Abstract
Three congeneric lizards from the southeastern United States (Plestiodon fasciatus, P. inexpectatus, and P. laticeps) exhibit a unique nested distribution. All three skink species inhabit the US Southeast, but two extend northward to central Ohio (P. fasciatus and P. laticeps) and P. fasciatus extends well into Canada. Distinct interspecific differences in microhabitat selection and behavior are associated with the cooler temperatures of the more Northern ranges. We hypothesized that interspecific differences in metabolic temperature sensitivity locally segregates them across their total range. Resting oxygen consumption was measured at 20°, 25° and 30°C. Plestiodon fasciatus, from the coolest habitats, exhibited greatly elevated oxygen consumption compared to the other species at high ecologically-relevant temperatures (0.10, 0.17 and 0.83 ml O2. g-1. h-1 at 20°, 25° and 30°C, respectively). Yet, P. inexpectatus, from the warmest habitats, exhibited sharply decreased oxygen consumption compared to the other species at lower ecologically-relevant temperatures (0.09, 0.27 and 0.42 ml O2. g-1. h-1 at 20°, 25° and 30°C, respectively). Plestiodon laticeps, from both open and closed microhabitats and intermediate latitudinal range, exhibited oxygen consumptions significantly lower than the other two species (0.057, 0.104 and 0.172 ml O2. g-1. h-1 at 20°, 25° and 30°C, respectively). Overall, Plestiodon showed metabolic temperature sensitivities (Q10s) in the range of 2–3 over the middle of each species’ normal temperature range. However, especially P. fasciatus and P. inexpectatus showed highly elevated Q10s (9 to 25) at the extreme ends of their temperature range. While morphologically similar, these skinks are metabolically distinct across the genus’ habitat, likely having contributed to their current distribution.
Introduction
Ecological physiologists have long questioned how species distribution is affected by local environment, phenotype and phylogenesis. Yet, few unifying principles have emerged. The five-lined skink lizards of the forests in the eastern United States comprise a highly tractable system to investigate these phenomena, given that they share close phylogenetic relationships, phenotypic similarities, regional sympatry, and latitudinally-stratified northern range boundaries. Among the skinks, the common five-lined skink (Plestiodon fasciatus), the broadhead skink (Plestiodon laticeps) and the southeastern five-lined skink (Plestiodon inexpectatus) are phenotypically similar during much of their development and also exhibit similarities in behavior and diet [1, 2]–so much so they were considered a single species prior to their formal designation as distinct species [3–5]. Adult P. laticeps represent an obvious exception to these generalities because they are considerably larger than adult P. fasciatus and P. inexpectatus, thus allowing them to exploit different resources [2, 6, 7]. The ecological physiology of these closely related species is still poorly known, but they are of interest because they occur in regional sympatry and may be using the same set of resources. This is an apparent violation of both the competitive exclusion hypothesis and Jordan’s Rule, which states that an organism’s closest relative would be found either in adjacent, dissimilar habitats or separate but similar habitats [8–10]. While an important point to consider, this contravention of Jordan’s Rule may be irrelevant if one considers fine scale differences in microhabitat rather than broader habitat generalizations.
Dimensions of the ecological niches (e.g. thermal, spatial, and temporal) of these Plestiodon species have not been shown to differ experimentally. However, environmental temperature has been implicated as a potential factor differentiating the niches of these species [9, 11], for three reasons. First, these species exhibit a latitudinal stratification in northernmost range limits. P. inexpectatus is restricted to the warmer southeastern United States, while P. laticeps extends northward to around 40° latitude, and P. fasciatus extends northward into Canada [2]. Secondly, P. fasciatus is a deep forest dweller in the warmer south, but occupies open meadows and forest edges in the cooler northern parts of its range [7] [12]). Lastly, in areas of syntopy, field observations indicate that P. inexpectatus inhabits more open, warmer, microhabitats than the other two species [9, 11]. Preferred temperatures of these species range from ~32.5°C (P. fasciatus) to ~34°C (P. laticeps) [11]. Further details of the range and habitat of these lizards can be found in Conant and Collins [2].
Despite these marked differences in the thermal characteristics of the habitats of various species of Plestiodon, no measurements of metabolic rate and its sensitivity to temperature have been made across a North American cohort of species for this genus. Consequently, we hypothesize that differences in preferred microhabitat temperature among these closely related lizard species are correlated with intrinsic differences in thermal sensitivity of their metabolic rate. We additionally hypothesize that such differences could explain the physiological constraints underlying the temperature-related limitations of these species’ range, as previously described [9]. To test these hypotheses, we measured how these species responded metabolically to a range of temperatures between 20°C and 30°C, which encompasses their range of measured field temperatures. Our findings indicate that there are distinct differences in metabolic temperature sensitivity over the tested ranges, and that these differences help explain the patterns of species distribution.
Materials and Methods
Animal Collection
Lizards were collected during the spring and summer of 2006 and 2007 throughout the Southeastern United States from areas where all three species are regionally sympatric. Specimens were collected from a relatively constrained latitudinal range (~30°N-33.5°N) in order to negate the potentially confounding effects of latitudinal acclimatization as described for Sceloporus [13].
Animal collection and experimentation was conducted under protocols #A06.017 and #A07.003 approved by the IACUC of the University of Texas at Arlington.
Animal Maintenance
Individuals were maintained in the laboratory for a minimum of 1 month prior to the beginning of metabolic (oxygen consumption) measurements. During this maintenance period, animals were kept in commercially-available plastic boxes with a shredded aspen substrate, a water bowl, and a ceramic tile for refuge. They were maintained with a 12-hour light/dark cycle (on at 0800h and off at 2000h CST) at room temperature, which uniformly fluctuated between 22.5–27.0°C. No thermal gradients were evident in the holding cages. Three to five crickets were offered twice weekly. All work was performed under The University of Texas at Arlington IACUC protocols #A06.017 and #A07.003.
Temperature Acclimation and Oxygen Consumption Measurement Protocol
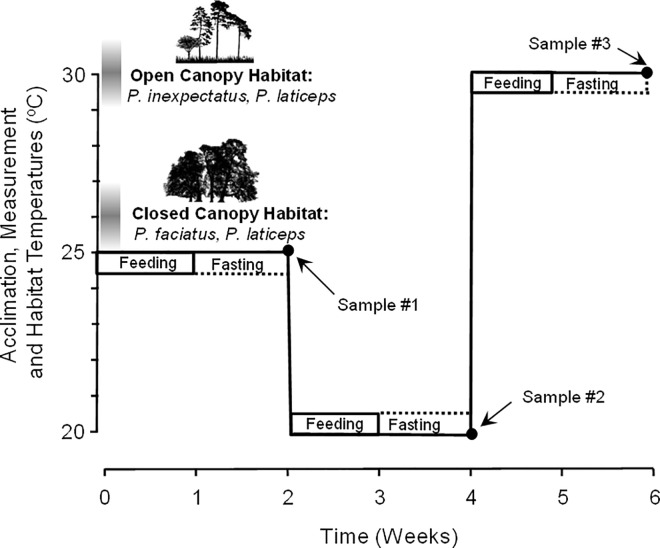
After the preliminary maintenance period in the laboratory, lizards in their enclosures were placed inside a walk-in environmental chamber with a 12 hour light/12 hour dark light cycle. There are a variety of protocols for subjecting animals to variation in ambient temperature, each with advantages and disadvantages. For example, temperature can be first raised, then lowered (or vice versa), or temperature can be changed in a stepwise or ramp fashion. Metabolic rate can be affected by the previous high or low acclimation temperature. There is no “standard” protocol for such experipmetns. During the course of animal husbandry, we determined that P. fasciatus may be especially sensitive to high temperature. Consequently, in this study, lizards were subjected to a rigidly controlled temperature acclimation and oxygen consumption measurement protocol where lizards were exposed to the highest temperature last (Fig 1). All lizards of each of the three species were first acclimated to 25±<0.5°C for two weeks (Fig 1), at the end of which their oxygen consumption () was individually measured (see below) and they were weighed. The temperature was then lowered to 20°C for two weeks, followed by a second measurement and weighing. The temperature was then raised to 30°C for a final period of two weeks, finishing with a final measurement and weighing. All measurements were made between 1500h and 1900h CST. The temperature range of 20–30°C for laboratory measurements was specifically selected for three reasons: 1) 20°C reflects the low ambient temperature of the general habitat of all species during the seasons in which they are active, 2) 25°C approximates the mean ambient daily temperature of the habitat frequented by Plestiodon fasciatus and P. laticeps, and 3) 30°C approximates the mean ambient daily summer temperature of the microhabitat frequented by P. inexpectatus and P. laticeps (30°C) [9, 11]. The acclimation and measurement temperatures, as well as the field temperatures determined from the studies indicated above, are shown on Fig 1 for the two habitats.
Fig 1. Protocol for temperature acclimation for oxygen consumption measurements in Plestiodon fasciatus, Plestiodon inexpectatus, and Plestiodon laticeps.
The left side of the panel depicts the daily habitat temperature range of the three species (after [9, 11], while the right side shows the time course of the temperature acclimation and sampling schedule for the measurements of resting oxygen consumption.
Specific dynamic action–the increase in metabolic rate after eating–can be both large and long-lasting in reptiles [14–17]. To avoid complex interactions of thermal acclimation and specific dynamic action, all lizards were fasted for one week prior to each experimental trial (i.e., for the second half of the acclimation period, as indicated in Fig 1) Lizards were provided water ad libitum throughout the acclimation/measurement protocol.
Respirometry
Resting values were measured via standard closed-system respirometry techniques described for terrestrial and aquatic animals [18–20]. Each lizard was placed within a 500 ml Erlenmeyer flask that was sealed with a gas-tight rubber stopper. Verification of air-tightness of the respirometry system was initially verified by submerging the sealed respirometer in water and inspecting for escaping air bubbles. Two ports in the stopper, an inlet and outlet, were each fitted with glass tubing and a three way stopcock. CO2 absorbant (Ascarite) in a small porous container was placed in each respirometer. The flasks were covered with aluminum foil to visually shield the lizards from the researchers. Movement of the lizard when first placed within the respirometer could be audibly detected by the researchers, but animals settled down within minutes with no further evidence of movement. Thus, it was assumed that the lizards were at rest during these trials. The respirometers, oxygen analysis equipment and acclimating animals were placed inside the environmental chamber. Each animal was placed from their temperature acclimation enclosure directly into a respirometer for 1 h, with air being pumped through the respirometer using a commercially available aquarium air pump. After this period of acclimation to the respirometer, the barometric pressure (in mmHg) was measured and the respirometers were sealed for measurement.
Lizards were left in the closed respirometers for 1–3 h, depending upon body size. Larger lizards use more oxygen at the same metabolic rate as smaller specimens due to the larger volume of respiring tissue. This effect is exacerbated by the lowered amount of gas available in the respirometer because of larger body size, resulting in a reduced volume of airavailable to breath. Therefore, larger specimens took less time to depleat the air of oxygen and produce a measurable result. Preliminary experiments revealed that this was a sufficient time period to reduce PO2 in the chambers by ~8–12 mmHg. This change was large enough to be measured accurately, but not so large as to cause significant hypoxia in the chambers. Upon the completion of each respirometry trial, which lasted from 1–3 h, a 4 ml sample of gas from inside the respirometer was drawn via a stopcock into a gas-tight glass syringe and then injected into a thermostatted PO2 electrode connected to a Radiometer PHM72 gas meter. Each animal was then weighed. was calculated using standard closed respirometry equations [18, 20, 21], with the following variables: decline in PO2 in the sealed respirometer over a known elapsed period of time, volume of the chamber, volume of the animal, and temperature and barometric pressure of gas in the respirometer. Mass-specific resting was expressed as ml O2. g-1. h-1 (STP).
Temperature coefficient (Q10) values for were calculated for the temperature intervals of 20–25°C and 25–30°C using the Van’t Hoff equation:
where R1 and R2 is the at T1 and T2, respectively.
Data Analysis and Statistics
Resting values were log-transformed to satisfy the assumption of normality. A One-way repeated measures ANOVA was carried out using species and acclimation temperature as factors. A post hoc Tukey test was used to determine specific differences among species at each acclimation temperature (α = 0.05). Q10 values were log transformed to satisfy the assumption of normality and analyzed with a one-way repeated measures ANOVA performed to test the differences among species at each of the two intervals. A post-hoc Fisher LSD test was used to determine significance among species (α = 0.05). All data are expressed as ± SE unless otherwise stated.
Results
Species-Specific Body Mass and Size
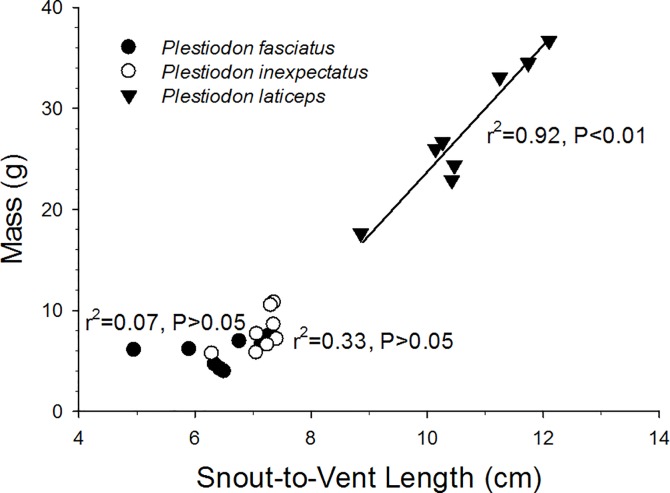
The distribution of mass and snout-to-vent length (SVL) of the three species of Plestiodon used in this study is presented in Fig 2. Adult Plestiodon laticeps were significantly longer and heavier (10.55 ± 0.36 mm, 27.73 ± 2.30 g) than adult P. fasciatus (6.41 ± 0.36 mm, 5.80 ± 0.47 g) and P. inexpectatus (10.55 ± 0.36 mm, 7.87 ± 0.69 g). There is extensive overlap in both mass and snout-to-vent length in the two smaller species. Only in P. laticeps was the relationship between mass and SVL significant. Thus, there are relatively large ranges in body length for adults of similar body mass in P. fasciatus and P. inexpectatus (Fig 2).
Fig 2. Size distribution, with individual regression lines for adult Plestiodon fasciatus, P. inexpectatus, and P. laticeps used to determine temperature sensitivity of oxygen consumption (Q10).
r2 and P values are indicated for each species–only P. laticeps showed a significant relationship between body mass and snout-to-vent length.
Resting Oxygen Consumption
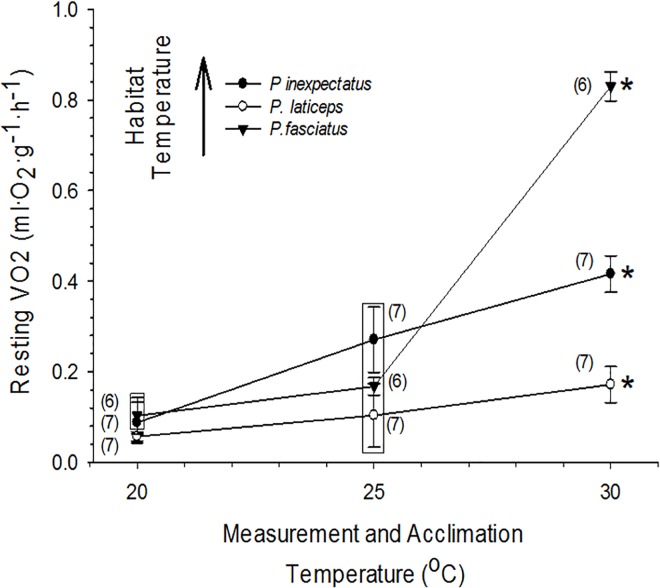
Resting at 20°C was approximately 0.06–0.1 mlO2.g-1.h-1 in all three species (Fig 3). The resting of P. laticeps was slightly but significantly lower than that of P. fasciatus and P. inexpectatus at 20°C. In fact, this trend continued at all acclimation/measurement temperatures, where the of P. laticeps was consistently significantly lower than that of P. fasciatus and P. inexpectatus.
Fig 3. Resting oxygen consumption at 20°, 25° and 30°C for the scincid lizards Plestiodon fasciatus, Plestiodon inexpectatus, and Plestiodon laticeps.
Means ± 1 SE and n (in parentheses) are given. An * indicates significant (P<0.05) temperature affect for each species. Boxes enclose statistically identical mean values at each temperature.
Not unexpectedly, increased with increasing temperature in all three species. However, as temperature increased, the three species showed distinctly different trends in rising (Fig 3). Resting of P. laticeps increased the least of the three species, rising from 0.057 ml O2.g-1.h-1 at 20°C to just 0.172 ml O2.g-1.h-1 at 30°C, a 2.3-fold increase. Over that same 10°C temperature range, the of P. inexpectatus rose from 0.088 ml O2.g-1.h-1 at 20°C to 0.416 ml O2.g-1.h-1 at 30°C, a 3.7-fold increase. P. fasciatus showed the greatest sensitivity of metabolism to temperature, with rising from 0.103 ml O2.g-1.h-1 at 20°C to 0.830 ml O2.g-1.h-1 at 30°C, a very large 7.1-fold increase (Fig 3). This large temperature-stimulated increase in metabolism in P. fasciatus was primarily the result of a large increase in from 25°C to 30°C, since P. fasciatus and P. inexpectatus exhibited no significant difference (P>0.10) in at either 20°C or 25°C. Indeed, at 30°C, resting was significantly different in all three species, with showing the following relationship: P. fasciatus > inexpectatus > P. laticeps (Fig 3).
Metabolic Temperature Sensitivity
We determined the metabolic temperature sensitivities of each species over the temperature range 20–25°C and 25–30°C, as expressed from the Q10 value calculated for from the Van’t Hoff equation (Fig 4, see Methods).
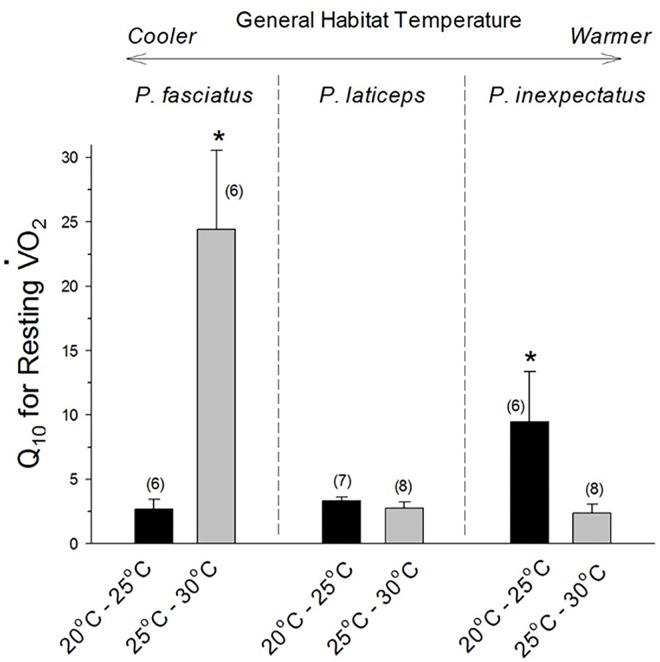
Fig 4. Q10 values for resting oxygen consumption for the scincid lizards Plestiodon fasciatus, Plestiodon inexpectatus, and Plestiodon laticeps.
The placement of each species is relative to the general habitat temperatures, indicated at the top of the graph. Thus, P. fasciatus experiences the coolest temperatures, and P. inexpectatus the warmest. The gray bars show the metabolic response to an increase in temperature from 25°C to 30°C, while the black bars show the metabolic response to a decrease in temperature from 25°C to 20°C. Means ± 1 SE and n (in parentheses) are given. An * indicates significant difference from the other species for Q10 for the temperature interval to the right of the graph.
Overall, each species exhibited Q10 values for in the range of 3–4 over the middle of their normal temperature range. P. laticeps, the species inhabiting the widest temperature range, maintained a relatively constant Q10 for both temperature intervals of 20–25°C and 25–30°C, with values of 4.1±0.4 (N = 7); and 3.3 ± 0.6 (N = 8) respectively. The other two species exhibited similar Q10 values toward the middle of their typical temperature range. Thus, P. fasciatus, which is found in cooler habitats, had a Q10 at 20–25°C of 3.9±1.2 (N = 6), while P. inexpectatus, which inhabits warmer habitats, had a Q10 of 3.2±1.0 (N = 7) at 25–30°C (Fig 4).
To understand how metabolic responses to temperature might be a determining factor in energy expenditure and thus species range, we also measured Q10 at the extremes of the typical temperature ranges for the three species. Both P. inexpectatus and P. fasciatus, the two species with the narrowest temperature ranges, exhibited extremely high and variable Q10 values towards these temperature extremes. Thus, at the lower range of acclimation and measurement temperatures of 20–25°C, P. inexpectatus (the species typically dwelling in warmer habitats), exhibited a Q10 of 9.5 ± 3.9. On the other hand, P. fasciatus (typically dwelling in cooler habitats) at the higher range of acclimation and temperature measurements of 25–30°C had a very high Q10 of 24.4 ± 6.2. Importantly, P. fasciatus exposed to 30°C for the 14-day acclimation period often refused food over the subsequent few weeks, lost weight, and eventually died over the course of a month or two. However, P. inexpectatus and P. laticeps were not adversely affected by the highest acclimation temperature.
Discussion
Size Relationships
Plestiodon laticeps is the most phenotypically distinct of the three Plestiodon species examined in this study. Individuals of P. laticeps are much larger (Fig 2) and exhibit a greater degree of sexual dimorphism [2, 5]. Furthermore, P. laticeps exhibits a statistically much stronger linear relationship between mass and snout-to-vent length compared to the other two species. These values are consistent with published maximum sizes for each species. For example, P. laticeps (SVL: 14.3cm) is more than 60% larger than both P. fasciatus (SVL: 8.6cm) and P. inexpectatus (SVL: 8.9cm) [2] and present study). These data are consistent with the much debated Hutchinsonian ratio, which is the body size ratio (1.3:1) that would theoretically allow two similar species to coexist [22]. The ratio of P. inexpectatus’ maximum SVL to P. fasciatus’ maximum SVL is only 1.03:1, at odds with Hutchinson’s ratio. Owing primarily to this difference in adult size, P. laticeps may not actually be in direct competition with the other two species and indeed P. laticeps may be partitioning their niche along different dimensions such as preferred prey, space, or microhabitat. This could potentially explain the observation that P. laticeps is commonly found in sympatry with each of the two smaller species [9, 11]. However, the large amount of size overlap by P. fasciatus and P. inexpectatus suggests that they may be in more direct competition for resources and therefore must partition their respective niche spaces. Therefore, physiological differences associated with temperature change may play an important role in niche segregation between these two species.
Resting Oxygen Consumption
Considerable variation in resting mass-specific exists in scincid lizards (Table 1 and S1 Table). Some of this variation may result from size differences, age differences and seasonal changes in metabolic rate. Additionally, prandial state has a major effect on metabolic rate as a result of specific dynamic action in many lizard species Additionally, and beyond scincid lizards, habitat has a notable effect on metabolic rate. For example, desert geckos in the genus Rhoptropus have unusually low mass-specific metabolic rates, which may correlate with both their habitat and life style [23]. Resting in Plestiodon, ranging between 0.1 and 0.3 ml O2. g-1. h-1 at 20°C, is consistent with values of resting oxygen consumption reported at comparable temperatures for other similarly sized lizards (Table 1), including mostly forest dwelling lizards such as Eulamprus tympanum [13], Eulamprus quoyii [24], Sceloporus undulatus [25, 26], Eremia lugubris [27] and Eumeces chinensis (now Plesiodon chinensis) [28].
Table 1. Mass-specific oxygen consumption in scincid lizards.
| Lizard | Body Mass(g) | Measurement Temperature (°C) | Mass-Specific Oxygen Consumption(ml. g-1. h-1) | Q10 (Across Maximum Reported Temperature Range) | Reference | |
|---|---|---|---|---|---|---|
| Genus | Species | |||||
| Plestiodon | chinensis | 21–38 | 30 | 5.0–9.0 | - | [28] |
| fasciatus | 5.0–7.3 | 20, 25, 30 | 0.10, 0.17, 0.83 | 8.3 | Present Study | |
| inexpectatus | 6.0–7.5 | 20, 25, 30 | 0.09, 0.27, 0.42 | 4.7 | Present Study | |
| laticeps | 9.0–12.2 | 20, 25, 30 | 0.06, 0.10, 0.17 | 2.8 | Present Study | |
| Eulamprus | quoyii | 28.8 | 30 | 0.12 | - | [24] |
| tympanum | Not reported | 30 | 0.21 | - | [13] | |
| Eremia | lineocellata | 3.3 | 26, 37 | 0.10, 0.25 | 2.0 | [27] |
| lugubris | 3.8 | 26, 34, 37 | 0.09, 0.21, 0.24 | 2.4 | [27] | |
| Oligosoma | nigriplantare polychroma | 3.3 | 13 | 0,05* | - | [29] |
| zelandicum | 3.8 | 13 | 0.04* | - | [29] | |
| Cyclodina | aenea | 2.5 | 13 | 0.05* | - | [29] |
| macgregori | 17.6 | 13 | 0,04* | - | [29] | |
| Scincella | lateralis | 0.9 | 24, 28, 32, 36 | 0.11, 0.19, 0.19, 0.27* | 2.1–3.3 | [30] |
*values calculated from reported means for body mass and whole animal oxygen consumption.
Interestingly, despite species-specific differences in body size,range and habitat characteristics (including temperature) in the species used for the present study, all three species of Plestiodon showed similar levels of when measured at 25°C. Moreover, even at higher or lower temperatures, of the three species showed similar rates of metabolism (except for P. fasciatus at 30°C).
Mass is, of course, an important “driver” of metabolic rate in animals, including lizards–for recent reviews see [31–33]. Scaling effects on metabolism have been studied in lizards. For example, a recent study on the agamid lizard Uromastyx philbyi [34] has shown profound effects of body mass (and, of course, temperature) on mass-specfic metabolic rate. However, the lizards used in that study varied in size by more than 1 log cycle (~15–175 g), making allometric (scaling) analysis and interpretation more feasible. In the present study the very small size range between individuals within a species and even between species of Plestiodon precludes meaningful allometric analysis. Future studies should involve measurement of metabolic rate in a larger range of individuals (e.g. newly hatched and juvenile individuals as well as adults), with the caveat that this will introduce ontogeny as well as body size into the factors affecting mass-specific metabolic rate.
Interspecific Differences in Metabolic Temperature Sensitivity
Many diurnal lizards have been reported to show Q10 values of ~ 2–2.5 [30, 35, 36]. However, closer examination of the metabolic temperature sensitivity reveals that there several factors affecting Q10, including season, nutritional state and proximity of the measurement temperature to the animals preferred body temperature. Metabolic thermal sensitivity in Plestiodon, as reflected in the Q10 for , ranged from approximately 3.1 to 3.9 when measured in the middle of the normal temperature range for P. fasciatus, P. inexpectatus and P. laticeps (Fig 4). These values are similar to those reported for the desert skink Scincus mitranus, whose Q10 values ranged from 2.5–3.8 [37] and the temperate skink Scincella lateralis, with reported overall values of ~3.5 (though this is an average of Q10s measured at both low and high temperatures–see below [30]. Q10s ranging from 1.37–8.58, 0.98–2.56 and 2.6–2.8 were also reported for Acanthodactylus boskianus and Diplometopon zarudnyi [37] andthe zebra-tailed lizard Callisaurus draconoides [38]. Generally, the present values are within the high end of the normal published range of temperature sensitivity for resting metabolic rate exhibited by vertebrate poikilotherms [39–44].
Changes in metabolic Q10 within a species’ tolerable temperature range can be both greatly exaggerated and highly non-linear, especially when measurements are made towards the extremes of its normal temperature range [30, 41, 43, 45]. Frequently, the relationship of metabolic Q10 to temperature is described by a U-shaped curve, with the lowest temperature sensitivity (lowest Q10) occurring near the animal’s preferred temperature. Although the present study did not examine metabolic Q10 over the entire range of tolerable temperatures for each species of Plestiodon, high Q10 values at extreme temperatures (for the species) were evident in both P. fasciatus (from cooler habitats) exposed to high temperatures and P. inexpectatus (from warmer habitats) exposed to lower temperatures (Fig 4). These metabolic temperature sensitivities are further supported by our personal observations that P. fasciatus and P. laticeps remained alert when transported at ~12–13°C, while P. inexpectatus became flaccid and visibly slowed its breathing at this cooler transport temperature. Similar data have been reported for the scincid lizard Scincella lateralis [37]. Metabolic rate in that species, when measured during both summer and winter, was most temperature sensitive between 24 and 28°C (Q10 = 5.8), but nearly temperature insensitive between 28 and 33°C (Q10 = 1.1).
In the present study, prolonged exposure of Plestiodon fasciatus to 30°C temperatures caused individuals of this species to quit eating and eventually die. This laboratory finding would seem contradictory with the fact that body temperatures of basking P. fasciatus often exceed 30°C in the field [8] (Watson, C. pers. obs.) and that their preferred temperature–acutely—has been experimentally quantified at 32.6°C [8], well above the highest acclimation temperature used in this study. This is also inconsistent with the findings for two nocturnal gecko species, Ptylodactylus hasselquistii and Bunopus tuberculatus, which exhibited a low metabolic thermal dependence at temperatures that approximate their preferred body temperatures [46]. However, P. fasciatus is a diurnal, actively-thermoregulating species in a habitat with limited basking opportunities [47]. Consequently, P. fasciatus almost certainly experiences different selection pressures in native environments. The closed canopy forest habitat of this species dictates that P. fasciatus must take advantage of direct solar radiation and associated elevated temperatures as a limited resource, so periodic and rapid elevation of metabolism via a shortened basking time may hold a fitness benefit. Brief, periodic basking would temporarily raise the body temperature to or above 30°C, allowing normal metabolic processes to take place while reducing time spent basking and the cost associated with time spent exposed to potential predators. However, prolonged exposure to this temperature appears physiologically untenable, even as it is acutely beneficial.
Ecological Implications of Interspecific Differences in Metabolic Thermal Sensitivity
Plestiodon laticeps can be found in sympatry with P. fasciatus and P. laticeps, but P. fasciatus and P. inexpectatus are rarely found together [9, 11]. This distribution could be explained, at least in part, by the metabolic thermal sensitivity of each species, as reflected in the metabolic Q10 patterns presented in the current study. Thus, P. fasciatus may be excluded from areas inhabited by P. inexpectatus that remain above the former’s critical temperatures for an extended period of time. P. inexpectatus and P. laticeps, which are metabolically stable at those same warm temperatures, could remain active throughout the day without having to continually bask. Conversely, P. inexpectatus would be excluded from those cooler habitats that exhibit low availability of higher temperatures (i.e. closed-canopy forest), while P. fasciatus and P. laticeps would be able to maintain activity in those microhabitats by remaining active with less time spent thermoregulating.
Established geographic distributions [2,7] suggest that the metabolic temperature sensitivity of these species strongly reflects the temperature characteristics of the local niche that each occupies, and may be an important mechanism underlying their distributions. Indeed, the concept of “metabolic niche” has been used to describe broad distributions of endotherms [13] and these findings support the application of this concept. Here, we extend this concept to include the occupation of metabolic niches of ectotherms based upon their metabolic responses to temperature. These findings support our hypotheses as all three species inhabit microhabitats that reflect their metabolic response to temperature and the two like-sized species show significantly different temperature responses that could limit their ability to co-exist. These conclusions depend upon data from sympatric populations, but it may be interesting in future studies to determine whether patterns of character release and/or displacement may occur.
Generally, metabolic rates are species-specific and, for ectothermic organisms, will vary across temperatures. The preferred temperature required to maintain a metabolic rate conducive to homeostasis therefore also varies among species. In the case of Plestiodon fasciatus and P. inexpectatus, there is a measurable difference in metabolism that corresponds to relatively fine-scale temperature differences between open and closed canopy habitats [9, 11]. Although they exhibit morphological similarities that have historically caused them to be considered a single species, the current study demonstrates P. fasciatus and P. inexpectatus to differ in their metabolic physiology. These differences could explain a mechanism by which they may be segregated in time and space across the landscape, therefore maintaining an allopatric relationship at the microhabitat scale while remaining regionally sympatric. Therefore, P. fasciatus and P. inexpectatus do not appear to be in conflict with the “competitive exclusion hypothesis” [48]. Furthermore, this highlights an instance where differential metabolic responses to temperature among closely-related organisms can possibly structure species’ distributions in a habitat that is thermally dynamic at the local scale.
Supporting Information
(DOCX)
Acknowledgments
We thank Corey Roelke, Robert Makowsky, John Morse, Christian Cox, Brian Fontenot, Rebbekah Watson, Chris Winne, Whit Gibbons, Leslie Rissler, Lora Smith, and Craig Guyer for assistance in laboratory and field activities. Laura Gough and the Gough laboratory at UTexas-Arlington were particularly important to the completion of this project by providing funding and advice throughout this entire project.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
National Science Foundation (www.nsf.gov) operating grant IOS-1025823 to WWB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hamilton WJ, Pollack JA. The food of some lizards from Fort Benning, Georgia. Herpetologica. 1961;17(2):99–106. [Google Scholar]
- 2.Conant R, Collins JT. A Field Guide to Reptiles and Amphibians of Eastern Central North America. New York: Houghton Mifflin; 1998. 616 p. [Google Scholar]
- 3.Taylor EH. Eumeces inexpectatus: A new American lizard of the family Scincidae. The University of Kansas Science Bulletin. 1932;20:251–8. [Google Scholar]
- 4.Taylor EH. Eumeces laticeps: a neglected species of skink. The University of Kansas Science Bulletin. 1932;20:263–72. [Google Scholar]
- 5.Taylor EH. A taxonomic study of the cosmopolitan scincoid lizards of the genus Eumeces with an account of the distribution and relationships of its species. The University of Kansas Science Bulletin. 1935;23:1–643. [Google Scholar]
- 6.Davis D. A study of the variation in North American lizards of the fasciatus group of the genus Eumeces (Scincidae): Duke University; 1968. [Google Scholar]
- 7.Mount RH. The Reptiles and Amphibians of Alabama. Auburn, AL: Auburn University Agricultural Experiment Station; 1975. 347 p. [Google Scholar]
- 8.Fitch HS. Life history and ecology of the five-lined skink, Eumeces fasciatus. University of Kansas Publications of the Museum of Natural History. 1954;8:1–156. [Google Scholar]
- 9.Watson CM, Gough L. The role of temperature in determining distributions and coexistence of three species of Plestiodon. J Therm Biol. 2012;37:374–9. [Google Scholar]
- 10.Jordan DS. The law of geminate species. Am Nat. 1908;42:73–80. [Google Scholar]
- 11.Watson CM, Formanowicz DR. A comparison of maximum sprint speed among the five-lined skinks (Plestiodon) of the Southeastern United States at ecologically relevant temperatures. Herp Conserv Biol. 2012;7(1):75–82. [Google Scholar]
- 12.Harding JH, Holman JA. Michigan Turtles and Lizards. East Lansing, MI, USA: Michigan State University Cooperative Extension Service; 1997. 94 p. [Google Scholar]
- 13.Robert KA, Thompson MB. Influence of feeding on the metabolic rate of the lizard, Eulamprus tympanum. Copeia. 2000;(3):851–5. [Google Scholar]
- 14.Secor SM, Boehm MC. Specific dynamic action of ambystomatid salamanders and the impact of meal size, meal type, and body temperature. Physiol Biochem Zool. 2006;79:720–35. 10.1086/505511 [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Burggren WW, Nobrega E. Metabolic, ventilatory, and acid-base responses associated with specific dynamic action in the toad Bufo marinus. Physiol Zool. 1995;68(2):192–205. [Google Scholar]
- 16.McCue MD, Bennett AF, Hicks JW. The effect of meal composition on specific dynamic action in burmese pythons (Python molurus). Physiol Biochem Zoology. [DOI] [PubMed] [Google Scholar]
- 17.Crocker-Buta SP, Secor SM. Determinants and repeatability of the specific dynamic response of the corn snake, Pantherophis guttatus. Comp Biochem Physiology A. 2014;169:60–9. [DOI] [PubMed] [Google Scholar]
- 18.Burggren WW, Infantino RL, Townsend DL. Developmental changes in cardiac and metabolic physiology of the direct-developing tropical frog Eleutherodactylus coqui. J Exp Biol. 1990; 152:129–47. [Google Scholar]
- 19.Gore M, Burggren WW. Cardiac and metabolic physiology of early larval zebrafish (Danio rerio) reflects parental swimming stamina. Front Physiol. 2012;3:35 10.3389/fphys.2012.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lighton JRB. Measuring Metabolic Rates: A Manual for Scientists. Oxford: Oxford University Press; 2008. [Google Scholar]
- 21.Vleck D. Measurement of O2 consumption, CO2 production, and water vapor production in a closed system. J Appl Physiol. 1987;62(5):2103–6. [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals. Am Nat. 1959;93:145–59. [Google Scholar]
- 23.Murray IW, Fuller A, Lease HM, Mitchell D, Woldf BO, Hefem RS. Low field metabolic rates for geckos of the genus Rhoptropus may not be surprising. J Arid Environ. 2015;113:35–42. [Google Scholar]
- 24.Iglesias S, Thompson MB, Seebacher F. Energetic cost of a meal in a frequent feeding lizard. Comp Biochem Phys A. 2003;135(3):377–82. [DOI] [PubMed] [Google Scholar]
- 25.Angilletta MJ. Variation in metabolic rate between populations of a geographically widespread lizard. Physiol Biochem Zool. 2001;74(1):11–21. 10.1086/319312 [DOI] [PubMed] [Google Scholar]
- 26.Zannoni JT. Measuring the Effects of Temperature and Nutritional State on Metabolic Rates of Eastern Fence Lizards, Sceloporus undulate. Akron, Ohio: University of Akron; 1997. [Google Scholar]
- 27.Nagy KA, Huey RB, Bennett AF. Field energetics and foraging mode in Kalahari lactertid lizards. Ecology. 1984;65(2):588–96. [Google Scholar]
- 28.Pan ZC, Ji X, Lu HL, Ma XM. Influence of food type on specific dynamic action of the Chinese skink Eumeces chinensis. Comp Biochem Physiol A. 2005;140(1):151–5. [DOI] [PubMed] [Google Scholar]
- 29.Hare KM, Pledger S, Thompson MB, Miller JH, Daugherty CH. Daily patterns of metabolic rate among New Zealand lizards (Reptilia: Lacertilia: Diplodactylidae and Scincidae). Physiol Biochem Zool. 2006;79(4):745–53. 10.1086/504618 [DOI] [PubMed] [Google Scholar]
- 30.Parker SL. Physiological ecology of the ground skink, Scincella lateralis in South Carolina: thermal biology, metabolism, water loss and seasonal patterns. Herp Conserv Biol. 2014;9(2):309–21. [Google Scholar]
- 31.White CR, Kearney MR. Metabolic scaling in animals: methods, empirical results, and theoretical explanations. Comprehensive Physiol. 2014. [DOI] [PubMed] [Google Scholar]
- 32.Glazier DS. Is metabolic rate a universal ‘pacemaker’for biological processes? Biol Reviews. 2015;90(2):377–407. [DOI] [PubMed] [Google Scholar]
- 33.Agutter PS, Tuszynski JA. Analytic theories of allometric scaling. J Exp Biol. 2011; 214(7):1055–62. [DOI] [PubMed] [Google Scholar]
- 34.Zari TA. Seasonal metabolic acclimatization in the herbivorous desert lizard Uromastyx philbyi (Reptilia: Agamidea) from western Saudi Arabia. J Therm Biol. 2016;60:180–5. 10.1016/j.jtherbio.2016.07.014 [DOI] [PubMed] [Google Scholar]
- 35.Bennett AF. The energetics of reptilian activity. Biology of the Reptilia. 1982;13:155–99. [Google Scholar]
- 36.Andrews RM, Pough FH. Metabolism of squamate reptiles: allometric and ecological relationships. Physiol Zool. 1985:214–31. [Google Scholar]
- 37.Al-Sadoon MK. Influence of a broad temperature range on the oxygen consumption rates of three desert lizard species. Comp Biochem Physiol A: 1986;84(2):339–44. [DOI] [PubMed] [Google Scholar]
- 38.Karasov WH, Anderson RA. Correlates of average daily metabolism of field-active zebra-tailed lizards (Callisaurus draconoides). Physiol Zool. 1998;71(1):93–105. [DOI] [PubMed] [Google Scholar]
- 39.Pelster B. Environmental influences on the development of the cardiac system in fish and amphibians. Comp Biochem Physiol A. 1999;124(4):407–12. [DOI] [PubMed] [Google Scholar]
- 40.Burggren W, Roberts J. 9 Respiration and Metabolism. Comparative Animal Physiology, Environmental and Metabolic Animal Physiology. 1991:353. [Google Scholar]
- 41.Clarke A, Johnston NM. Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol. 1999;68(5):893–905. [Google Scholar]
- 42.Hölker F. The metabolic rate of roach in relation to body size and temperature. J Fish Biol. 2003;62(3):565–79. [Google Scholar]
- 43.Barrionuevo W, Burggren W. O2 consumption and heart rate in developing zebrafish (Danio rerio): influence of temperature and ambient O2. Am J Physiol. 1999;276(2):R505–R13. [DOI] [PubMed] [Google Scholar]
- 44.Hochscheid S, Bentivegna F, Speakman JR. Long‐Term Cold Acclimation Leads to High Q10 Effects on Oxygen Consumption of Loggerhead Sea Turtles Caretta caretta. Physiol Biochem Zool. 2004;77(2):209–22. 10.1086/381472 [DOI] [PubMed] [Google Scholar]
- 45.Carey C. Effect of constant and fluctuating temperatures on resting and active oxygen consumption of toads, Bufo boreas. Oecologia. 1979;39(2):201–12. [DOI] [PubMed] [Google Scholar]
- 46.Al-Sadoon MK, Abdo NM. Temperature effects on oxygen consumption of two nocturnal geckos, Ptyodactylus hasselquistii (Donndorff) and Bunopus tuberculatus (Blanford) (Reptilia: Gekkonidae) in Saudi Arabia. J Comp Physiol B 1989;159:1–4. [Google Scholar]
- 47.Watson CM L. G. The role of temperature in determining distributions and coexistence of three species of Plestiodon. J Therm Biol. 2012;37(5):374–9. [Google Scholar]
- 48.Gause GF. The Struggle for Existence. Baltimore, MD, USA: William and Wilkins; 1934. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.