Abstract
Background
Rift Valley fever virus (RVFV) causes a viral zoonosis, with discontinuous epizootics and sporadic epidemics, essentially in East Africa. Infection with this virus causes severe illness and abortion in sheep, goats, and cattle as well as other domestic animals. Humans can also be exposed through close contact with infectious tissues or by bites from infected mosquitoes, primarily of the Aedes and Culex genuses. Although the cycle of RVFV infection in savannah regions is well documented, its distribution in forest areas in central Africa has been poorly investigated.
Methodology/Principal Findings
To evaluate current circulation of RVFV among livestock and humans living in the Central African Republic (CAR), blood samples were collected from sheep, cattle, and goats and from people at risk, such as stock breeders and workers in slaughterhouses and livestock markets. The samples were tested for anti-RVFV immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies. We also sequenced the complete genomes of two local strains, one isolated in 1969 from mosquitoes and one isolated in 1985 from humans living in forested areas. The 1271 animals sampled comprised 727 cattle, 325 sheep, and 219 goats at three sites. The overall seroprevalence of anti-RVFV IgM antibodies was 1.9% and that of IgG antibodies was 8.6%. IgM antibodies were found only during the rainy season, but the frequency of IgG antibodies did not differ significantly by season. No evidence of recent RVFV infection was found in 335 people considered at risk; however, 16.7% had evidence of past infection. Comparison of the nucleotide sequences of the strains isolated in the CAR with those isolated in other African countries showed that they belonged to the East/Central African cluster.
Conclusion and significance
This study confirms current circulation of RVFV in CAR. Further studies are needed to determine the potential vectors involved and the virus reservoirs.
Author Summary
Rift valley fever virus (RVFV) is an arthropod-borne virus that causes serious illness in both animals and humans. RVFV is transmitted by direct contact with infectious tissues or by the bites of infected mosquito species of the Aedes and Culex genuses. Its distribution in tropical forests in central Africa is poorly documented. We assessed the current circulation of RVFV among livestock and humans in the Central African Republic (CAR) by detecting anti-RVFV immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies in sheep, cattle and goats and in people living in Bangui who were considered at risk. We also sequenced the complete genomes of two local strains, one isolated in 1969 from mosquitoes and one isolated in 1985 from humans living in forested areas. Sheep were the most frequently infected ruminants. IgM antibodies were found only during the rainy season; the frequency of IgG antibodies did not differ according to season. No evidence of recent RVFV infection was found in humans at risk; however, 16.7% had evidence of past infection. Phylogenetic analysis showed a perfect match of CAR strains with the East/Central African cluster. Our results confirm current circulation of RVFV in CAR. Further studies should be conducted to determine the vectors involved and the virus reservoirs.
Introduction
Rift Valley fever (RVF) is a viral zoonosis that affects mainly animals but is also found in humans. It is caused by an RNA virus of the Phlebovirus genus (Bunyaviridae family), the genome consisting of three RNA segments: large, medium, and small [1,2]. RVFV is transmitted mainly by infected mosquitoes of the Aedes and Culex genuses, but humans can be contaminated by direct contact with blood (e.g. aerosols, absorption) or tissues (e.g. placenta of stillborns from infected animals) [3,4].
The virus was first identified in 1930 during an epidemic that caused deaths and sudden abortions among sheep on the shores of Lake Naivasha in the Great Rift Valley in Kenya [5,6]. Since then, the virus has spread to most African countries. The disease occurs in endemic and epidemic forms along the east and south coasts of Africa, in West Africa, in Madagascar [7,8] and as far north as Egypt, with a recent outbreak in the Arabian Peninsula [9,10]. Severe episodes of RVF have been reported among humans and animals in southern Africa [11,12,13,14]. The animals most frequently infected are sheep and cattle, followed by goats, with heavy economic losses due to abortions and high mortality rates among juvenile animals [15,16]. RVFV antibodies have been detected in many wild animal species, including ungulates in Kenya [17,18], bats in Guinea [19], and small vertebrates in Senegal and South Africa [20,21]; however, their role in maintenance of the virus in the ecosystem during inter-epidemic periods and their contribution to amplifying outbreaks remain unknown.
RVFV was first isolated in the Central African Republic (CAR) in 1969 from a pool of Mansonia africana mosquitoes [22]. It was identified as the causative virus of RVF in 1983 [23,24]. RVFV-specific antibodies have since been detected in humans, and 15 strains of RVFV have been isolated from humans and sylvatic mosquitoes in CAR [25,26], although no RVF outbreak has been reported. Current circulation of RVFV in the CAR is unknown, after a gap of two decades without surveillance; however, as animal breeding plays a large part in the economy of the CAR, an epidemic involving humans and animals is possible.
We undertook a study to assess the current circulation of RVFV in livestock and humans in the CAR. We also sequenced the genomes of local strains isolated in 1969 from wild mosquitoes and in 1985 from humans in a forested area of the country in order to determine the genetic diversity of these strains which will serve as reference for the future.
Materials and Methods
We performed a prospective cross-sectional study in two phases between November 2010 and November 2012.
Ethics statement
The national ethical and scientific committees in charge of validating study design in the CAR approved the study design (No. 9/UB/FACSS/CSCVPRE/13). The study was described orally before blood samples were collected from human participants, and participants were included only if they gave written consent; for participants aged ≤ 18 years, a parent or guardian provided written informed consent. The informed consent form included a clause permitting use of the participants’ biological specimens for future research. No endangered or sheltered animal species were used in the survey. Verbal consent for testing their animals was acquired from farmers after the objectives of the study had been explained. Once permission was obtained for blood sample collection, an experienced veterinarian bled the animals gently.
Sample collection
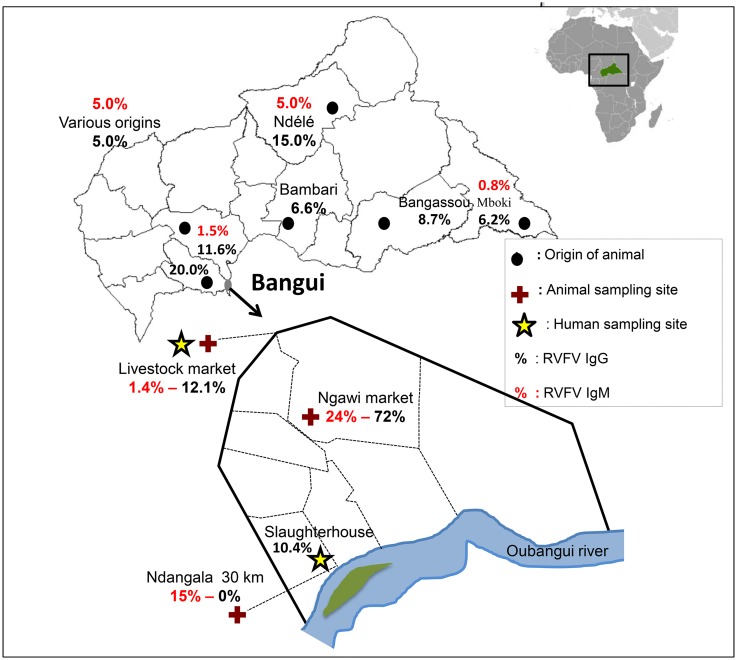
Samples were taken during the dry season in the first year and at the same sites during the rainy season in the second year. Blood was collected from sheep, cattle, and goats at three localities: the livestock market situated 13 km north of Bangui for cattle, the Ngawi market in Bangui commercial centre, and Ndangala village located 30 km south of Bangui for sheep and goats (Fig 1). The sex and age of each animal were noted. All animals under 3 years of age were considered juveniles and those over 3 years as adults. Blood samples were also collected from people at risk, such as stock breeders and people working in slaughterhouses and livestock markets. From both animals and humans, venous blood samples were collected in 5-mL Vacutainer tubes (Becton Dickinson, Franklin Lakes, New Jersey, USA), which were placed in a cooler, transported to the laboratory, and centrifuged at 2–8°C for 10 min at 2000 rpm. Each serum sample was separated on collection into two aliquots and stored at –20°C until analysis.
Fig 1. Origin of animals and sampling sites.
Each person who agreed to participate in this study completed an anonymous questionnaire that included demographics.
The mosquitoes were collected in sylvan environments in 1969, identified, and grouped into pools of up to 30 individuals per species per site; samples were stored at –20°C for a maximum of 4 days in the field, transported to the Institut Pasteur in Bangui, and stored at –80°C until virus isolation. The virus was isolated and amplified by four serial passages in suckling mice brain, as described by Saluzzo and colleagues [27]. The brain suspensions were then lyophilized and stored in sealed glass vials at room temperature until use.
Serological analysis
Serum samples were analysed for the presence of immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies to RVF with a SPU-02 RVF IgM and IgG Biological Diagnostic Supplies Ltd (ELISA) kit according to the manufacturer’s instructions. Briefly, plates were coated with a recombinant nucleocapsid RVFV antigen diluted 1:1000 in sodium bicarbonate buffer (pH = 9.6), covered with plate seals and incubated at 4°C overnight. Unbound antigen was removed by washing three times for 15 s each with PBS-T. Plates were then blocked with 10% skimmed milk in PBS (PBS-SM) at 37°C for 1 h and then washed. Test sera were added in duplicate at a dilution of 1:400 in 2% PBS-SM and incubated for 1 h at 37°C. The plates were washed once more, and HRP-conjugated anti-human IgG antibody, diluted 1:25 000 in 2% PBS-SM, was added to each well and incubated for 1 h at 37°C. After a final wash, chromagenic detection of HRP and absorbance measurement were performed as described previously. Negative and positive control sera were included for each plate. Sera samples were considered positive if their calculated optical density (OD) was ≥ 0.29 (net OD serum/net mean OD positive control).
Genetic analysis
RVFV was isolated from three samples: two strains isolated in 1969 from mosquitoes (ArB1986 and HB74P59) and one from human serum in 1985 (HB1752). It was amplified by inoculation into the brains of newborn mice in a laboratory of biosafety level 3. A brain suspension was prepared, lyophilized, and stored in glass vials at room temperature, and viral RNA was extracted with a QIAmp viral RNA Mini kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions [28]. The extracted RNA was treated with Turbo DNase (Life Technologies Inc., Carlbad, California, USA) and then retro-transcribed into cDNA with a SuperScript III First Strand Synthesis kit in the presence of random hexamers. The cDNA generated was amplified with Phi29 enzyme as described previously [29]. A fixed amount of amplified DNA was sequenced in an Illumina Hi-seq 2000 sequencer. An average of 30 × 106 single reads with 100 bases was obtained for each sample [28]. The quality of reads was assessed by FastQC, and the sequences were selected according to their quality. All reads corresponding to the mouse genome sequence were filtered by mapping with Bowtie 2.0 software on a Mus musculus Mn10 sequence. The viral reads corresponding to the RVFV genome were selected by a similarity approach with BLASTN search tools [30]. All selected viral reads were assembled with Ray software, with k = 25, to obtain the full-length viral genomes [28].
In order to validate our approach for obtaining the complete sequence of RVFV by high-throughput sequencing, the RNA was extracted from three viral strains (HB74P59, HB1752, and ArB1986), and fragments of the small, medium, and large segments were amplified and sequenced. The sequence obtained from HB74P59 was compared with those obtained previously by Bird et al. in 2007 [31]; no difference was found in three segments obtained by high-throughput sequencing and classical Sanger sequencing. Moreover, no difference was found in the three segments of strain ArB1986 isolated from an Aedes palpalis mosquito and that of a strain isolated in the same city, Loko-Zinga, in 1969 but from another arthropod, Mansonia africana.
Statistics
The distribution of serological results for RVFV was analysed by species, season, and human age and sex and presented as proportions. The effect of each variables on the RVFV positivity was examined using the chi-squared test. P values < 0.05 were considered statistically significant. The variables were then put in a Logistic regression model in stepwise manner. The likelihood ratio test was used to compare the model with and without the variable. In case there was no evidence that the variable fitted, it was dropped (parsimonious model). Statistical analyses were performed with STATA software version 11.
Results
Animal samples
A total of 1271 animals were sampled, comprising 727 cattle, 325 sheep, and 219 goats (Table 1). The overall seroprevalence in animals was 1.9% for anti-RVFV IgM antibodies and 8.6% for IgG antibodies. The seroprevalence varied significantly by ruminant. The IgM antibody titre, which indicates recent circulation of RVFV, was 4.3% in sheep, 1.4% in goats, and 1.1% in cattle (P < 0.0001), whereas the IgG seroprevalence was 12.9% in sheep, 7.8% in cattle, and 5.0% in goats (Table 2). However, the multivariate analysis showed that IgM and IgG seropositivity rates in sheep were higher than other species (OR = 1.8, 95% CI = 1.1–3.0) (Table 1). No significant difference in seropositivity was found between male and female animals (Table 3). The IgG seropositivity rate was 9.6% in adults and 5.1% in juveniles (P < 0.02), but no significant difference in positivity for anti-RVFV IgM antibodies was found between juveniles and adults (Table 3). The IgM and IgG seropositivity rates varied significantly according to the origin of the sample. No animals positive for IgG antibodies were found in Ndangala, whereas most of those positive for IgM antibodies were originated from this site, and cattle market are less likely to be IgM positive (OR = 0.1, 95% CI = 0.0–0.5) (Tables 1 and 3). All animals with positive IgM were found in the rainy season, and IgG seropositivity was more pronounced during dry season (Table 3). None of the livestock owners reported cases of abortion or death in the months before sampling that would indicate RVFV infection in their herds.
Table 1. Multivariate analysis of risk factors for RVF virus seropositivity in animals.
| No. | Positive IgM | Positive IgG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | P | Multivariate analysis | No. | % | P | Multivariate analysis | ||||
| OR | 95% CI | OR | 95% CI | ||||||||
| Species | |||||||||||
| Cattle | 727 | 8 | 1.1 | 0.002 | Reference | 57 | 7.8 | 0.003 | Reference | ||
| Goat | 219 | 3 | 1.4 | 0.3 | 0.0–2.2 | 11 | 5.0 | 0.8 | 0.4–1.7 | ||
| Sheep | 325 | 14 | 4.3 | 0.9 | 0.2–4.7 | 42 | 12.9 | 1.8 | 1.1–3.0 | ||
| Sex | |||||||||||
| Male | 551 | 6 | 1.1 | 0.07 | - | 39 | 7.1 | 0.204 | - | ||
| Female | 719 | 19 | 2.6 | - | 71 | 10.0 | - | ||||
| Age | |||||||||||
| Juvenile | 273 | 3 | 1.1 | 0.36 | - | 14 | 5.1 | 0.019 | - | ||
| Adult | 998 | 22 | 2.2 | - | 96 | 9.6 | - | ||||
| Origin | |||||||||||
| Ndangala | 69 | 6 | 8.7 | < 0.0001 | Reference | 0 | 0.0 | < 0.0001 | Reference | ||
| Ngawi | 119 | 9 | 7.6 | 0.7 | 0.2–2.4 | 22 | 18.5 | 0.5 | 0.8–2.8 | ||
| Cattle market | 1083 | 10 | 2.0 | 0.1 | 0.0–0.5 | 88 | 8.1 | N/A | |||
| Season | |||||||||||
| Rainy | 732 | 25 | 3.4 | NA | 88 | 12.0 | < 0.001 | Reference | |||
| Dry | 539 | 0 | 0.0 | < 0.001 | NA | 22 | 2.1 | 0.3 | 0.1–0.5 | ||
NA, not applicable; OR, odd ratio; CI, confidence interval; IgM, Immunoglobulin M; IgG, Immunoglobulin
Table 2. Serological results for RVFV by species and season in the CAR.
| No. | Positive IgM | Positive IgG | |||||
|---|---|---|---|---|---|---|---|
| No. | % | P | No. | % | P | ||
| Species | |||||||
| Human | 335 | 0 | 0.0 | 56 | 16.7 | ||
| Cattle | 727 | 8 | 1.1 | 0.002* | 57 | 7.8 | 0.003 |
| Goat | 219 | 3 | 1.4 | 11 | 5.0 | ||
| Sheep | 325 | 14 | 4.3 | 42 | 12.9 | ||
| Season* | |||||||
| Dry | 539 | 0 | 0.0 | <0.001 | 22 | 2.1 | <0.001 |
| Rainy | 732 | 25 | 3.4 | 88 | 12.0 | ||
IgM, Immunoglobulin M; IgG, Immunoglobulin G; No., number positive
*For animals only
Table 3. Prevalence of RVFV in ruminants.
| No. | Positive IgM | Positive IgG | |||||
|---|---|---|---|---|---|---|---|
| No. | % | P | No. | % | P | ||
| Sex | |||||||
| Male | 551 | 6 | 1.0 | 0.07 | 39 | 7.1 | 0.09 |
| Female | 719 | 19 | 2.6 | 71 | 10.0 | ||
| Age | |||||||
| Juvenile | 273 | 3 | 1.1 | 0.36 | 14 | 5.1 | 0.03 |
| Adult | 998 | 22 | 2.2 | 96 | 9.6 | ||
| Origin | |||||||
| Ndangala | 69 | 6 | 8.7 | 0.001 | 0 | 0 | 0.001 |
| Gawi | 119 | 9 | 7.6 | 22 | 18.5 | ||
| Cattle market | 1083 | 10 | 2.0 | 88 | 8.1 | ||
IgM, Immunoglobulin M; IgG, Immunoglobulin G
Human samples
Blood samples were collected from 335 people who were regularly in contact with blood from the animals. The mean age (±SD) was 36.3 years (±18.1), and the sex ratio (M/F) was 6.0/1 (287/48). No evidence of recent RVFV infection (absence of IgM) was found in human samples; however, 16.7% had evidence of past infection (IgG alone). Of these, 7.7% were stock breeders, 6.6% were butchers, 1.5% were slaughterhouse workers, and 0.9% were veterinarians (Table 4). A higher positivity rate was observed among people over 25 years of age (P = 0.04), and 17.8% (51/287) of males and 10.4% (5/48) of females were positive for IgG (P = 0.29) (Table 4).
Table 4. Prevalence of RVFV in people at risk.
| Positive IgG | ||||
|---|---|---|---|---|
| Characteristic | No. | No. | % | P |
| Sex | ||||
| Male | 287 | 51 | 17.8 | 0.29 |
| Female | 48 | 5 | 10.4 | |
| Age (years) | ||||
| < 15 | 23 | 0 | 0.0 | 0.04 |
| 15–24 | 39 | 4 | 10.4 | |
| 25–34 | 90 | 18 | 20.0 | |
| 35–44 | 88 | 21 | 23.9 | |
| > 44 | 95 | 13 | 13.7 | |
| Trade* | ||||
| Veterinarian | 28 | 3 | 10.7 | 0.72 |
| Slaughterhouse worker | 41 | 5 | 12.2 | |
| Butcher | 41 | 9 | 22.0 | |
| Breeder | 77 | 10 | 13.0 | |
IgG, Immunoglobulin G; N, number positive
* Differences between groups are due to the missing data
Genetic analysis of complete genome isolated from mosquitoes
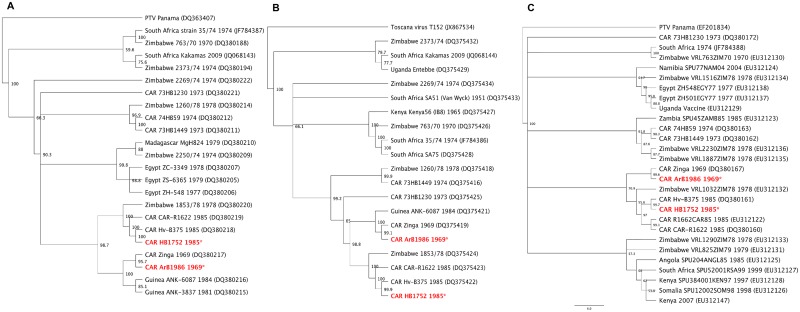
A third RVFV strain obtained by high-throughput sequencing was isolated in Bangui at the end of December 1984 (HB1752). Genomic analysis of three segments showed that it was identical to a strain isolated in Bangui 3 months earlier; however, RVFV strains isolated from vectors in 1969 and from human cases in the CAR several decades later had different nucleic sequences, even though they belonged to the same cluster (Fig 2). The complete genome sequence was made available to GenBank (HB1752 strain small, medium, and large accession numbers KJ782452, KJ782453, and KJ782454 respectively; ArB1986 strain small, medium, and large accession numbers KJ782455, KJ782456, and KJ782457, respectively).
Fig 2. Phylogenetic tree of selected RVFV segments: (A) small (S), (B) medium (M) and (C) large (L).
Analysis at nucleic acid level with sequences available in GenBank. The tree was generated by the neighbor-joining method with Geneious software for Mac (Geneious version 6.1 created by Biomatters) by the boostrapping approach with 1000 replicates. Values are showed as percentages.
Discussion
This study, the first in the CAR since RVFV was isolated in 1985, shows that the virus continues to circulate in central Africa. The overall prevalence in animals in this study was lower than that reported in Comoros in 2009 (39% in sheep and 33.5% in goats) [32], in Madagascar in 2008 (24.7% in small ruminants) [33], and in Mozambique (35.8% in sheep and 21.2% in goats) [34]. The same ELISA kits were used in all these studies, suggesting that the differences are due to climatic factors, entomological parameters, agro-ecological conditions, or sampling strategies.
The higher prevalence in sheep is consistent with previous work, indicating that this species is preferentially infected with RVFV [15]. Most infected animals, especially sheep, were found in Ndangala, a rural forested area south of Bangui that has more rainfall than the rest of the country, with lower temperatures and constant humidity in the rainy season, during which time there is little husbandry. The high prevalence observed at this site, with the presence of IgM antibodies, suggests endemic virus circulation, which would be maintained by a sylvatic cycle involving wild animals and mosquitoes, as suggested by Olive et al. [35]. In a previous study in a forested area of the CAR, RVFV was isolated from wild mosquitoes, including Ae. palpalis [26]. In the rainy season, there are many potential breeding sites, which increases the density of vectors and subsequently increases transmission of arboviral diseases such as RVFV. IgM, which indicates recent infection, was present only during the rainy season, but IgG was also significantly associated with the rainy season. These finding are consistent with those in Mauritania and Senegal that indicate that the risk factors for RVF are linked to heavy rainfall and the presence of large temporary masses of surface water [36,37]. In a previous study, seropositivity for RVFV was associated with increased numbers of mosquito vectors [38]. Although we did not conduct entomological surveys, recent entomological surveillance for yellow fever identified several species of Aedes mosquito, including Ae. cuminsii, Ae. circumluteolus, and Ae. palpalis [39], which are known vectors of RVFV [40].
The seroprevalence of RVFV was higher in adult than in juvenile animals. Similar results were reported in Mauritania and Senegal [32,33], supporting the hypothesis of endemic circulation of the virus, as older animals would have longer exposure than younger ones [34]. The presence of IgG in young animals (< 3 years) in this study suggests recent circulation of the virus. This result is compatible with the IgM titres in each species, with high titres in samples taken from sheep in Ndangala (Table 2). The absence of IgG in animals from this region indicates that introduction of the virus south of Bangui is recent. Recent introduction of the virus associated with environmental modifications such as deforestation, population displacement due to the socio-political crisis, and introduction of a new vector competent for RVFV [41] could increase the risk for emergence of an epidemic.
As no epidemic of RVF or obvious clinical signs of the disease (such as abortions) was observed, the infections were minor or sub-clinical [34]. In a study in Madagascar, circulation of RVFV during the dry season did not result in clinical cases [33]. Viral activity may be maintained in mosquitoes near rivers that do not dry up during the dry season, resulting in a low level of transmission among domestic animals. These observations and the recent epidemics in East Africa illustrate the risk for introduction of pathogenic strains of RVFV to CAR from countries such as South Sudan, which shares a long border with CAR and has had many epidemics and epizootics of RVF.
The absence of anti-RVFV IgM antibodies among people regularly exposed to animals would appear to indicate that contact with the virus is uncommon and the public health risk is low. Nevertheless, the presence of IgG among breeders and butchers, who are in contact with the blood of these animals, should alert the authorities to strengthen surveillance of circulation of this virus. Studies to isolate the virus in the vector (mosquitoes) and longitudinal studies in sheep, goats, and cattle should be conducted to detect clinical cases, particularly among sheep and herders in Ndangala village, where evidence of current circulation of the virus was found. As most of the inhabitants of the rural areas in which the small ruminants were sampled are farmers who share the same environment as their animals and may have the same exposure to mosquitoes, a study should also be carried out to determine the prevalence of RVFV antibodies and to establish whether RVF occurs regularly in these zones and is thus a neglected cause of morbidity and mortality.
Our study was limited to Bangui and neighbouring areas because animals are brought to the capital from all regions of the country. As we were unable to isolate recent strains of the virus, we could not establish the precise geographical origin of the viral strains currently circulating in the CAR. Nevertheless, on the basis of genomic data for old strains, the same strain may be circulating relatively freely in several vectors in a defined geographical area over a long period. Furthermore, although IgG responses persist for several years in continually exposed people, we were unable to obtain a second sample at an interval of 2 weeks and therefore could not demonstrate seroconversion, which would support recent infection. The low IgG titres and high IgM titres found in the Ndangala region suggest recent introduction of the virus into this forested area, probably associated with illegal movement of sheep and goats from the Democratic Republic of Congo.
In view of the highly precarious situation in CAR, a large-scale molecular study will not be possible in the short term; furthermore, the socio-political upheaval in the country may change socioeconomic and environmental conditions and the time of infection before the study is conducted.
Comparison of the sequences of strains isolated from vectors and humans in the CAR with those isolated in other African counties show that they belong to the East/Central African cluster, confirming RVFV strain exchanges among geographical areas. Propagation of RVFV from East Africa to other regions was noted in Saudi Arabia and Yemen in 2000–2001 [42] and in Chad in 2001 [43] during previous RVF outbreaks.
Conclusion
The results of this first study conducted in both humans and animals in the CAR are important for public health. They shows a high prevalence of RVFV in an area where neither epidemics nor clinical cases of RVF have been reported previously. These results are also important because, in the forested area south of Bangui where there is little husbandry, there is nevertheless low virus noise, which might suggest the presence of a reservoir that has come nearer to human habitats. Unexpectedly, we found low IgM titres in regions of previous intensive animal husbandry, because animal density in these areas has fallen sharply due to the migration of herders in response to the continuing instability in the CAR.
Other studies are required to elucidate and measure the environmental risk factors for infection with RVFV in order to predict epidemics, and entomological studies should be performed to identify all the potential vector species to better understand the ecological and climate factors that favor the distribution of RVFV.
Supporting Information
(DOC)
Acknowledgments
We thank Benjamin Selekon and Xavier Namkona for technical assistance. We also thank the reviewers for correcting the manuscript.
Data Availability
All relevant data are within the paper and in GeneBank (HB1752 strain small, medium, and large accession numbers KJ782452, KJ782453, and KJ782454 respectively; ArB1986 strain small, medium, and large accession numbers KJ782455, KJ782456, and KJ782457, respectively).
Funding Statement
This study was supported by the Pasteur Institute of Bangui and the Programme Transversal de Recherche (CEVACAR No. 385) financed by the Institut Pasteur (Paris, France). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, et al. (1995) Family Bunyaviridae, p. 300–315. In Virus Taxonomy. Classification and Nomenclature of Viruses. Sixth report of the international committee on taxonomy of viruses. Springer-Verlag, Wien, New York. [Google Scholar]
- 2.Jackel S, Eiden M, El Mamy BO, Isselmou K, Vina-Rodriguez A, et al. (2013) Molecular and serological studies on the Rift Valley fever outbreak in Mauritania in 2010. Transbound Emerg Dis 60 Suppl 2: 31–39. 10.1111/tbed.12142 [DOI] [PubMed] [Google Scholar]
- 3.Flick R, Bouloy M (2005) Rift Valley fever virus. Curr Mol Med 5: 827–834. [DOI] [PubMed] [Google Scholar]
- 4.Balenghien T, Cardinale E, Chevalier V, Elissa N, Failloux AB, et al. (2013) Towards a better understanding of Rift Valley fever epidemiology in the south-west of the Indian Ocean. Vet Res 44: 78 10.1186/1297-9716-44-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daubney R, Hudson JR, Garnham PC (1931) Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep, cattle and man from East Africa. Journal of Pathology Bacteriology 34: 545–579. [Google Scholar]
- 6.Daubney R, Hudson JR (1932) Rift Valley fever. Lancet i: 611–612. [Google Scholar]
- 7.Gerdes GH (2004) Rift Valley fever. Rev Sci Tech 23: 613–623. [DOI] [PubMed] [Google Scholar]
- 8.Clements AC, Pfeiffer DU, Martin V, Otte MJ (2007) A Rift Valley fever atlas for Africa. Prev Vet Med 82: 72–82. 10.1016/j.prevetmed.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 9.Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, et al. (2003) Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis 37: 1084–1092. 10.1086/378747 [DOI] [PubMed] [Google Scholar]
- 10.Abdo-Salem S, Waret-Szkuta A, Roger F, Olive MM, Saeed K, et al. (2011) Risk assessment of the introduction of Rift Valley fever from the Horn of Africa to Yemen via legal trade of small ruminants. Trop Anim Health Prod 43: 471–480. 10.1007/s11250-010-9719-7 [DOI] [PubMed] [Google Scholar]
- 11.Sang R, Kioko E, Lutomiah J, Warigia M, Ochieng C, et al. (2010) Rift Valley fever virus epidemic in Kenya, 2006/2007: the entomologic investigations. Am J Trop Med Hyg 83: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Easterday BC, Mc GM, Rooney JR, Murphy LC (1962) The pathogenesis of Rift Valley fever in lambs. Am J Vet Res 23: 470–479. [PubMed] [Google Scholar]
- 13.Samui KL, Inoue S, Mweene AS, Nambota AM, Mlangwa JE, et al. (1997) Distribution of Rift Valley fever among cattle in Zambia. Jpn J Med Sci Biol 50: 73–77. [DOI] [PubMed] [Google Scholar]
- 14.Nderitu L, Lee JS, Omolo J, Omulo S, O'Guinn ML, et al. (2011) Sequential Rift Valley fever outbreaks in eastern Africa caused by multiple lineages of the virus. J Infect Dis 203: 655–665. 10.1093/infdis/jiq004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easterday BC (1965) Rift valley fever. Adv Vet Sci 10: 65–127. [PubMed] [Google Scholar]
- 16.Eisa M (1984) Preliminary survey of domestic animals of the Sudan for precipitating antibodies to Rift Valley fever virus. J Hyg (Lond) 93: 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans A, Gakuya F, Paweska JT, Rostal M, Akoolo L, et al. (2008) Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol Infect 136: 1261–1269. 10.1017/S0950268807009806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britch SC, Binepal YS, Ruder MG, Kariithi HM, Linthicum KJ, et al. (2013) Rift Valley fever risk map model and seroprevalence in selected wild ungulates and camels from Kenya. PLoS One 8: e66626 10.1371/journal.pone.0066626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oelofsen MJ, Van der Ryst E (1999) Could bats act as reservoir hosts for Rift Valley fever virus? Onderstepoort J Vet Res 66: 51–54. [PubMed] [Google Scholar]
- 20.Pretorius A, Oelofsen MJ, Smith MS, van der Ryst E (1997) Rift Valley fever virus: a seroepidemiologic study of small terrestrial vertebrates in South Africa. Am J Trop Med Hyg 57: 693–698. [DOI] [PubMed] [Google Scholar]
- 21.Gora D, Yaya T, Jocelyn T, Didier F, Maoulouth D, et al. (2000) The potential role of rodents in the enzootic cycle of Rift Valley fever virus in Senegal. Microbes Infect 2: 343–346. [DOI] [PubMed] [Google Scholar]
- 22.Digoutte JP, Cordellier R, Robin Y, Pajot FX, Geoffroy B (1974) [Zinga virus (Ar B 1976), a new arbovirus isolated in central africa (author's transl)]. Ann Microbiol (Paris) 125B: 107–118. [PubMed] [Google Scholar]
- 23.Meegan JM, Digoutte JP, Peters CJ, Shope RE (1983) Monoclonal antibodies to identify Zinga virus as Rift Valley Fever virus. Lancet 1: 641 [DOI] [PubMed] [Google Scholar]
- 24.Georges AJ, Wahid SA, Meunier DY, Georges MC, Saluzzo JF, et al. (1983) Serological evidence of endemic Zinga virus and Rift Valley Fever virus in Central African Republic. Lancet 1: 1338. [DOI] [PubMed] [Google Scholar]
- 25.Digoutte JP, Jacobi JC, Robin Y, Gagnard VJ (1974) [Zinga virus infection in man]. Bull Soc Pathol Exot Filiales 67: 451–457. [PubMed] [Google Scholar]
- 26.Cordellier R, Geoffroy B (1976) Les moustiques de la République Centrafricaine. Distribution, abondance et fréquence des culicidés dans l'ouest du pays. Les arbovirus isolés. Cah ORSTOM Sér Entomol Méd Parasitol 49: 105. [Google Scholar]
- 27.Saluzzo JF, Gonzalez JP, Herve JP, Georges AJ (1980) [Epidemiological study of arboviruses in the Central African Republic: demonstration of Chikungunya virus during 1978 and 1979]. Bull Soc Pathol Exot Filiales 73: 390–399. [PubMed] [Google Scholar]
- 28.Berthet N, Nakoune E, Kamgang B, Selekon B, Descorps-Declere S, et al. (2014) Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis 14: 862–865. 10.1089/vbz.2014.1607 [DOI] [PubMed] [Google Scholar]
- 29.Berthet N, Paulous S, Coffey LL, Frenkiel MP, Moltini I, et al. (2013) Resequencing microarray method for molecular diagnosis of human arboviral diseases. J Clin Virol 56: 238–243. 10.1016/j.jcv.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 30.Berthet N, Nakoune E, Gessain A, Manuguerra JC, Kazanji M (2016) Complete Genome Characterization of the Arumowot Virus (Unclassified Phlebovirus) Isolated from Turdus libonyanus Birds in the Central African Republic. Vector Borne Zoonotic Dis 16: 139–143. 10.1089/vbz.2015.1830 [DOI] [PubMed] [Google Scholar]
- 31.Bird BH, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST (2007) Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J Virol 81: 2805–2816. 10.1128/JVI.02095-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roger M, Girard S, Faharoudine A, Halifa M, Bouloy M, et al. (2011) Rift valley fever in ruminants, Republic of Comoros, 2009. Emerg Infect Dis 17: 1319–1320. 10.3201/eid1707.102031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeanmaire EM, Rabenarivahiny R, Biarmann M, Rabibisoa L, Ravaomanana F, et al. (2011) Prevalence of Rift Valley fever infection in ruminants in Madagascar after the 2008 outbreak. Vector Borne Zoonotic Dis 11: 395–402. 10.1089/vbz.2009.0249 [DOI] [PubMed] [Google Scholar]
- 34.Fafetine J, Neves L, Thompson PN, Paweska JT, Rutten VP, et al. (2013) Serological evidence of Rift Valley fever virus circulation in sheep and goats in Zambezia Province, Mozambique. PLoS Negl Trop Dis 7: e2065 10.1371/journal.pntd.0002065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olive MM, Goodman SM, Reynes JM (2012) The role of wild mammals in the maintenance of Rift Valley fever virus. J Wildl Dis 48: 241–266. 10.7589/0090-3558-48.2.241 [DOI] [PubMed] [Google Scholar]
- 36.Lancelot R, Gonzalez JP, Le Guenno B, Diallo BC, Gandega Y, et al. (1990) [Descriptive epidemiology of Rift Valley fever in small ruminants in Southern Mauritania after the 1988 rainy season]. Rev Elev Med Vet Pays Trop 42: 485–491. [PubMed] [Google Scholar]
- 37.Clements AC, Pfeiffer DU, Martin V, Pittliglio C, Best N, et al. (2007) Spatial risk assessment of Rift Valley fever in Senegal. Vector Borne Zoonotic Dis 7: 203–216. 10.1089/vbz.2006.0600 [DOI] [PubMed] [Google Scholar]
- 38.Digoutte JP, Peters CJ (1989) General aspects of the 1987 Rift Valley fever epidemic in Mauritania. Res Virol 140: 27–30. [DOI] [PubMed] [Google Scholar]
- 39.Ngoagouni C, Kamgang B, Manirakiza A, Nangouma A, Paupy C, et al. (2012) Entomological profile of yellow fever epidemics in the Central African Republic, 2006–2010. Parasit Vectors 5: 175 10.1186/1756-3305-5-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, et al. (1998) New vectors of Rift Valley fever in West Africa. Emerg Infect Dis 4: 289–293. 10.3201/eid0402.980218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gratz NG (2004) Critical review of the vector status of Aedes albopictus. Med Vet Entomol 18: 215–227. 10.1111/j.0269-283X.2004.00513.x [DOI] [PubMed] [Google Scholar]
- 42.Fagbo SF (2002) The evolving transmission pattern of Rift Valley fever in the Arabian Peninsula. Ann N Y Acad Sci 969: 201–204. [DOI] [PubMed] [Google Scholar]
- 43.Durand JP, Richecoeur L, Peyrefitte C, Boutin JP, Davoust B, et al. (2002) [Rift Valley fever: sporadic infection of French military personnel outside currently recognized epidemic zones]. Med Trop (Mars) 62: 291–294. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and in GeneBank (HB1752 strain small, medium, and large accession numbers KJ782452, KJ782453, and KJ782454 respectively; ArB1986 strain small, medium, and large accession numbers KJ782455, KJ782456, and KJ782457, respectively).