Squared tests. The CSS was calculated from the date of diagnosis to the date of death from breast cancer. Survival curves were generated using Kaplan-Meier methods, and the log-rank test was performed to evaluate the survival differences between groups. The 5- and 10-year CSS rates were calculated using the Life-Table method. Multivariable analyses were performed with Cox regression models and adjusted hazard ratios (HRs) along with 95% confidence intervals (CIs) to adjust for prognostic variables. In the multivariable analysis, the T- and N-classification and ER/PR status variables, rather than the stage and HoR status variables, were included to avoid multicollinearity.
A nomogram was constructed based on the results of the Cox proportional hazard model and by using the rms package in R software (http://www.r-project.org/). For inclusion into the final nomogram, the effect of the continuous variable, age, was explored using restricted cubic splines with five knots, resulting in a satisfactory sensitivity. The nomogram was internally validated by bootstrapping with 1000 resamples as quantified by the concordance index (C-index). Calibration curves, which plot the average Kaplan-Meier estimate against the corresponding nomogram for 5- or 10- year CSS, are provided to evaluate the nomogram performance.
The probability of CSS in every variable was predicted as a point by the nomogram. The risk score of CSS was calculated for each patient by totaling the points for every variable. Using two cut-off values from the X-tile program, the cohort was classified as three subgroups: low risk = scoring 0–158, medium risk = scoring 159–205 and high risk = scoring 205–416.
In the MBC cohort, the cumulative incidence of breast cancer special death (BCSD) was calculated based on a competing risk regression model [19]. The BCSD was considered as the failure event and non-BCSD as the competing event. The stacked cumulative incidence function plot was used to describe the actual prognosis of specific causes of death [20].
When the two-sided P value was less than 0.05, the difference was considered statistically significant. Analyses were performed using statistical software STATA/SE 12.0 (StataCorp LP, TX, USA) and R software (version 3.0.1).
Results
Clinicopathological characteristics
A total of 139611 eligible patients with early breast cancer were included in the study. The medium age of the 136569 (97.82%) patients with IDC was 53 years, and it was 75 years in the 3042 (2.18%) patients with MBC. The detailed clinicopathological characteristics according to the histological types are summarized in Table 1. Patients with MBC had a higher percentage than IDL in cases with patients over 70 years old (P < 0.001). MBC was more common in women of another race (P < 0.001). Furthermore, patients with MBC had less lymph node involvement (89.84% vs. 65.29%, P < 0.001), an earlier stage (stage I) (68.54% vs. 49.74%, P < 0.001), and well-differentiated lesions (59.57% vs. 17.40%, P < 0.001). MBC were associated with a higher frequency of ER-positive status (96.75% vs. 84.52%, P < 0.001) as well as a dramatically higher frequency of HoR-positive status (97.14%).
Table 1. The characteristics of 139611 patients with resectable breast cancer.
ER: Estrogen receptor; HoR: Hormone receptor; PR: prognosis receptor; IDC: Infiltrating duct carcinoma; and MBC: mucinous breast cancer.
| Risk Factors | n (%) | IDC, n (%) | MBC, n (%) | P * |
|---|---|---|---|---|
| Total | 139611 | 136569(97.82) | 3042(2.18) | |
| Age | <0.001 | |||
| Younger | 115213 | 113163(82.86) | 2050(67.39) | |
| Older | 24398 | 23406(17.14) | 992(32.61) | |
| Marital status | ||||
| Married | 87769 | 86038(63.00) | 1731(56.90) | <0.001 |
| Single | 17613 | 17210(12.60) | 403(13.25) | |
| Divorced | 34229 | 33321(24.40) | 908(29.85) | |
| Race | ||||
| White | 114544 | 112092(82.08) | 2452(80.60) | <0.001 |
| Black | 12886 | 12656(9.27) | 230(7.56) | |
| Other | 12181 | 11821(8.66) | 360(11.83) | |
| Location | <0.001 | |||
| Central portion of breast | 11403 | 11051(8.09) | 352(11.57) | |
| Upper-inner quadrant | 23561 | 23054(16.88) | 507(16.67) | |
| Lower-inner quadrant | 12292 | 11844(8.67) | 448(14.73) | |
| Upper-outer quadrant | 77704 | 76391(55.94) | 1313(43.16) | |
| Lower-outer quadrant | 14651 | 14229(10.42) | 422(13.87) | |
| Differentiated grade | ||||
| Well | 25570 | 23758(17.40) | 1812(59.57) | <0.001 |
| Moderate | 57856 | 56792(41.58) | 1064(34.98) | |
| Poor | 56185 | 56019(41.02) | 166(5.46) | |
| T-classification | ||||
| T1 | 90953 | 88742(64.98) | 2211(72.68) | |
| T2 | 41656 | 40925(29.97) | 731(24.03) | |
| T3 | 4798 | 4709(3.45) | 89(2.93) | <0.001 |
| T4 | 2204 | 2193(1.61) | 11(0.36) | |
| N-classification | ||||
| N0 | 91896 | 89163(65.29) | 2733(89.84) | |
| N1 | 33160 | 32908(24.10) | 252(8.28) | |
| N2 | 9906 | 9862(7.22) | 44(1.45) | |
| N3 | 4649 | 4636(3.39) | 13(0.43) | <0.001 |
| Stage a | ||||
| I | 70018 | 67933(49.74) | 2085(68.54) | |
| II | 52219 | 51349(37.60) | 870(28.60) | |
| III | 17374 | 17287(12.66) | 87(2.86) | |
| ER | <0.001 | |||
| Negative | 34915 | 34816(25.49) | 99(3.25) | |
| Positive | 104696 | 101753(74.51) | 2943(96.75) | |
| PR | <0.001 | |||
| Negative | 48284 | 47813(35.01) | 471(15.48) | |
| Positive | 91327 | 88756(64.99) | 2571(84.52) | |
| HoR | <0.001 | |||
| Negative | 31916 | 31829(23.31) | 87(2.86) | |
| Positive | 107695 | 104740(76.69) | 2955(97.14) |
* P values obtained from the χ2 test. All statistical tests were two-sided.
Survival analysis
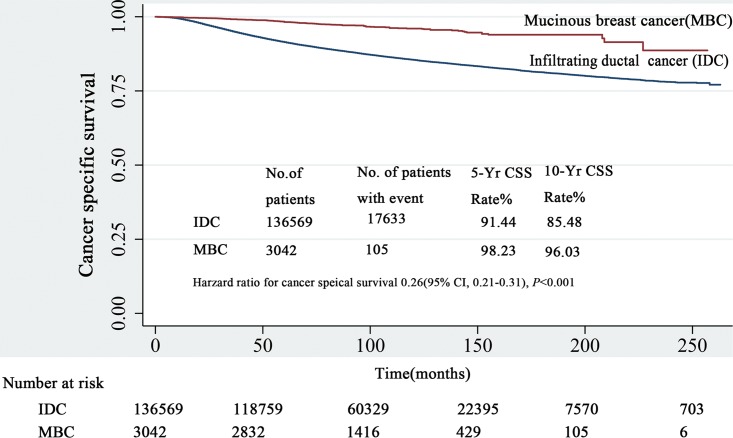
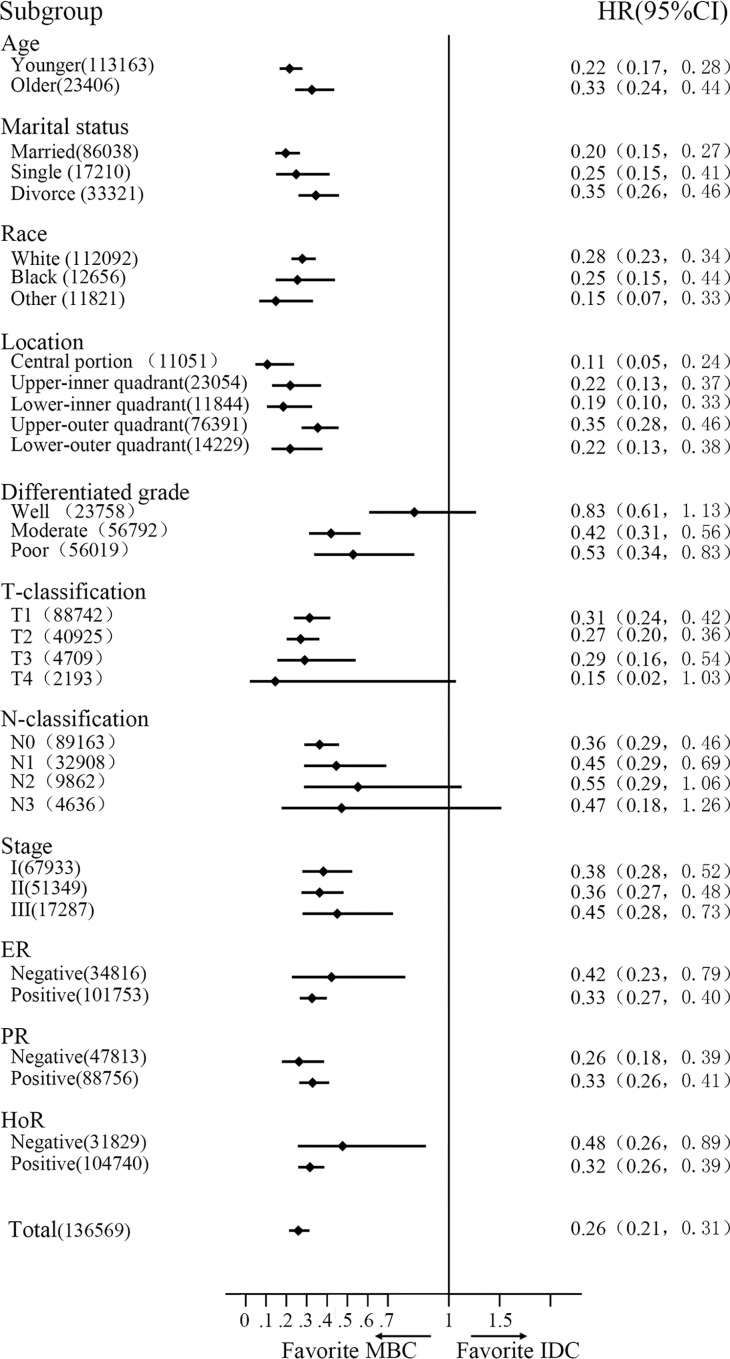
The median follow-up was 91 months (range 1–263 months). Patients with MBC obviously had better survival (HR = 0.26; 95% CI, 0.21–0.31, P < 0.001). The 5- and 10-year CSS rates of MBC were 98.23% and 96.03%, respectively, while 91.44 and 85.48% were observed for patients with IDC (Fig 1). Multivariate analysis with the Cox regression model showed that MBC was an independently better prognostic factor (HR = 0.62; 95% CI, 0.51–0.75; P < 0.001). Furthermore, we stratified the entire cohort by histological type and analyzed CSS according to patient and tumor characteristics (Fig 2). The forest plot of subgroup analysis revealed that except for the well-differentiated type, T4-classification or N3-classification subgroup, patients with MBC had favorite outcomes compared with patients with IDC.
Fig 1. The survival of patients with MBC and IDC by Kaplan-Meier analysis.
Patients with MBC obviously had better survival (HR = 0.26; 95% CI, 0.21–0.31, and P < 0.001) with 5- and 10-year CSS rates of 98.23% and 96.03% versus 91.44% and 85.48% in patients with IDC, respectively. IDC: infiltrative ductal cancer; MBC: mucinous breast cancer; and CSS: cancer specific survival.
Fig 2. The forest plot of subgroup analysis.
Except for the well-differentiated type, T4-classification or N3-classification subgroup, patients with MBC had better outcomes than patients with IDC. IDC: infiltrative ductal cancer; MBC: mucinous breast cancer; HR: hazard risk; and CI: confidence index.
Prognostic factors in MBC
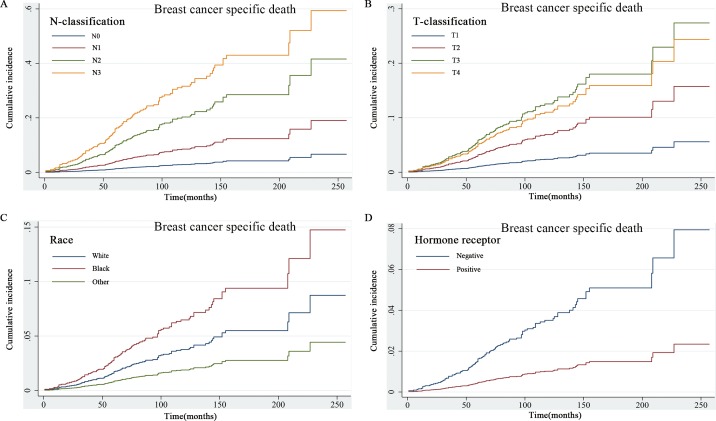
The competing risk regression model was used to explore the prognostic factors for patients with MBC. Univariate analysis revealed that age, marital status, tumor location, differentiated grade, T and N-classification, and ER/PR status were statistically significant prognostic factors for survival (Table 2, Fig 3). Based on the results of univariate analysis, further multivariate analysis showed that old age, poorly differentiated tumor and advanced T and N-classification were significantly associated with worse prognosis.
Table 2. The characteristics of 3042 patients with resectable mucinous breast cancer.
ER: Estrogen receptor; HoR: Hormone receptor; and PR: prognosis receptor.
| Risk Factors | Univariate analysis * | Multivariate analysis * | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | ||||
| Younger | - | - | - | - |
| Older | 1.56(1.06–2.3) | 0.023 | 1.97(1.32–2.95) | 0.001 |
| Marital status | ||||
| Married | 1 | - | 1 | - |
| Single | 1.67(0.93–3) | 0.089 | 1.57(0.87–2.85) | 0.134 |
| Divorced | 2.18(1.44–3.3) | <0.001 | 1.95(1.26–3) | 0.003 |
| Race | ||||
| White | 1 | - | 1 | - |
| Black | 1.74(0.97–3.13) | 0.062 | 1.18(0.64–2.17) | 0.594 |
| Other | 0.5(0.22–1.13) | 0.097 | 0.49(0.21–1.13) | 0.093 |
| Location | ||||
| Central portion of breast | 1 | - | 1 | - |
| Upper-inner quadrant | 1.66(0.64–4.32) | 0.296 | 2.16(0.8–5.82) | 0.129 |
| Lower-inner quadrant | 1.61(0.61–4.29) | 0.338 | 2.26(0.82–6.19) | 0.114 |
| Upper-outer quadrant | 2.7(1.17–6.23) | 0.020 | 2.92(1.23–6.93) | 0.015 |
| Lower-outer quadrant | 1.83(0.7–4.81) | 0.221 | 2.41(0.87–6.7) | 0.092 |
| Differentiated grade | ||||
| Well | 1 | - | 1 | - |
| Moderate | 1.72(1.12–2.63) | 0.013 | 1.35(0.87–2.1) | 0.185 |
| Poor | 4.98(2.89–8.59) | <0.001 | 2.86(1.51–5.41) | 0.001 |
| T-classification | ||||
| T1 | 1 | - | 1 | - |
| T2 | 2.98(1.99–4.47) | <0.001 | 2.07(1.31–3.25) | 0.002 |
| T3 | 5.57(2.81–11.06) | <0.001 | 3.12(1.34–7.27) | 0.008 |
| T4 | 4.86(0.71–33.33) | 0.108 | 3.16(0.35–28.88) | 0.308 |
| N-classification | ||||
| N0 | 1 | - | 1 | - |
| N1 | 3.07(1.87–5.04) | <0.001 | 2.23(1.31–3.79) | 0.003 |
| N2 | 7.81(3.85–15.85) | <0.001 | 4.1(1.62–10.37) | 0.003 |
| N3 | 13.08(4.72–36.3) | <0.001 | 6.46(1.91–21.82) | 0.003 |
| Stage | ||||
| I | 1 | - | - | - |
| II | 3.2(2.11–4.87) | <0.001 | - | - |
| III | 11.38(6.43–20.14) | <0.001 | - | - |
| ER | ||||
| Negative | 1 | - | 1 | - |
| Positive | 0.33(0.17–0.65) | 0.001 | 0.57(0.24–1.34) | 0.197 |
| PR | ||||
| Negative | 1 | - | 1 | - |
| Positive | 0.57(0.36–0.89) | 0.014 | 0.71(0.42–1.21) | 0.210 |
| HoR | ||||
| Negative | 1 | - | - | - |
| Positive | 0.29(0.15–0.56) | <0.001 | - | - |
* Univariate and multivariate analyses were conducted using the competing risk regression model.
Fig 3. Univariate analysis based on the competing risk regression model.
Factors of N-classification (3A), T-classification (3B), Race (3C) and HoR status (3D) were significant prognostic factors for survival.
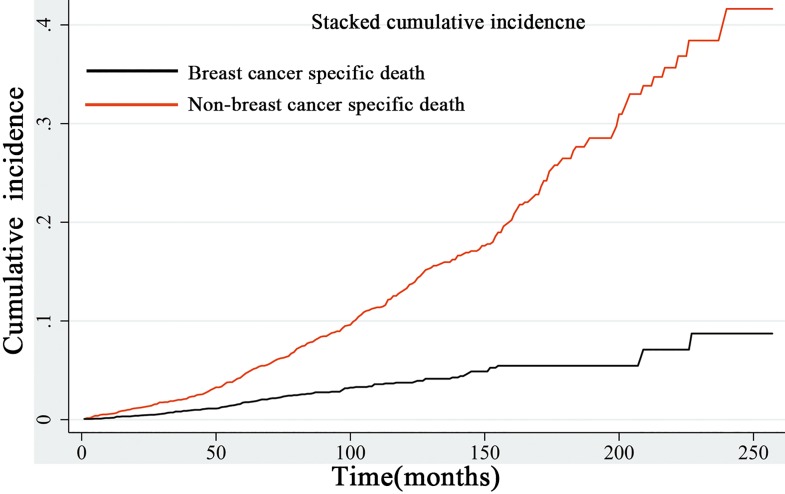
The stacked incidence cumulative plot showed that non-BCSD had a predominant impact on survival. The risk of BCSD was exceeded by non-BCSD in the early course of follow-up, and the trend was increasingly obvious with the passage of time (Fig 4).
Fig 4. Stacked cumulative incidence plot of breast cancer-specific survival and non-breast cancer-specific survival.
Non-breast cancer specific survival had a predominant impact on the survival.
Construction and validation of a prognostic nomogram model in MBC
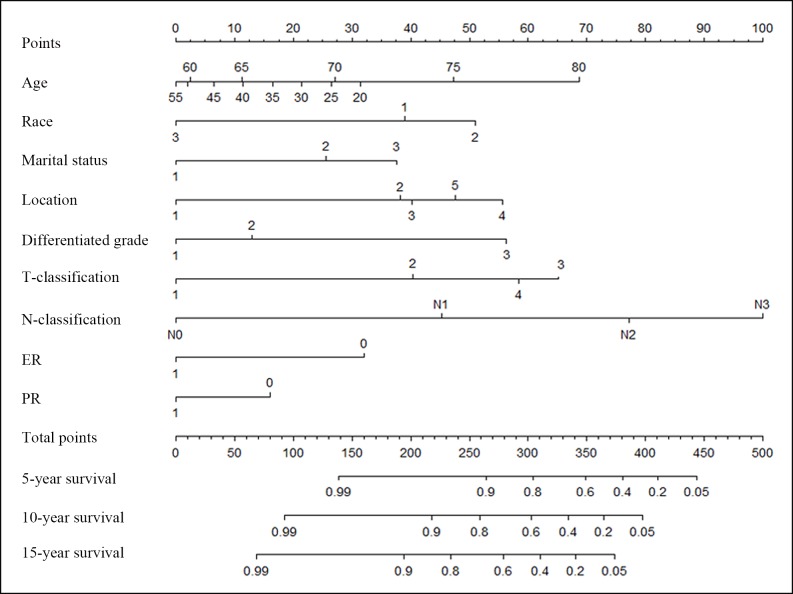
Significant factors identified by the Cox model were used to build a nomogram to predict the probability of CSS in MBC patients (Fig 5). The tumor location, differentiation grade, T and N-classification, and ER and PR statuses were included. Baseline characteristics, such as the age, race and marital status were also incorporated into the model. The nomogram illustrated T and N-classification as the largest contributor to the prognosis, which was followed by the differentiation grade. Each subtype for all variables was assigned a score on the point scale (Table 3). By summing the total score and locating it on the total point scale, we were easily able to draw a straight line down to estimate 5-, 10- or 15- year predicted CSS rate.
Fig 5. Nomogram for predicting the CSS of patients with MBC.
The nomogram is used by summing the points identified on the top scale for each independent covariate. The total points projected to the bottom scale indicate the % probability of the 5-, 10-, and 15-year survival. Race: 1 = white, 2 = black and 3 = other; Marital status: 1 = married; 2 = single (never married or having a domestic partner) and 3 = divorced (separated, divorced, or widowed). Location: 1 = central portion of the breast, 2 = upper-inner quadrant of the breast, 3 = lower-inner quadrant of the breast, 4 = upper-outer quadrant of the breast and 5 = lower-outer quadrant of breast. T and N-classification according to the 7th AJCC TNM system. ER = estrogen receptor: 1 = positive and 0 = negative. PR = progesterone receptor: 1 = positive and 0 = negative. CSS: cancer specific survival and MBC: mucinous breast cancer.
Table 3. Point assignment and prognostic score in the nomogram.
CSS: cancer specific survival.
| Variable | Score | Estimated 5-year CSS rate (%) |
|---|---|---|
| Age (years) | ||
| 20 | 31 | |
| 25 | 26 | |
| 30 | 21 | |
| 35 | 16 | |
| 40 | 11 | |
| 45 | 6 | |
| 50 | 2 | |
| 55 | 0 | |
| 60 | 2 | |
| 65 | 11 | |
| 70 | 27 | |
| 75 | 47 | |
| 80 | 69 | |
| Marital status | ||
| Married | 0 | |
| Single | 26 | |
| Divorced | 38 | |
| Race | ||
| White | 39 | |
| Black | 51 | |
| Other | 0 | |
| Location | ||
| Central portion of breast | 0 | |
| Upper-inner quadrant | 38 | |
| Lower-inner quadrant | 40 | |
| Upper-outer quadrant | 56 | |
| Lower-outer quadrant | 48 | |
| Differentiated grade | ||
| Well | 0 | |
| Moderate | 13 | |
| Poor | 56 | |
| T-classification | ||
| T1 | 0 | |
| T2 | 40 | |
| T3 | 65 | |
| T4 | 58 | |
| N-classification | ||
| N0 | 0 | |
| N1 | 45 | |
| N2 | 77 | |
| N3 | 100 | |
| ER | ||
| Negative | 32 | |
| Positive | 0 | |
| PR | ||
| Negative | 16 | |
| Positive | 0 | |
| Total prognostic score | ||
| > = 444 | 0.05 | |
| 410–443 | 0.2 | |
| 380–409 | 0.4 | |
| 349–379 | 0.6 | |
| 305–348 | 0.8 | |
| 264–304 | 0.9 | |
| 139–263 | 0.99 |
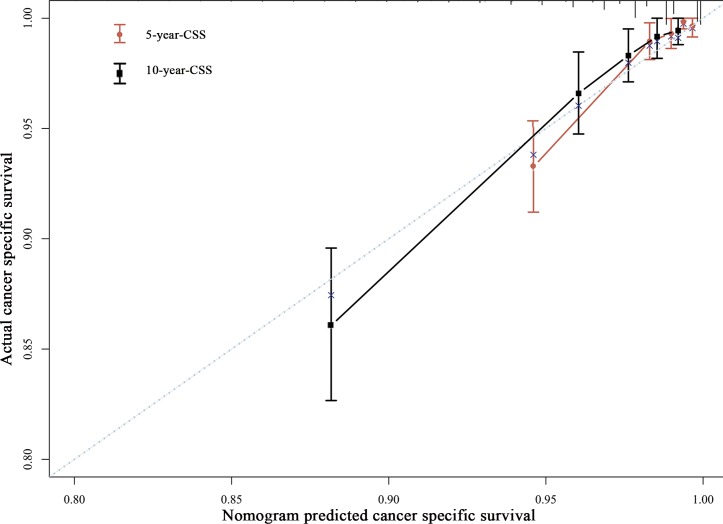
The internal validation using the bootstrap method showed the nomogram can accurately predict the CSS with a C-index of 0.816 (95% CI, 0.773–0.859). The calibration plots demonstrated an excellent agreement between the nomogram prediction and actual observation for the 5- and 10- year CSS rates (Fig 6).
Fig 6. The calibration curve for predicting patient CSS rates at 5 and 10 years.
The nomogram-predicted probability of CSS is plotted on the x-axis; the actual CSS is plotted on the y-axis. CSS: cancer specific survival and MBC: mucinous breast cancer.
The risk score developed from the nomogram as a continuous variable acted as a prognostic factor for CSS (HR = 1.02, P < 0.001). Then, the MBC cohort was classified into three subgroups (low risk, score < 158; medium risk, score of 158–205 and high risk, score > 205) according to the risk score by two cutoff values from the X-tile program (S2 Fig). The univariate analysis showed that the Risk score was significantly associated with the prognosis; the 5- and 10-year CSS rates were 99.53% and 98.87% in the low risk subgroup, 87.92% and 94.02% in the medium risk subgroup, and 90.14% and 80.68% in the high risk subgroup, respectively. The C-index of the model based on the Risk score to predict CSS was 0.789, which was statistically higher than the AJCC TNM staging system (C-index = 0.704, P < 0.001).
Discussion
MBC is a rare histological cancer type with lower malignancy and a comparatively better prognosis. A series of previous studies reported that MBC had different characteristics than other histological breast cancer types. However, some studies showed that there was no longer a survival difference between the patients with MBC and IDC after adjusting for the tumor size and lymph node status [3,21]. In our study, patients with MBC had an obviously better prognosis than patients with IDC, even after adjusting for clinicopathological factors.
The features related to the prognosis of patients with MBC remain controversial. The validity of the intrinsic subtype as a prognostic factor is widely accepted by clinicians. The current NCCN guideline recommends using the ER and PR statuses as the most important factors for making clinical decisions. However, in our study, multivariate analysis showed that ER and PR cannot independently predict the MBC prognosis. This is partly because the rate of ER and PR positivity is too high in our study, which will make it difficult to define the prognostic effect of the ER and PR statuses. Additionally, the HoR positivity (97.14%) is dramatically high and the remaining 3% of patients who are HoR negative are difficult to further stratify. It is not practicable to guide clinical practice according to the ER or PR status.
The gene signature is widely used to predict the prognosis of patents with ER positive and lymph node negative status (most MBC patients have such characteristics) [22,23]. However, for MBC tumor, abundant mucinous content in the tumor will impact the RNA quality of RNA. As a result, the 21-gene assay and MammaPrint based on real-time PCR will no longer be suitable for testing [24].
In previous studies, lymph node involvement was recognized as the most important prognostic factor [1,25,26]. In the current study, the effect of lymph node involvement was predominate in the nomogram. For MBC patients with lymph node involvement (10%), only 1.45% of patients had 1–3 lymph node metastases, and 0.43% of patients had more than three lymph node metastases. Therefore, sentinel lymph node biopsy may be sufficient to evaluate axillary lymph node metastasis.
The prognostic significance of the tumor size is an interesting but controversial issue in MBC patients. In the past, the NCCN guidelines recommended that patients with a tumor larger than 2 cm should receive adjuvant chemotherapy. However, the guidelines have been modified; now, lymph node involvement alone is considered as an indication for chemotherapy, regardless of the T-classification. Although the tumor size is associated with a delay in diagnosis in tumors with low invasion, the prognostic significance of the tumor size is questioned because the production of abundant extracellular mucin is included in the size measurement. As a result, the measured tumor size might fail to reflect the actual tumor size. This complicates the predictive role of the tumor size for MBC. One small size sample study showed that the MBC is associated with a larger tumor compared with IDC [27]. Additionally, one study showed that lymph node involvement is not associated with the tumor size [28]. In our study, the nomogram showed that T3 and T4 tumors had a worse prognosis than T1 and T2 tumors. Therefore, tumor size larger than 5 cm could be considered a poor prognostic factor.
Additionally, a poor differentiation grade is a poorer prognostic factor in the current study. The subgroup showed that MBC had a similar prognosis as IDC in the well-differentiated tumor subgroup, which may improve the prognosis for well-differentiated IDC tumor.
Two published studies in which the MBC prognosis was analyzed using a SEER dataset revealed that patients with MBC were more likely to be older women, had less lymph node involvement and had a better prognosis than IDC patients[5,29]. These findings are consistent with our study. In their studies, a considerable number of patients were diagnosed before 1990 without details about the ER and PR statuses or T- and N-classification. In the current study, detailed information on the T- and N- classification and ER or PR status are presented. Subgroup analysis with these factors was also performed to identify the impact of MBC on survival. Furthermore, we constructed a nomogram to individually predict the CSS. This could more directly help clinicians determine the probability of specific death for individual patients. To the best of our knowledge, this is the first large-population study to construct a nomogram for patients with early MBC.
A stacked cumulative incidence plot surprisingly showed that non-BCSD was predominant events of death. In the previous studies, non-BCSD was never considered in the survival analysis. Based on the results, we further proposed that for patients with early MBC, a competing risk regression model (non-BCSD as competing events) is more rational for analyzing the survival outcome. De Glas also recommended using a competing risk regression model rather than a Cox proportional hazard model that would otherwise overestimate the absolute risk of death in studies of mainly older patients with HoR+ breast cancer [30]. To the best of our knowledge, we first analyzed the prognosis of MBC using a competing risk model.
Our study has several potential limitations. First, we failed to differentiate between MBC subtypes, such as types A, B and AB. Then, the details about other prognostic factors, such the HER2 status and Ki67, as well as information about adjuvant therapy are lacking. Furthermore, external validation of the nomogram was not performed in our study. Despite these limitations, our study shed new light on the impact of MBC on the prognosis of breast cancer patients. Currently, there are no effective tools for predicting the prognosis of patients with MBC. Our study is the first to develop a clinical nomogram that could help clinicians in daily practice. More importantly, the competing risk regression model is recommended as a substitute for the traditional Cox model to decrease the bias of non-BCSD in the MBC survival analysis.
Supporting Information
(A) The optimal cut-off value highlighted by the black circle in the rectangular X-tile plot. (B) The histogram of the entire cohort. (C) The Kaplan-Meier plot: The cancer-specific survival (CSS) curve of young, older and oldest patients. The young and older groups have similar survival. The age of 70 is chosen as the optimal cut-off value. (D) The relative risks (RRs) for all cut-off values from low to high (left to right, x-axis). The RRs are calculated as: events in the older group / event risk in the younger group.
(TIF)
(A) The optimal cut-off value is highlighted by the black circle in the triangular X-tile plot. (low risk group, score<158; medium risk group, score of 158–205 and high risk group, score >205). (2) The histogram of the entire cohort. (C) The Kaplan-Meier plot: The cancer-specific survival curve of younger and older group have similar survival. The age of 70 is chosen as the optimal cut-off value. (D) The relative risks (RRs) for all cut-off values from low to high (left to right, x-axis). RRs are calculated as the events in the older group / event risk in the younger group.
(TIF)
Acknowledgments
We acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program registries for creating the SEER database. We also thank Wei Fu, Johns Hopkins University School of Medicine, for reviewing our statistical methods and his professional advice. Also, the work was sponsored by the Zheng Shu Medical Elite Scholarship Fund.
Abbreviations
- MBC
mucinous breast cancer
- IDC
infiltrative ductal breast cancer
- HoR
hormone receptor
- ER
estrogen receptor
- PR
progesterone receptor
- CSS
cancer specific survival
- BCSD
breast cancer special death
- IQR
inter quartile range
- HRs
hazard ratios
- CIs
confidence intervals
- AJCC
American Joint Committee on Cancer
- SEER
Surveillance, Epidemiology, and End Results
- BCSD
Breast cancer special death
- NCCN
National Comprehensive Cancer Network
Data Availability
All SEER files are available from the SEER database (http://seer.cancer.gov/) (accession number:10263-Nov2015).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Fentiman IS, Millis RR, Smith P, Ellul JP, Lampejo O (1997) Mucoid breast carcinomas: histology and prognosis. Br J Cancer 75: 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komaki K, Sakamoto G, Sugano H, Morimoto T, Monden Y (1988) Mucinous carcinoma of the breast in Japan. A prognostic analysis based on morphologic features. Cancer 61: 989–996. [DOI] [PubMed] [Google Scholar]
- 3.Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM (1999) Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol 17: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 4.Andre S, Cunha F, Bernardo M, Meneses e Sousa J, Cortez F, Soares J (1995) Mucinous carcinoma of the breast: a pathologic study of 82 cases. J Surg Oncol 58: 162–167. [DOI] [PubMed] [Google Scholar]
- 5.Di Saverio S, Gutierrez J, Avisar E (2008) A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat 111: 541–547. 10.1007/s10549-007-9809-z [DOI] [PubMed] [Google Scholar]
- 6.Louwman MW, Vriezen M, van Beek MW, Nolthenius-Puylaert MC, van der Sangen MJ, Roumen RM, et al. (2007) Uncommon breast tumors in perspective: incidence, treatment and survival in the Netherlands. Int J Cancer 121: 127–135. 10.1002/ijc.22625 [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen BB (1985) Human mucinous breast carcinomas and their lymph node metastases. A histological review of 247 cases. Pathol Res Pract 180: 377–382. 10.1016/S0344-0338(85)80110-2 [DOI] [PubMed] [Google Scholar]
- 8.Scopsi L, Andreola S, Pilotti S, Bufalino R, Baldini MT, Testori A, et al. (1994) Mucinous carcinoma of the breast. A clinicopathologic, histochemical, and immunocytochemical study with special reference to neuroendocrine differentiation. Am J Surg Pathol 18: 702–711. [PubMed] [Google Scholar]
- 9.Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, et al. (2010) Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol 222: 282–298. 10.1002/path.2763 [DOI] [PubMed] [Google Scholar]
- 10.Weigelt B, Geyer FC, Horlings HM, Kreike B, Halfwerk H, Reis-Filho JS (2009) Mucinous and neuroendocrine breast carcinomas are transcriptionally distinct from invasive ductal carcinomas of no special type. Mod Pathol 22: 1401–1414. 10.1038/modpathol.2009.112 [DOI] [PubMed] [Google Scholar]
- 11.Liu F, Yang M, Li Z, Guo X, Lin Y, Lang R, et al. (2015) Invasive micropapillary mucinous carcinoma of the breast is associated with poor prognosis. Breast Cancer Res Treat 151: 443–451. 10.1007/s10549-015-3413-4 [DOI] [PubMed] [Google Scholar]
- 12.Lacroix-Triki M, Lambros MB, Geyer FC, Suarez PH, Reis-Filho JS, Weigelt B (2010) Absence of microsatellite instability in mucinous carcinomas of the breast. Int J Clin Exp Pathol 4: 22–31. [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii H, Anbazhagan R, Bornman DM, Garrett ES, Perlman E, Gabrielson E (2002) Mucinous cancers have fewer genomic alterations than more common classes of breast cancer. Breast Cancer Res Treat 76: 255–260. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. (2013) Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 31: 1188–1195. 10.1200/JCO.2012.41.5984 [DOI] [PubMed] [Google Scholar]
- 15.Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, et al. (2016) Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J Clin Oncol 34: 2157–2164. 10.1200/JCO.2015.65.9128 [DOI] [PubMed] [Google Scholar]
- 16.Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. (2015) Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 33: 861–869. 10.1200/JCO.2014.56.6661 [DOI] [PubMed] [Google Scholar]
- 17.Iasonos A, Schrag D, Raj GV, Panageas KS (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26: 1364–1370. 10.1200/JCO.2007.12.9791 [DOI] [PubMed] [Google Scholar]
- 18.Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10: 7252–7259. 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 19.Howlader N, Mariotto AB, Woloshin S, Schwartz LM (2014) Providing clinicians and patients with actual prognosis: cancer in the context of competing causes of death. J Natl Cancer Inst Monogr 2014: 255–264. 10.1093/jncimonographs/lgu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinchliffe SR, Lambert PC (2013) Flexible parametric modelling of cause-specific hazards to estimate cumulative incidence functions. BMC Med Res Methodol 13: 13 10.1186/1471-2288-13-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao AY, He M, Liu ZB, Di GH, Wu J, Lu JS, et al. (2012) Outcome of pure mucinous breast carcinoma compared to infiltrating ductal carcinoma: a population-based study from China. Ann Surg Oncol 19: 3019–3027. 10.1245/s10434-012-2322-6 [DOI] [PubMed] [Google Scholar]
- 22.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351: 2817–2826. 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 23.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. (2015) Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 373: 2005–2014. 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347: 1999–2009. 10.1056/NEJMoa021967 [DOI] [PubMed] [Google Scholar]
- 25.Skotnicki P, Sas-Korczynska B, Strzepek L, Jakubowicz J, Blecharz P, Reinfuss M, et al. (2016) Pure and Mixed Mucinous Carcinoma of the Breast: A Comparison of Clinical Outcomes and Treatment Results. Breast J. [DOI] [PubMed] [Google Scholar]
- 26.Komenaka IK, El-Tamer MB, Troxel A, Hamele-Bena D, Joseph KA, Horowitz E, et al. (2004) Pure mucinous carcinoma of the breast. Am J Surg 187: 528–532. 10.1016/j.amjsurg.2003.12.039 [DOI] [PubMed] [Google Scholar]
- 27.Ranade A, Batra R, Sandhu G, Chitale RA, Balderacchi J (2010) Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol 63: 1043–1047. 10.1136/jcp.2010.082495 [DOI] [PubMed] [Google Scholar]
- 28.Paramo JC, Wilson C, Velarde D, Giraldo J, Poppiti RJ, Mesko TW (2002) Pure mucinous carcinoma of the breast: is axillary staging necessary? Ann Surg Oncol 9: 161–164. [DOI] [PubMed] [Google Scholar]
- 29.Northridge ME, Rhoads GG, Wartenberg D, Koffman D (1997) The importance of histologic type on breast cancer survival. J Clin Epidemiol 50: 283–290. [DOI] [PubMed] [Google Scholar]
- 30.de Glas NA, Kiderlen M, Vandenbroucke JP, de Craen AJ, Portielje JE, van de Velde CJ, et al. (2016) Performing Survival Analyses in the Presence of Competing Risks: A Clinical Example in Older Breast Cancer Patients. J Natl Cancer Inst 108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The optimal cut-off value highlighted by the black circle in the rectangular X-tile plot. (B) The histogram of the entire cohort. (C) The Kaplan-Meier plot: The cancer-specific survival (CSS) curve of young, older and oldest patients. The young and older groups have similar survival. The age of 70 is chosen as the optimal cut-off value. (D) The relative risks (RRs) for all cut-off values from low to high (left to right, x-axis). The RRs are calculated as: events in the older group / event risk in the younger group.
(TIF)
(A) The optimal cut-off value is highlighted by the black circle in the triangular X-tile plot. (low risk group, score<158; medium risk group, score of 158–205 and high risk group, score >205). (2) The histogram of the entire cohort. (C) The Kaplan-Meier plot: The cancer-specific survival curve of younger and older group have similar survival. The age of 70 is chosen as the optimal cut-off value. (D) The relative risks (RRs) for all cut-off values from low to high (left to right, x-axis). RRs are calculated as the events in the older group / event risk in the younger group.
(TIF)
Data Availability Statement
All SEER files are available from the SEER database (http://seer.cancer.gov/) (accession number:10263-Nov2015).