Abstract
Aims
To assess protective efficacy of genetically modified Escherichia coli Nissle 1917 (EcN) on metabolic effects induced by chronic consumption of dietary fructose.
Materials and Methods
EcN was genetically modified with fructose dehydrogenase (fdh) gene for conversion of fructose to 5-keto-D-fructose and mannitol-2-dehydrogenase (mtlK) gene for conversion to mannitol, a prebiotic. Charles foster rats weighing 150–200 g were fed with 20% fructose in drinking water for two months. Probiotic treatment of EcN (pqq), EcN (pqq-glf-mtlK), EcN (pqq-fdh) was given once per week 109 cells for two months. Furthermore, blood and liver parameters for oxidative stress, dyslipidemia and hyperglycemia were estimated. Fecal samples were collected to determine the production of short chain fatty acids and pyrroloquinoline quinone (PQQ) production.
Results
EcN (pqq-glf-mtlK), EcN (pqq-fdh) transformants were confirmed by restriction digestion and functionality was checked by PQQ estimation and HPLC analysis. There was significant increase in body weight, serum glucose, liver injury markers, lipid profile in serum and liver, and decrease in antioxidant enzyme activity in high-fructose-fed rats. However the rats treated with EcN (pqq-glf-mtlK) and EcN (pqq-fdh) showed significant reduction in lipid peroxidation along with increase in serum and hepatic antioxidant enzyme activities. Restoration of liver injury marker enzymes was also seen. Increase in short chain fatty acids (SCFA) demonstrated the prebiotic effects of mannitol and gluconic acid.
Conclusions
Our study demonstrated the effectiveness of probiotic EcN producing PQQ and fructose metabolizing enzymes against the fructose induced hepatic steatosis suggesting that its potential for use in treating fructose induced metabolic syndrome.
Introduction
Fructose, present in high fructose corn syrup, is known to induce metabolic syndrome leading to type 2 diabetes mellitus, cardiovascular disease and mortality [1, 2]. Fructose is converted to triglycerides (TG) through de novo lipogenesis when taken in excess amount building up lipids in the liver [3]. These lead to elevated blood lipid levels resulting into inflammation, insulin resistance, increased oxidative stress, and blood glucose levels.
High-fructose fed rats develop clinical features of metabolic syndrome and therefore they are used for assessing beneficial effects of various treatments against metabolic syndrome [4]. In rats fed with high fructose diet, probiotic treatment has been shown to significantly reduce oxidative stress, insulin resistance and lipogenesis [5]. Prebiotic such as short chain fatty acids (SCFA) stimulates fatty acid oxidation, inhibits lipogenesis, and glucose production through inhibition of gluconeogenic gene expression [6, 7]. Mannitol, a known prebiotic, converted to SCFA such as butyrate has been demonstrated to confer protection against the development of colon cancer; in the prevention and treatment of the metabolic syndrome [8, 9]. The combination of prebiotic and probiotic known as “synbiotic” synergistically promotes the growth and survival of existing beneficial bacteria along with the newly added probiotic strains in the colon [10].
Pyrroloquinoline quinone (PQQ) is a powerful antioxidant compound synthesized by many Gram negative bacteria but not by humans and human microbiota [11, 12]. It can induce nerve cells regeneration, enhance mitochondrial functions and reproductive capabilities as well as maintain mitochondrial and neuronal function [13]. Our previous work demonstrated that probiotic E. coli CFR 16 expressing Vitreoscilla haemoglobin (VHb) and secreting PQQ protected against CCl4 and dimethyl hydrazine induced damage by reducing liver and colon damage mediated by their antioxidant abilities [14, 15] This probiotic treatment also restored neurotransmitter levels which alter in response to oxidative damage [16]. Additionally, probiotic EcN producing PQQ was found to be more effective than orally given PQQ against alcohol and rotenone induced oxidative stress [17, 18].
Uptake of fructose in E. coli is mediated by phosphotranferase system (PTS) leading to phosphorylated D-fructose in the cytoplasm [19]. On the other hand, Zymomonas mobilis encodes a glucose facilitator protein (GLF) which allows efficient uptake of unphosphorylated D-fructose [20]. We used the genes corresponding to enzymes that catabolize fructose to mannitol and 5-keto-D-fructose in EcN. Heterofermentative Lactic acid bacteria, Lactobacillus brevis catalyze the conversion of D-fructose directly to D-mannitol by cytosolic mannitol-2-dehydrogenase (MTLK) [21]. Likewise, Gluconobacter japonicas NBRC3260 possess membrane bound Fructose dehydrogenase (FDH; EC 1.1.99.11) which catalyzes the oxidation of D-fructose to 5-keto-D-fructose. FDH is used in the diagnosis and food analysis due to its high substrate specificity to D-fructose [22]. In this study, we genetically modified EcN producing PQQ to convert fructose to 5-KF and mannitol in gut by incorporating fructose metabolizing enzymes, FDH and MTLK along with GLF protein, respectively (S1 and S2 Tables). The potential of these modified probiotics, EcN (pqq-fdh) and EcN (pqq-glf-mtlK) producing PQQ and fructose metabolizing enzymes was investigated for ameliorating high fructose induced metabolic syndrome.
Material and Methods
Plasmid, Bacterial strains and culture condition
EcN was obtained from Dr.Ulrich Sonnenborn (Ardeypharm GmbH, Loerfeldstrabe 20, Herdecke, Germany) as a generous gift. All plasmid constructs and bacterial strains used in the present study are summarized in S1 and S2 Tables. Routine DNA manipulations were done in E. coli DH10B (Invitrogen, USA) using standard molecular biology protocols from Sambrook et al [23]. For supplementation to different rat groups, probiotics with different metabolic efficacy were grown overnight in Luria Broth at 37°C, re-inoculated in fresh L.B tubes to achieve final colony forming unit (CFU) of 109 per ml. One ml of fresh culture (CFU of 109/ml) was taken from the tube, centrifuged and washed twice with normal saline before oral administration to rats.
Construction of pJET with fdh-pqq under constitutive tac promoter
For the constitutive expression of fdh under tac promoter, tac promoter was obtained by polymerase chain reaction amplification using primers listed in S3 Table. tac forward primer contains a modification in tac promoter at lac repressor binding site and tac reverse primer contains a portion of tac promoter and complementary region of fdh. PCR amplification was done using XT20 polymerase (Thermo Scientific, USA) from plasmid pMALp2. Then, fdh gene was amplified using gene specific primers from the genome of Gluconobacter frauteuri IFO3260 (S3 Table). The amplicons of size 0.2 kb and 3.7 kb were gel eluted, purified mixed and again amplified using tac forward primer and fdh reverse primer yielding the final amplicon containing fdh under constitutive tac promoter (ctac*-fdh) of size 3.9kb. The ctac*-fdh amplicon was gel eluted, purified and ligated in pJET plasmid which gave pAN2. Construct was confirmed by restriction digestion pattern. The activity of the clone was confirmed by fructose dehydrogenase enzyme assay upon transforming in EcN:: vgb-gfp. For the constitutive expression of pqq operon, pqq was amplified by polymerase chain reaction amplification using primers from the genome of G. suboxydans. This amplicon ctac*-pqq was gel eluted, purified and ligated in pJET plasmid. Further, pAN2 was linearized using XhoI and end-filled. Digestion of pAN1 with BglII gave pqq operon which was gel eluted, purified and ligated in XhoI digested and gel purified pAN2 which gave pAN7 construct. The construct was confirmed by restriction digestion pattern and PCR amplification. This was followed by transformation of the final construct pAN7 in EcN:: vgb-gfp. The construct was confirmed by restriction digestion pattern and PQQ quantification in the bacterial supernatant using fluorometer (Hitachi High-Technologies Corporation, Japan).
Construction of pJET with pqq-glf-mtlK under constitutive tac promoter
The strategy for constitutive expression of mtlK gene was similar to that of fdh gene. The promoter was first amplified by polymerase chain reaction using primers listed in S3 Table. Then, mtlK gene was obtained using polymerase chain reaction by primes listed in S3 Table from pRSETmtlK. The amplicon ctac*-mtlK gene was gel eluted, purified and ligated in pJET vector which gave pAN4. The glf gene was amplified from Zymomonas mobilis using primers listed in S3 Table. The ctac*-glf gene was then gel eluted, purified and ligated in pJET vector which gave pAN3. Digestion of pAN1 by BglII gave ctac*-pqq gene cluster which was gel eluted, purified and ligated in XhoI digested, gel purified pAN3. Construct was confirmed by restriction digestion pattern. Finally, the ctac*-pqq and ctac*-glf genes were amplified together and the product containing ctac*pqq-ctac*glf was inserted in XhoI digested, gel eluted purified vector pAN4 to give pAN6. This was followed by transformation of the final construct pAN6 in EcN:: vgb-gfp. Functionality of construct was confirmed by mannitol-2-dehydrogenase enzyme assay. Briefly the cells grown in M9 minimal medium [23] were harvested aseptically at stationary phase by centrifugation at 9,200 g for 2 min at 4°C. Cell-free extract was prepared by sonication [24]. Mannitol-2-dehydrogenase activity was assayed by measuring the rate of oxidation of NAD(P)H using fructose as substrate according to Liu et al. [21]. The culture supernatant collected at the end was used for gluconic acid estimation produced by EcN (pAN7) and EcN (pAN6) by HPLC [24].
PQQ quantification
PQQ was extracted from E.coli strains and liver tissue as described in Suzuki et al. [25] and Singh et al. [17]. Briefly the cells were grown overnight in M9 minimal medium containing glucose. These cells were harvested, and the supernatant was used for PQQ extraction. Culture supernatant was incubated with 50% acetonitrile at 65°C for 2 hours followed by centrifugation at 15,000xg for 10 minutes. The clear supernatant attained was dried using concentrator in vacuum. The residues were dissolved in 50% n-butanol (1 mg/ml) and incubated at 50°C till it dries. Finally, the residues attained were dissolved in water and filtered with 0.2 micron filter. Quantification was done fluorimetrically as described by Suzuki and colleagues (1990) using Hitachi fluorescence spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan) with excitation 375 nm and emission 465 nm (uncorrected). Standard plot for area under curve was drawn using 6 different concentration of standard PQQ ranging from 0.2 to 20 μM. Liver tissue and colonic contents were homogenized in phosphate-buffered saline 20 and 10% (w/v), respectively followed by centrifugation at 10,000 xg for 20 minutes to obtain supernatant for PQQ extraction followed by quantification as described above.
Animals
Adult albino male Charles Foster rats (180–200 g) were used for animal studies. All rats were housed in plastic cages and maintained in controlled condition as per committee guidelines i.e. temperature (25 ± 1°C), relative humidity (45.5%) and photoperiod cycle (12 h light: 12 h dark)). Free access to food and water was provided as per recommended by committee for the purpose of control and supervision of experiments on animals (CPCSEA) guidelines of animal ethical committee of M. S. University of Baroda, India, Registration number 938/a/06/CPCSEA. The presented research was approved by Animal Ethical Committee of Department of Biochemistry, The M. S. University of Baroda, Gujarat, India (Approval No. 938/a/06/CPCSEA), and CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals). The rats were acclimated to laboratory conditions, monitored daily twice for welfare without disturbing and checked whether each rat is feeding and drinking. Rats were checked for any red staining around the eyes and physically examined by running fingers over their body to check whether they are normal. Rat body weight and food consumption were measured weekly.
Experimental design
For present study, rats were divided into seven different groups (n = 6) as follows: Control group received pellet diet; Fructose group received pellet diet and 20% fructose in drinking water; F + EcN-2 group received pellet diet, 20% fructose in drinking water and EcN-2; F + EcN (pqq) group received pellet diet, 20% fructose in drinking water and EcN (pqq); F + EcN (pqq-glf) group received pellet diet, 20% fructose in drinking water and EcN (pqq-glf); F + EcN (pqq-glf-mtlK) group received pellet diet, 20% fructose in drinking water and EcN (pqq-glf-mtlK); F + EcN (pqq-fdh) group received pellet diet, 20% fructose in drinking water and EcN (pqq-fdh). All groups except Control group and Fructose Control group were gavaged with probiotics (109 cfu) once per week till two months as per Singh et al. [17].
Colonic SCFA extraction and quantification
Colonic SCFA extraction and quantification was carried out as described in Singh et al. [17]. In brief, after dissection of rats colonic content was collected immediately and stored at -80°C till use. For quantification, samples were taken from -80°C and suspended in sterile deionized water containing 0.015 M H2SO4 and detected using Shimadzu HPLC system with C-18 column. (Shimadzu Analytical (India) Pvt.Ltd, Mumbai, India.
Preparation of cell lysate and tissue homogenates
Blood was collected by orbital sinus puncture under general anaesthesia with ether in EDTA coated and normal tubes followed by centrifugation at 1500 g for 10 minutes. Plasma and serum were collected separately in fresh tubes and stored in -80°C till use. Pack cell volume (PCV) was washed thrice with normal saline prior to lysis in ice cold water. Cell lysate so obtained was centrifuged at 15,000 g for 10 min and fresh supernatant was used for enzyme assays. Liver was collected and washed with PBS immediately after sacrificing rats by cervical dislocation Liver homogenates were prepared in different buffers for antioxidant enzyme activity.
Biochemical assays
Superoxide dismutase (SOD) activity was measured by a method which is based on auto-oxidation of pyrogallol monitored spectrophotometrically at 420 nm [26]. Catalase (CAT) activity were monitored by measuring rate of disappearance of hydrogen peroxide (H2O2) spectrophotometrically at 240 nm [27]. SOD and CAT activities were reported as units/mg protein. Reduced glutathione (GSH) was performed as described in Beutler et al. [28]. Lipid peroxidation was estimated by measuring levels of malondialdehyde at 412 nm as described in Buege et al. [29].
Liver enzyme test, kidney function test, and lipid estimation
Aspartate transaminase(AST), Alkaline phosphatase(ALP), and Alanine transaminase(ALT),Total bilirubin, triglyceride, HDL, VLDL and total cholesterol content in blood plasma were measured using kits as per manufacturer protocol (Beacon Diagnostics Pvt. Ltd. Navsari, India).
mRNA expression and qRT-PCR
RNA was extracted with Trizol (Invitrogen Bio Services India Pvt. Ltd., Bangalore, India) and cDNAs were generated from 1 μg total RNA (Reverse Transcription Kit; Applied Bio systems, Foster City, CA) following the manufacturer’s instructions. The primers for fatty acid synthase and acyl coenzyme A gene were ACCTCATCACTAGAAGCCACCAG (forward) and GTGGTACTTGGCCTTGGGTTTA (reverse), and CCCAAGACCCAAGAGTTCATTC (forward) and TCACGGATAGGGACAACAAAGG (reverse), respectively. PCR was performed using ABI Quant-StudioTM 12K flex Real Time PCR system coupled with SYBR Green technology (Applied Biosystems) and following cycling parameters. The linearity of the dissociation curve was analysed using the software provided with the thermo cycler (QuantStudioTM). Each sample was analysed in duplicate. The mean cycle time of the linear part of the curve was designated Ct.
Histopathological changes
Liver tissue was fixed in 10% neutral buffered formalin. Histological sections were stained with hematoxylin and eosin and evaluated by pathologist unaware with experimental codes.
Statistical analysis
All values are expressed as mean ± SEM. Differences in lipid peroxidation and antioxidant enzymes (SOD, CAT and GSH) among six different groups were evaluated using the one-way ANOVA followed by Bonferroni comparisons. Differences were considered significant at P<0.05. Calculations were performed using commercially available statistical software packages (Graph Pad PRISM Version 5.0 La Jolla, CA 92037 USA).
Results
Cloning and expression of pqq-glf-mtlK and pqq-fdh in EcN::vgb-gfp
The pAN1 plasmid possessing G. suboxydans pqq operon, the pAN2 plasmid containing G. frauteuri IFO3260 fdh gene cluster, the pAN3 plasmid harbouring Z. mobilis glf gene, the pAN4 plasmid containing L. brevis mtlK gene, the pAN5 plasmid possessing pqq operon and glf gene together, the pAN6 plasmid containing pqq operon, glf gene and mtlK together, the pAN7 plasmid harbouring pqq operon and fdh together under constitutive tac promoter were cloned in pJET vector (S1 Fig). Functional confirmation of EcN transformants harbouring pAN1, pAN5, and pAN6 and pAN7 plasmids was done by growth and acidification on Tris-buffered medium containing methyl red as pH indicator [30].
In M9 medium, EcN containing pAN1, pAN5, pAN6 and pAN7 produced 6.5 ± 0.15 μg / ml, 6.29 ± 0.22 μg / ml, 5.78 ± 0.18 μg / ml and 5.23 ± 0.32μg / ml of PQQ/ml along with 34.44± 0.09 mM, 31.44± 0.17 mM, 30.56± 0.24 mM and 31.34 ± 0.19 mM of gluconic acid in the culture medium. Acidification of Tris-buffered medium with methyl red as indicator is due to secretion of gluconic acid by PQQ dependent glucose dehydrogenase (Data not provided). In M9 medium, EcN harbouring mtlK and pqq-glf-mtlK showed 0.23± 0.22 and 0.2± 0.16 μmoles of NADPH oxidized per min per mg of protein enzyme activity.
Effect of probiotic treatment on body weight, hyperglycemia
The body weight gain of fructose fed group significantly increased compared to control group. After administration of probiotic producing fructose metabolizing enzymes, EcN (pqq-glf-mtlK) and EcN (pqq-fdh) body weight gain significantly decreased and reached the levels similar to the control group (Table 1). The fasting serum concentrations of glucose were also significantly higher in fructose fed group compared to control group. However, the increase in serum concentrations of glucose, in fructose fed rats were significantly decreased after administration of probiotic producing fructose metabolizing enzymes (Table 1).
Table 1. Effect of different probiotic treatment on body weight gain, food intake, fasting glucose, serum insulin levels of rats.
| Groups | Body weight gain (g) | Food intake (g/day) | Fasting glucose (mg/dl) | Serum insulin (μg/l) |
|---|---|---|---|---|
| Control (No F) | 43.33±8.45 | 18.15±1.19 | 98.04±3.59 | 1.10±0.21 |
| Fructose control (F) | 92.6±8.902** | 17.24±1.73 | 188.48±6.83*** | 3.14 ± 0.25** |
| F+ EcN-2 | 87±9.08** | 17.66±1.14 | 185.57±4.81*** | 3.28± 0.35** |
| F+ EcN (pqq) | 67.75±9.12** | 17.77±1.25 | 154.33±5.34*** | 2.91± 0.15** |
| F+ EcN (pqq-glf) | 66.75±9.23** | 17.69±1.58 | 149.82±10.31*** | 2.88 ± 0.21** |
| F+ EcN (pqq-glf-mtlK) | 52.4±7.31# # | 17.33±1.93 | 105.23±10.49**# # | 1.23± 0.35# # |
| F+ EcN (pqq-fdh) | 60.75±8.93# # | 17.97±1.25 | 120.29±6.50**# # | 1.57± 0.35# # |
F, Fructose.
Values are expressed as mean ± SEM (n = 6 each group).
** P ≤ 0.01 and
*** P ≤ 0.001 compared with control group.
# # P ≤ 0.01 compared with fructose control group.
Effect of probiotic treatment on hepatic injury markers
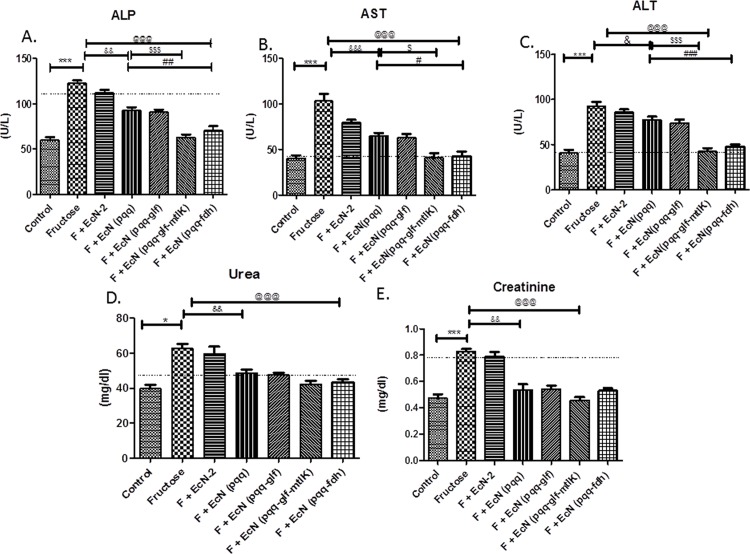
Increased serum levels of Aspartate transaminase (AST), Alkaline phosphatase (ALP), and Alanine transaminase (ALT) were found in fructose fed group (Fig 1A–1C). These results showed that fructose induced damage to liver cells. However, supplementation of probiotic producing fructose metabolizing enzymes significantly decreased ALP, AST and ALT levels in fructose fed group.
Fig 1. Effect of probiotic treatment on liver enzyme tests.
(A) ALP (B) AST (C) ALT activity and kidney function tests (D) Urea (E) Creatinine of serum. Values are expressed as mean ± SEM (n = 5–6 each group). ***P≤ 0.001 compared with fructose control, @@@P ≤ 0.001 compared to fructose control, $ $ $ P ≤ 0.001, $ $ P ≤ 0.01 compared to EcN (pqq) group. F: Fructose.
Effect of probiotic treatment on kidney function
There is a noticeable increase in urea and creatinine in kidneys of rats fed with fructose fed group compared to control group. Treatment with probiotic producing fructose metabolizing enzymes, EcN(pqq-glf-mtlK) and EcN(pqq-fdh) decreased urea and creatinine in probiotic treated rats as compared to fructose fed group (Fig 1D and 1E).
Effect of probiotic treatment on dyslipidemia
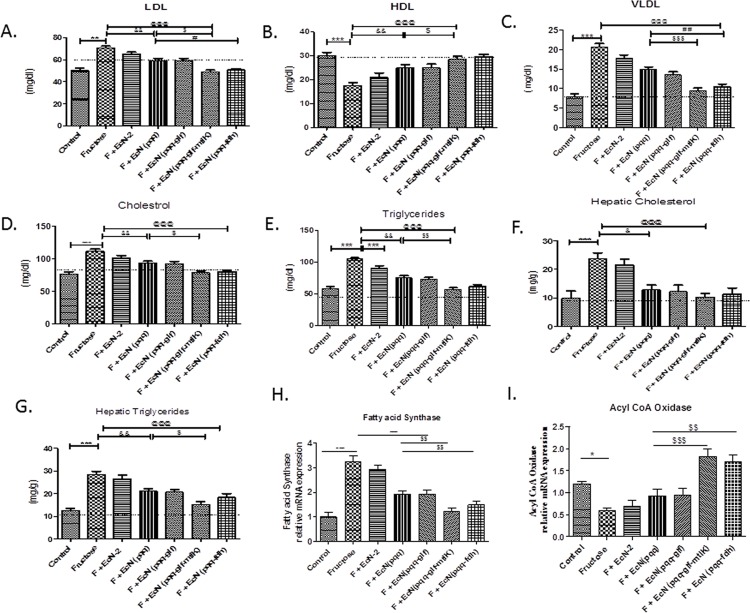
Higher serum levels of LDL, VLDL, cholesterol and TG were noticed in fructose fed group as compared to control group (Fig 2A, 2C, 2D and 2E). Administration of probiotic producing fructose metabolizing enzymes, significantly reduced the increased levels of LDL, TG, VLDL and cholesterol as compared to fructose fed group. Our results demonstrated that higher levels of hepatic TG and cholesterol were found in fructose fed group as compared to control group. Feeding of probiotic producing fructose metabolizing enzymes significantly suppressed the increased hepatic TG and cholesterol levels compared to fructose fed group which showed that probiotic can suppress the formation of hepatic steatosis (Fig 2F and 2G).
Fig 2. Effect of probiotic treatment on serum lipid profile.
(A) LDL, (B) HDL, (C) VLDL, (D) Cholesterol and (E) Triglycerides; on hepatic lipid profile (F) Cholesterol and (G) Triglycerides; (H) mRNA of Fatty acid synthase and (I) mRNA of Acyl Coenzyme A oxidase. Values are expressed as mean ± SEM (n = 5–6 each group). ***P≤ 0.001 compared with fructose control, @@@P ≤ 0.001 compared to fructose control, $ $ $ P ≤ 0.001, $ $ P ≤ 0.01 compared to EcN (pqq) group, ### ≤ 0.001. Values are expressed in mg/dl. F: Fructose.
Effect of probiotic treatment on Hepatic Fatty Acid Synthase (FAS) and Acyl Coenzyme A Oxidase (ACOx) mRNA Expression
Finally, mRNA expression of FAS and ACOx showed increased and decreased expression, respectively, in fructose fed group (Fig 2H and 2I). This expression was reversed in groups supplemented with probiotic producing fructose metabolizing enzymes.
Effect of probiotic treatment on oxidative stress
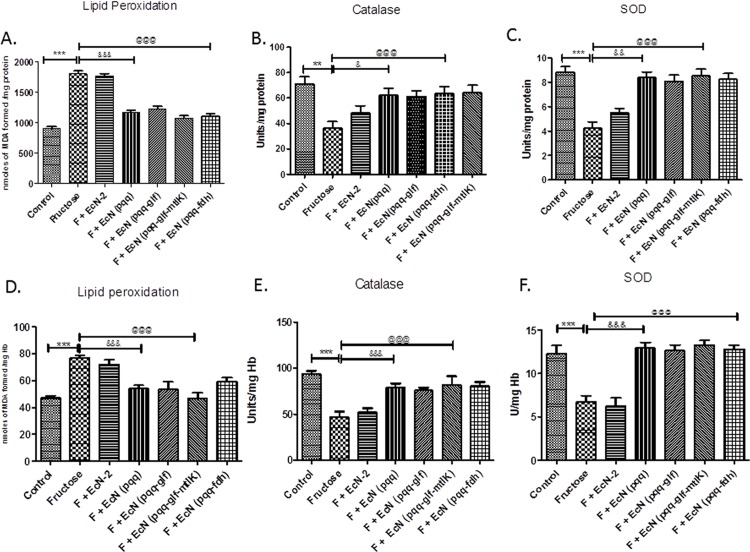
Fructose control group rats showed markedly high levels of lipid peroxidation as compared to control rats (Fig 3A) After administration of probiotic producing fructose metabolizing enzymes, the levels of MDA were significantly lowered to near normal levels as compared to untreated fructose fed rats group. Catalase and SOD activities in rat livers were decreased in fructose fed group, as compared to control group (Fig 3B and 3C). Probiotic treatment significantly up regulated these two antioxidant enzyme activities, which were decreased in fructose fed group, in rat liver. Glutathione levels decreased in fructose fed rats compared to control group (Table 2). Treatment with probiotic producing fructose metabolizing enzymes increased glutathione levels as compared to fructose fed rats.
Fig 3.
Effect of probiotic treatment on antioxidant status of liver (A), (B) and (C) and blood (D), (E) and (F) in rats. (A and D) Lipid peroxidation (LPO), (B and E) Catalase and (C and F) SOD. Values are expressed as mean ± SEM (n = 5–6 each group). ***P≤ 0.001 compared with fructose control, @@@P ≤ 0.001 compared to fructose control, $ $ $ P ≤ 0.001, $ $ P ≤ 0.01 compared to EcN (pqq) group. F: Fructose.
Table 2. Effect of probiotic treatment on Colonic SCFA, PQQ concentration in liver tissue and fecal samples and liver GSH levels in rats.
| Groups | SCFA levels | PQQ levels | Liver GSH(μmoles/g tissue) | |||
|---|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | Feacal (n moles /g faecal wet weight) | Liver (picomoles / g tissue) | ||
| Control (No F) | 98.43±3.28 | 18.52±4.24 | 9.52±1.41 | 0.698±0.10 | 30.93±1.13 | 22.41±1.15 |
| Fructose control (F) | 80.32±6.94* | 20.54±3.67 | 11.10±1.41 | 0.656±0.09 | 34.23±2.34 | 11.66±1.24** |
| F+ EcN-2 | 76.45 ±4.89 | 25.53 ± 5.11 | 10.99±2.91 | 0.701±0.11 | 39.23±1.54 | 14.9 ±3.1** |
| F+ EcN (pqq) | 123.12±7.61## | 28.16±3.65# | 18.54±2.56# | 4.181±0.13** | 58.24±4.08** | 17.54 ±2.06** |
| F+ EcN (pqq-glf) | 120.32 ±4.5## | 29.95618# | 19.24±4.34# | 3.633±0.04** | 57.89±.94** | 16.94±1.75** |
| F+ EcN (pqq-glf-mtlK) | 125.27±3.45## | 30.57512# | 20.56±3.4# | 3.51± 0.09** | 60.32±1.97** | 19.23±1.86## |
| F+ EcN (pqq-fdh) | 126.12±6.61## | 28.96±3.65# | 19.84±3.9# | 3.69± 0.09** | 52.43±0.97** | 18.93±1.66## |
F, Fructose; SCFA, Short chain fatty acid; GSH, Reduced glutathione; PQQ, pyrroloquinoline quinone.
Values are expressed as mean ± SEM (n = 6 each group).
*P ≤ 0.05 and
** P ≤ 0.01 compared with control group.
# P ≤ 0.05 and
##P ≤ 0.001 compared with Fructose control group.
Effect of probiotic treatment on colonic SCFAs concentration
Additionally, levels of colonic SCFA i.e. acetate, propionate and butyrate were found to be increased in EcN (pqq), EcN (pqq-glf), EcN (pqq-glf-mtlK) and EcN (pqq-fdh) fed rats in comparison with control rats and fructose fed rats (Table 2).
Effect of probiotic treatment on PQQ concentration in faecal matter and liver
PQQ concentration estimated in faecal matter and in liver of EcN (pqq), EcN (pqq-glf), EcN (pqq-glf-mtlK) and EcN (pqq-fdh) co-treated rats, on the day of sacrifice, was found to be increased than control and fructose-treated rats (Table 2). Rats treated with EcN-2 and fructose fed rats had similar PQQ concentration.
Histopathological analysis
High degree of lipid droplet accumulation was found in Group 2 rats while Group 6 and 7 rats, supplemented with probiotic producing fructose metabolizing enzymes, showed significant reduction in lipid droplet accumulation which was correlated with decreased serum and hepatic triglyceride levels (S2 Fig).
Discussion
Fructose which is highly lipogenic has now become a major constituent of our modern diet even though it was absent in our diet few hundred years ago [31]. In several studies it has been observed that acute fructose ingestion contributes to the synthesis of hepatic triose-phosphate leading to fatty acid synthesis [32].
S3 and S4 Figs. show the proposed mechanism of conversion of fructose by probiotic producing fructose metabolizing enzymes, EcN (pqq-glf-mtlK) and EcN (pqq-fdh). Fructose is taken up by probiotic EcN (pqq-glf-mtlK) through GLF in unphosphorylated form whereby the cytosolic MTLK then converts it to mannitol which is exported outside the EcN (pqq-glf-mtlK). The membrane bound fructose dehydrogenase converts the fructose to 5-KF which is then exported outside the EcN (pqq-fdh).
Fructose is well known for inducing metabolic syndrome. 20% fructose increased fasting glucose similar to the report of Mamikutty et al. [33]. The efficiency of probiotics in ameliorating metabolic disorders has been revealed in a high-fructose-fed rat model [14]. Probiotic producing fructose metabolizing enzymes treatment maintained body weight and fasting glucose level in comparison with fructose control group. In our present data, the serum levels of two critical markers of liver injury, ALT and AST, were increased in fructose control rats. The activities of hepatic antioxidant enzymes, SOD and catalase were decreased in fructose control rats. These results demonstrated that high fructose led to production of enhanced oxidative stress in rats, which then resulted in liver damage in fructose fed rats seen in our study. After treatment with probiotic, up regulation of hepatic activities of antioxidant enzymes and down regulation of serum AST and ALT levels in high fructose rats were noticed. These findings implied that consumption of probiotic significantly reduced both liver damage and oxidative stress in fructose fed rats by enhancing hepatic antioxidants expressions and uptake and conversion of fructose in the intestine by probiotic.
High fructose increased plasma TGs, most probably by up regulation of hepatic de novo lipogenesis and secretion of TGs [34]. Administration of probiotic producing fructose metabolizing enzymes significantly reduced the levels of important components of metabolic disorder, including LDL, TG, and cholesterol, enhanced by fructose. In addition to serum levels, the increased hepatic lipid accumulation by fructose was also found to be suppressed by oral administration of probiotic producing fructose metabolizing enzymes. The levels of SCFA increased in probiotic treated group suggest that fructose is converted to mannitol which is further converted to SCFA in intestine by colonic flora. This is supported by the fact that mannitol treatment increased levels of butyrate in large intestine of pigs [12]. Presence of PQQ enables glucose dehydrogenase to produce gluconic acid which in turn is utilized by Bifidobacteria and Lactobacilli species leading to the formation of SCFA [35].
EcN is known to have many probiotic properties, acts as a safe carrier for localized delivery of biomolecules in intestine for human use [36]. S5 Fig shows the proposed mechanism of action of probiotic producing PQQ and fructose metabolizing enzyme, EcN (pqq-glf-mtlK). EcN possessing MTLK converts fructose to mannitol while secreted PQQ acts as an antioxidant molecule as well as a co-factor for glucose dehydrogenase enzyme which catalyzes the production of gluconic acid. Thus, EcN (pqq-glf-mtlK) facilitates the formation of two prebiotic molecules, mannitol and gluconic acid. Mannitol and gluconic acid are metabolized by lactic acid bacteria in lower part of gastrointestinal tract resulting in production of SCFA [12, 35]. Vgb produced by EcN could improve the survival in intestine as seen in probiotic E. coli CFR16 strain [14]. Overall metabolic effects observed in the present study demonstrates the synergistic effects of EcN, PQQ, Vgb and SCFA formed due to in situ generation of prebiotic gluconic acid and mannitol. Thus, these EcN probiotics act as synbiotics in the intestine.
Recent studies have recommended that the incorporation of a synbiotic with antioxidants can help in alleviating certain disease states via synergistically improved intestinal microflora [37, 38, 39]. Use of genetically modified probiotics is more beneficial than wild-type probiotics by not only acting as a suitable vehicle for the delivery of small molecules but also colonize more efficiently in the gut as most probiotic bacteria do not colonize properly in unhealthy individuals [40]. This is supported by the fact that EcN secreting PQQ was very efficient in preventing adverse effects of chronic ethanol consumption [18]. Thus, this approach may be very useful in developing novel synbiotics for treatment of chronic medical conditions including obesity.
Our study supports the concept of sustained delivery of molecules for treatment of fructose induced hepatic steatosis. This will decrease the need for daily administration of molecules whereby it can be effective strategy in the treatment of metabolic syndrome.
Supporting Information
Agarose gel analysis of PCR amplicons of the recombinant plasmids (A) pAN1, (B) pAN5, (C) pAN6 and (D) pAN7.
(TIF)
Blue arrows indicate the accumulation of lipid droplets in Hepatocytes of fructose fed rat (Fructose control). Images were taken by LEICA DME microscope at 40 X magnification (A) Control, (B) Fructose control, (C) EcN-2, (D) EcN (pqq-glf-mtlK) and (E) EcN (pqq-fdh).
(TIF)
PTS: Phosphotransferase system, MtlK: Mannitol dehydrogenase.
(TIF)
5-KF: 5-Ketofructose, FDH: Fructose dehydrogenase.
(TIF)
(TIF)
(DOCX)
(DOCX)
aPrimers used for construction of ptac*-pqq-fdh.bPrimers used for construction of ptac*-pqq—glf-mtlK.
(DOCX)
Acknowledgments
We thank Dr. Rer. Nat. Ulrich Sonnenborn, Ardeypharm GmbH, Loerfeldstrabe 20, Herdecke (Germany) for providing Escherichia coli Nissle 1917, and the DBT-ILSPARE Program for carrying out real-time PCR and HPLC experiments. Chaudhari Archana Somabhai and Ruma Raghuvanshi are supported by Senior Research Fellowship of DBT and ICMR, New Delhi,India respectively.
Abbreviations
- EcN
Escherichia coli Nissle 1917
- TG
Triglycerides
- Fdh
Fructose dehydrogenase
- MTLK
mannitol-2-dehydrogenase
- PQQ
pyrroloquinoline quinone
- SCFA
short chain fatty acids
- GLF
glucose facilitator protein
- CFU
colony forming unit
- SOD
Superoxide dismutase
- PCV
Pack cell volume
- CAT
Catalase
- GSH
glutathione
- AST
Aspartate transaminase
- ALP
Alkaline phosphatase
- ALT
Alanine transaminase
- vgb
Vitreoscilla haemoglobin
- H2O2
Hydrogen peroxide
- MDA
Malondialdehyde
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Chaudhari Archana Somabhai and Ruma Raghuvanshi are supported by Senior Research Fellowship of DBT and ICMR, New Delhi, India respectively.
References
- 1.Busserolles J, Gueux E, Rock E, Mazur A, Rayssiguier Y . High fructose feeding of magnesium deficient rats is associated with increased plasma triglyceride concentration and increased oxidative stress. Magnesium Research. 2003. March 1; 16(1):7–12. [PubMed] [Google Scholar]
- 2.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocrine Reviews. 2008. December; 29(7):777–822. 10.1210/er.2008-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park DY, Ahn YT, Huh CS, McGregor RA, Choi MS. Dual probiotic strains suppress high fructose-induced metabolic syndrome. World Journal of Gastroenterology: WJG. 2013. January 14; 19(2):274 10.3748/wjg.v19.i2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nature Reviews Endocrinology. 2011. November 1; 7(11):639–46. 10.1038/nrendo.2011.126 [DOI] [PubMed] [Google Scholar]
- 5.Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond). 2013. April 17; 10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochemical Society Transactions. 2002. November; 30(Pt 6):1064–70. [DOI] [PubMed] [Google Scholar]
- 7.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metabolism. 2009. May 6; 9(5):407–16. 10.1016/j.cmet.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 8.Shen B, Jensen RG, Bohnert HJ. Mannitol protects against oxidation by hydroxyl radicals. Plant Physiology. 1997. October 1; 115(2):527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maekawa M, Ushida K, Hoshi S, Kashima N, Ajisaka K,. Butyrate and propionate production from D-mannitol in the large intestine of pig and rat. Microbial Ecology in Health and Disease. 2005. January 1; 17(3):169–76. [Google Scholar]
- 10.Anandharaj M, Sivasankari B, Parveen Rani R. Effects of probiotics, prebiotics, and synbiotics on hypercholesterolemia: a review. Chinese Journal of Biology. 2014. February 27; 2014. [Google Scholar]
- 11.Rucker R, Chowanadisai W, Nakano M. Potential physiological importance of pyrroloquinoline quinone. Alternative Medicine Review. 2009. September 1; 14(3):268 [PubMed] [Google Scholar]
- 12.Misra HS, Rajpurohit YS, Khairnar NP. Pyrroloquinoline quinone and its versatile roles in biological processes. Journal of Biosciences. 2012. June 1; 37(2):313–25. [DOI] [PubMed] [Google Scholar]
- 13.Kumazawa T, Hiwasa T, Takiguchi M, Suzuki O, Sato K. Activation of Ras signaling pathways by pyrroloquinoline quinone in NIH3T3 mouse fibroblasts. International Journal of Molecular Medicine. 2007. May 1; 19(5):765–70. [PubMed] [Google Scholar]
- 14.Kumar P, Ranawade AV, Kumar NG. Potential probiotic Escherichia coli 16 harboring the Vitreoscilla hemoglobin gene improves gastrointestinal tract colonization and ameliorates carbon tetrachloride induced hepatotoxicity in rats. BioMed research international. 2014. June 19; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey S, Singh A, Kumar P, Chaudhari A, Nareshkumar G. Probiotic Escherichia coli CFR 16 producing pyrroloquinoline quinone (PQQ) ameliorates 1, 2-dimethylhydrazine-induced oxidative damage in colon and liver of rats. Applied Biochemistry and Biotechnology. 2014. June 1; 173(3):775–86. 10.1007/s12010-014-0897-z [DOI] [PubMed] [Google Scholar]
- 16.Pandey S, Singh A, Chaudhari N, Nampoothiri LP, Kumar GN. Protection against 1,2-Di-methylhydrazine-induced systemic oxidative stress and altered brain neurotransmitter status by probiotic Escherichia coli CFR 16 secreting pyrroloquinoline quinone. Current Microbiology. 2015. May 1; 70(5):690–7. 10.1007/s00284-014-0763-9 [DOI] [PubMed] [Google Scholar]
- 17.Singh AK, Pandey SK, Saha G, Gattupalli NK. Pyrroloquinoline quinone (PQQ) producing Escherichia coli Nissle 1917 (EcN) alleviates age associated oxidative stress and hyperlipidemia, and improves mitochondrial function in ageing rats. Experimental Gerontology. 2015. June 30; 66:1–9. 10.1016/j.exger.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Singh AK, Pandey SK, Naresh Kumar G. Pyrroloquinoline quinone‐secreting probiotic Escherichia coli Nissle 1917 ameliorates ethanol‐induced oxidative damage and hyperlipidemia in rats. Alcoholism: Clinical and Experimental Research. 2014. July 1; 38(7):2127–37. [DOI] [PubMed] [Google Scholar]
- 19.Kornberg HL, Lambourne LT, Sproul AA. Facilitated diffusion of fructose via the phosphoenolpyruvate/glucose phosphotransferase system of Escherichia coli. Proceedings of the National Academy of Sciences. 2000. February 15; 97(4):1808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saier MH, Crasnier M. Inducer exclusion and the regulation of sugar transport. Research in Microbiology. 1996. September 30; 147(6):482–9. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Saha B, Cotta M. Cloning, expression, purification, and analysis of mannitol dehydrogenase gene mtlK from Lactobacillus brevis. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals 2005 Jan 1 (pp. 391–401). Humana Press. [PubMed]
- 22.Kaur J. A comprehensive review on metabolic syndrome. Cardiology Research and Practice. 2014. March 11; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Sambrook J, Maccallum P, Russel D. Molecular cloning: A laboratory manual, Cold Springs Harbor Press, NY: ISBN 0-87969-577-3: 2344; 2001. [Google Scholar]
- 24.Buch AD, Archana G, Kumar GN. Enhanced citric acid biosynthesis in Pseudomonas fluorescens ATCC 13525 by overexpression of the Escherichia coli citrate synthase gene. Microbiology. 2009. August 1; 155(8):2620–9. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki O, Kumazawa T, Seno H, Urakami T, Matsumoto T. Extractions of pyrroloquinoline quinone from crude biological samples. Life Sciences. 1990. December 31; 47(23):2135–41. [DOI] [PubMed] [Google Scholar]
- 26.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974. September 16; 47(3):469–74. [DOI] [PubMed] [Google Scholar]
- 27.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952. March 1; 195(1):133–40. [PubMed] [Google Scholar]
- 28.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. The Journal of Laboratory and Clinical Medicine. 1963. May; 61:882–8. [PubMed] [Google Scholar]
- 29.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in Enzymology. 1978. December 31; 52:302–10. [DOI] [PubMed] [Google Scholar]
- 30.Gyaneshwar P., Parekh L.J., Archana G., Poole P.S., Collins M.D., Hutson R.A., Kumar G.N., 1999. Involvement of a phosphate starvation inducible glucose dehydrogenase in soil phosphate solubilization by Enterobacter asburiae. FEMS Microbiol. Lett. 171, 480 223–229. [Google Scholar]
- 31.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiological Reviews. 2010. January 1; 90(1):23–46. 10.1152/physrev.00019.2009 [DOI] [PubMed] [Google Scholar]
- 32.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. The Journal of Nutrition. 2008. June 1; 138(6):1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamikutty N, Thent ZC, Sapri SR, Sahruddin NN, Mohd Yusof MR, Haji Suhaimi F. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats. BioMed Research International. 2014. June 18; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, Roncal C. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocrine Reviews. 2009. February; 30(1):96–116. 10.1210/er.2008-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kameue C, Tsukahara T, Yamada K, Koyama H, Iwasaki Y, Nakayama K, Ushida K. Dietary sodium gluconate protects rats from large bowel cancer by stimulating butyrate production. The Journal of Nutrition. 2004. April 1; 134(4):940–4. [DOI] [PubMed] [Google Scholar]
- 36.Westendorf AM, Gunzer F, Deppenmeier S, Tapadar D, Hunger JK, Schmidt MA, Buer J, Bruder D. Intestinal immunity of Escherichia coli NISSLE 1917: a safe carrier for therapeutic molecules. FEMS Immunology & Medical Microbiology. 2005. March 1; 43(3):373–84. [DOI] [PubMed] [Google Scholar]
- 37.Frece J, Kos B, Svetec IK, Zgaga Z, Beganović J, Leboš A, Šušković J. Synbiotic effect of Lactobacillus helveticus M92 and prebiotics on the intestinal microflora and immune system of mice. Journal of Dairy Research. 2009. February 1; 76(01):98–104. [DOI] [PubMed] [Google Scholar]
- 38.Greenway F, Wang S, Heiman M. A novel cobiotic containing a prebiotic and an antioxidant augments the glucose control and gastrointestinal tolerability of metformin: a case report. Beneficial Microbes. 2013. May 17; 5(1):29–32. [DOI] [PubMed] [Google Scholar]
- 39.Singh DP, Khare P, Zhu J, Kondepudi KK, Singh J, Baboota RK, Boparai RK, Khardori R, Chopra K, Bishnoi M. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. International Journal of Obesity. 2015. September 23. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, Morris LC, Matafonova E, Stien X, Kang L, Coulon D. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. The Journal of Clinical Investigation. 2014. August 1; 124(8):3391 10.1172/JCI72517 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Agarose gel analysis of PCR amplicons of the recombinant plasmids (A) pAN1, (B) pAN5, (C) pAN6 and (D) pAN7.
(TIF)
Blue arrows indicate the accumulation of lipid droplets in Hepatocytes of fructose fed rat (Fructose control). Images were taken by LEICA DME microscope at 40 X magnification (A) Control, (B) Fructose control, (C) EcN-2, (D) EcN (pqq-glf-mtlK) and (E) EcN (pqq-fdh).
(TIF)
PTS: Phosphotransferase system, MtlK: Mannitol dehydrogenase.
(TIF)
5-KF: 5-Ketofructose, FDH: Fructose dehydrogenase.
(TIF)
(TIF)
(DOCX)
(DOCX)
aPrimers used for construction of ptac*-pqq-fdh.bPrimers used for construction of ptac*-pqq—glf-mtlK.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.