Abstract
In Mediterranean subtidal rocky reefs, Cystoseira spp. (Phaeophyceae) form dense canopies up to 1 m high. Such habitats, called ‘Cystoseira forests’, are regressing across the entire Mediterranean Sea due to multiple anthropogenic stressors, as are other large brown algae forests worldwide. Cystoseira forests are being replaced by structurally less complex habitats, but little information is available regarding the potential difference in the structure and composition of fish assemblages between these habitats. To fill this void, we compared necto-benthic (NB) and crypto-benthic (CB) fish assemblage structures between Cystoseira forests and two habitats usually replacing the forests (turf and barren), in two sampling regions (Corsica and Menorca). We sampled NB fish using Underwater Visual Census (UVC) and CB fish using Enclosed Anaesthetic Station Vacuuming (EASV), since UVC is known to underestimate the diversity and density of the ‘hard to spot’ CB fish. We found that both taxonomic diversity and total density of NB and CB fish were highest in Cystoseira forests and lowest in barrens, while turfs, that could be sampled only at Menorca, showed intermediate values. Conversely, total biomass of NB and CB fish did not differ between habitats because the larger average size of fish in barrens (and turfs) compensated for their lower densities. The NB families Labridae and Serranidae, and the CB families Blenniidae, Cliniidae, Gobiidae, Trypterigiidae and Scorpaenidae, were more abundant in forests. The NB taxa Diplodus spp. and Thalassoma pavo were more abundant in barrens. Our study highlights the importance of using EASV for sampling CB fish, and shows that Cystoseira forests support rich and diversified fish assemblages. This evidence suggests that the ongoing loss of Cystoseira forests may impair coastal fish assemblages and related goods and services to humans, and stresses the need to implement strategies for the successful conservation and/or recovery of marine forests.
Introduction
Habitat degradation, including the loss of structural complexity (e.g. loss of structural components such as boulders, trees or corals) [1], is recognized as a major threat to terrestrial, aquatic and marine ecosystems [2, 3]. This may affect ecological processes underlying abundances and distributions of organisms, community structures, ecosystem functions and ecosystem resistance and resilience. Ultimately, this may reduce the potential of the ecosystem to sustainably provide goods and services to humans [3–5].
In temperate subtidal seascapes worldwide, some macrophytes (seaweeds and seagrasses) may form structurally complex benthic habitats, such as kelp forests on hard bottoms and seagrass meadows on soft bottoms. These macrophyte-formed habitats are usually characterized by high biodiversity and high production rates [6]. However, these habitats are being degraded or lost worldwide due to a broad spectrum of anthropogenic and natural causes [7, 8]. This process has negative impacts on associated communities [9], including species that are of ecological and socio-economic importance, such as some fish [10, 11].
Mediterranean algal forests are formed in subtidal rocky reefs by Cystoseira (and some Sargassum) species (Phaeophyceae), forming a dense canopy up to 1 m high (depending on the species, site and season, e.g. [12, 13–15]). These habitats are suffering degradation as well [16], and past and ongoing losses of Cystoseira forests have been recorded throughout the Mediterranean Sea [17, 18–21]. Depending on the identity and intensity of natural and/or anthropogenic stressors, Cystoseira forests can be replaced by structurally less complex macroalgal habitats. For instance, in some areas with degraded water quality (e.g. eutrophication, increased turbidity, waste water discharge, other pollutants), Cystoseira forests can be replaced by turfs [22, 23] or shrubland-like habitats (hereafter ‘shrubs’), formed by Dictyotales, Sphacelariales and/or articulated Corallinales [24, 25]. In addition, herbivory can be a major cause of Cystoseira forest loss. For example, in areas where sea urchins are abundant (due to natural [26] and/or anthropogenic [27–29] stressors), they can over-graze erect macrophyte assemblages (including Cystoseira forests) and produce barren grounds, i.e. bare rocks covered only by encrusting corallinales, hereafter called 'barrens' [19, 26–29] (Fig 1).
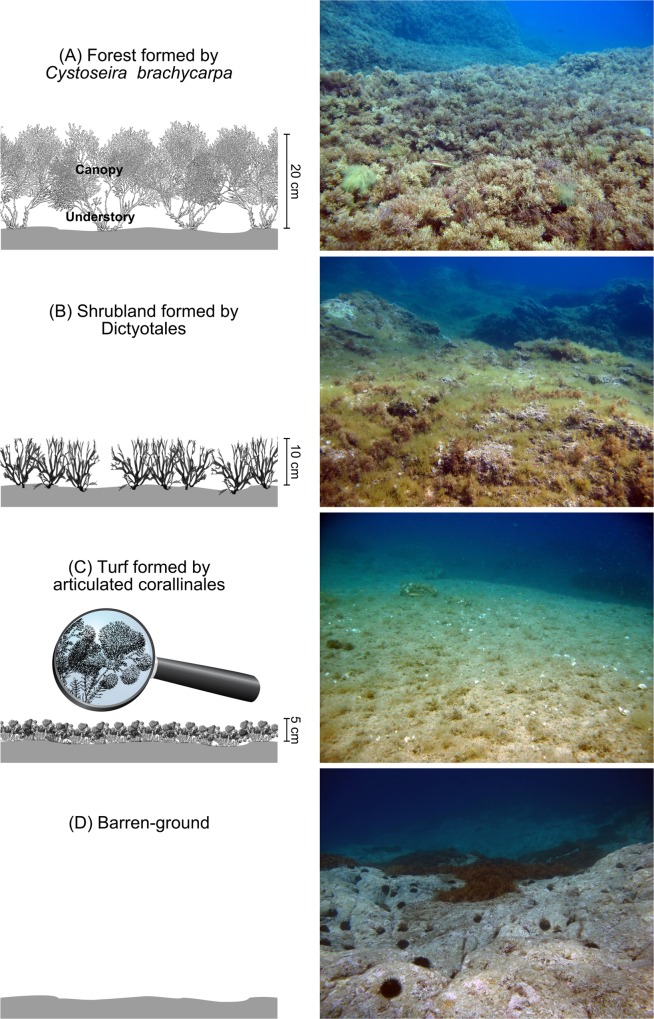
Fig 1. Four habitat types in North-Western Mediterranean subtidal rocky reefs.
(A) forest formed by the locally threatened species Cystoseira brachycarpa var. balearica, and 3 habitat types that may replace lost Cystoseira forests: (B) shrubs formed by Dictyotales and Sphacelariales, (C) turf formed by articulated corallinales, and (D) barren characterized by the absence of erect macrophytes. Upper panel: schematic representations of the habitat structure provided by the dominant macrophytes. Habitat complexity decreases from A to D. Lower panel: pictures taken in Corsica during summer 2011, at 8 m depth. Foregrounds span around 2 m width. Modified from Thiriet et al. [30].
Although the impact of kelp forest loss on coastal ecosystems worldwide is well known [6, 31], effects of Mediterranean Cystoseira forest losses on the associated assemblages remain poorly understood. This is mainly because 1) time series analyses are usually not feasible due to a general lack of historical data on Mediterranean subtidal rocky reef ecosystem structures (but see [19]), and 2) Cystoseira forest large-scale removal experiments may not be acceptable from a conservation point of view since the recovery of the Cystoseira forest would be slow (> 10 years) or even null [18, 32]. Using the ‘space for time’ approach therefore appears a likely solution to gain insights into the possible effects of Mediterranean Cystoseira forest losses although it cannot control all the alternative hypotheses that may explain the results obtained through this approach.
Sala et al. [33] compared fish assemblage structure within and outside marine reserves in rocky habitats (8–12 m deep, in different algal assemblages) throughout the Mediterranean Sea. The authors identified 4 main ecosystem states. These included one ‘predator dominated’ state with high fish biomass and extensive shrubs, occurring inside the well-enforced marine reserves which prohibit fishing of sea urchin predators, and 3 states occurring outside the well-enforced marine reserves which were poorly protected or unprotected. The authors expected Cystoseira forest to be indicative of ‘healthy’ rocky reefs and to be associated with high fish biomasses in well-enforced marine reserves. However, most of the Cystoseira forests were found in unprotected (fished) localities and therefore fish biomass in forests was lower than that recorded in well-protected (unfished) localities, generally characterized by shrubs.
A few other studies [34–36] have compared fish assemblage structure between Cystoseira forest and other habitats. Despite potential biases related to possible confounding effects from variability in abiotic features known to affect fish assemblages (e.g. depth, substrate nature and rugosity [37–39]), results suggested the importance of Cystoseira forests for some fish taxa, at least for some life stages. One study [40] resolved confounding effects by comparing juvenile fish assemblage structure between patches of Cystoseira forests and patches of shrubs sharing the same abiotic features within the same localities/protection levels, and highlighted that juvenile Symphodus spp. densities were higher in patches of Cystoseira forests while juvenile Coris julis densities were lower, and juveniles of all other fish taxa showed no significant difference between habitats.
The above-mentioned studies estimated fish assemblage structure using Underwater fish Visual Census (UVC). UVC has the main advantage of being non-destructive, and is particularly suitable for assessing necto-benthic (NB) fish, which are conspicuous fish swimming just above the substrate [41]. However, UVC underestimates richness and densities of crypto-benthic (CB) fish (e.g. Blenniidae and Gobiidae), which are 'hard to spot' due to some morphological (small body-size and/or camouflage) and/or behavioural traits (motionless and/or hiding within shelter) [42–45]. Consequently, the sole use of UVC may result in an incomplete picture of fish assemblage composition and density patterns biased towards conspicuous NB fish. CB fish assemblage structure can reliably be assessed only by using harvesting methods (e.g. using anesthetic such as quinaldine or piscicide such as rotenone: [42, 43–48]).
Kovačić et al. [47], for the first time in the Mediterranean Sea, used a quantitative harvesting method specifically designed to sample CB fish by using quinaldine within a 1 m² sampling area. This enabled sampling of a higher number of CB species compared to previous studies using UVC [49, 50], and assessment of CB fish densities, which is an improvement on previous qualitative harvesting methods (e.g. [51]). Thus, Kovačić et al. [47] highlighted the high diversity and densities of CB fish inhabiting various benthic habitat types (from 1 to 20 m depth, in the Adriatic Sea). Unfortunately, this study [47] and the previous ones on CB fish [49–51] did not include Cystoseira forests. CB fish assemblages associated with Cystoseira forests remain therefore mostly unknown, although they may have important roles in ecosystem functioning [46].
To fill this gap and to assess the potential role of the Cystoseira forest for fish assemblages, we carried out a spatial comparison of small-medium (total length < 30 cm) fish assemblage structure between Cystoseira forest and two structurally less complex habitat types usually replacing the forests (turf and barren). In each of the 3 habitat types investigated, we sampled NB fish using UVC, and for the first time, CB fish using Enclosed Anaesthetic Station Vacuuming (EASV).
Material and Methods
Sampling design
We sampled fish and macrophyte assemblages within 2 regions of the North-Western Mediterranean Sea: Corsica (10 sites, May 2011) and Menorca (13 sites, July 2011). For logistical reasons, we did not sample the two regions at the same time. However, we sampled both regions during the period of maximum temporal stability of macrophyte biomass (late spring to early summer [15]) to minimize the potential effects of variation in habitat structures, which may have impacted fish assemblages. Within each of the two region-time combinations (Corsica-May and Menorca-July), we sampled two localities (L): one protected (within a marine protected area, L1 and L3 respectively in Corsica and Menorca) and one unprotected (outside marine protected area, L2 and L4 respectively in Corsica and Menorca) (Fig 2). We aimed to sample all habitat types (forest, shrub, turf and barren) within each locality in order to avoid possible confounding effects between the putative effects of habitat types and inter-locality variations related to natural variations and/or potential protection effects (which are at present not distinguishable [52]). Within each locality, we found 1 to 4 sampling sites (750 to 1000 m² areas) of both forest and barren. Turf was only sampled at 4 sites in Menorca (within L4). We did not find suitable areas for sampling shrub (Fig 2, and S2 Table for geographical coordinates of all sites). This was due to our stringent procedure of sampling site selection, which was as follows. Within each locality, sampling sites were randomly chosen among the 750 to 1000 m² areas that fulfilled two criteria: (1) at least 80% of the area was covered by one of the 4 targeted habitat types, and (2) the whole area was between 4 m to 9 m in depth, presenting only monolithic rock (as opposed to blocks, pebbles etc.), with gentle slope (0° to 15°) and low substrate rugosity (i.e. holes, steps, crevasses and overhangs were avoided). These abiotic features known to affect fish assemblage structure [39] were constrained in order to avoid possible confounding effects. The surface area of sampling sites (750 to 1000 m²) was chosen as a trade-off between (1) a surface area small enough so that it was possible to find sufficient areas fulfilling all of the above criteria for each habitat type within each locality, and (2) a surface area large enough so that it may be regarded as a habitat rather than a patch, at least for low mobility organisms (see below).
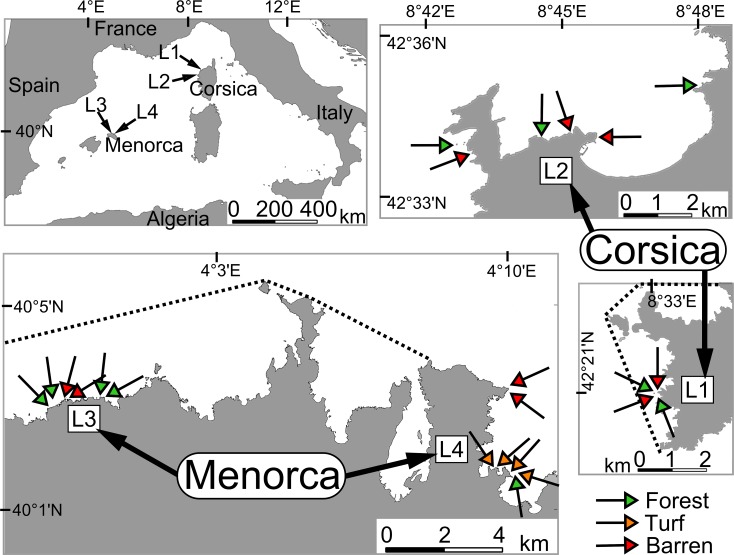
Fig 2. Location of the sampling sites.
Green filled arrows indicate forest sites, red filled arrows indicate barren sites, orange filled arrows indicate turf sites. Localities 'L1' and 'L3' were within the Marine Protected Areas (MPA) Scandola Marine Reserve and Norte de Menorca Marine Reserve, respectively. Dotted lines indicate MPA boundaries. Localities 'L2' and 'L4' were both outside MPAs. See also S2 Table for geographical coordinates of all sites. Public domain source of backgrounds maps: OpenStreetMap contributors, available under ODbL licence at http://www.openstreetmap.org/. Figure modified from Thiriet et al. [30].
Data collection
Ethics statement
Small surfaces (625 cm²) of macrophyte communities were harvested (using chisel and hammer) to perform species identification and biomass assessment in the laboratory. Removal of algae was necessary since non-destructive methods (e.g. visual estimation of percent cover) did not allow for quantification of the understory macrophyte assemblages. Even though knowledge regarding the role of Cystoseira species in coastal ecosystems is improving, as is the awareness that they may be locally threatened (for this reason most Cystoseira species are listed in the Bern Convention and in the Aspim Protocol), no conservation measures have yet been adopted at the national or international level. Independently of regulations, the surface area sampled and the number of samples were kept to the minimum. Care was taken to avoid sampling isolated populations or damaged / declining forests.
The non-destructive methodology UVC was used to gather data on NB fish. However, UVC is not suitable for gathering data on CB fish. Thus, we harvested CB fish by scuba diving using EASV (see below). Fish were anesthetized before being collected by spraying locally (1 m²) 2 L of 5 ppm quinaldine solution (0.01 L of quinaldine, 0.1 L of acetone and 1.89 L of seawater). After each dive, collected fish, still anesthetized, were killed immediately by anaesthesia overdose (immersion in a 2 L tank filled with 25 ppm quinaldine solution: 0.05 L of quinaldine, 0.5 L of acetone and 1.45 L of seawater), following Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. The EASV protocol did not require animal ethics committee approval since fish were killed in the field directly after collection (no housing, husbandry nor experiments) using anaesthesia overdose. No negative effects of EASV on the benthic community were recorded during the sampling. A few mobile macroinvertebrates were caught unintentionally on rare occasions. They were released alive and unharmed after each dive. Sessile benthic organisms did not show any damage related to the EASV sampling procedure. The whole experimental protocol was approved by the relevant regulatory bodies of each sampling locality. Permission for sampling in Locality 1 (Corsica, inside the MPA Réserve Naturelle de Scandola) and Locality 2 (Corsica, unprotected) was issued by the Direction Interrégionale de la Mer Méditerranée in the form of the prefectural ruling Décision du 8 avril 2011. Additional permission for sampling in Locality 1 (inside MPA) was issued by the Parc Naturel Regional de Corse (the institution managing the MPA). The permission for sampling in Locality 3 (Menorca, inside the MPA Reserva del Nord de Menorca) and Locality 4 (Menorca, unprotected) was issued by the Direccio General de Pesca, Govern de Illes Balears (the Spanish administration in charge of Maritime affairs in Menorca, managing the MPA).
Macrophyte Assemblage
We measured biomass of macrophytes in order to verify a posteriori that sampling sites (visually selected) were appropriately classified into meaningful and objective habitat types, and to describe the macrophyte assemblages. While scuba diving, we scraped (using chisel and hammer) all non-encrusting macrophytes in three replicate 25 x 25 cm² quadrats at each site. Each sample was placed in an individual zip-lock bag. After the dive, macrophyte samples were individually removed from their bags, wrapped in a terrycloth soaked with 70% alcohol, packed again in a hermetic bag and stored in a cooler until we reached the field laboratory where we stored samples in a freezer. Macrophyte biomass was measured within 3 days after collection. Excess water and alcohol were removed from samples by centrifuging them using a salad spinner for 30 seconds [33]. Samples were individually sorted and weighed using operational taxonomic units (S1 Table). In order to characterize fish habitat types, macrophytes were pooled into 6 functional groups before data analyses: (1) canopy-forming macrophytes (mostly Cystoseira brachycarpa var. balearica, with sometimes less than 5% of C. compressa and/or Sargassum spp.), (2) large erect macrophytes (e.g. Dictyota spp.), (3) small erect macrophytes (e.g. Acetabularia acetabulum), (4) turf-forming articulated corallinales, (5) turf-forming filamentous macrophytes, and (6) massive macrophytes (i.e. Codium bursa) (S1 Table).
Fish assemblage
Given the extent of our sampling sites (750 to 1000 m²), we did not take into account some NB fish that clearly move on broader spatial scales, such as transient predators (e.g. Dentex dentex), shoaling species (e.g. Chromis chromis, Oblada melanura, Sarpa salpa) and also large (Total Length, TL > 30 cm) resident fish (e.g. (sub-) adult Epinephelus marginatus, large-sized Diplodus spp.). We restricted our fish surveys to fish individuals that were a priori more sedentary at the scale of our sampling sites, hereafter referred as ‘small-medium resident fish’, which were juveniles of all CB and NB fish species, along with all older life stages for the small-medium species (maximum TL <30 cm) or only some of the older stages for larger species, depending on their maximum TL. Hence, in the present study, ‘all fish’ refers only to all individuals of small-medium resident fish. Likewise, ‘total density’ and ‘total biomass’ also refer to small-medium resident fish.
We used EASV to sample CB fish, which were defined in the present study as the 'hard to spot' fish individuals (see [47] for other definitions), including (1) early juveniles of NB species, which are small-sized individuals (TL < 25 mm for Labridae, < 35 mm for Serranidae) spending most of their time hidden within macrophytes [53], and (2) all life stages of CB fish species (e.g. Blenniidae and Gobiidae). During daylight (10AM–4PM), we conducted 3 replicate EASV samples of 1 m² at each site. The 1 m² sample area was enclosed by a perimeter fence and all fish inside were collected using anaesthetic and an air-lift pump (Fig 3). The perimeter fence (0.56 m in radius, 1 m in height) was a circular 1 mm nylon mesh mounted on a metal hoop. The base of the perimeter fence was extended by a tissue strip (0.25 m in width) weighted with galvanized chain so that the base of the perimeter fence could be moulded to the substrate shape. Two litres of anaesthetic solution (5 ppm quinaldine solution: 1 cl of quinaldine, 10 cl of acetone, 189 cl of seawater) were sprayed 15 cm above the substrate [47]. One minute later, fish were collected by vacuuming using an air-lift pump (with a 1mm mesh collecting bag). The pump head was moved all around the 1 m² sample area for 2 minutes. After the dive, fish samples, still anesthetised, were killed by an anaesthetic overdose and stored in plastic tubes filled with 70% alcohol. In the laboratory, samples were sorted, and individuals were measured (to the nearest mm), weighed (mg), and identified to the species level whenever possible, or alternatively to the family level. EASV samples contained both CB and NB fish individuals. NB fish were removed in order to prevent overlap with NB sampled using UVC.
Fig 3. Quantitative sampling of crypto-benthic fish using Enclosed Anaesthetic Station Vacuuming.
Steps include: (A) Setting-up the perimeter fence by arriving vertically from 2 m above the substrate, and moulding the base of the perimeter fence (weighted with galvanized chain) to the substrate in order to avoid fish escapes; (B) Spraying of the anaesthetic and waiting for 1 minute; (C) Vacuuming for 2 minutes using an air-lift sampler; (D) Closing the collecting bag as soon as the vacuuming session ends. Modified from Thiriet et al. [30].
We used UVC for sampling small-medium resident NB fish, which are late juveniles of NB species (≥ 25 mm for Labridae, ≥ 35 mm for other taxa) that reached the NB behaviour stage [53] and (sub-) adult fish individuals (TL <30 cm) belonging to NB species, hereafter referred as ‘NB fish’. We did not use the standard method 5 x 25 m² transect [41] because it would have not been possible to fit multiple 125 m² replicates within our sampling sites (750 to 1000 m²) and to meet the independence assumption. Instead, we used 9-m² stationary-point snapshot-count, conducted during daylight (10AM–4PM). Six random replicates, which were at least 10 m apart from each other, were done at each site. The 9-m² sampling area was a semicircle 2.5 m in radius in front of the diver, excluding the inner semicircle 0.7 m in radius nearest to the diver (S1 Fig). The diver did a snapshot count of every NB fish individual inside the sampling area at the time the census started, by estimating the species and the body size (total length to the nearest 0.5 cm for fish ≤ 5cm, to the nearest 1 cm for larger fish). Fish biomass was estimated using the existing length—weight relationship from the literature [54, 55].
Data analyses
Data pre-processing
In order to analyse relationships between macrophytes and NB and CB fish, data for algae, CB and NB fish (each stored in a database) were aggregated at the site level, which was the smallest sampling unit shared by the 3 databases. Biomass (and/or densities) was averaged over replicates and mean values (for each site) were stated in grams (and/or number of individuals) per 1 m² for macrophytes and per 10 m² for fish. These values were used for all statistical analyses. Using sites as statistical units did not lower the power of the analyses of variances (see below) when comparing inter-habitat variability over intra-habitat (inter-site within habitat) variability [56].
Habitat types
In order to verify a posteriori that sampled sites were appropriately classified into meaningful and objective habitat types (forest, turf, or barren), biomass of the 6 macrophyte functional groups was used for clustering sites into internally homogenous groups of habitat types, by running the PRIMER routine combining hierarchical clustering (group-average) and Type 1 SIMPROF test (defining the most appropriate number of clusters), on Bray-Curtis dissimilarity matrices with square root transformed data [57, 58].
Fish assemblages
To compare multiple aspects of fish assemblage structure between habitat types, we considered 9 multivariate descriptors, combining 3 sets of fish category (only CB fish sampled by EASV, only NB fish sampled by UVC and all fish) and 3 types of metrics (presence/absence, density and biomass). Jaccard similarity was used on presence/absence, and Bray-Curtis dissimilarity was used on square root transformed densities and biomasses. Similarly, nine univariate descriptors were also used: number of taxa, total density and total biomass for each of the 3 sets of fish category.
Based on each descriptor (multivariate or univariate), we tested for putative differences between forest and barren, by using 3-way permutational (multivariate or univariate) analyses of variance (PERMANOVAs): factor region-time ('RT', fixed, 2 levels: Corsica-May and Menorca-July), factor locality-protection ('LP', fixed, 2 levels nested within each 'RT' level), factor habitat ('HA', fixed, 2 levels: forest, barren). The habitat turf was excluded from the PERMANOVA design because turf sites were sampled only within locality L4 of Menorca-July, this would have induced a large amount of empty cells in the design. Because the design was still unbalanced, we used Type III sum of squares (SS). P-values were obtained by 9999 permutations of residuals under a reduced model. Post-hoc pair-wise comparisons were used when appropriate. Univariate PERMANOVA were based on Euclidean distances which makes this a non-parametric test that is equivalent to a parametric ANOVA but free from the assumption of normality of residuals [59].
To identify groups of fish taxa responding similarly to factors evidenced as significant by PERMANOVA on densities of all fish, we performed Type 2 and Type 3 SIMPROF tests. Densities were averaged for each level of the factor combining all significant factors (i.e. the combination of habitat and region-time, see ‘Results‘). Type 2 SIMPROF test tested the null hypothesis of 'no associations among taxa'. Type 3 SIMPROF test was used to identify statistically distinct groups of taxa, by combining hierarchical clustering (group-average) of taxa and Type 2 SIMPROF test (see [58] for more details). Only taxa that occurred in at least 4 out of the 23 sampling sites were retained since the method is sensitive to the inclusion of the rarest taxa [58]. For this test (very conservative since it controls experiment-wise type I error rate [58]), we used the threshold 0.1 as significance level instead of the common threshold 0.05, since we aimed to explore ecological trends rather than to test ecological inferences. For all other statistical significance tests (of inferences), we used 0.05 as threshold.
To visualize multivariate patterns, Principal Coordinates Analyses (PCoA) were used on the 3 dissimilarity matrices involving all fish taxa. Due to high variability among replicates that prevented getting reliable visualizations of dissimilarities in 2D (first two PCoA axes), we used only centroids of each level of the factor combining all factors (HA x RT x LP(RT)).
All SIMPROF tests and PERMANOVAs were performed using the PRIMER 6 and PERMANOVA + B20 package [60, 61]. All graphical visualizations were performed in R Environment [62] using the libraries vegan [63] and ggplot2 [64].
As a supplement, we investigated fish body-size (total length) distributions in order to (1) assess the relative contributions of CB and NB fish to total fish density and total fish biomass, and (2) visualize putative differences among habitats. Methods and results of this complementary analysis are reported in S1 Text and S2 Fig.
Results
Habitat types
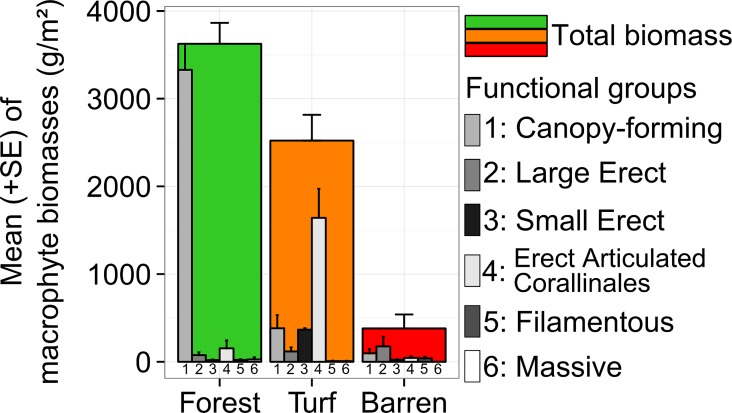
Biomass of the 6 macrophyte functional groups was not homogeneous among sites (Type 1 SIMPROF test, π = 2.186, p = 0.02). The combined clustering/SIMPROF analysis showed that 3 groups of sites were significantly different from each other but internally homogeneous. The clustering matched with our a priori grouping of sampling sites by habitat types (presented in Fig 2). The habitat forest exhibited the highest total macrophyte biomass (Fig 4). It was dominated by Fucales (> 90% of Cystoseira brachycarpa var. balearica in both Corsica and Menorca) forming a dense canopy (around 15 to 20 cm in height). The habitat turf exhibited lower total macrophyte biomass (70% of forest's biomass). It was dominated by erect articulated Corallinales forming a dense layer of turf (around 5 cm thick). The turf layer sometimes smothered short-sized individuals of Fucales and/or was sparsely epiphyted by some other erect macrophytes. The habitat barren exhibited very low total macrophyte biomass (10% of forest's biomass). In some barren sites, short-sized individuals of Fucales (< 5cm) and/or of erect non canopy-forming macrophyte (mostly Padina sp.) were sparsely present.
Fig 4. Macrophyte assemblage structures discriminating the 3 habitat types.
Mean total macrophyte biomass (+SE) and mean biomass (+SE) of the 6 macrophyte functional groups for each of the 3 habitat types sampled (see also Fig 1A, 1C and 1D). Modified from Thiriet et al. [30].
Multivariate descriptors of fish assemblage structure
All 9 multivariate descriptors considered were significantly different between forest and barren, and between Corsica-May and Menorca-July (Table 1). This showed that (1) the differences in the whole fish assemblage structure were due to both the subsets of CB and NB fish, and (2) the differences in fish assemblage structure were in terms of taxa composition, and possibly also in terms of densities and biomass.
Table 1. Results of multivariate PERMANOVAs comparing fish assemblage structure between forest and barren.
| All fish | Crypto-benthic fish | Necto-benthic fish | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Data and dissimilarity measure used | Source | df | SS | F | SS | F | SS | F | |||
| Jaccard on presence / absence | HA | 1 | 7334.8 | 5.17 | *** | 4254.2 | 3.50 | * | 9762.7 | 8.06 | *** |
| RT | 1 | 7428.1 | 5.23 | *** | 6140.4 | 5.06 | ** | 7296.3 | 6.02 | *** | |
| LP(RT) | 2 | 3296.0 | 1.16 | ns | 1022.6 | 0.42 | ns | 3809.2 | 1.57 | ns | |
| RTxHA | 1 | 2056.1 | 1.45 | ns | 2626.2 | 2.16 | ° | 997.2 | 0.82 | ns | |
| LP(RT)xHA | 2 | 3570.9 | 1.26 | ns | 1508.7 | 0.62 | ns | 4356.3 | 1.80 | ° | |
| Residual | 11 | 15613.0 | 13359.0 | 13332.0 | |||||||
| Bray-Curtis on square root transformed densities | HA | 1 | 5139.7 | 4.08 | ** | 4389.9 | 4.14 | * | 8452.9 | 10.36 | *** |
| RT | 1 | 7498.9 | 5.96 | *** | 7209.2 | 6.81 | *** | 5860.0 | 7.18 | *** | |
| LP(RT) | 2 | 3023.3 | 1.20 | ns | 1076.5 | 0.51 | ns | 3456.5 | 2.12 | ° | |
| RTxHA | 1 | 2872.2 | 2.28 | ° | 2369.0 | 2.24 | ° | 1598.7 | 1.96 | ns | |
| LP(RT)xHA | 2 | 3177.1 | 1.26 | ns | 1354.1 | 0.64 | ns | 3640.2 | 2.23 | ° | |
| Residual | 11 | 13849.0 | 11652.0 | 8977.9 | |||||||
| Bray-Curtis on square root transformed biomasses | HA | 1 | 5621.5 | 5.75 | *** | 6097.4 | 2.99 | * | 5629.7 | 6.10 | *** |
| RT | 1 | 6475.2 | 6.62 | *** | 5491.8 | 2.70 | * | 6576.4 | 7.13 | *** | |
| LP(RT) | 2 | 5776.4 | 2.96 | ** | 3036.8 | 0.75 | ns | 5685.4 | 3.08 | *** | |
| RTxHA | 1 | 2272.3 | 2.32 | ° | 3810.9 | 1.87 | ° | 2234.4 | 2.42 | ° | |
| LP(RT)xHA | 2 | 4075.9 | 2.09 | ° | 2987.4 | 0.73 | ns | 4184.4 | 2.27 | ° | |
| Residual | 11 | 10751.0 | 22401.0 | 10149.0 | |||||||
HA: habitat; RT: region-time; LP: locality-protection.
ns not significant
° p < 0.1
* p < 0.05
** p < 0.01
*** p < 0.001.
When considering fish assemblage composition (presence/absence of all fish), the inter-habitat and inter- region-time differences were additive (Table 1 and Fig 5A). When considering fish densities, inter-habitat differences appeared slightly higher within Menorca-July than within Corsica-May (Table 1 and Fig 5B). In contrast, when considering fish biomass, inter-habitat differences appeared slightly lower within Menorca-July than within Corsica-May (Table 1 and Fig 5C).
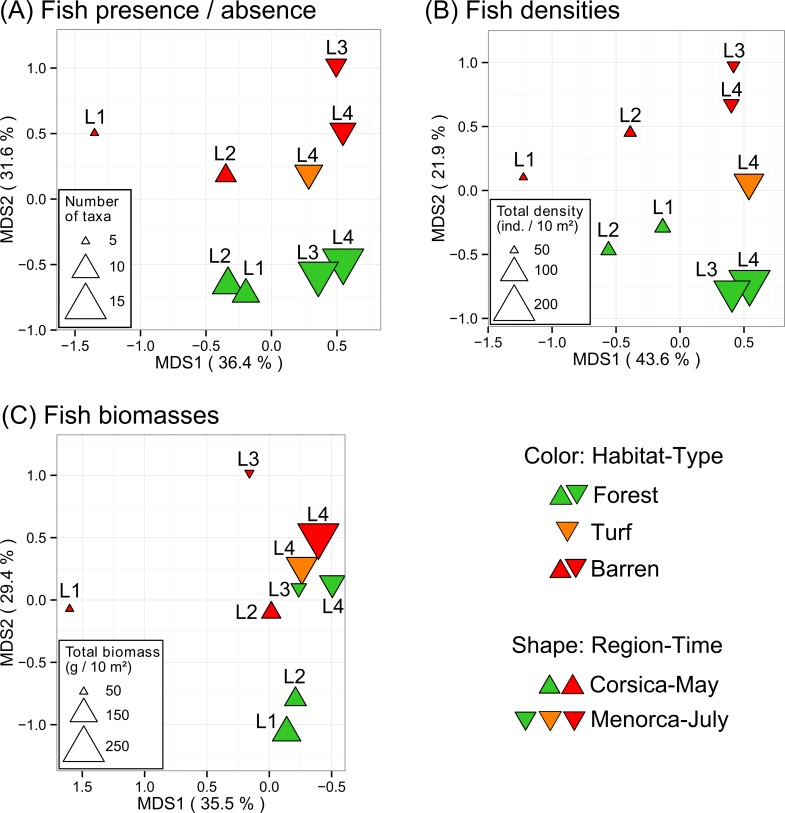
Fig 5.
Fish assemblage structure compared among habitats and regions-times, in terms of (A) presence / absence, (B) densities, and (C) biomasses of all crypto- and necto- benthic fish. Principal coordinates analyses (PCoA) were built using dissimilarities among centroids of each levels of the combined factor habitat X locality-protection (region-time), which were computed using Jaccard dissimilarity for presence / absence data and Bray-Curtis dissimilarity for both square root transformed densities and biomass data. First two axes (MDS 1 and 2) are plotted and percentages of explained variance are indicated within brackets. Labels refer to the 4 locality-protection levels (see Fig 2). Modified from Thiriet et al. [30].
Considering the habitat turf (not included in PERMANOVAs, see Material & Methods section) sampled only within Locality 4 (L4), the centroid of turf X L4 was positioned between the centroids of barren X L4 and forest X L4, particularly on PCoA biplot based on presence/absence (Fig 5A) and densities (Fig 5B).
Univariate descriptors of fish assemblage structure
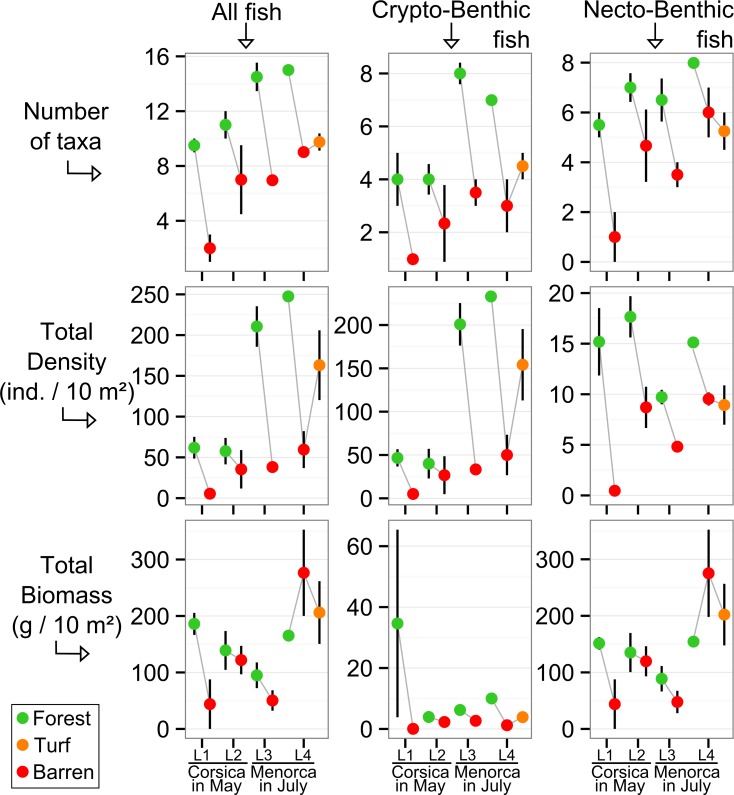
The number of taxa of all fish, of the subset CB fish and of the subset NB fish were all significantly higher in forest than in barren (Table 2 and Fig 6). The number of taxa of all fish and of the subset CB fish were both significantly higher in Menorca-July than in Corsica-May (Table 2 and Fig 6). The number of taxa of all fish, of the subset CB fish and of the subset NB fish, were similar between the turf sites and the barren sites of the same locality (see L4 in Fig 6).
Table 2. Results of univariate PERMANOVAs comparing fish assemblage structure between forest and barren.
| All fish | Crypto-benthic fish | Necto-benthic fish | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Response variable | Source | df | SS | F | SS | F | SS | F | |||
| Number of taxa | HA | 1 | 159.6 | 29.50 | *** | 44.3 | 23.00 | *** | 35.8 | 13.72 | ** |
| RT | 1 | 65.4 | 12.08 | ** | 26.4 | 13.72 | ** | 8.7 | 3.33 | ° | |
| LP(RT) | 2 | 28.1 | 2.60 | ns | 2.1 | 0.54 | ns | 23.1 | 4.44 | * | |
| RTxHA | 1 | 1.0 | 0.19 | ns | 3.8 | 1.95 | ns | 0.9 | 0.33 | ns | |
| LP(RT)xHA | 2 | 8.4 | 0.77 | ns | 1.2 | 0.31 | ns | 3.3 | 0.63 | ns | |
| Residual | 11 | 59.5 | 21.2 | 28.7 | |||||||
| Total density | HA | 1 | 492.8 | 39.16 | *** | 420.5 | 34.95 | *** | 2.9 | 39.54 | *** |
| RT | 1 | 399.7 | 31.77 | *** | 406.8 | 33.81 | *** | 0.0 | 0.43 | ns | |
| LP(RT) | 2 | 19.2 | 0.76 | ns | 12.1 | 0.50 | ns | 1.1 | 7.49 | ** | |
| RTxHA | 1 | 203.0 | 16.13 | ** | 223.5 | 18.57 | ** | 0.5 | 6.77 | * | |
| LP(RT)xHA | 2 | 8.1 | 0.32 | ns | 5.9 | 0.25 | ns | 0.2 | 1.38 | ns | |
| Residual | 11 | 138.4 | 132.7 | 0.8 | |||||||
| Pairwise tests Forest vs Barren | Corsica: t = 2.05°; Menorca: t = 5.86** | Corsica: t = 1.50ns; Menorca: t = 5.70 *** | Corsica: t = 5.27**; Menorca: t = 5.51** | ||||||||
| Total biomass | HA | 1 | 21.8 | 0.70 | ns | 6.0 | 3.42 | ns | 4.9 | 0.16 | ns |
| RT | 1 | 23.5 | 0.76 | ns | 1.1 | 0.62 | ns | 34.7 | 1.11 | ns | |
| LP(RT) | 2 | 395.3 | 6.38 | * | 4.9 | 1.38 | ns | 404.5 | 6.48 | * | |
| RTxHA | 1 | 130.6 | 4.22 | ° | 1.5 | 0.82 | ns | 104.6 | 3.35 | ° | |
| LP(RT)xHA | 2 | 202.1 | 3.26 | ° | 6.6 | 1.88 | ns | 166.7 | 2.67 | ns | |
| Residual | 11 | 340.7 | 19.4 | 343.0 | |||||||
HA: habitat; RT: region-time; LP: locality-protection.
ns not significant
° p < 0.1
* p < 0.05
** p < 0.01
*** p < 0.001.
Fig 6. Univariate descriptors of fish assemblage structure compared among habitats and regions-times.
Mean values (+/- SE) of the number of taxa (observed per site), the total density and the total biomass of all fish, only crypto-benthic fish and only necto-benthic fish, for each habitat x locality-protection (region-time) level combination. Modified from Thiriet et al. [30].
The total densities of all fish and of CB fish in Menorca-July were significantly higher in forest than in barren. This trend was almost significant within Corsica-May (Table 2 and Fig 6). The higher densities of CB fish in forests of Menorca-July compared to forests of Corsica-May were mainly driven by very small sized individuals (TLs between 5 and 15 mm) that were highly abundant in Menorca-July (see below description of group 6, and S1 Text and S2 Fig). The total NB fish density was significantly higher in forest than in barren within both regions, but this was more pronounced within Corsica-May (Table 2 and Fig 6). The total densities of (1) all fish and (2) only CB fish recorded in turf were intermediate between densities recorded in barrens (low) and in forests (high) of the same locality (Fig 6). Contrastingly, the total NB fish density recorded in turfs was similar to that recorded in barrens (Fig 6). None of the 3 total biomass variables (all fish, only CB and only NB) showed significant difference between habitat types or between regions-times (Table 2 and Fig 6).
Total CB fish density represented on average 92% of all small-medium resident fish density, and total CB fish biomass represented on average 17% of all small-medium resident fish biomass. This was related to the fact that total-lengths (and correlated body-weight) of CB fish were on average smaller than total-lengths of NB fish (Table 3, S1 Text and S2 Fig).
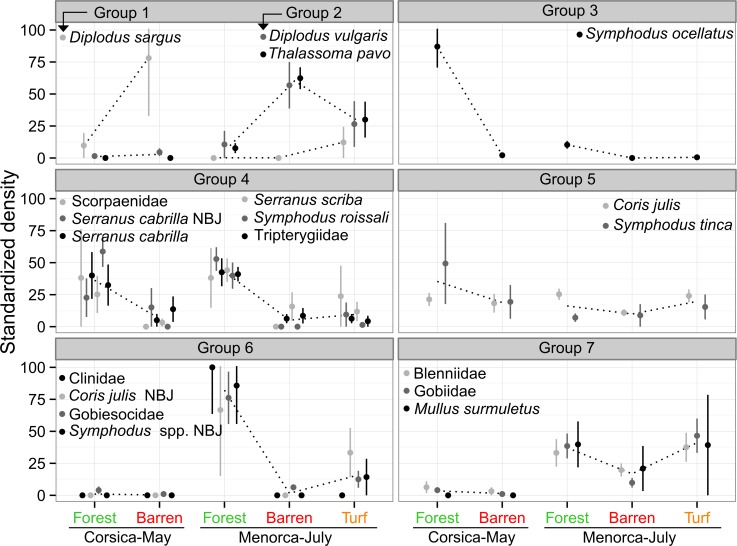
Table 3. Groups of fish sharing the same density variations across habitats and regions-times.
| G | Family | Taxa (and life history traits) | Size range in mm | Mean Size (SE) | Mean densities (SE) (indiv./ 10 m²) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Corsica-May | Menorca-July | ||||||||
| Forest (n = 5) | Barren (n = 5) | Forest (n = 5) | Barren (n = 4) | Turf (n = 4) | |||||
| 1 | Sparidae | Diplodus sargus (NB) | [80,300] | 144 (20.2) | 0.04 (0.04) | 0.3 (0.17) | - | - | 0.05 (0.05) |
| 2 | Labridae | Thalassoma pavo (NB) | [50,160] | 92.7 (3.1) | - | - | 0.3 (0.15) | 2.41 (0.33) | 1.16 (0.54) |
| Sparidae | Diplodus vulgaris (NB) | [60,160] | 109.5 (3.4) | 0.04 (0.04) | 0.11 (0.07) | 0.26 (0.26) | 1.39 (0.44) | 0.65 (0.44) | |
| Total G2 | 0.04 (0.04) | 0.11 (0.07) | 0.56 (0.38) | 3.8 (0.77) | 1.81 (0.67) | ||||
| 3 | Labridae | Symphodus ocellatus (NB) | [30,120] | 59.8 (1) | 7.52 (1.42) | 0.19 (0.19) | 0.89 (0.26) | - | 0.05 (0.05) |
| 4 | Labridae | Symphodus roissali (NB) | [25,150] | 70.6 (3.2) | 1.96 (0.4) | - | 1.33 (0.34) | - | 0.05 (0.05) |
| Scorpaenidae | [15,43] | 29 (5.1) | 1.33 (1.33) | - | 1.33 (0.82) | - | 0.83 (0.83) | ||
| Serranidae | Serranus cabrilla (NBJ) | [12,32] | 20 (2.1) | 2 (1.33) | 1.33 (1.33) | 4.67 (0.82) | - | 0.83 (0.83) | |
| Serranus cabrilla (NB) | [35,180] | 94.9 (6.7) | 0.59 (0.27) | 0.07 (0.07) | 0.63 (0.16) | 0.09 (0.05) | 0.09 (0.05) | ||
| Serranus scriba (NB) | [60,240] | 135.3 (6.8) | 0.3 (0.17) | 0.04 (0.04) | 0.52 (0.11) | 0.19 (0.13) | 0.14 (0.09) | ||
| Tripterygiidae (CBS) | [13,56] | 20.2 (1) | 12.67 (6.27) | 5.33 (3.89) | 16 (2.21) | 3.33 (2.36) | 1.67 (1.67) | ||
| Total G4a | 18.85 (6.69) | 6.78 (5.18) | 24.48 (2.53) | 3.61 (2.36) | 3.61 (1.29) | ||||
| 5 | Labridae | Coris julis (NB) | [25,250] | 88.9 (1.8) | 4.96 (1.19) | 4.26 (1.7) | 5.89 (1.02) | 2.55 (0.62) | 5.6 (1.18) |
| Symphodus tinca (NB) | [35,300] | 126.6 (9.3) | 1.04 (0.67) | 0.41 (0.28) | 0.15 (0.07) | 0.19 (0.19) | 0.32 (0.21) | ||
| Total G4b | 6 (1.01) | 4.67 (1.93) | 6.04 (1) | 2.73 (0.75) | 5.93 (1.31) | ||||
| 6 | Clinidae (CBS) | [10,23] | 17.9 (1.7) | - | - | 4.67 (1.7) | - | - | |
| Gobiesocidae (CBS) | [5,14] | 8 (0.2) | 2.67 (1.94) | 0.67 (0.67) | 50.67 (13.6) | 4.17 (1.6) | 8.33 (4.41) | ||
| Labridae | Coris julis (NBJ) | [15,21] | 18.7 (0.8) | - | - | 3.33 (2.58) | - | 1.67 (0.96) | |
| Symphodus spp. (NBJ) | [7,20] | 10.4 (1.1) | - | - | 10 (3.5) | - | 1.67 (1.67) | ||
| Total G5 | 2.67 (1.94) | 0.67 (0.67) | 68.67 (10.98) | 4.17 (1.6) | 11.67 (4.19) | ||||
| 7 | Blenniidae (CBS) | [15,43] | 21.5 (1) | 2.67 (1.94) | 1.33 (1.33) | 14 (4.52) | 8.33 (2.15) | 15.83 (4.79) | |
| Gobiidae (CBS) | [7,95] | 13.9 (0.6) | 10.67 (3.4) | 2.67 (1.94) | 101.33 (25.55) | 25.83 (9.85) | 122.5 (35.26) | ||
| Mullidae | Mullus surmuletus (NB) | [50,100] | 75 (2) | - | - | 0.7 (0.32) | 0.37 (0.31) | 0.69 (0.69) | |
| Total G6 | 13.33 (4.94) | 4 (2.45) | 116.04 (28.85) | 34.54 (8.56) | 139.03 (39.24) | ||||
G: groups delimited by Type 3 SIMPROF test (see also Fig 7); NB: late juveniles and (sub-) adult of necto-benthic species sampled by UVC; NBJ: early juveniles of necto-benthic species sampled by EASV; CBS: all life stages of crypto-benthic species sampled by EASV. Size are fish total lengths expressed in mm.
Groups of fish
The null hypothesis of 'no associations among species' was rejected (Type 2 SIMPROF test, π = 0.043, p = 0.021). Seven groups of taxa were identified as significantly different from each other but internally homogeneous (p-values < 0.1) in terms of their trends in density variations across habitat and regions-times (results of Type 3 SIMPROF tests in Fig 7 and Table 3).
Fig 7. Groups of fish sharing the same density variations across habitats and regions-times.
Mean standardized density (+/-SE) indicates variations of every fish taxon on a common scale even if their respective absolute densities may be different. NBJ: early juveniles of necto-benthic species sampled by EASV, while late juveniles and (sub-)adults were sampled by UVC. See Table 3 for detailed information about body-size and absolute densities of each fish taxon. Modified from Thiriet et al. [30].
Groups 1 and 2 were composed of NB species that were more abundant in barren but species were segregated by regions-times. Diplodus sargus (group 1) was recorded almost only in barren in Corsica-May. Diplodus vulgaris and Thalassoma pavo (group 2) were recorded almost only in Menorca-July, with higher densities in barren, intermediate in turf, and lower in forest.
Groups 3 to 6 were composed of fish generally more abundant in forest in at least one region-time. The NB Symphodus ocellatus (forming group 3) was highly abundant in forests of Corsica-May and was also abundant in forests of Menorca-July, while almost never recorded in barren and turf, irrespective of the region-time.
Group 4 was the larger and more diversified group, composed of both CB and NB fish with sizes ranging from 1 to 24 cm (Table 3), which were abundant in forest but rarely observed in other habitats, consistently across regions-times.
Group 5 was composed of the labrids Coris julis and Symphodus tinca, which were slightly more abundant in forest (especially for S. tinca), although densities were high in every habitat (Table 3).
Group 6 was composed essentially of CB juvenile fish, belonging to CB taxa (Clinidae and Gobiesocidae juveniles) and NB taxa (early juveniles of Coris julis and Symphodus spp. at the CB stage). They were almost exclusively recorded in Menorca-July (excepting Gobiesocidae) where they were more abundant in forest than in turf and almost absent in barren. The few individuals of Gobiesocidae recorded in Corsica-May (relatively to the other region-time, Table 3) were also more abundant in forest.
Group 7 was composed of CB fish belonging to the family Blenniidae and Gobiidae and of juveniles of the NB species Mullus surmuletus. They were mainly recorded in Menorca-July, with higher densities in forest and turf than in barren. The few individuals of Blenniidae and Gobiidae recorded in Corsica-May were more abundant in forest (Table 3).
Discussion
Taxonomic diversity and total density of small-medium resident fish were highest in Cystoseira forest and lowest in barren. Turf showed intermediate values, but this finding should be regarded with caution as turf was sampled only at 4 sites in Menorca. Total fish biomass did not differ between habitats because the larger average size of fish in barrens compensated for their lower densities. Effects of habitat were consistent between regions-times in terms of direction, but showed variability in their magnitude. This was mainly due to high densities of very small new settlers of Gobiesocidae, Clinidae, Blenniidae, Gobiidae, Coris julis and Symphodus spp. in Cystoseira forests of Menorca-July. This suggests that Cystoseira forests, at least in Menorca, act as nursery habitat for these species, as previously suggested for Symphodus spp. in Corsica [40]. Settler densities were considerably lower at Corsica-May probably due to the sampling period (i.e. May) that was too early to detect settlement peaks for these species (from late spring to autumn [30, 40, 65–68]). Therefore, observed difference between regions were likely due, at least in part, to seasonal variability.
Small-medium resident fish were also more diverse in Cystoseira forests in terms of trophic groups. Fish with the highest densities in Cystoseira forests included both juvenile and adult fish belonging to (1) the CB taxa Blenniidae, Gobiidae, Trypterigiidae and Cliniidae, which are omnivores, micro- or meso- carnivorous (depending on taxa and/or life stage), (2) the NB Labridae, which are mainly mesocarnivorous, and (3) the CB Scorpaenidae and NB Serranidae, which are meso- or macro- carnivorous (depending on life stages) whose food items include small-sized CB and/or NB fish [30, 69, 70]. In contrast, the only fishes that displayed the highest densities in barrens were the NB sea urchin feeders Diplodus spp. and Thalassoma pavo [28] (See S2 Text for more details).
Small-sized fish and large-sized macrocarnivorous fish cohabit at higher densities in Cystoseira forests. This may be due to lower mortality in Cystoseira forest (starvation- and/or predation- induced) and/or net immigration from other habitats (due to habitat selection toward Cystoseira forests) [71]. Lower mortality and habitat selection could both be related to: 1) increased food resources in Cystoseira forest (invertebrates for small-sized fish, and invertebrates and small-sized fish for macrocarnivorous fish [72, 73]) and/or 2) forest structural complexity providing shelters against predators (small sized-fish threatened by macrocarnivorous fish, and macrocarnivorous fish threatened by higher order predators such as Epinephelus marginatus or Dentex dentex [71]).
CB fish included omnivorous and micro-carnivorous fish while NB fish did not. Considering their relatively high densities (92% of small-medium resident fish density) and their exclusiveness at intermediate trophic positions (among small-medium resident fish), CB fish may play a crucial role with regard to the trophic functioning of Cystoseira forest-dominated ecosystems. This highlights the need for further research on CB fish assemblages, as they are underestimated by UVC sampling [46] and therefore have been largely understudied. As demonstrated in this study, EASV is an effective quantitative harvesting method that can be used in complex habitats to study CB assemblages.
Our finding of higher diversity and densities of small-medium resident fish in Cystoseira forest than in barren corroborates broad patterns of higher fish diversity and density in high complexity biotic habitats compared to adjacent, structurally less complex habitats. Examples include seagrass meadows compared to adjacent bare sediments [74–78], mangrove roots compared to adjacent mud flats [79–81], algal forests [59, 82] or other erect or turf-forming algae [27] compared to adjacent bare rocks. Studies that compared some components of the whole fish assemblage between Cystoseira forest and various habitat types [33–36, 40] also reported higher fish diversity and/or density and/or biomass in Cystoseira forests, at least for some fish taxa and/or at some of their life stages (see S2 Text). Although all the studies assessed potential variability using the same variables (i.e. diversity, density, biomass), the response of each single variable was inconsistent among the studies. Such discrepancies among studies (including ours) may be primarily related to: (1) differences in the fish assemblages studied, which were either the whole NB fish assemblage including transient and large resident fish [33–35], only NB juvenile fish [40], or the assemblage of small-medium resident CB and NB fish (the present study), and/or (2) the inaccurate sampling of CB fish using UVC [36], and/or (3) sampling designs where the results are confounded with protection levels [33] or abiotic variations [34–36].
Our study has significantly contributed to the knowledge of CB and NB small-medium resident fish assemblage structure in Cystoseira forests and barrens (and turf to some extent). Based on the differences between habitat types, we can speculate that Cystoseira forest degradation into barrens (and likely turfs) may reduce the density and diversity of small-medium resident fish. This includes both juvenile fish which are important for population replenishment as well as socio-economically important fish species such as Scorpaena spp. and Serranus spp. However, we note that our evidence relates to a relatively short observational period (May or July) in two different localities and that our findings are based on a space-for-time approach that does not allow consideration of all alternative hypotheses. A manipulative approach would enable a broader range of inference, but would need to include large-scale removal experiments of Cystoseira spp, which may not be appropriate given its conservation status. Hence, to better assess the community-wide impact of Cystoseira forest losses, it is crucial to set up long-term monitoring of Mediterranean subtidal macroalgal habitats and associated communities [19]. In addition, further space-for-time studies would be of value (1) to compare the assemblage structure of large and/or highly mobile NB fish between habitats and (2) to extend sampling locations and periods (all seasons during several years) in order to draw a more robust picture of the impact of changes in marine vegetation on fish assemblage structure.
Supporting Information
The 9m² sampling area was the semicircle 2.5 m in radius in front of the diver, without considering the inner part, semicircle 0.7 m in radius.
(TIFF)
Curves are smoothed histograms (Kernel density estimations) of total lengths of all sampled fish (crypto- or necto- benthic) within each level of the combined factor habitat X locality-protection (region-time). The surfaces below the curves (the integrals) are proportional to fish densities (abundance per sampling unit). Total length distributions are also presented using Tukey's boxplots. (See also S1 Text). Modified from Thiriet et al. [30].
(TIFF)
(DOCX)
Latitude (North) and Longitude (East) are in decimal degrees (See also Fig 2).
(DOCX)
(See also S2 Fig)
(DOCX)
A discussion enriched by previous studies on density patterns and fish life history traits found in the literature.
(DOCX)
Acknowledgments
The authors are grateful to all the staff of the 3 organizations that provided accommodation and logistical support (boat, driver, compressor) during field sampling: (1) the management board of the Marine Protected Area Réserve Naturelle de Scandola (part of the Parc Naturel Régional de Corse), directed by Jean-Marie Dominici, (2) the Station de Recherches Océanographiques et Sous-marines (STARESO), a private marine research station directed by Pierre Lejeune, and (3) the Estación de Investigación Jaume Ferrer, a marine research station (part of the Instituto Espanol de Oceanografia) that was directed by Joan Moranta at the time of our sampling campaign. The authors warmly thank Anne-Marie Corre of the Oceanographic Observatory of Villefranche-sur-Mer for her invaluable help in building the perimeter fences used for the Enclosed Anaesthetic Station Vacuuming. The authors are grateful to Prof. Bob Clarke (PRIMER-E and Plymouth Marine Laboratory Fellow) for his precious help with experimental design and statistical analyses. The authors also thank Thierry Thibaut for his help in determining macrophytes, and Eléonore Cambra for her help with map editing. The authors are grateful to Riccardo Cattaneo-Vietti for his help in the preliminary design of this study, and also warmly thank Philippe Lenfant and Gilles Lepoint for their helpful comments on an earlier version of the manuscript. The authors also thank Michael Paul for his help in English language editing.
The authors wish to thank the academic editor and two anonymous reviewers for their useful comments which have helped us to improve the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
PT Phd thesis was funded by the University Nice Sophia Antipolis; the work was partially realized in the framework of (and funded by) the Coconet European project (grant number 287844) and FOREFISH project funded by Total Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Beck MW. Separating the elements of habitat structure: independent effects of habitat complexity and structural components on rocky intertidal gastropods. Journal of Experimental Marine Biology and Ecology. 2000;249(1):29–49. 10.1016/s0022-0981(00)00171-4 . [DOI] [PubMed] [Google Scholar]
- 2.Keith DA, Rodríguez JP, Rodríguez-Clark KM, Nicholson E, Aapala K, Alonso A, et al. Scientific Foundations for an IUCN Red List of Ecosystems. PLoS One. 2013;8(5):e62111 10.1371/journal.pone.0062111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, et al. Consequences of changing biodiversity. Nature. 2000;405(6783):234–42. 10.1038/35012241 . [DOI] [PubMed] [Google Scholar]
- 4.Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. http://www.nature.com/nature/journal/v486/n7401/abs/nature11148.html#supplementary-information. 10.1038/nature11148 [DOI] [PubMed] [Google Scholar]
- 5.Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, et al. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annual Review of Ecology, Evolution, and Systematics. 2004;35:557–81. 10.1146/annurev.ecolsys.35.021103.105711 [DOI] [Google Scholar]
- 6.Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, et al. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation. 2002;29(4):436–59. 10.1017/s0376892902000322 . [DOI] [Google Scholar]
- 7.Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences. 2009;106(30):12377–81. 10.1073/pnas.0905620106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodie J, Williamson CJ, Smale DA, Kamenos NA, Mieszkowska N, Santos R, et al. The future of the northeast Atlantic benthic flora in a high CO2 world. Ecology and Evolution. 2014;4(13):2787–98. 10.1002/ece3.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smale DA, Burrows MT, Moore P, O'Connor N, Hawkins SJ. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecology and Evolution. 2013;3(11):4016–38. 10.1002/ece3.774 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmlund CM, Hammer M. Ecosystem services generated by fish populations. Ecol Econ. 1999;29(2):253–68. 10.1016/S0921-8009(99)00015-4 [DOI] [Google Scholar]
- 11.Jackson EL, Rees SE, Wilding C, Attrill MJ. Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conservation Biology. 2015:n/a-n/a. 10.1111/cobi.12436 [DOI] [PubMed] [Google Scholar]
- 12.Ballesteros E. Structure and dynamics of the Cystoseira caespitosa Sauvageau (Fucales, Phaeophyceae) community in the North-Western Mediterranean. Sci March 1990;54:155–68. [Google Scholar]
- 13.Ballesteros E, Garrabou J, Hereu B, Zabala M, Cebrian E, Sala E. Deep-water stands of Cystoseira zosteroides C. Agardh (Fucales, Ochrophyta) in the Northwestern Mediterranean: Insights into assemblage structure and population dynamics. Estuarine, Coastal and Shelf Science. 2009;82(3):477–84. [Google Scholar]
- 14.Ballesteros E, Sala E, Garrabou J, Zabala M. Community structure and frond size distribution of a deep water stand of Cystoseira spinosa (Phaeophyta) in the Northwestern Mediterranean. European Journal of Phycology. 1998;33(2):121–8. [Google Scholar]
- 15.Hoffmann L, Renard R, Demoulin V. Phenology, growth, and biomass of Cystoseira balearica in Calvi (Corsica). Marine Ecology-Progress Series. 1992;80(2–3):249–54. . [Google Scholar]
- 16.Claudet J, Fraschetti S. Human-driven impacts on marine habitats: A regional meta-analysis in the Mediterranean Sea. Biological Conservation. 2010;143(9):2195–206. 10.1016/j.biocon.2010.06.004 [DOI] [Google Scholar]
- 17.Thibaut T, Pinedo S, Torras X, Ballesteros E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Marine Pollution Bulletin. 2005;50(12):1472–89. 10.1016/j.marpolbul.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 18.Gianni F, Bartolini F, Airoldi L, Ballesteros E, Francour P, Guidetti P, et al. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine Protected Areas. Advances in Oceanography and Limnology. 2013;4(2):83–101. 10.1080/19475721.2013.845604 [DOI] [Google Scholar]
- 19.Bianchi CN, Corsini-Foka M, Morri C, Zenetos A. Thirty years after—dramatic change in the coastal marine habitats of Kos Island (Greece), 1981–2013. Mediterranean Marine Science. 2014;15(3):482–97. [Google Scholar]
- 20.Thibaut T, Blanfune A, Boudouresque C-F, Verlaque M. Decline and local extinction of Fucales in French Riviera: the harbinger of future extinctions? 2014.
- 21.Tsiamis K, Panayotidis P, Salomidi M, Pavlidou A, Kleinteich J, Balanika K, et al. Macroalgal community response to re-oligotrophication in Saronikos Gulf. Marine Ecology Progress Series. 2013;472:73–85. 10.3354/meps10060 [DOI] [Google Scholar]
- 22.Strain EMA, Thomson RJ, Micheli F, Mancuso FP, Airoldi L. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob Change Biol. 20(11):3300–12. 10.1111/gcb.12619 [DOI] [PubMed] [Google Scholar]
- 23.Connell SD, Foster MS, Airoldi L. What are algal turfs? Towards a better description of turfs. Marine Ecology Progress Series. 2014;495:299–307. 10.3354/meps10513 [DOI] [Google Scholar]
- 24.Airoldi L, Beck M. Loss, status and trends for coastal marine habitats of Europe. Oceanography and Marine Biology: an annual review. 2007;45:345–405. 10.1201/9781420050943.ch7 [DOI] [Google Scholar]
- 25.Mangialajo L, Chiantore M, Cattaneo-Vietti R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Marine Ecology-Progress Series. 2008;358:63–74. 10.3354/meps07400 . [DOI] [Google Scholar]
- 26.Cardona L, Moranta J, Reñones O, Hereu B. Pulses of phytoplanktonic productivity may enhance sea urchin abundance and induce state shifts in Mediterranean rocky reefs. Estuarine, Coastal and Shelf Science. 2013;133(0):88–96. 10.1016/j.ecss.2013.08.020 [DOI] [Google Scholar]
- 27.Guidetti P, Fraschetti S, Terlizzi A, Boero F. Effects of desertification caused by Lithophaga lithophaga (Mollusca) fishery on littoral fish assemblages along rocky coasts of southeastern Italy. Conservation Biology. 2004;18(5):1417–23. 10.1111/j.1523-1739.2004.00343.x . [DOI] [Google Scholar]
- 28.Guidetti P. Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecological Applications. 2006;16(3):963–76. 10.1890/1051-0761(2006)016[0963:mrrlpi2.0.co;2 . [DOI] [PubMed] [Google Scholar]
- 29.Hereu B, Zabala M, Sala E. Multiple controls of community structure and dynamics in a sublittoral marine environment. Ecology. 2008;89(12):3423–35. 10.1890/07-0613.1 [DOI] [PubMed] [Google Scholar]
- 30.Thiriet P. Comparisons of fish assemblage structure and underlying ecologigal processes, between Cystoseira forests and less structurally complex habitats, in North-Western Mediterranean subtidal rocky reefs. PhD Thesis, Université Nice Sophia Antipolis. 2014. Available: Agence bibliographique de l’Enseignement supérieur, http://www.theses.fr/en/2014NICE4061.
- 31.Graham MH. Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems. 2004;7(4):341–57. 10.1007/s10021-003-0245-6 . [DOI] [Google Scholar]
- 32.Mangialajo L, Chiantore M, Susini M-L, Meinesz A, Cattaneo-Vietti R, Thibaut T. Zonation patterns and interspecific relationships of fucoids in microtidal environments. Journal of Experimental Marine Biology and Ecology. 2012;412(0):72–80. 10.1016/j.jembe.2011.10.031 [DOI] [Google Scholar]
- 33.Sala E, Ballesteros E, Dendrinos P, Di Franco A, Ferretti F, Foley D, et al. The Structure of Mediterranean Rocky Reef Ecosystems across Environmental and Human Gradients, and Conservation Implications. PLoS One. 2012;7(2):e32742 10.1371/journal.pone.0032742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giakoumi S, Cebrian E, Kokkoris GD, Ballesteros E, Sala E. Relationships between fish, sea urchins and macroalgae: The structure of shallow rocky sublittoral communities in the Cyclades, Eastern Mediterranean. Estuar Coast Shelf Sci. 2012;109:1–10. 10.1016/j.ecss.2011.06.004 . [DOI] [Google Scholar]
- 35.Giakoumi S, Kokkoris GD. Effects of habitat and substrate complexity on shallow sublittoral fish assemblages in the Cyclades Archipelago, North-eastern Mediterranean sea. Mediterranean Marine Science. 2013;14(1):58–68. 10.12681/mms.v0i0.318 . [DOI] [Google Scholar]
- 36.Orlando Bonaca M, Lipej L. Factors affecting habitat occupancy of fish assemblage in the Gulf of Trieste (Northern Adriatic Sea). Marine Ecology. 2005;26(1):42–53. 10.1111/j.1439-0485.2005.00037.x [DOI] [Google Scholar]
- 37.Ruitton S, Francour P, Boudouresque CF. Relationships between Algae, Benthic Herbivorous Invertebrates and Fishes in Rocky Sublittoral Communities of a Temperate Sea (Mediterranean). Estuarine, Coastal and Shelf Science. 2000;50(2):217–30. 10.1006/ecss.1999.0546 [DOI] [Google Scholar]
- 38.Consoli P, Romeo T, Giongrandi U, Andaloro F. Differences among fish assemblages associated with a nearshore vermetid reef and two other rocky habitats along the shores of Cape Milazzo (northern Sicily, central Mediterranean Sea). Journal of the Marine Biological Association of the United Kingdom. 2008;88(02):401–10. 10.1017/S0025315408000489 [DOI] [Google Scholar]
- 39.Letourneur Y, Ruitton S, Sartoretto S. Environmental and benthic habitat factors structuring the spatial distribution of a summer infralittoral fish assemblage in the north-western Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom. 2003;83(1):193–204. 10.1017/s0025315403006970h . [DOI] [Google Scholar]
- 40.Cheminée A, Sala E, Pastor J, Bodilis P, Thiriet P, Mangialajo L, et al. Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. Journal of Experimental Marine Biology and Ecology. 2013;442(0):70–9. 10.1016/j.jembe.2013.02.003 [DOI] [Google Scholar]
- 41.Harmelin-Vivien ML, Harmelin JG, Chauvet C, Duval C, Galzin R, Lejeune P, et al. Evaluation visuelle des peuplements et populations de poissons: méthodes et problèmes Paris, FRANCE: Société nationale de protection de la nature et d'acclimatation de France; 1985. [Google Scholar]
- 42.Willis TJ. Visual census methods underestimate density and diversity of cryptic reef fishes. Journal of Fish Biology. 2001;59(5):1408–11. 10.1006/jfbi.2001.1721 . [DOI] [Google Scholar]
- 43.Smith-Vaniz WF, Jelks HL, Rocha LA. Relevance of cryptic fishes in biodiversity assessments: A case study at Buck Island Reef National Monument, St. Croix. Bulletin of Marine Science. 2006;79(1):17–48. . [Google Scholar]
- 44.Bozec Y-M, Kulbicki M, Laloë F, Mou-Tham G, Gascuel D. Factors affecting the detection distances of reef fish: implications for visual counts. Marine Biology. 2011;158(5):969–81. 10.1007/s00227-011-1623-9 [DOI] [Google Scholar]
- 45.Ackerman JL, Bellwood DR. Reef fish assemblages: a re-evaluation using enclosed rotenone stations. Marine Ecology-Progress Series. 2000;206:227–37. 10.3354/meps206227 . [DOI] [Google Scholar]
- 46.Depczynski M, Bellwood DR. The role of cryptobenthic reef fishes in coral reef trophodynamics. Marine Ecology Progress Series. 2003;256:183–91. 10.3354/meps256183 [DOI] [Google Scholar]
- 47.Kovačić M, Patzner RA, Schliewen U. A first quantitative assessment of the ecology of cryptobenthic fishes in the Mediterranean Sea. Marine Biology. 2012;159(12):2731–42. 10.1007/s00227-012-2030-6 [DOI] [Google Scholar]
- 48.Jordan F, Coyne S, Trexler JC. Sampling Fishes in Vegetated Habitats: Effects of Habitat Structure on Sampling Characteristics of the 1-m2 Throw Trap. Transactions of the American Fisheries Society. 1997;126(6):1012–20. [DOI] [Google Scholar]
- 49.La Mesa G, Di Muccio S, Vacchi M. Structure of a Mediterranean cryptobenthic fish community and its relationships with habitat characteristics. Marine Biology. 2006;149(2):149–67. 10.1007/s00227-005-0194-z . [DOI] [Google Scholar]
- 50.La Mesa G, Micalizzi M, Giaccone G, Vacchi M. Cryptobenthic fishes of the “Ciclopi Islands” marine reserve (central Mediterranean Sea): assemblage composition, structure and relations with habitat features. Marine Biology. 2004;145(2):233–42. 10.1007/s00227-004-1315-9 [DOI] [Google Scholar]
- 51.Patzner R. Habitat Utilization and Depth Distribution of Small Cryptobenthic Fishes (Blenniidae, Gobiesocidae, Gobiidae, Tripterygiidae) in Ibiza (Western Mediterranean Sea). Environmental Biology of Fishes. 1999;55(3):207–14. 10.1023/a:1007535808710 [DOI] [Google Scholar]
- 52.Guidetti P. The importance of experimental design in detecting the effects of protection measures on fish in Mediterranean MPAs. Aquatic Conservation: Marine and Freshwater Ecosystems. 2002;12(6):619–34. 10.1002/aqc.514. 10.1002/aqc.514 [DOI] [Google Scholar]
- 53.Cheminée A. Ecological functions, transformations and management of infralittoral rocky habitats from the North-western Mediterranean: the case of fish (Teleostei) nursery habitats. [PhD]. Nice: University Nice Sophia—Antipolis; 2012.
- 54.Morey G, Moranta J, Massutí E, Grau A, Linde M, Riera F, et al. Weight–length relationships of littoral to lower slope fishes from the western Mediterranean. Fisheries Research. 2003;62(1):89–96. 10.1016/S0165-7836(02)00250-3 [DOI] [Google Scholar]
- 55.Froese R, Pauly D. FishBase. World Wide Web electronic publication; www.fishbase.org. 2012. [Google Scholar]
- 56.Murtaugh PA. Simplicity and complexity in ecological data analysis. Ecology. 2007;88(1):56–62. 10.1890/0012-9658(2007)88[56:sacied]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 57.Clarke KR, Somerfield PJ, Gorley RN. Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology. 2008;366(1–2):56–69. 10.1016/j.jembe.2008.07.009 . [DOI] [Google Scholar]
- 58.Somerfield PJ, Clarke KR. Inverse analysis in non-parametric multivariate analyses: distinguishing groups of associated species which covary coherently across samples. Journal of Experimental Marine Biology and Ecology. 2013;449(0):261–73. 10.1016/j.jembe.2013.10.002 [DOI] [Google Scholar]
- 59.Anderson MJ, Millar RB. Spatial variation and effects of habitat on temperate reef fish assemblages in northeastern New Zealand. Journal of Experimental Marine Biology and Ecology. 2004;305(2):191–221. 10.1016/j.jembe.2003.12.011 [DOI] [Google Scholar]
- 60.Anderson MJ, Gorley RN, Clarke KR. PERMANOVA for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth; 2008. [Google Scholar]
- 61.Clarke K, Gorley R. PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth; 2006. [Google Scholar]
- 62.R Development Core Team. R: A Language and Environment for Statistical Computing. 2013.
- 63.Oksanen J, Blanchet G, Kindt R, Legendre P, Minchin P, O'Hara R, et al. vegan: Community Ecology Package. 2012.
- 64.Wickham H. ggplot2: elegant graphics for data analysis: Springer; New York; 2009. [Google Scholar]
- 65.Beldade R, Erzini K, Goncalves EJ. Composition and temporal dynamics of a temperate rocky cryptobenthic fish assemblage. Journal of the Marine Biological Association of the United Kingdom. 2006;86(5):1221–8. 10.1017/s0025315406014226 . [DOI] [Google Scholar]
- 66.Felix-Hackradt FC, Hackradt CW, Trevino-Oton J, Segovia-Viadero M, Perez-Ruzafa A, Garcia-Charton JA. Environmental determinants on fish post-larval distribution in coastal areas of south-western Mediterranean Sea. Estuar Coast Shelf Sci. 2013;129:59–72. 10.1016/j.ecss.2013.05.029 . [DOI] [Google Scholar]
- 67.Raventos N, Macpherson E. Environmental influences on temporal patterns of settlement in two littoral labrid fishes in the Mediterranean Sea. Estuar Coast Shelf Sci. 2005;63(4):479–87. 10.1016/j.ecss.2004.11.018 . [DOI] [Google Scholar]
- 68.Lejeune P. Etude écoéthologique des comportements reproducteurs et sociaux des Labridae méditerranéens des genres Symphodus (Rafinesque 1810) et Coris (Lacepede 1802). Cahiers d’Ethologie Appliquée. 1985; 5:1–208. [Google Scholar]
- 69.Stergiou KI, Karpouzi VS. Feeding habits and trophic levels of Mediterranean fish. Reviews in Fish Biology and Fisheries. 2002;11(3):217–54. 10.1023/a:1020556722822 [DOI] [Google Scholar]
- 70.Harmelin-Vivien ML, Kaim-Malka RA, Ledoyer M, Jacob-Abraham SS. Food partitioning among scorpaenid fishes in Mediterranean seagrass beds. Journal of Fish Biology. 1989;34(5):715–34. 10.1111/j.1095-8649.1989.tb03352.x [DOI] [Google Scholar]
- 71.Thiriet P, Cheminée A, Mangialajo L, Francour P. How 3D Complexity of Macrophyte-Formed Habitats Affect the Processes Structuring Fish Assemblages Within Coastal Temperate Seascapes? In: Musard O, Le Dû-Blayo L, Francour P, Beurier J-P, Feunteun E, Talassinos L, editors. Underwater Seascapes: Springer International Publishing; 2014. p. 185–99. [Google Scholar]
- 72.Gozler AM, Kopuz U, Agirbas E. Seasonal changes of invertebrate fauna associated with Cystoseira barbata facies of Southeastern Black Sea coast. African Journal of Biotechnology. 2010;9(51):8852–9. . [Google Scholar]
- 73.Chemello R, Milazzo M. Effect of algal architecture on associated fauna: some evidence from phytal molluscs. Marine Biology. 2002;140(5):981–90. 10.1007/s00227-002-0777-x . [DOI] [Google Scholar]
- 74.Bostrom C, Jackson EL, Simenstad CA. Seagrass landscapes and their effects on associated fauna: A review. Estuar Coast Shelf Sci. 2006;68(3–4):383–403. 10.1016/j.ecss.2006.01.026 . [DOI] [Google Scholar]
- 75.Guidetti P. Differences Among Fish Assemblages Associated with Nearshore Posidonia oceanica Seagrass Beds, Rocky–algal Reefs and Unvegetated Sand Habitats in the Adriatic Sea. Estuarine, Coastal and Shelf Science. 2000;50(4):515–29. 10.1006/ecss.1999.0584 [DOI] [Google Scholar]
- 76.Horinouchi M, Mizuno N, Jo Y, Fujita M, Sano M, Suzuki Y. Seagrass habitat complexity does not always decrease foraging efficiencies of piscivorous fishes. Marine Ecology-Progress Series. 2009;377:43–9. 10.3354/meps07869 . [DOI] [Google Scholar]
- 77.Schultz S, Kruschel C. Frequency and success of ambush and chase predation in fish assemblages associated with seagrass and bare sediment in an Adriatic lagoon. Hydrobiologia. 2010;649(1):25–37. 10.1007/s10750-010-0256-1 [DOI] [Google Scholar]
- 78.Schultz ST, Kruschel C, Bakran-Petricioli T. Influence of seagrass meadows on predator-prey habitat segregation in an Adriatic lagoon. Marine Ecology-Progress Series. 2009;374:85–99. 10.3354/meps07779 . [DOI] [Google Scholar]
- 79.Laegdsgaard P, Johnson C. Why do juvenile fish utilise mangrove habitats? Journal of Experimental Marine Biology and Ecology. 2001;257(2):229–53. 10.1016/s0022-0981(00)00331-2 [DOI] [PubMed] [Google Scholar]
- 80.Manson FJ, Loneragan NR, Skilleter GA, Phinn SR. An Evaluation of the Evidence for Linkages between Mangroves and Fisheries Oceanography and Marine Biology. Oceanography and Marine Biology—An Annual Review: CRC Press; 2005. p. 483–513. [Google Scholar]
- 81.Nanjo K, Nakamura Y, Horinouchi M, Kohno H, Sano M. Predation risks for juvenile fishes in a mangrove estuary: A comparison of vegetated and unvegetated microhabitats by tethering experiments. Journal of Experimental Marine Biology and Ecology. 2011;405(1–2):53–8. 10.1016/j.jembe.2011.05.016 [DOI] [Google Scholar]
- 82.Jones GP. Population ecology of the temperate reef fish Pseudolabrus celidotus Bloch & Schneider (Pisces: Labridae). I. Factors influencing recruitment. Journal of Experimental Marine Biology and Ecology. 1984;75(3):257–76. 10.1016/0022-0981(84)90170-9 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 9m² sampling area was the semicircle 2.5 m in radius in front of the diver, without considering the inner part, semicircle 0.7 m in radius.
(TIFF)
Curves are smoothed histograms (Kernel density estimations) of total lengths of all sampled fish (crypto- or necto- benthic) within each level of the combined factor habitat X locality-protection (region-time). The surfaces below the curves (the integrals) are proportional to fish densities (abundance per sampling unit). Total length distributions are also presented using Tukey's boxplots. (See also S1 Text). Modified from Thiriet et al. [30].
(TIFF)
(DOCX)
Latitude (North) and Longitude (East) are in decimal degrees (See also Fig 2).
(DOCX)
(See also S2 Fig)
(DOCX)
A discussion enriched by previous studies on density patterns and fish life history traits found in the literature.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.