Abstract
Identifying the metabolic differences in the livers of modern broilers and local chicken breeds is important for understanding their biological characteristics, and many proteomic changes in their livers are not well characterized. We therefore analyzed the hepatic protein profiles of a commercial breed, Arbor Acres (AA) broilers, and a local dual purpose breed, Big Bone chickens, using two-dimensional electrophoresis combined with liquid chromatography-chip/electrospray ionization-quadruple time-of-flight/mass spectrometry (LC-MS/MS). A total of 145 proteins were identified as having differential abundance in the two breeds at three growth stages. Among them, 49, 63 and 54 belonged to 2, 4, and 6 weeks of age, respectively. The higher abundance proteins in AA broilers were related to the energy production pathways suggesting enhanced energy metabolism and lipid biosynthesis. In contrast, the higher abundance proteins in Big Bone chickens showed enhanced lipid degradation, resulting in a reduction in the abdominal fat percentage. Along with the decrease in fat deposition, flavor substance synthesis in the meat of the Big Bone chickens may be improved by enhanced abundance of proteins involved in glycine metabolism. In addition, the identified proteins in nucleotide metabolism, antioxidants, cell structure, protein folding and transporters may be critically important for immune defense, gene transcription and other biological processes in the two breeds. These results indicate that selection pressure may have shaped the two lines differently resulting in different hepatic metabolic capacities and extensive metabolic differences in the liver. The results from this study may help provide the theoretical basis for chicken breeding.
Introduction
Over the past few decades, China’s broiler industry has transformed from traditional farming to the intensive farming model. At the same time, the rapid development of the broiler industry is associated with poor meat quality and negative feedback. Commercial broilers are characterized by fast growth and a high feed-to-weight conversion rate; however, excessive abdominal fat deposition and poor meat quality are becoming the major concerns in the poultry industry [1]. Fortunately, indigenous chicken breeds produce better quality meat and the cross between indigenous chickens and broilers may provide a way to overcome these problems [2].
Broiler chickens demonstrate differences in fat deposition, indicating the importance of genetic factors in fat deposition [3]. Unlike mammals, de novo fatty acid synthesis (lipogenesis) in birds occurs mainly in the liver [4]. Previous studies have revealed that fat and lean lines of broilers differed in the metabolism of very low-density lipoprotein [5, 6] and fatty acid in the liver [7, 8]. Moreover, the liver is a vital organ in chickens which carries out multiple metabolic roles. Profiling of liver proteins abundance can aid in the understanding of variations in poultry liver metabolism.
The Arbor Acres (AA) broiler is a well-known commercial breed in the poultry industry. It is featured by a large size, rapid-growth rate, high feed-conversion rate and strong adaptability, but possesses less favorable meat quality and excessive abdominal fat [9]. Gene expression analysis demonstrated that the AA breed had dysregulated lipid metabolism and other cellular pathways compared to a Chinese breed with high meat quality [10]. In contrast, Big Bone Chickens, which originated in the Liaoning province of China, is a meat-and-egg dual-purpose local breed and famous for its big size and large eggs as well as delicious and nutritious meat [11]. Thus, these two breeds can offer suitable models to study the difference in the relationship between liver metabolism and meat quality. This study aims to identify the molecular mechanism of metabolic differences between modern broilers and local chicken breeds, in an attempt to provide the theoretical basis for improving meat quality in commercial chickens.
Materials and Methods
AA broilers and Zhuanghe Big Bone chickens were raised, sampled and slaughtered in the Nankou experimental farm of the Feed Research Institute, Chinese Academy of Agricultural Sciences (CAAS), Beijing, China. The care and use of all birds in this experiment was approved by the Animal Care and Use Committee of the Feed Research Institute of CAAS. The proteomics analysis was conducted in the Feed Research Institute, CAAS.
Reagents
Tris-base, ammonium persulfate, sodium dodecyl sulfate (SDS), N,N,N’,N’-tetramethylethylenediamine (EDTA), sodium bicarbonate (NH4HCO3), glycine, agarose, urea trichloroacetic acid (TCA), and formic acid were purchased from Sigma (St. Louis, USA). Bio-lyte was purchased from Bio-Rad (Hercules, CA, USA). Acrylamide, N, N’-methylenebisacrylamide, bromophenol blue, coomassie brilliant blue G-250, thiourea, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), glycerol, and bovine serum albumin were purchased from Amresco (Solon, OH). Dithiothreitol (DTT) and iodoacetamide were purchased from Merck (Darmstadt, Germany). Trypsin was purchased from Roche (Basel, Switzerland), and trifluoroacetic acid and acetonitrile were from J. T. Baker (Phillipsburg, NJ). Lastly, dipotassium phosphate (K2HPO4), monopotassium phosphate (KH2PO4), sodium chloride (NaCl), sodium hydroxide (NaOH), and acetone were purchased from Beijing Shiji Co. (Beijing, China).
Care and management of chickens
Seventy-two each of AA broilers and Zhuanghe Big Bone chickens (1-day-old) were purchased from the Xingwang Chicken Company. (Liaoning, China). Each group had 6 replicates and each replicate had 12 individuals. The chickens were reared from 0 to 42 days and fed with corn-soybean starter (days 0–21) and grower (days 22–42) diets. All chickens were subject to 23 h light and 1 h dark on days 0–7, and 20 h light and 4 h dark thereafter in accordance with the AA broiler and Zhuanghe Big Bone Chicken Management Guides. The room temperature was maintained at 33–35°C on days 0–3, at 32–34°C on days 4–7 and gradually reduced to the maintenance temperature of 20°C by day 42. The relative humidity was kept at 70% during the first week and thereafter at about 60%.
Liver protein extraction
At the age of 2 weeks, 4 weeks, and 6 weeks, twelve chickens from each group (two birds each replicate) were randomly selected, electrically stunned, and manually slaughtered within 5 min [12] Livers were collected and washed in cold saline solution (0.9% NaCl) to clear blood and other contaminants, and were frozen immediately in liquid nitrogen and stored at -80°C for further processing.
Protein preparation was performed as described previously with some modifications [13]. To avoid erroneous conclusions due to individual variations, the same quantity of proteins from the liver of four chickens were pooled as a biological replicate, and three biological replicates were acquired for each group. Briefly, the frozen liver tissues were manually ground into fine powders using a mortar and pestle in liquid nitrogen, and were then homogenized in PBS (pH 7.6) containing 32.5 mM K2HPO4, 2.6 mM KH2PO4, and 400 mM NaCl. The mixture was sonicated for 2 min and centrifuged at 14,000 g for 10 min at 4°C. The supernatant was stored for later use. The pellets were washed in PBS (pH 7.6) and were homogenized in lysis buffer (LB, 9 M urea, 2 M thiourea, 4% CHAPS, 20 mM Tris-base, 30 mM DTT, and 2% Bio-lyte, pH 3–10). The homogenate was then sonicated for 2 min and centrifuged at 14,000 g, 4°C for 10 min. The supernatant was transferred to a tube containing PBS protein extract. TCA was added to a final concentration of 10% and then the mixture was kept on ice for 10 min for protein precipitation and desalting. Subsequently, the mixture was centrifuged twice at 14,000 g for 10 min at 4°C and the pellets were suspended in loading buffer, sonicated for 1 min and adjusted to pH 7.0. About four-times volume of acetone was added into the protein mixture, the mixture stored at -20°C for 2 hours for protein precipitation and desalting. Next, the mixture was centrifuged and the protein pellets were resolved in LB. The protein extract was stored at -70°C for further use. Protein concentration was determined according to the Bradford method [14] against a bovine serum albumin standard curve, at 595 nm in a spectrophotometer DU800 (Beckman Coulter, Los Angeles, CA).
Two dimensional gel electrophoresis
Three gels were independently carried out with each biological replicate sample. Each 500 μg protein sample suspended in lysis buffer was mixed with rehydration buffer (8 M urea, 2% CHAPS, 0.001% bromophenol blue, 45 mM DTT, 0.2% Bio-lyte pH 3–10). The mixture was loaded onto a 17 cm IPG strip (pH 3–10, linear, Bio-Rad). Isoelectric focusing (IEF) was performed at 18°C according to manufacturer’s instructions (Protean IEF Cell, Bio-Rad). Before the second dimension of electrophoresis, the IPG strips were first equilibrated in equilibration buffer I [6 M urea, 0.375 M Tris-HCl (pH 8.8), 20% glycerol, 2% SDS, 2% DTT] for 15 minutes and then in equilibration buffer II [6 M urea, 0.375M Tris-HCl (pH 8.8), 20% glycerol, 2% SDS, 2.5% iodoacetoamide] for another 15 minutes. After equilibration, the strip was transferred to a SDS polyacrylamide gel, 12% T separating gel (1.00 mm). The second dimension of electrophoresis was performed in a Protean II Xi Cell (Bio-Rad) at 25 mA/gel for about 5 h.
Image acquisition and statistical analysis
Gels were fixed in 50% (v/v) ethanol and 10% (v/v) acetic acid solution overnight and then stained with coomassie brilliant blue G-250. Three technical replicate gels were scanned to acquire images and evaluate reproducibility. Images were imported into PD Quest V 8.0 (Bio-Rad) for further analysis. The abundance of each spot was expressed as %Vol (spot volume / total volumes of all spots resolved in the gel). The average values from three independent experiments were calculated and considered to be statistically significant with p<0.05 and at least a 2-fold change.
Mass spectrometry analysis
The protein spots were excised and digested with 10 ng/μl trypsin (Roche) for MS analysis as previously reported [15]. The peptides were analyzed by liquid chromatography-chip/electrospray ionization-quadruple time-of-flight/mass spectrometry (QTOF G6520, Agilent Technologies). Tandem mass spectra were retrieved using MassHunter software (Version B. 02. 01, Agilent Technologies). Before MS/MS data searching, a peak-list was generated by Mascot Distiller software (Version 3. 2. 1. 0, Matrix Science). MS/MS data were searched with Mascot 2.2 (Matrix Science) against NCBInr (release date on March, 2015). Carbamidomethylation (C) and oxidation (M) parameters were selected as fixed and variable modifications, respectively. The other parameters were: taxonomy, all entries; enzyme, trypsin; missed cleavages, 1; peptide tolerance, ± 20 ppm; MS/MS tolerance, ±0.02 Da. A total of 6,649,798 sequences and 2,279,950,795 residues in the database were searched. When the identified peptides matched to multiple members of a protein family, the match was determined based on a higher Mascot score, and differential patterns of protein spots on 2-DE gels. Protein identifications were accepted if they had a probability score greater than 95% and contained at least two identified peptides with maximum peptide coverage (S1 Table).
Bioinformatics analysis of differentially expressed proteins in the liver between AA broiler and Big Bone chickens
The ClueGo plug-in of Cytoscape software (http://cytoscape.org/) with the Gene Ontology (GO) database (released June 2015) and the Kyoto encyclopedia of genes and genomes (KEGG) database (released October 2015) were used for functional and pathway enrichment analysis of the identified differential abundance proteins. The significantly enriched GO terms were performed by the right-side hypergeometric statistical test, which compares the background set of GO annotations in the whole genome of Gallus gallus database. Its probability value was corrected by the Bonferroni method [16]. The protein-protein biological interaction network (BIN) of differential proteins was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING) 10.0 (http://string-db.org/) [17]. The network nodes represent proteins, and the edges represent the predicted functional associations.
Validation of proteins with differential abundance by qPCR (Real-time Quantitative PCR Detecting System)
To further understand the relationship between proteins and their encoding genes, qPCR was run for differential abundance proteins at the mRNA level. Specific primers for target genes of the identified proteins were designed using the primer BLAST of NCBI and nucleotide information in GenBank (S2 Table). Total RNA was prepared from the liver of control and treated groups using SGTriEx (SinoGene Scientific Co., Beijing, China). RNA quality and concentration were detected using Biophotometer (Eppendorf, Hamburg, Germany) and agarose gel electrophoresis (S1 Fig). cDNA synthesis with 5 μg of RNA was performed using Thermo First cDNA Synthesis Kit (SinoGene). qPCR was conducted using the StepOnePLUS(Applied Biosystems, Massachusetts, USA). The PCR was performed in a 20-μL reaction system containing 1 μL of cDNA, 0.4 μL of each primer (10 μM), 10 μL of SG Green qPCR Mix (SinoGene) and 8.2 μL of water. The fold-change was calculated with the 2 −ΔΔCt method [18]. All operation for qPCR was followed by the MIQE guide-lines [19].
Results
Abdominal fat and liver weight comparison between AA broiler and Big Bone chickens
Average abdominal fat percentage was significantly higher in AA broilers than that in Big Bone chickens on day 28 (p < 0.05, Table 1). Abdominal fat percentage was not significantly different between female and male Big Bone chickens, but female AA broilers had higher abdominal fat than male AA broilers of the same age (day 28). Female AA broilers had significantly higher abdominal fat than male AA broilers and Big Bone chickens of the same body weight (3.0±0.13kg). The same weight Big Bone chickens and male AA broilers showed no significant difference in abdominal fat percentage.
Table 1. Carcass index contrast between AA broiler and Big Bone chickens.
| Big Bone chickens | AA broiler chickens | |||
|---|---|---|---|---|
| male | female | male | female | |
| Abdominal fat percentage, % | ||||
| Same age (day 28) | 5.53±1.03a | 5.20±0.91a | 18.25±2.93b | 24.82±2.84c |
| Same body weight (3.0±0.13kg) | 6.97±2.57a | 7.12±1.21a | 8.08±2.82a | 14.03±1.81b |
| Liver percentage, % | ||||
| Same age (day 28) | 37.49±3.53a | 34.45±1.22a | 26.17±0.74b | 26.23±1.36b |
| Same body weight (3.0±0.13kg) | 23.77±2.19a | 24.37±1.12a | 28.59±1.56a | 27.66±1.76a |
Notes: There were significant difference between different letters of groups in the same row (P< = 0.05), but no significant difference with the same letter (p>0.05).
There was no significant difference in the liver weight between female and male chickens of these two breeds on the same day or the same body weight. Big Bone chickens and AA broilers of the same weight showed no significant difference in liver weight percentage. However, the liver weight was significantly higher in Big Bone chickens than that in AA broilers on the same day (day 28).
Identification of proteins with differential abundance
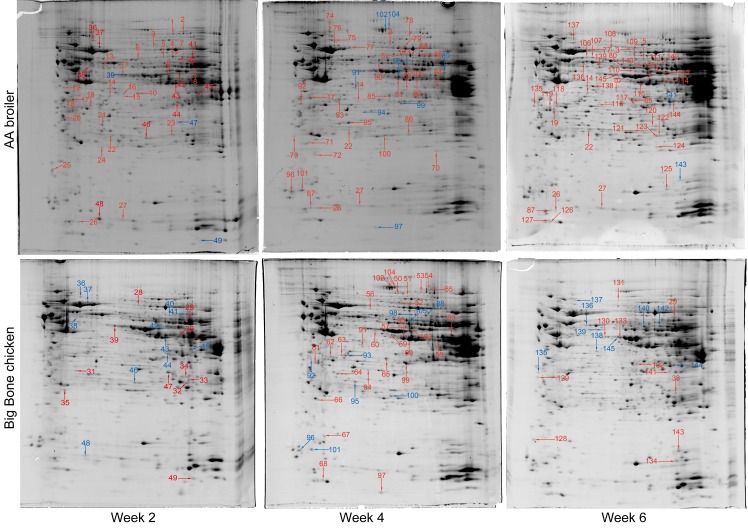
We compared the liver proteome between AA broiler and Big Bone chickens at three age stages using 2-DE and LC-MS/MS analysis. There were 264 to 263, 242 to 235 and 275 to 200 protein spots detected on 2-DE gels at 2 weeks, 4 weeks, and 6 weeks of age, in AA broiler to Big Bone chickens, respectively. Among them, 78, 84, and 87 protein spots showed significantly differential abundance (> 2 fold, p < 0.05) at 2, 4, and 6 weeks respectively. Subsequently, 49, 63, and 54 protein spots were identified after MS analysis at 2, 4, and 6 weeks respectively (Fig 1, Table 2). The remaining unidentified differential protein spots on 2-DE images could be due to their low abundance to produce enough spectra or their less than 95% search scores in the databases.
Fig 1. 2-DE hepatic protein profiles of AA broilers and Big Bone chickens.
Protein spots showing significant differences (2-fold, p < 0.05) were manually excised and identified by LC-Chip-ESI-QTOF-MS. Proteins of differential abundance with known identities were numbered and marked red or blue for increased or decreased abundance, respectively.
Table 2. Protein spots of differential abundance identified in the liver of AA broiler and Big Bone chickens.a.
| Spot no. | Protein name | Accession no.(NCBInr) | Symbol ID | Theoretical Mr (kDa)/pI | Sequence coverage (%) | Matched/searched | Mascot score | Log 2ratio (treatment/control) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 weeks | 4 weeks | 6 weeks | ||||||||
| Carbohydrate metabolism and energy production | ||||||||||
| 4 | NADP-dependent malic enzyme | gi|45383538 | ME1 | 62.53/6.45 | 63 | 32/62 | 785 | 8.93 | ||
| 5 | NADP-dependent malic enzyme | gi|45383538 | ME1 | 62.53/6.45 | 50 | 16/37 | 331 | 8.65 | 8.85 | 8.74 |
| 7 | malic enzyme | gi|45383538 | ME1 | 62.53/6.45 | 33 | 10/28 | 142 | 9.86 | ||
| 10 | sulfotransferase | gi|45384226 | CHST3 | 36.33/5.89 | 68 | 22/56 | 739 | 8.50 | 8.17 | |
| 11 | ubiquinol—cytochrome c reductase | gi|50754375 | UQCRFS1 | 53.41/6.58 | 6 | 2/5 | 58 | 9.38 | ||
| 14 | alpha-enolase | gi|46048768 | ENO1 | 47.62/6.17 | 26 | 8/25 | 320 | 8.38 | 6.82 | 8.25 |
| 16 | sulfotransferase | gi|45384226 | CHST3 | 36.33/5.89 | 30 | 3/10 | 115 | 7.65 | ||
| 17 | sulfotransferase | gi|118088279 | CHST3 | 36.79/5.50 | 8 | 1/4 | 62 | 9.79 | 9.01 | |
| 18 | phosphoglycolate phosphatase | gi|71894743 | PGP | 33.55/5.53 | 19 | 4/5 | 121 | 8.57 | ||
| 22 | sepiapterin reductase | gi|50767570 | SPR | 29.28/5.82 | 45 | 6/13 | 635 | 8.42 | 8.00 | 8.82 |
| 23 | phosphoglycerate mutase 1 | gi|71895985 | PGAM1 | 29.05/7.03 | 72 | 11/30 | 396 | 9.42 | ||
| 29 | electron transfer flavoprotein-ubiquinone oxidoreductase, mitochondrial | gi|71895853 | ETFDH | 53.61/8.99 | 42 | 7/35 | 969 | -8.19 | -9.63 | |
| 44 | malate dehydrogenase, cytoplasmic | gi|57530355 | MDH1 | 36.75/6.92 | 54 | 6/32 | 724 | 1.10 | ||
| 45 | fructose 1,6-bisphosphatase | gi|50762393 | FBP2 | 37.05/8.07 | 65 | 16/52 | 1132 | 1.02 | ||
| 47 | voltage-dependent anion-selective channel protein 1 | gi|76443696 | VDAC1 | 30.74/6.85 | 32 | 2/15 | 295 | -1.15 | ||
| 80 | sorting and assembly machinery component 50 homolog | gi|118083116 | SAMM50 | 52.47/5.99 | 30 | 2/17 | 540 | 7.37 | 8.84 | |
| 83 | galactose mutarotase (aldose 1-epimerase) | gi|118087781 | GALM | 38.18/6.19 | 18 | 1/6 | 166 | 7.53 | ||
| 84 | alcohol dehydrogenase [NADP+] | gi|57529654 | ADH5 | 37.34/7.66 | 35 | 11/17 | 168 | 7.90 | ||
| 85 | L-lactate dehydrogenase B chain | gi|45383766 | LDHB | 36.69/7.07 | 40 | 10/13 | 249 | 8.73 | 9.06 | |
| 87 | ubiquinol-cytochrome c reductase hinge protein | gi|118094600 | UQCRH | 9.45/5.17 | 43 | 1/6 | 125 | 8.30 | 8.15 | |
| 88 | phosphoglucomutase-1 | gi|84619526 | PGM1 | 67.06/8.98 | 65 | 43/61 | 1142 | 1.16 | ||
| 89 | fumarate hydratase, mitochondrial | gi|57530433 | FH | 54.49/9.20 | 49 | 27/31 | 1098 | -1.08 | ||
| 98 | NADP-dependent malic enzyme | gi/45383538 | ME1 | 62.53/6.45 | 43 | 27/68 | 657 | 1.73 | ||
| 102 | pyruvate carboxylase | gi|45383466 | PCX | 128.03/6.26 | 61 | 81/135 | 2199 | -1.32 | ||
| 104 | pyruvate carboxylase | gi|45383466 | PCX | 128.03/6.26 | 53 | 49/99 | 1099 | -1.09 | ||
| 120 | carbonic anhydrase II | gi|833606 | CA2 | 28.82/6.51 | 41 | 8/12 | 218 | 7.31 | ||
| 121 | phosphomannomutase 2 | gi|71895479 | PMM2 | 28.59/5.79 | 9 | 1/2 | 56 | 9.06 | ||
| 122 | triosephosphate isomerase | gi|45382061 | TPI1 | 26.83/6.71 | 55 | 6/14 | 105 | 8.70 | ||
| 123 | triosephosphate isomerase | gi|45382061 | TPI1 | 26.83/6.71 | 75 | 13/23 | 276 | 8.66 | ||
| 130 | alpha-enolase | gi|46048768 | ENO1 | 47.62/6.17 | 39 | 9/22 | 943 | -8.76 | ||
| 132 | malate dehydrogenase, cytoplasmic | gi|57530355 | MDH1 | 36.75/6.92 | 27 | 1/11 | 237 | -8.76 | ||
| 133 | ubiquinol-cytochrome c reductase | gi|50754375 | UQCRH | 53.41/6.58 | 26 | 2/16 | 430 | -9.99 | ||
| 144 | L-lactate dehydrogenase A chain | gi|45384208 | LDHA | 36.78/7.75 | 36 | 6/15 | 271 | 1.14 | ||
| 145 | alpha-enolase | gi|46048769 | ENO1 | 47.62/6.17 | 49 | 15/33 | 644 | 1.35 | ||
| Protein and amino acid metabolism | ||||||||||
| 1 | elongation factor 2 | gi|45382453 | EEF2 | 96.34/6.40 | 39 | 24/53 | 387 | 8.34 | ||
| 2 | glycine dehydrogenase [decarboxylating], mitochondrial precursor | gi|45383510 | GLDC | 111.78/7.55 | 19 | 9/22 | 246 | 7.93 | ||
| 3 | methylcrotonoyl-CoA carboxylase subunit alpha, mitochondrial | gi|57529595 | MCCC1 | 78.88/6.51 | 17 | 3/14 | 95 | 6.33 | 6.75 | 7.20 |
| 8 | cytosol aminopeptidase | gi|71897015 | LAP3 | 56.92/8.38 | 27 | 7/14 | 239 | 10.50 | ||
| 21 | guanidinoacetate N-methyltransferase | gi|118103242 | GAMT | 17.46/6.59 | 48 | 13/23 | 551 | 9.36 | ||
| 26 | SH3 domain-binding glutamic acid-rich-like protein | gi|60302796 | Q5F3C9 | 12.79/5.07 | 90 | 1/22 | 325 | 8.54 | 7.76 | 7.81 |
| 35 | cathepsin B precursor | gi|46195455 | CTSB | 38.48/5.74 | 27 | 6/21 | 530 | -9.02 | ||
| 43 | glutamine synthetase | gi|45382781 | GLUL | 42.75/6.38 | 11 | 1/8 | 108 | 1.39 | ||
| 50 | sarcosine dehydrogenase, mitochondrial | gi|363740469 | SARDH | 102.26/6.42 | 27 | 4/30 | 633 | 8.34 | -7.10 | |
| 51 | dimethylglycine dehydrogenase, mitochondrial | gi|363744224 | DMGDH | 96.68/7.33 | 40 | 3/41 | 1091 | 7.93 | -6.91 | |
| 52 | histidine ammonia-lyase | gi|45383354 | HAL | 73.54/6.17 | 31 | 3/26 | 590 | -7.52 | ||
| 53 | elongation factor 2 | gi|45382453 | EEF2 | 96.34/6.40 | 25 | 2/27 | 499 | -7.73 | ||
| 54 | glycine dehydrogenase [decarboxylating], mitochondrial precursor | gi|45383510 | GLDC | 113.26/7.55 | 23 | 7/21 | 122 | -6.86 | ||
| 55 | elongation factor 2 | gi|45382453 | EEF2 | 96.34/6.40 | 21 | 6/19 | 90 | -7.63 | ||
| 56 | ovoinhibitor precursor | gi|71895337 | SPINK5 | 54.39/6.16 | 18 | 6/9 | 137 | -7.53 | ||
| 57 | S-adenosylmethionine synthase isoform type-1 | gi|313760551 | MAT1A | 44.24/6.28 | 30 | 7/14 | 137 | -7.73 | ||
| 58 | fumarylacetoacetase (Fumarylacetoacetate hydrolase) | gi|50753071 | FAA | 46.80/7.31 | 43 | 18/49 | 259 | -9.88 | ||
| 64 | guanidinoacetate N-methyltransferase | gi|118103242 | GAMT | 17.46/6.59 | 35 | 4/ 12 | 249 | -8.71 | ||
| 69 | glutamine synthetase | gi|45382781 | GLUL | 42.75/6.38 | 29 | 6/15 | 171 | -9.73 | ||
| 74 | alanyl-tRNA synthetase, cytoplasmic | gi|57524852 | AARS | 102.00/5.68 | 38 | 25/50 | 623 | 8.85 | ||
| 78 | trifunctional purine biosynthetic protein adenosine-3 | gi|47825387 | GART | 107.56/7.51 | 26 | 6/26 | 132 | 7.07 | ||
| 81 | glutamine synthetase | gi|45382781 | GLUL | 42.75/6.38 | 40 | 8/24 | 171 | 9.64 | ||
| 90 | 4-hydroxyphenylpyruvate dioxygenase | gi|363739843 | HPD | 45.05/6.41 | 59 | 5/27 | 374 | -1.43 | ||
| 93 | eukaryotic translation initiation factor 3 subunit I | gi|256419027 | EIF3I | 36.87/5.38 | 32 | 2/13 | 338 | 1.02 | ||
| 94 | 3-hydroxyanthranilate 3,4-dioxygenase | gi|118087959 | HAAO | 33.74/6.27 | 18 | 1/4 | 131 | -1.82 | ||
| 95 | guanidinoacetate N-methyltransferase | gi|118103242 | GAMT | 17.46/6.59 | 44 | 7/18 | 370 | 1.17 | ||
| 97 | pterin-4-alpha-carbinolamine dehydratase | gi|45382483 | PCBD1 | 12.05/6.04 | 69 | 4/20 | 415 | -1.36 | ||
| 103 | S-adenosylmethionine synthase isoform type-1 | gi|313760551 | MAT1A | 44.24/6.28 | 55 | 28/57 | 664 | -2.67 | ||
| 105 | S-adenosylmethionine synthase isoform type-1 | gi|313760551 | MAT1A | 44.24/6.28 | 10 | 3/5 | 94 | 1.11 | ||
| 108 | lysyl-tRNA synthetase | gi|71895483 | KARS | 68.33/5.89 | 36 | 3/28 | 809 | 6.775 | ||
| 110 | phenylalanine-4-hydroxylase | gi|47604920 | PAH | 51.51/6.49 | 53 | 8/42 | 1247 | 8.80 | ||
| 139 | cytosolic non-specific dipeptidase | gi|57530409 | CNDP2 | 53.39/5.71 | 39 | 4/21 | 690 | 1.66 | ||
| 140 | aldehyde dehydrogenase 9 family, member A1 | gi|118094103 | ALDH9A1 | 57.00/7.81 | 65 | 34/53 | 1215 | 1.13 | ||
| Nucleotide metabolism | ||||||||||
| 19 | adenosine 5-diphosphosugar pyrophosphatase | gi|118081976 | GMPS | 35.43/6.36 | 28 | 9/15 | 312 | 8.78 | 8.38 | |
| 39 | adenosine kinase | gi|57529848 | ADK | 40.46/6.06 | 20 | 3/8 | 96 | -1.21 | ||
| 40 | dihydropyrimidinase | gi|118087274 | DPYS | 69.50/6.42 | 19 | 4/16 | 108 | 1.48 | ||
| 61 | adenosine 5-diphosphosugar pyrophosphatase | gi|118081976 | GMPS | 35.43/6.36 | 22 | 6/12 | 504 | -9.03 | ||
| 62 | ribokinase | gi|118088003 | RBKS | 36.72/5.39 | 22 | 3/7 | 378 | -8.01 | ||
| 99 | ribose-phosphate pyrophosphokinase 2 | gi|57525515 | PRPS2 | 36.04/6.37 | 58 | 31/53 | 865 | -1.35 | ||
| 125 | nucleoside diphosphate kinase | gi|45384260 | NME5 | 17.45/7.72 | 79 | 16/36 | 264 | 8.59 | ||
| 128 | nucleolar protein B23/No38 | gi|212456 | NPM1 | 10.77/4.38 | 57 | 1/6 | 205 | -8.94 | ||
| 135 | p32 subunit of splicing factor SF2 | gi|5509946 | C1QBP | 23.78/4.41 | 23 | 2/5 | 238 | 1.14 | ||
| 141 | thiosulfate sulfurtransferase | gi|268370289 | TST | 33.09/6.80 | 52 | 22/34 | 503 | -1.20 | ||
| 143 | nucleoside diphosphate kinase | gi|2827446 | NME5 | 17.54/7.11 | 66 | 32/45 | 1004 | -1.33 | ||
| Fatty acid metabolism | ||||||||||
| 27 | fatty acid-binding protein | gi|45384320 | FABP7 | 15.03/5.61 | 56 | 2/6 | 85 | 8.68 | 8.52 | 9.24 |
| 30 | long-chain specific acyl-CoA dehydrogenase, mitochondrial | gi|57529797 | ACADL | 48.26/8.34 | 32 | 10/17 | 159 | -10.58 | ||
| 33 | dodecenoyl-Coenzyme A delta isomerase | gi|118098151 | ECI1 | 34.56/9.30 | 33 | 18/32 | 379 | -8.52 | -7.68 | |
| 42 | 3-hydroxy-3-methylglutaryl-coenzyme A synthase | gi|118094097 | HMGCS1 | 52.98/6.57 | 31 | 11/26 | 195 | 1.69 | ||
| 48 | fatty acid-binding protein | gi|45384320 | FABP7 | 15.03/5.61 | 71 | 9/16 | 276 | 2.16 | ||
| 65 | sulfotransferase 1B | gi|118090275 | SULT1B1 | 32.01/6.00 | 48 | 1/16 | 351 | -6.71 | ||
| 70 | phosphatidylethanolamine-binding protein 1 | gi|310772215 | PEBP1 | 21.12/6.96 | 66 | 4/17 | 419 | 8.23 | ||
| 82 | 3-ketoacyl-CoA thiolase, mitochondrial | gi|57529492 | HADHA | 42.17/8.02 | 18 | 3/7 | 89 | 10.03 | ||
| 111 | 3-hydroxy-3-methylglutaryl-coenzyme A synthase | gi|118094097 | HMGCS1 | 52.98/6.57 | 30 | 16/25 | 383 | 10.33 | ||
| 112 | 3-hydroxy-3-methylglutaryl-coenzyme A synthase | gi|118094097 | HMGCS1 | 52.98/6.57 | 32 | 15/27 | 462 | 9.48 | ||
| 114 | SEC14-like 2 | gi|50756739 | SEC14L3 | 46.94/6.73 | 60 | 24/43 | 520 | 10.40 | ||
| 116 | phosphatidylinositol transfer protein beta isoform | gi|86129444 | PITPNB | 30.86/5.63 | 71 | 12/27 | 224 | 7.01 | ||
| Antioxidants | ||||||||||
| 6 | epoxide hydrolase 2 | gi|45384320 | EPHX2 | 63.72/5.89 | 45 | 17/29 | 448 | 8.37 | ||
| 46 | peroxiredoxin-6 | gi|57529797 | PRDX6 | 25.08/5.72 | 58 | 4/35 | 793 | 1.00 | ||
| 68 | thioredoxin | gi|118098151 | TXNRD1 | 11.98/5.10 | 49 | 1/12 | 285 | -8.97 | ||
| 76 | serum albumin precursor | gi|118094097 | ALB | 71.87/5.51 | 26 | 1/14 | 362 | 8.70 | ||
| 77 | serum albumin precursor | gi|45384320 | ALB | 71.87/5.51 | 48 | 18/31 | 455 | 9.23 | 9.39 | |
| 86 | glutathione S-transferase 2 | gi|118090275 | GSTM2 | 26.05/6.85 | 80 | 9/33 | 746 | 8.86 | ||
| 118 | thioredoxin-like protein | gi|310772215 | TXNL1 | 32.73/4.94 | 40 | 5/18 | 131 | 8.55 | ||
| 124 | peroxiredoxin-1 isoform 1 | gi|57529492 | PRDX1 | 22.53/8.24 | 40 | 2/15 | 390 | 8.19 | ||
| 126 | thioredoxin | gi|118094097 | TXNRD1 | 11.98/5.10 | 49 | 2/6 | 61 | 8.82 | ||
| 127 | thioredoxin | gi|118094097 | TXNRD1 | 11.98/5.10 | 32 | 1/3 | 67 | 8.22 | ||
| 136 | protein disulfide-isomerase A3 precursor | gi|50756739 | PDIA3 | 56.55/5.76 | 32 | 2/16 | 507 | 2.25 | ||
| 142 | retinal dehydrogenase 1 | gi|86129444 | ALDH1A1 | 56.40/7.49 | 51 | 24/35 | 615 | 1.30 | ||
| Cell structure | ||||||||||
| 12 | alpha-tropomyosin 2 | gi|27465053 | TPM1 | 32.85/4.65 | 40 | 8/17 | 164 | 7.73 | ||
| 15 | protein syndesmos | gi|45382147 | NUDT16L1 | 33.98/5.74 | 48 | 12/33 | 277 | 7.24 | ||
| 28 | lamin-A | gi|45384214 | LMNA | 73.35/6.50 | 14 | 1/11 | 373 | -8.00 | ||
| 36 | mitochondrial inner membrane protein | gi|57530041 | IMMT | 79.54/5.72 | 31 | 2/31 | 745 | 1.24 | ||
| 37 | mitochondrial inner membrane protein | gi|57530041 | IMMT | 79.54/5.72 | 24 | 2/28 | 479 | 1.02 | ||
| 38 | desmin | gi|2959450 | DES | 51.69/5.30 | 53 | 12/35 | 272 | 1.46 | ||
| 63 | F-actin-capping protein subunit alpha-1 | gi|45382905 | CAPZA1 | 33.11/5.43 | 53 | 3/15 | 499 | -8.42 | ||
| 66 | translationally-controlled tumor protein homolog | gi|45382329 | TPT1 | 19.69/4.90 | 90 | 12/23 | 372 | -7.95 | ||
| 67 | glia maturation factor beta | gi|71894963 | GMFB | 16.88/5.19 | 28 | 1/4 | 144 | -8.10 | ||
| 71 | translationally-controlled tumor protein homolog | gi|45382329 | TPT1 | 19.69/4.90 | 50 | 8/24 | 190 | 8.19 | ||
| 75 | mitochondrial inner membrane protein | gi|57530041 | IMMT | 79.54/5.72 | 40 | 15/36 | 1360 | 6.91 | ||
| 79 | lamin-A | gi|45384214 | LMNA | 73.35/6.50 | 58 | 24/44 | 512 | 7.76 | ||
| 92 | alpha-tropomyosin | gi|211109 | TPM1 | 32.81/4.75 | 15 | 2/4 | 177 | 1.61 | ||
| 109 | coronin-1C | gi|86129440 | CORO1C | 53.74/6.22 | 39 | 20/34 | 244 | 6.78 | ||
| Protein folding | ||||||||||
| 49 | 10 kDa heat shock protein, mitochondrial | gi|45384204 | HSPE1 | 11.13/8.68 | 82 | 1/20 | 438 | -1.5892 | ||
| 59 | cyclophilin | gi|118089782 | PPIA | 39.80/5.61 | 27 | 4/16 | 95 | -7.12 | ||
| 106 | T-complex protein 1 subunit alpha | gi|57530301 | TCP1 | 61.06/5.66 | 34 | 9/18 | 181 | 8.83 | ||
| 107 | T-complex protein 1 subunit alpha | gi|57530301 | TCP1 | 61.06/5.66 | 43 | 11/32 | 1071 | 9.28 | ||
| 131 | hsc70-interacting protein | gi|71896903 | ST13 | 40.36/5.07 | 36 | 8/22 | 646 | -6.97 | ||
| 134 | peptidylprolyl isomerase A | gi|261490820 | PPIA | 18.08/8.29 | 67 | 9/28 | 194 | -9.24 | ||
| Transporter | ||||||||||
| 24 | heme-binding protein 1 | gi|71896913 | HEBP1 | 21.26/5.76 | 34 | 3/7 | 88 | 7.85 | ||
| 31 | chloride intracellular channel protein 2 | gi|71895359 | CLIC2 | 28.46/5.39 | 42 | 4/19 | 492 | -8.06 | ||
| 119 | coatomer subunit epsilon | gi|57530593 | COPE | 34.52/4.99 | 35 | 4/31 | 371 | 9.29 | ||
| 129 | clathrin light chain A | gi|86129544 | CLTA | 23.86/4.42 | 40 | 6/12 | 189 | -8.33 | ||
| 137 | transitional endoplasmic reticulum ATPase | gi|113206112 | VCP | 89.95/5.14 | 54 | 21/60 | 496 | 1.04 | ||
| Unknown function | ||||||||||
| 9 | MGC82288 protein | gi|50729534 | 50.12/6.35 | 44 | 14/23 | 264 | 10.66 | |||
| 13 | protein PRRC1 | gi|71894751 | PRRC1 | 45.84/5.52 | 10 | 2/4 | 80 | 7.89 | ||
| 20 | hypothetical protein RCJMB04_5n23 | gi|53129586 | TPM3 | 28.80/4.69 | 47 | 12/24 | 305 | 9.33 | ||
| 25 | hypothetical protein | gi|118083300 | 10.04/4.17 | 14 | 2/4 | 58 | 9.29 | |||
| 32 | ES1 protein homolog, mitochondrial | gi|71895261 | C1H21orf33 | 27.87/8.54 | 46 | 17/45 | 213 | -9.54 | ||
| 34 | hypothetical protein | gi|118098539 | 28.31/7.63 | 42 | 3/28 | 647 | -8.71 | |||
| 41 | hypothetical protein RCJMB04_1j22 | gi|53127216 | 60.28/8.09 | 51 | 20/40 | 412 | 1.23 | |||
| 60 | hypothetical protein | gi|118087385 | 44.65/5.82 | 16 | 4/6 | 185 | -8.23 | |||
| 72 | sorcin | gi|124249424 | SRI | 22.21/5.37 | 55 | 9/17 | 216 | 8.01 | ||
| 73 | sorcin | gi|124249425 | SRI | 22.21/5.37 | 24 | 2/5 | 51 | 8.76 | ||
| 91 | hypothetical protein RCJMB04_1g3 | gi|53126716 | 33.27/5.60 | 46 | 5/19 | 579 | -1.06 | |||
| 96 | hypothetical protein | gi|50800573 | 11.84/4.42 | 66 | 8/11 | 348 | 1.06 | |||
| 100 | LOC495096 protein isoform 3 | gi|50734923 | 28.44/6.45 | 34 | 2/13 | 297 | 1.26 | |||
| 101 | thyroid hormone responsive spot 14 beta 2 | gi|45826439 | THRSPB | 14.52/5.10 | 20 | 1/3 | 98 | 1.13 | ||
| 113 | hypothetical protein | gi|50728520 | 48.21/6.29 | 42 | 15/30 | 430 | 10.66 | |||
| 115 | LOC495029 protein | gi|118098511 | 45.06/6.55 | 63 | 26/45 | 716 | 9.86 | |||
| 117 | MGC83663 protein | gi|118093845 | 51.57/6.30 | 30 | 6/18 | 730 | 8.70 | |||
| 138 | MGC82230 protein | gi|50756617 | 43.26/5.76 | 61 | 11/33 | 1036 | 1.03 | |||
a Spot no. corresponds to the number of protein spots in Fig 1. Protein name is given when proteins were identified by LC-Chip ESI-QTOF MS. Accession no. is the unique number given to mark the entry of a protein in the database NCBInr. Theoretical molecular weight (Mr) and isoelectric point (pI) of the identified proteins are retrieved from the protein database of NCBInr (S1 Table). Sequence coverage is the ratio of the number of amino acids in every peptide that matches with the mass spectrum divided by the total number of amino acids in the protein sequence. Matched peptide is the number of paring an experimental fragmentation spectrum to a theoretical segment of protein and searched peptide is the total searched peptide. Mascot scores are derived from ion scores as a non-probabilistic basis for ranking protein hits.
Qualitative comparisons of proteins with differential abundance
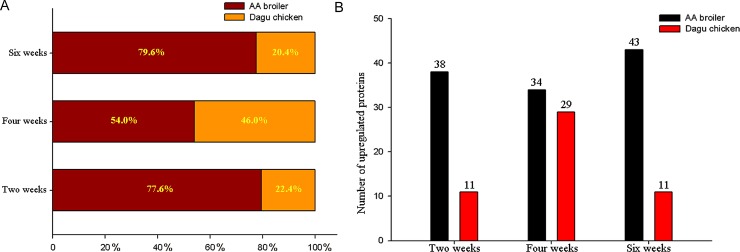
In general, the liver protein profiles between AA broiler and Big Bone chickens during three age stages were similar. However, some protein spots displayed obvious differences in abundance. As mentioned previously, a total of 49, 63, and 54 proteins were differentially expressed in AA broiler and Big Bone chickens at 2, 4, and 6 weeks, respectively. From the 49 identified proteins at 2 weeks, 38 (77.6%) and 11 (22.4%) had increased abundance in the AA broiler and the Big Bone chickens, respectively. Similarly at 4 weeks, 34 (54.0%) and 29 (46.0%) proteins had higher abundance in the AA broiler and the Big Bone chickens, respectively. Also at 6 weeks, the AA broiler and the Big Bone chickens differentially expressed 43 (79.6%) and 11(20.4%) proteins, respectively (Fig 2A and 2B). On average, the abundance for 115 (69.3%) proteins was higher in the AA broiler and that for 51 (30.7%) was higher in the Big Bone chickens.
Fig 2. Comparisons proteins with higher abundance in the livers between the AA broiler and Big Bone chickens at 2, 4 and 6 weeks, respectively.
A represents the percentage of proteins with increased abundance, B represents the numbers of proteins with increased abundance.
Classification of proteins with differential abundance
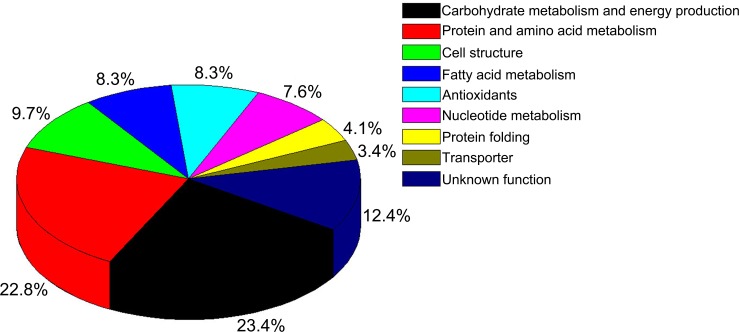
Based on biological processes in the database annotations, the identified differential proteins between the AA broiler and the Big Bone chickens were classified into nine main functional categories, including carbohydrate metabolism and energy production (23.4%), protein and amino acid metabolism (22.8%), cell structure (9.7%), fatty acid metabolism (8.3%), antioxidants (8.3%), nucleotide metabolism (7.6%), protein folding (4.1%), transporters (3.4%), and unknown function (12.4%) (Fig 3).
Fig 3. Functional classification of the proteins with differential abundance identified in the livers of AA broiler and Big Bone chickens at 2, 4 and 6 weeks.
Qualitative comparisons of differentially expressed proteins
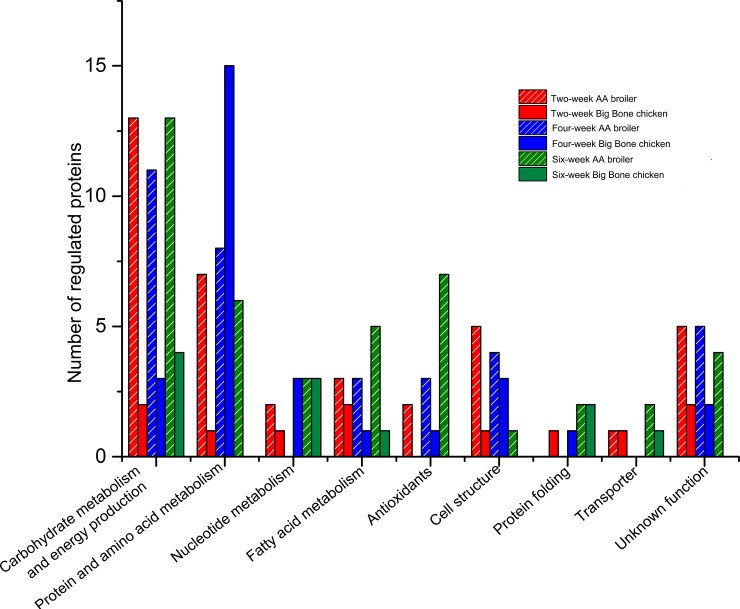
Interestingly, the higher abundance proteins at the different stages in the two breeds showed distinct functional categories (Fig 4). The number of proteins with higher abundance in the AA broilers liver was greater than in the Big Bone chickens liver at three age stages, which were mainly involved in carbohydrate metabolism and energy production, fatty acid metabolism, antioxidants and cell structure.
Fig 4. Comparisons of functional classification of proteins with higher abundance in the livers between the AA broiler and Big Bone chickens at 2, 4 and 6 weeks.
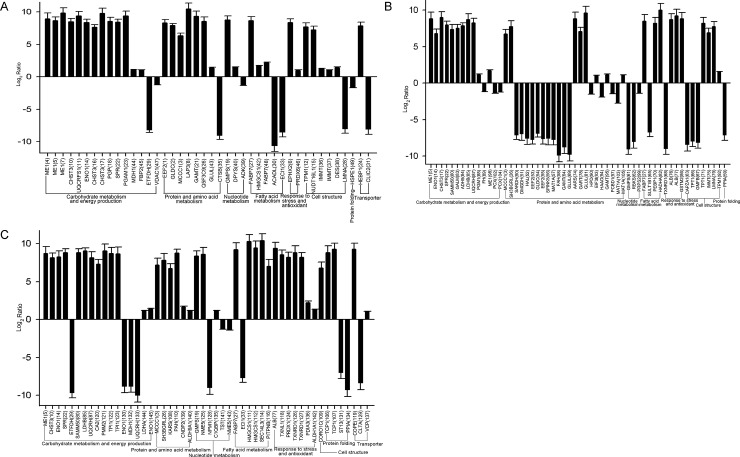
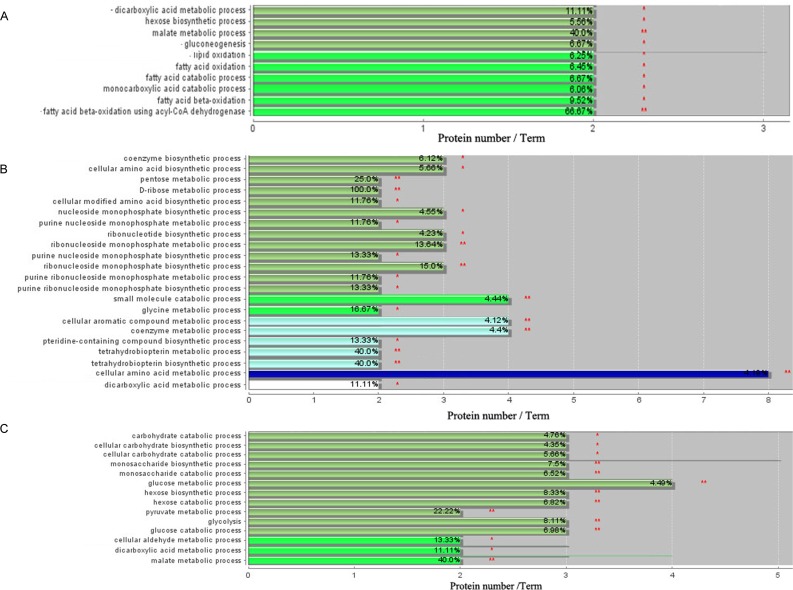
The relative abundance between the two breeds was shown by Fig 5A, 5B and 5C. Enrichment analysis of the proteomes at 2 weeks showed that two major functional groups, namely carbohydrate metabolism and fatty acid metabolism, were significantly enriched (Fig 6A). Similarly, two main functional groups, including protein and amino acid metabolism and nucleotide metabolism, were enriched at 4 weeks (Fig 6B). At 6 weeks, the functional group carbohydrate metabolism was significantly enriched (Fig 6C).
Fig 5. Quantitative comparisons of differentially expressed proteins in the livers of AA broiler and Big Bone chickens at 2, 4 and 6 weeks.
The ratios of the protein abundance (AA broilers to Big Bone chickens) are transformed, and the protein spots with |log2 ratio|≥1 (p≤0.05) are selected as the differentially expressed proteins. A, B and C represent differentially expressed proteins at 2, 4 and 6 weeks, respectively.
Fig 6. Functional enrichment analysis of the proteins of differential abundance in the livers between AA broiler and Big Bone chickens at 2, 4 and 6 weeks using ClueGO software.
* and ** mean p < 0.05 and p < 0.01 levels of significance. A, B and C represent enrichment analysis of differentially expressed proteins at 2, 4 and 6 weeks, respectively.
Biological network analysis
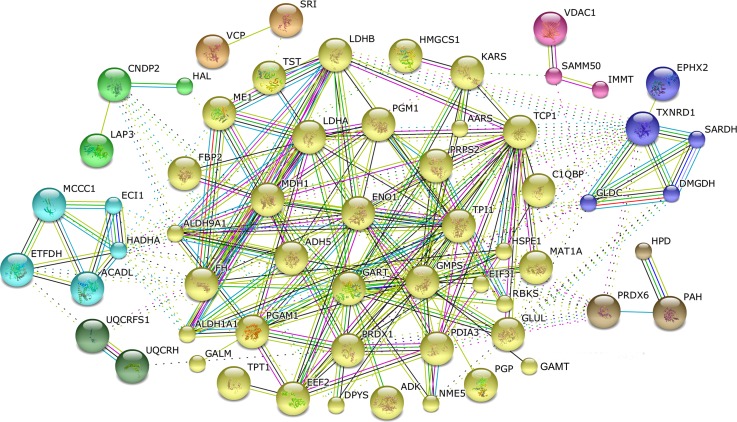
Proteins are fundamental parts of living cells and cellular functions are mainly carried out by protein complexes. Using the online tool STRING 10.0, sixty proteins were recognized as nodes in biological interaction networks, in which eight clusters were enriched and connected by cytosolic non-specific dipeptidase (CNDP2), 3-ketoacyl-CoA thiolase (HADHA), electron transfer flavoprotein-ubiquinone oxidoreductase (ETFDH), ubiquinol—cytochrome c reductase (UQCRFS1), ubiquinol-cytochrome c reductase hinge protein (UQCRH), peroxiredoxin-6 (PRDX6), glycine dehydrogenase (GLDC), thioredoxin (TXNRD1), sorting and assembly machinery component 50 (SAMM50), fructose 1,6-bisphosphatase (FBP2) and aldehyde dehydrogenase 9 family (ALDH9A1) (Fig 7). The biggest cluster mainly involved in glycolysis/gluconeogenesis, carbon metabolism and amino acid metabolism pathway by the interaction between 37 proteins.
Fig 7. Biological interaction network of the proteins of differential abundance in the livers of AA broiler and Big Bone chickens at 2, 4 and 6 weeks.
Red lines indicate fusion evidence, green lines indicate neighborhood evidence, blue lines indicate co-occurrence evidence, purple lines indicate experimental evidence, yellow lines indicate text mining evidence, light blue lines indicate database evidence and black lines indicate co-expression evidence.
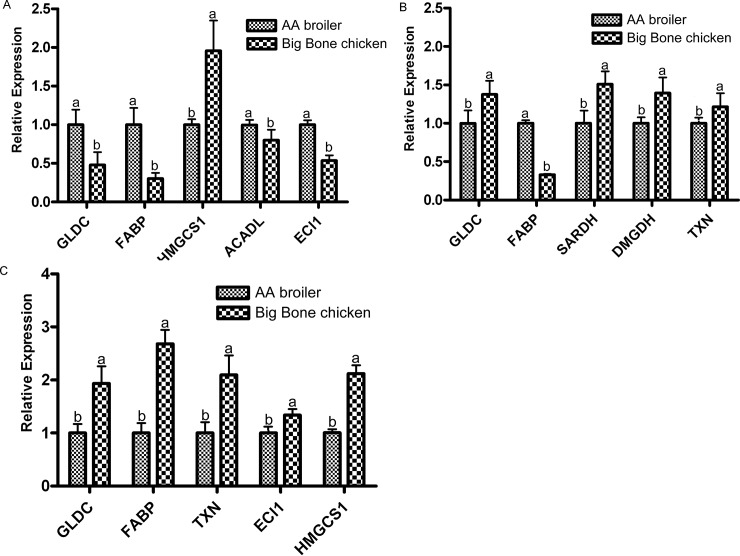
Validation of proteins of differential abundance by qPCR
Of the liver proteins with differential abundance at 2 weeks, 4 weeks and 6 weeks, proteins that played an important role in nutrient metabolism (amino acid and lipid metabolism) and antioxidants were selected to validate their expression at the level of mRNA (Fig 8). At 2 weeks (Fig 8A), GLDC (spot 2) and FABP (spots 27 and 48) at the protein levels were consistent with their mRNA expression levels; but HMSC1 (spot 42), ACADL (spot 30) and ECI1 (spot 33) showed an inconsistent pattern between the mRNA expression and protein abundance level. At 4 weeks (Fig 8B), GLDC (spot 54), FABP (spot 27), SARDH (spot 50), DMGDH (spot 51) and TXNRD1 (spot 168) were consistent with their mRNA expression levels. The similar expression pattern at the transcript level indicates a prospective opportunity for reverse genetic research through gene manipulation. At 6 weeks (Fig 8C), ECI1 (spot 33) at the protein level were consistent with their mRNA expression levels; however, FABP (spot 27), HMSC1 (spots 111 and 112) and TXNRD1 (spots 126 and 127) showed an inconsistent pattern between the mRNA expression and protein abundance level.
Fig 8. Validation using qPCR of proteins of differential abundance at the mRNA level in the livers between the AA broiler and Big Bone chickens at 2, 4 and 6 weeks.
Samples were normalized with the reference genes β-actin. A, B and C represent differentially expressed proteins at the mRNA level at 2, 4 and 6 weeks, respectively.
Discussion
This study compared differences in the proteome and the weight of the liver between AA broiler and Big Bone chickens at three different age stages. Average abdominal fat percentage and the liver weight showed significant differences between two breeds at four weeks of age. Moreover, AA broilers also showed the most differential proteome at four weeks compared to Big Bone chickens. Additionally, the number of proteins with increased abundance related to carbohydrate metabolism and energy production were higher in AA broilers than Big Bone chickens at three stages of age, which indicates breed differentiation of the two strains may be associated with differential abdominal fat deposition.
Malic enzyme (ME1, spot 4, 5, 7 and 98), ubiquinol-cytochrome C reductase (UQCRFS1, spot 11), enolase 1 (ENO1, spot 14), phosphoglycerate mutase 1 (PGAM1, spot 23), malate dehydrogenase (MDH1, spot 44), fructose-1, 6-bisphosphatase (FBP2, spot 45), phosphoglucomutase 1 (PGM1, spot 88), and triosephosphate isomerase (TPI1, spot 122 and 123) are key proteins which showed higher abundance in AA broiler chickens to mediate energy production. MDH1, FBP2, PGM1 and phosphoglycerate mutase 1 (PGAM1, spot 23) participate in the TCA cycle and UQCRFS1 (spot 11) is an electron transporter chain protein of the mitochondria to promote energy production. Although the TCA cycle is mainly involved in energy metabolism, it also produces intermediates to be converted to glucose, fatty acids, or non-essential amino acids [20]. Previously, malic enzyme gene expression in hepatic metabolism was found to be enhanced in fatter chicken varieties over lean chicken varieties [21], which was consistent with increased abundance for ME1 protein in AA broilers. Moreover, the higher abundance for pyruvate carboxylase (PCX, spot 102 and 104) and fumarate hydratase (FH, spot 89) in AA broilers at 4 weeks are the key enzymes involved in energy production and gluconeogenesis. Therefore, these differentially expressed proteins strongly suggest higher energy metabolism and enhanced lipid biosynthesis in AA broilers than that in Big Bone chicken.
The liver is a vital organ for dietary protein and amino acid metabolism. Protein synthesis is subject to regulation by eukaryotic initiation factor 2 and elongation factor 2 (EEF2, spot 1, 53 and 55) [22, 23]. The abundance of EEF2 was higher in the liver of AA broilers at two weeks of age and in Big Bone chickens at four weeks. The differential EEF2 abundance at different stages in the two breeds may affect the protein synthesis. At the same time, AA broilers had more proteins with increased abundance involved in amino acid metabolism than Big Bone chickens at two weeks, but less than Big Bone chicken at four weeks. Guanidinoacetate N-methyltransferase (GAMT, spot 21) was higher at -two weeks and GAMT (spot 64) was lower at four weeks in AA broilers, however the abundance of GAMT (spot 95) was significantly differential between Big Bone chickens and AA broilers at four weeks of age. These protein spots were differentially modified and played different roles in creatine biosynthesis between two breeds [24]. Furthermore at four weeks, Big Bone chickens had increased levels of glycine dehydrogenase (GLDC, spot 54), sarcosine dehydrogenase (SARDH, spot 50) and dimethylglycine dehydrogenase (DMGDH, spot 51), which are all involved in glycine metabolism [25, 26]. This enhanced glycine metabolism may contribute to the better meat quality in Big Bone chickens because glycine is one of most important flavor substances.
Previous studies demonstrated differential lipid metabolism between fat and lean broiler breeds [5, 9, 27]. Specifically, this study found that AA broilers had enhanced levels of fatty acid-binding protein (FABP7, spot 27 and 48), enzyme HMG-CoA synthase 1 (HMGCS1, spot 42, 111 and 112), phosphatidylinositol transfer protein (PITPNB, spot 116) and lipid binding protein SEC14-Like 2 (SEC14L3, spot 114). This fatty acid-binding protein is involved in intracellular fatty acid movement and its abundance has been associated with fat deposition in chickens [28–30]. HMGCS1 is a rate-limiting enzyme for ketone body formation from fatty acids in mitochondria [31]. PITPNB catalyzes the transfer of phosphatidylinositol and phosphatidylcholine between membranes for lipid delivery [32]. SEC14L3 stimulates squalene monooxygenase in the cholesterol biosynthetic pathway [33]. The increased abundance of these proteins suggest that AA broilers have enhanced lipid metabolism. On the contrary, Big Bone chickens had higher levels of acyl-CoA dehydrogenase long chain (ACADL, spot 30) and enoyl CoA isomerase 1 (ECI1, spot 33), both of which are involved in the beta-oxidation of fatty acids [34, 35]. Thus the enhanced lipid degradation in Big Bone chickens may be related to their reduced abdominal fat percentage.
Nucleotides are used in a wide variety of cellular metabolism and are fundamental for cellular functions [36]. The abundance for ribose-phosphate pyrophosphokinase 2 (PRPS2, spot 99) was increased in AA broilers liver at two weeks and four weeks of age. PRPS2 catalyzes the synthesis of phosphoribosyl pyrophosphate in purine nucleotide synthesis [37, 38]. In contrast, adenosine kinase (ADK, spot 39) was higher abundance in Big Bone chickens at two weeks of age and its function is known to be associated with liver disease [39]. Moreover, ribokinase (RBKS, spot 62) was higher abundance in Big Bone chickens at four weeks. RBKS belongs to the transferase family and participates in the pentose phosphate pathway. Nucleolar protein B23/No38 (NPM1, spot 128) and thiosulfate sulfurtransferase (TST, spot 141) were higher abundance in Big Bone chickens at six weeks. Thus, the differential nucleotide metabolism may contribute to variation in body weight between AA broiler and Big Bone chickens.
Additionally, differential abundance of liver proteins was observed, including those involved in the antioxidant system, protein folding, cytoskeleton, and transport proteins. These proteins may also play a role in regulating the size of the liver and the body weight of different breeds. Nevertheless, proteins function together in the context of networks through protein-protein interactions [40]. Protein associations involved in the development of the two breeds was analyzed by STRING. The majority of proteins identified were related to carbohydrate metabolism and energy production; approximately 31% of the biological interaction networks, followed by proteins in amino acid and protein metabolism (29%), antioxidants (13%) and nucleotide metabolism (13%). Moreover, proteins associated with protein folding, cell structure and fatty acid metabolism were nodes in the biological interaction networks, indicating that these proteins play a role in the construction and function of the liver of chickens. Some of the key node proteins that were highly linked in the BIN were validated at a gene level. GLDC, FABP, SARDH, DMGDH and TXNRD1 at different age stages were consistent or in consistent with mRNA levels may represent potential targets for genetic manipulation.
In conclusion, proteomic analysis of two unique chicken breeds was performed and differences in multiple metabolic pathways were identified between modern broilers and a local chicken breed. These findings can be used in the future to improve meat quality in commercial chicken supplies as the demand increases for not only greater amounts of food but better tasting meat.
Supporting Information
(DOCX)
(XLSX)
(DOCX)
Acknowledgments
We thank Jiancheng Yang from Shenyang Agricultural University for sample analyses. This work was supported by China Agriculture Research System (CARS-42) and the National Natural Science Foundation (31101731) of China.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funders are China Agriculture Research System (http://www.zgrj.org/, CARS-42) and the National Natural Science Foundation (http://www.nsfc.gov.cn/,31101731) of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Griffiths L, Leeson S, Summers JD. Studies on Abdominal Fat with Four Commercial Strains of Male Broiler Chicken. Poultry Science. 1978;57(5):1198–203. 10.3382/ps.0571198 [DOI] [Google Scholar]
- 2.Guan RF, Lyu F, Chen XQ, Ma JQ, Jiang H, CG X. Meat quality traits of four Chinese indigenous chicken breeds and one commercial broiler stock. Journal of Zhejiang University Science B. 2013;14(10):896–902. 10.1631/jzus.B1300163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumova E, Teimouri A. Fat deposition in the broiler chicken: a review. Scientia Agriculturae Bohemica. 2010; 41(2):121–8. [Google Scholar]
- 4.Hermier D. Lipoprotein metabolism and fattening in poultry. J Nutr. 1997;127(5 Suppl):805S–8S. Epub 1997/05/01. . [DOI] [PubMed] [Google Scholar]
- 5.Leclercq B, Hermier D, G G. Metabolism of very low density lipoproteins in genetically lean or fat lines of chicken. Reproduction Nutrition Development. 1990;30(6): 701–15. [DOI] [PubMed] [Google Scholar]
- 6.Saadoun A, Leclercq B. Comparison of in vivo fatty acid synthesis of the genetically lean and fat chickens. Comp Biochem Physiol B. 1983;75(4):641–4. Epub 1983/01/01. . [DOI] [PubMed] [Google Scholar]
- 7.Hermier D, Chapman MJ. [Plasma lipoproteins and fattening: description of a model in the domestic chicken, Gallus domesticus]. Reprod Nutr Dev. 1985;25(1B):235–41. Epub 1985/01/01. . [PubMed] [Google Scholar]
- 8.Legrand P, Hermier D. Hepatic delta 9 desaturation and plasma VLDL level in genetically lean and fat chickens. Int J Obes Relat Metab Disord. 1992;16(4):289–94. Epub 1992/04/01. . [PubMed] [Google Scholar]
- 9.Huang J, Tang X, Ruan J, Ma H, Zou S. Use of comparative proteomics to identify key proteins related to hepatic lipid metabolism in broiler chickens: evidence accounting for differential fat deposition between strains. Lipids. 2010; 45(1):81–9. 10.1007/s11745-009-3373-8 [DOI] [PubMed] [Google Scholar]
- 10.Cui HX, Liu RR, Zhao GP, Zheng MQ, Chen JL, Wen J. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens. BMC Genomics. 2012;13:213 Epub 2012/06/01. 1471-2164-13-213 [pii] 10.1186/1471-2164-13-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li HF, Han W, Zhu YF, Shu JT, Zhang XY, Chen KW. Analysis of genetic structure and relationship among nine indigenous Chinese chicken populations by the Structure program. J Genet. 2009;88(2):197–203. Epub 2009/08/25. . [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Yue HY, Wu SG, Xu L, Zhang HJ, Yan HJ, et al. Transport stress in broilers. II. Superoxide production, adenosine phosphate concentrations, and mRNA levels of avian uncoupling protein, avian adenine nucleotide translocator, and avian peroxisome proliferator-activated receptor-gamma coactivator-1alpha in skeletal muscles. Poult Sci. 2010;89(3):393–400. Epub 2010/02/26. 89/3/393 [pii] 10.3382/ps.2009-00281 . [DOI] [PubMed] [Google Scholar]
- 13.Zheng A, Liu G, Zhang Y, Hou S, Chang W, Zhang S, et al. Proteomic analysis of liver development of lean Pekin duck (Anas platyrhynchos domestica). J Proteomics. 2012;75(17):5396–413. Epub 2012/07/10. S1874-3919(12)00485-X [pii] 10.1016/j.jprot.2012.06.019 . [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. Epub 1976/05/07. S0003269776699996 [pii]. . [DOI] [PubMed] [Google Scholar]
- 15.Zheng A, Chang W, Hou S, Zhang S, Cai H, Chen G, et al. Unraveling molecular mechanistic differences in liver metabolism between lean and fat lines of Pekin duck (Anas platyrhynchos domestica): a proteomic study. J Proteomics. 2014;98:271–88. Epub 2014/01/15. S1874-3919(13)00656-8 [pii] 10.1016/j.jprot.2013.12.021 . [DOI] [PubMed] [Google Scholar]
- 16.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–3. Epub 2009/02/25. btp101 [pii] 10.1093/bioinformatics/btp101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Research. 2015;43(Database issue):D447–D52. 10.1093/nar/gku1003 PubMed PMID: PMC4383874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-Time quantitative PCR and the 2-ΔΔCT method. Methods 2001;25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 19.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–22. Epub 2009/02/28. clinchem.2008.112797 [pii] 10.1373/clinchem.2008.112797 . [DOI] [PubMed] [Google Scholar]
- 20.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277(34):30409–12. Epub 2002/06/28. 10.1074/jbc.R200006200 R200006200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 21.Daval S, Lagarrigue S, Douaire M. Messenger RNA levels and transcription rates of hepatic lipogenesis genes in genetically lean and fat chickens. Genet Sel Evol. 2000;32(5):521–31. Epub 2004/01/23. 10.1051/gse:2000134g320505 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269(22):5360–8. Epub 2002/11/09. 3290 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23.Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16(1):3–12. Epub 2005/01/22. S1084-9521(04)00106-5 [pii] 10.1016/j.semcdb.2004.11.004 . [DOI] [PubMed] [Google Scholar]
- 24.http://www.uniprot.org/uniprot/Q14353.
- 25.Fujiwara K, Okamura-Ikeda K, Motokawa Y. Amino acid sequence of the phosphopyridoxyl peptide from P-protein of the chicken liver glycine cleavage system. Biochem Biophys Res Commun. 1987;149(2):621–7. Epub 1987/12/16. 0006-291X(87)90413-X [pii]. . [DOI] [PubMed] [Google Scholar]
- 26.Porter DH, Cook RJ, Wagner C. Enzymatic properties of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver. Arch Biochem Biophys. 1985;243(2):396–407. Epub 1985/12/01. . [DOI] [PubMed] [Google Scholar]
- 27.Whitehead CC, Griffin HD. Development of divergent lines of lean and fat broilers using plasma very low density lipoprotein concentration as selection criterion: the first three generations. Br Poult Sci. 1984;25(4):573–82. Epub 1984/10/01. 10.1080/00071668408454899 . [DOI] [PubMed] [Google Scholar]
- 28.Besnard P, Niot I, Poirier H, Clement L, A B. New insights into the fatty acid-binding protein (FABP) family in the small intestine. Molecular and cellular biochemistry. 2002;239(1–2):139–47. [PubMed] [Google Scholar]
- 29.Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47(1):39–48. Epub 2006/01/21. 313 [pii] 10.1007/BF03194597 . [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Li H, Li N, Leng L, Wang Y. Tissue expression and association with fatness traits of liver fatty acid-binding protein gene in chicken. Poult Sci. 2006;85(11):1890–5. Epub 2006/10/13. 85/11/1890 [pii]. . [DOI] [PubMed] [Google Scholar]
- 31.Miziorko HM, Clinkenbeard KD, Reed WD, Lane MD. 3-Hydroxy-3-methylglutaryl coenzyme A synthase. Evidence for an acetyl-S-enzyme intermediate and identification of a cysteinyl sulfhydryl as the site of acetylation. J Biol Chem. 1975;250(15):5768–73. Epub 1975/08/10. . [PubMed] [Google Scholar]
- 32.Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771(6):677–91. Epub 2007/05/11. S1388-1981(07)00076-5 [pii] 10.1016/j.bbalip.2007.03.009 . [DOI] [PubMed] [Google Scholar]
- 33.Singh DK, Mokashi V, Elmore CL, Porter TD. Phosphorylation of supernatant protein factor enhances its ability to stimulate microsomal squalene monooxygenase. J Biol Chem. 2003;278(8):5646–51. Epub 2002/11/28. 10.1074/jbc.M211750200 M211750200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 34.Chegary M, Brinke H, Ruiter JP, Wijburg FA, Stoll MS, Minkler PE, et al. Mitochondrial long chain fatty acid beta-oxidation in man and mouse. Biochim Biophys Acta. 2009;1791(8):806–15. Epub 2009/05/26. S1388-1981(09)00131-0 [pii] 10.1016/j.bbalip.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssen U, Fink T, Lichter P, Stoffel W. Human mitochondrial 3,2-trans-enoyl-CoA isomerase (DCI): gene structure and localization to chromosome 16p13.3. Genomics. 1994;23(1):223–8. Epub 1994/09/01. S0888-7543(84)71480-7 [pii] 10.1006/geno.1994.1480 . [DOI] [PubMed] [Google Scholar]
- 36.Lane AN, Fan TW. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015;43(4):2466–85. Epub 2015/01/30. gkv047 [pii] 10.1093/nar/gkv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee D, Nandagopal K. Phylogenetic analysis and in silico characterization of the GARS-AIRS-GART gene which codes for a tri-functional enzyme protein involved in de novo purine biosynthesis. Mol Biotechnol. 2009;42(3):306–19. Epub 2009/03/21. 10.1007/s12033-009-9160-1 . [DOI] [PubMed] [Google Scholar]
- 38.Fridman A, Saha A, Chan A, Casteel DE, Pilz RB, Boss GR. Cell cycle regulation of purine synthesis by phosphoribosyl pyrophosphate and inorganic phosphate. Biochem J. 2013;454(1):91–9. Epub 2013/06/06. BJ20130153 [pii] 10.1042/BJ20130153 . [DOI] [PubMed] [Google Scholar]
- 39.Boison D, Scheurer L, Zumsteg V, Rulicke T, Litynski P, Fowler B, et al. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci U S A. 2002;99(10):6985–90. Epub 2002/05/09. 10.1073/pnas.092642899 092642899 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phizicky EM, Fields S. Protein-protein interactions: methods for detection and analysis. Microbiol Rev. 1995;59(1):94–123. Epub 1995/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.