Update to: European Journal of Human Genetics (2012) 20,; doi:10.1038/ejhg.2012.28; published online 29 February 2012
1. Disease characteristics
1.1 Name of the Disease (Synonyms):
Biotinidase deficiency (late-onset multiple carboxylase deficiency; late-onset biotin-responsive multiple carboxylase deficiency; juvenile-onset multiple carboxylase deficiency; BTD deficiency)
1.2 OMIM# of the Disease:
253260
1.3 Name of the Analysed Genes or DNA/Chromosome Segments:
BTD1
1.4 OMIM# of the Gene(s):
609019
1.5 Variant Spectrum:
Biotinidase deficiency is an autosomal recessive metabolic disorder characterized by neurocutaneous manifestations. Individuals with a biotinidase deficiency have either homozygous or compound heterozygous variants of BTD. One hundred and sixty-two variants of BTD that alter biotinidase activity have been reported so far. All types of variants have been observed: 115 missense, 12 nonsense, one splice-site, and 32 frameshifting indels, one intronic variant altering mRNA expression, and one contiguous gene deletion involving BTD, HCL1 and COLQ.2, 3, 4, 5, 6 In addition, 31 variants of unknown significance have been identified, including 14 suspected to affect function. No hotspot variant region has been observed and alterations have occurred throughout the coding sequence. Based on a worldwide screening of biotinidase deficiency performed in 1991,7 it was estimated that five variants account for about 60% of the genetic abnormalities encountered in individuals who are symptomatic or were identified by newborn screening (according to NCBI cDNA reference sequence NM_000060.3): c.98_104delGCGGCTGinsTCC (p. Cys33Phefs*36), c.1368A>C (p. Gln456His), c.1612C>T (p. Arg538Cys), c.1330G>C (p. Asp444His), and the combined allelic alteration c.[511G>A;1330G>C] (p.[Ala171Thr;Asp444His]).8, 9, 10 Frequency and repartition of the mutations may vary across countries, more especially those that were not included in the 1991 survey, but no comparable worldwide epidemiological investigation was reported since then. Regularly updated lists of published variants of BTD altering biotinidase activity and variants of unknown significance are available in the public BTD database hosted by ARUP Laboratories and the University of Utah Department of Pathology5 (http://www.arup.utah.edu/database/BTD/BTD_welcome.php), and/or in the LOVD database dedicated to BTD (www.LOVD.nl/BTD).
1.6 Analytical methods:
Two analytical strategies may be followed. The first strategy targets the most frequent variants that affect BTD function, and the second one encompasses the entire coding region of the gene. In populations of European descent, site-specific variant analysis can focus on the five most frequent variants cited above using real-time PCR.10, 11 The most recurrent variants observed may however vary across countries, due, for instance, to a founder effect; the targeted strategy should then be adapted accordingly. Yet, besides the five most frequent variants reported, other variants affecting function or even variants of unknown significance can be found along the entire coding region of BTD, including exon 1 which was shown to be involved in a contiguous gene deletion reported recently.6 It seems therefore more cost-effective to analyze the entire coding region at a time. A widely used method is the bi-directional gene sequencing of all four BTD exons and their flanking intronic sequences, which increases the detection rate to 99%4, 10 this method is based either on Sanger sequencing of polymerase chain reaction products or high-throughput sequencing (HTS) targeting the regions of interest with a recommended minimal read depth of 30X and preferably of at least 100X. Theoretically, whole-exome sequencing may also be used for variant detection, provided the sequence quality meets diagnostic requirements (Küry et al. unpublished data). Alternatively, dHPLC has been proposed for the screening of BTD variants,12 but its use is not yet generally used for diagnostic purposes, and the identification of variants by this method requires confirmation by sequencing. Screening of large rearrangements is also relevant, as such an alteration was reported recently.6 Recently, quantitative real-time reverse-transcription PCR was used to assess the role of an intronic variant altering BTD expression.5
1.7 Analytical Validation
Using real-time PCR, the validation of the results is accomplished by comparing the results of positive and negative controls. Using direct gene sequencing, validation is performed by sequencing both DNA strands, thereby excluding possible artifacts. Finding known variants can be correlated with the biochemical enzymatic results (see 3.1.2 for explanations on classification of biotinidase deficiency according to enzymatic activity).13, 14 Severe variants, such as c.98_104delGCGGCTGinsTCC (p. Cys33Phefs*36), c.1368A>C (p. Gln456His) or c.1612C>T (p. Arg538Cys), are associated with profound biotinidase deficiency when they are homozygous or compound heterozygous with another severe variant. In contrast, the severity of the decrease in serum biotinidase enzyme activity due to milder variant c.1330G>C (p. Asp444His) depends on the variants to which it is combined: it results in 45–50% of mean normal serum biotinidase enzyme activity when homozygous, 20–25% of mean normal serum biotinidase enzyme activity or partial biotinidase deficiency when in trans with a variant with very low biotinidase enzyme activity or profound biotinidase deficiency. When p.Asp444His is in cis configuration p.[Ala171Thr;Asp444His] with p.Ala171Thr, it results in an allele causing profound biotinidase deficiency; an individual who presents the combination p.[Ala171Thr;Asp444His] on one allele and a severe variant on the other allele has profound biotinidase deficiency and requires biotin therapy.15 When a suspected novel variant is identified on both alleles, it can be validated by correlating the suspected variant with the individual's enzymatic activity, the presence of the variants in the parents and correlating with their respective enzymatic activities, and by comparing the alteration to variants found in previously confirmed individuals with the disorder often available in public databases (e.g., dbSNP). Potential functional effects of the variant can be assessed by correlating the suspected variant of BTD with enzymatic activity.
1.8 Estimated frequency of the disease
(Incidence at birth (‘birth prevalence') or population prevalence)
In 2004, the incidence of biotinidase deficiency in Europe was estimated to 1:47 486, according to statistics data collected through newborn screening programmes in seven countries (Austria, Belgium, Germany, Italy, Spain, Sweden, and Switzerland).16 In 1991, the worldwide incidence for combined profound and partial biotinidase deficiency was approximately 1:60 000 based on 8.5 million newborn infants identified by newborn screening in 14 countries of which the majority were of European descent.7
1.9 If applicable, prevalence in the ethnic group of investigated person
Approximately 1:60 000 for combined profound and partial biotinidase deficiency, according to extrapolations of prevalence made by the US Census Bureau in 2004.
1.10 Diagnostic setting:

Comment: Biotinidase deficiency is an autosomal recessively inherited disorder that, if untreated, usually manifests in children from one week of age to adolescence, with most exhibiting symptoms between 3 to 6 months of age.4, 17 Diagnosis can be established by the concomitant presence of characteristic clinical and biochemical features. Clinically, a child with undetected and/or untreated profound biotinidase deficiency can exhibit symptoms shared with other metabolic disorders, such as seizures, hypotonia, respiratory problems, developmental delay and vision problems, and those more specific of biotinidase deficiency, such as eczematous skin rash, alopecia, conjunctivitis, candidiasis, ataxia, myelopathy and bilateral optic neuropathy.10, 18, 19 Symptoms occurring in older children or adolescents include limb weakness, paresis, and scotomata. Symptomatic affected individuals usually exhibit biochemical features, such as ketolactic acidosis, organic aciduria and hyperammonemia. A milder expression of the above symptoms may occur in individuals with untreated partial biotinidase deficiency;4, 10, 20, 21 such an event remains however anecdotal,4, 20 and its genuine frequency is difficult to assess, since no comprehensive study has been conducted to date to address this question. All symptomatic affected individuals improve with oral pharmacological doses of the vitamin, biotin. Asymptomatic Individuals having partial biotinidase deficiency or even sometimes profound biotinidase deficiency may be clinically asymptomatic.22 In rare cases, due to the absence of any biotin therapy, stress might trigger development of symptoms in these individuals, including principally hypotonia, skin rash, and hair loss;4, 10, 20, 21 more similar observations would be necessary however to assess the genuine risk for developing biotinidase deficiency associated with stress. Biotin treatment prevents the development of symptoms in affected children identified before they have clinical findings or are identified by newborn screening.18 Biotin therapy is lifelong.
The diagnostic biochemical finding of the disorder is decreased or undetectable biotinidase enzyme activity in serum or plasma. Biotinidase is essential for recycling the vitamin, biotin (also known as vitamin H or B8). Biotinidase enzyme activity can be determined by colorimetric or fluorimetric enzymatic assays in serum or plasma. The degree of enzyme deficiency can distinguish individuals with profound biotinidase deficiency (enzyme activity less than 10% of mean normal serum biotinidase enzyme activity) and those with partial biotinidase deficiency (enzyme activity between 10 and 30% of mean normal serum biotinidase enzyme activity).20 Clinical and biochemical findings may be sufficient to differentially diagnose nutritional biotin deficiency, other causes of sensorineural hearing loss, or ataxia. Biochemical and/or mutational analysis of the BTD gene may be helpful to exclude other diagnostic possibilities, such as an isolated carboxylase deficiency, holocarboxylase synthetase deficiency, zinc deficiency (acrodermatitis enteropathica), or essential fatty acid deficiency.10 Measuring plasma or urinary biotin concentrations is usually of little or no value in the diagnosis of biotinidase deficiency, but may be useful to confirm compliance of biotin treatment.
2. Test characteristics
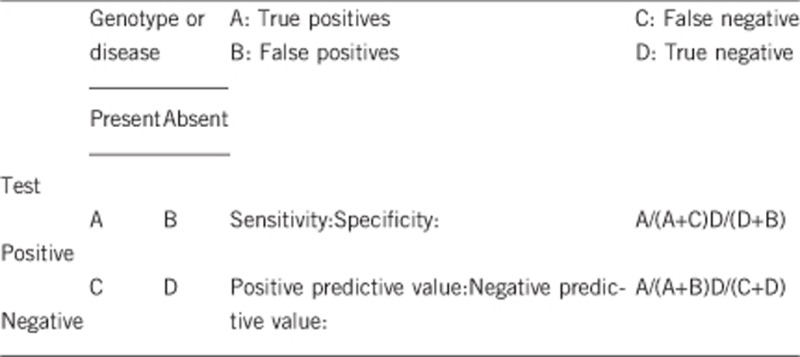
2.1 Analytical sensitivity
(proportion of positive tests if the genotype is present)
Close to 100% by direct gene sequencing and about 60% by targeted real-time PCR.
2.2 Analytical specificity
(proportion of negative tests if the genotype is not present)
100%.
2.3 Clinical sensitivity
(proportion of positive tests if the disease is present)
The clinical sensitivity can be dependent on variable factors, such as age or family history. In such cases a general statement should be given, even if a quantitation can only be made case by case.
It is almost 100%. Failure to identify variants on both alleles of the biotinidase (BTD) gene has usually resulted from incorrect evaluation of enzymatic activity. Yet, in a few rare cases, variants may be located in unexplored regions of the BTD gene (introns, 5'UTR, 3'UTR, or upstream regulating regions), as has been observed in Austrian patients.23
2.4 Clinical specificity
(proportion of negative tests if the disease is not present)
The clinical specificity can be dependent on variable factors, such as age or family history. In such cases a general statement should be given, even if a quantitation can only be made case by case.
100%.
2.5 Positive clinical predictive value
(life time risk to develop the disease if the test is positive)
Almost 100% of the children identified with two alleles of profound biotinidase deficiency will develop symptoms or are considered at high risk of becoming symptomatic if they are not treated with biotin. Without biotin treatment, even adults with the enzyme deficiency who have never been symptomatic can develop clinical features during stress, such as a prolonged infection.10
2.6 Negative clinical predictive value
(Probability not to develop the disease if the test is negative)
Assume an increased risk based on family history for a non-affected person. Allelic and locus heterogeneity may need to be considered.
Index case in that family had been tested:
When both alleles of the BTD gene are abnormal in an index case, the negative predictive value is 100% in relatives who are found non-carriers or heterozygous carriers. Individuals in both groups will remain asymptomatic.
Index case in that family had not been tested:
Not applicable.
3. Clinical Utility
3.1 (Differential) diagnostics: The tested person is clinically affected
(To be answered if in 1.10 ‘A' was marked)
3.1.1 Can a diagnosis be made other than through a genetic test?

3.1.2 Describe the burden of alternative diagnostic methods to the patient
It is noteworthy that, in matter of biotinidase deficiency screening, the gold standard method is represented by the biochemical analyses for measuring levels of biotinidase enzyme activity. Thus, the role of alternative method would rather be played by the genetic testing, which is used for the confirmation of clinico-biological diagnosis. The burden of methods alternative to genetic testing varies according to the strategy used for diagnosis.
In a propositus exhibiting clinical and/or biological features of profound biotinidase deficiency, there is theoretically no extra-burden of alternative methods to the patient. Repeated measurements of serum biotinidase enzyme activity are mandatory to confirm the diagnosis, which will be further confirmed by the identification of biallelic BTD causative variants.
When a newborn has been incorporated in a national screening programme, diagnostic methods are biochemical. Most of the times, cases of profound biotinidase deficiency are unambiguously diagnosed, but the differentiation between infants with actual partial deficiency and those who have false-positive tests may be more arduous18. Whereas the false positive rate in the US/worldwide pilot newborn screening programmes was very low (0.001),24 it dramatically increased these last years, as illustrated by three independent studies from Sweden, Brazil and Turkey of which the inferred positive predictive value for the screening test could ranged from above 50% to less than 10%.25, 26, 27 In such case of a false positive result, repeated measurements of serum biotinidase enzyme activities are mandatory, not only in the child, but also in his parents to help resolve the problem, which constitutes a real burden to the patient and his family. This situation warrants genetic testing as a confirmatory diagnostic tool. A possible cause for the increase in the false positive rate of newborn screening programmes is due to modifications, compared with the initial pilot programmes, to newer screening test methods based on commercial kits that measure biotinidase enzyme activity in blood-saturated filter paper samples.4 An inappropriate preparation of the sample and the prematurity of the newborn to be tested are also among the most frequent causes of false-positive screening test results.4
In case the biochemical assay uses biotinyl-p-aminobenzoate as the substrate, false-negative results may occur in infants if they are being treated with sulfa medications.4, 28 An increase of triglyceride concentration following the administration of intravenous lipids, or an immunoglobulin concentration can also induce false-negative results.4 In several cases, newborn screening failed to detect a profound biotinidase deficiency in children who ultimately developed symptoms of disorder.4 Repeated enzymatic testing allowed to confirm the diagnosis of profound biotinidase deficiency suggested by clinical symptoms. On the other hand, confirmation of normal biotinidase activity thorugh repeated testing could suggest a holocarboxylase synthetase deficiency.
Burden of disease is, however, much greater in infants whose biotinidase deficiency was not identified early through a national newborn screening campaign, but much later based upon clinical criteria. In many cases, clinicians, geneticists and metabolic specialists fail to identify biotinidase deficiency before occurrence of irreversible symptoms and lesions, such as developmental delay, hearing loss and optic atrophy, which complications all imply lifelong and very expensive medical care.18 Diagnostic difficulty is all the greater in patients with untreated profound biotinidase deficiency that the presentation of their disease is more likely to be atypical, which may delay further treatment initiation. In addition, clinical assessment is usually inefficient to detect partial biotinidase deficiency, in which the affected individual is usually asymptomatic, but who may become symptomatic during infancy or adulthood.18
3.1.3 How is the cost effectiveness of alternative diagnostic methods to be judged?
This question coincides with the debate over the usefulness of programmes of newborn screening of biotinidase deficiency based on measurement of biotinidase enzyme activity. One can distinguish between two categories of countries: those which chose to develop a systematic newborn screening programme for early diagnosis of biotinidase deficiency, such as the USA,29 some European countries,16 Turkey30 or Brazil,31 and those which did not choose such an option and preferred to rely on clinical diagnosis to identify affected individuals. From an economical perspective, the choice of one option over the other is guided by the balance between the overall cost of a massive screening involving thousands of newborns per year and the cost of more complex medical healthcare regarding much fewer infants clinically diagnosed with biotinidase deficiency. However, once some symptoms occur and biotin treatment initiated, they may not be reversible. This must be factored into the cost for consideration of newborn screening.
Several cost-analyses studies were conducted in the United States and in European countries which all concluded the positive cost-effectiveness of newborn screening strategies for biotinidase deficiency.18, 32, 33 On the contrary, in countries where generalized screening strategies have been excluded because the incidence of biotinidase deficiency is assumed to be too rare, no pilot screening programs have been conducted to determine the incidence of biotinidase deficiency.18
3.1.4 Will disease management be influenced by the result of a genetic test?

3.2 Predictive Setting: The tested person is clinically unaffected but carries an increased risk based on family history
(To be answered if in 1.10 ‘B' was marked)
3.2.1 Will the result of a genetic test influence lifestyle and prevention?

3.2.2 Which options in view of lifestyle and prevention does a person at-risk have if no genetic test has been done (please describe)?
Biotin treatment can prevent the occurrence of irreversible symptoms, such as developmental delay, hearing loss and optic atrophy, if they have not occurred in an individual with biotinidase deficiency. Confirmatory testing is recommended to avoid possible, as yet unknown, side-effects of the treatment.
3.3 Genetic risk assessment in family members of a diseased person
(To be answered if in 1.10 ‘C' was marked)
3.3.1 Does the result of a genetic test resolve the genetic situation in that family?
Yes, because the disorder is inherited as an autosomal recessive trait, other asymptomatic relatives can be evaluated and subsequent children born to the family can be evaluated immediately after birth for the disorder, particularly in those locations that do not perform newborn screening for biotinidase deficiency.
3.3.2 Can a genetic test in the index patient save genetic or other tests in family members?
Yes. If no variant of BTD that alters enzymatic activity is found in the index case, then genetic and other tests are unnecessary in relatives.
3.3.3 Does a positive genetic test result in the index patient enable a predictive test in a family member?
Yes.
3.4 Prenatal diagnosis
(To be answered if in 1.10 ‘D' was marked)
3.4.1 Does a positive genetic test result in the index patient enable a prenatal diagnosis?
It is possible, but it is not common, because biotinidase deficiency is a readily treatable disease.
4. If applicable, further consequences of testing
Please assume that the result of a genetic test has no immediate medical consequences. Is there any evidence that a genetic test is nevertheless useful for the patient or his/her relatives? (Please describe).
Finding variants in both of an index case's alleles is helpful in identifying at-risk individuals and those who are carriers in the family. Testing will have impact on genetic counselling and may consequently influence reproductive decisions.
Acknowledgments
This work was supported by EuroGentest2 (Unit 2: ‘Genetic testing as part of health care'), a Coordination Action under FP7 (Grant Agreement Number 261469) and the European Society of Human Genetics. In addition, this work was supported by the Safra Research Fund at Henry Ford Hospital.
The authors declare no conflict of interest.
References
- Pomponio RJ, Reynolds TR, Cole H, Buck GA, Wolf B: Mutational hotspot in the human biotinidase gene causes profound biotinidase deficiency. Nat Genet 1995; 11: 96–98. [DOI] [PubMed] [Google Scholar]
- Pindolia K, Jordan M, Wolf B: Analysis of mutations causing biotinidase deficiency. Human mutation 2010; 31: 983–991. [DOI] [PubMed] [Google Scholar]
- Procter M, Wolf B, Crockett DK, Mao R: The Biotinidase Gene Variants Registry. A Paradigm Public Database. G3 (Bethesda) 2013; 3: 727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B: Biotinidase deficiency: ‘if you have to have an inherited metabolic disease, this is the one to have'. Genetics in medicine: official journal of the American College of Medical Genetics 2012; 14: 565–575. [DOI] [PubMed] [Google Scholar]
- Li H, Spencer L, Nahhas F et al: Novel mutations causing biotinidase deficiency in individuals identified by newborn screening in Michigan including an unique intronic mutation that alters mRNA expression of the biotinidase gene. Molecular genetics and metabolism 2014; 112: 242–246. [DOI] [PubMed] [Google Scholar]
- Senanayake DN, Jasinge EA, Pindolia K et al: First contiguous gene deletion causing biotinidase deficiency: The enzyme deficiency in three Sri Lankan children. Molecular Genetics and Metabolism Reports 2015; 2: 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B: Worldwide survey of neonatal screening for biotinidase deficiency. Journal of inherited metabolic disease 1991; 14: 923–927. [DOI] [PubMed] [Google Scholar]
- Norrgard KJ, Pomponio RJ, Swango KL et al: Mutation (Q456H) is the most common cause of profound biotinidase deficiency in children ascertained by newborn screening in the United States. Biochem Mol Med 1997; 61: 22–27. [DOI] [PubMed] [Google Scholar]
- Pomponio RJ, Hymes J, Reynolds TR et al: Mutations in the human biotinidase gene that cause profound biotinidase deficiency in symptomatic children: molecular, biochemical, and clinical analysis. Pediatr Res 1997; 42: 840–848. [DOI] [PubMed] [Google Scholar]
- Wolf BBiotinidase deficiencyin:Pagon RA, Bird TD, Dolan CR(eds): GeneReviews [Internet]. University of Washington: Seattle: Seattle (WA), 1993–2013, [Last Update 2013]. [Google Scholar]
- Dobrowolski SF, Angeletti J, Banas RA, Naylor EW: Real time PCR assays to detect common mutations in the biotinidase gene and application of mutational analysis to newborn screening for biotinidase deficiency. Molecular genetics and metabolism 2003; 78: 100–107. [DOI] [PubMed] [Google Scholar]
- Iqbal F, Item CB, Vilaseca MA et al: The identification of novel mutations in the biotinidase gene using denaturing high pressure liquid chromatography (dHPLC). Molecular genetics and metabolism 2010; 100: 42–45. [DOI] [PubMed] [Google Scholar]
- Cowan TM, Blitzer MG, Wolf B: Technical standards and guidelines for the diagnosis of biotinidase deficiency. Genetics in medicine: official journal of the American College of Medical Genetics 2010; 12: 464–470. [DOI] [PubMed] [Google Scholar]
- Thodi G, Molou E, Georgiou V et al: Mutational analysis for biotinidase deficiency of a Greek patients' cohort ascertained through expanded newborn screening. J Hum Genet 2011; 56: 861–5. [DOI] [PubMed] [Google Scholar]
- Norrgard KJ, Pomponio RJ, Swango KL et al: Double mutation (A171T and D444H) is a common cause of profound biotinidase deficiency in children ascertained by newborn screening the the United States. Mutations in brief no. 128. Online. Human mutation 1998; 11: 410. [DOI] [PubMed] [Google Scholar]
- Loeber JG: Neonatal screening in Europe; the situation in 2004. Journal of inherited metabolic disease 2007; 30: 430–438. [DOI] [PubMed] [Google Scholar]
- Wolf B, Grier RE, Allen RJ, Goodman SI, Kien CL: Biotinidase deficiency: the enzymatic defect in late-onset multiple carboxylase deficiency. Clin Chim Acta 1983; 131: 273–281. [DOI] [PubMed] [Google Scholar]
- Wolf B: Why screen newborns for profound and partial biotinidase deficiency? Molecular genetics and metabolism 2015; 114: 382–387. [DOI] [PubMed] [Google Scholar]
- Bottin L, Prud'hon S, Guey S et al: Biotinidase deficiency mimicking neuromyelitis optica: initially exhibiting symptoms in adulthood. Mult Scler 2015; 21: 160–167. [DOI] [PubMed] [Google Scholar]
- McVoy JR, Levy HL, Lawler M et al: Partial biotinidase deficiency: clinical and biochemical features. J Pediatr 1990; 116: 78–83. [DOI] [PubMed] [Google Scholar]
- Suormala TM, Baumgartner ER, Wick H, Scheibenreiter S, Schweitzer S: Comparison of patients with complete and partial biotinidase deficiency: biochemical studies. Journal of inherited metabolic disease 1990; 13: 76–92. [DOI] [PubMed] [Google Scholar]
- Baykal T, Gokcay G, Gokdemir Y et al: Asymptomatic adults and older siblings with biotinidase deficiency ascertained by family studies of index cases. Journal of inherited metabolic disease 2005; 28: 903–912. [DOI] [PubMed] [Google Scholar]
- Muhl A, Moslinger D, Item CB, Stockler-Ipsiroglu S: Molecular characterisation of 34 patients with biotinidase deficiency ascertained by newborn screening and family investigation. Eur J Hum Genet 2001; 9: 237–243. [DOI] [PubMed] [Google Scholar]
- Wolf B: Clinical issues and frequent questions about biotinidase deficiency. Molecular genetics and metabolism 2010; 100: 6–13. [DOI] [PubMed] [Google Scholar]
- Neto EC, Schulte J, Rubim R et al: Newborn screening for biotinidase deficiency in Brazil: biochemical and molecular characterizations. Brazilian journal of medical and biological research=Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 2004; 37: 295–299. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Guthenberg C, Holme E, von Dobeln U: Profound biotinidase deficiency: a rare disease among native Swedes. Journal of inherited metabolic disease 2010; 33 (Suppl 3): S175–S180. [DOI] [PubMed] [Google Scholar]
- Tanzer F, Sancaktar M, Buyukkayhan D: Neonatal screening for biotidinidase deficiency: results of a 1-year pilot study in four cities in central Anatolia. Journal of pediatric endocrinology & metabolism: JPEM 2009; 22: 1113–1116. [DOI] [PubMed] [Google Scholar]
- Heard GS, Secor McVoy JR, Wolf B: A screening method for biotinidase deficiency in newborns. Clinical chemistry 1984; 30: 125–127. [PubMed] [Google Scholar]
- Jay AM, Conway RL, Feldman GL, Nahhas F, Spencer L, Wolf B: Outcomes of individuals with profound and partial biotinidase deficiency ascertained by newborn screening in Michigan over 25 years. Genetics in medicine: official journal of the American College of Medical Genetics 2015; 17: 205–209. [DOI] [PubMed] [Google Scholar]
- Tezel B, Dilli D, Bolat H et al: The development and organization of newborn screening programs in Turkey. Journal of clinical laboratory analysis 2014; 28: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara MT, Gurgel-Giannetti J, Aguiar MJ et al: High Incidence of Biotinidase Deficiency from a Pilot Newborn Screening Study in Minas Gerais, Brazil. JIMD reports 2015; 24: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AE, Downs SM: Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics 2006; 117: S287–S295. [DOI] [PubMed] [Google Scholar]
- Schoos R, Verloes A, Bourguignon JP, Koulischer L: [Programs of systematic screening in neonatology. Pharmaco-economic aspects]. Revue medicale de Liege 1998; 53: 311–315. [PubMed] [Google Scholar]
- Swango KL, Demirkol M, Huner G et al: Partial biotinidase deficiency is usually due to the D444H mutation in the biotinidase gene. Human genetics 1998; 102: 571–575. [DOI] [PubMed] [Google Scholar]
- Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK: Marginal biotin deficiency is teratogenic in ICR mice. The Journal of nutrition 2003; 133: 2519–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said HM: Biotin: biochemical, physiological and clinical aspects. Sub-cellular biochemistry 2012; 56: 1–19. [DOI] [PubMed] [Google Scholar]
- Watanabe T: Teratogenic effects of biotin deficiency in mice. The Journal of nutrition 1983; 113: 574–581. [DOI] [PubMed] [Google Scholar]
- Zempleni J, Mock DM: Marginal biotin deficiency is teratogenic. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine 2000; 223: 14–21. [DOI] [PubMed] [Google Scholar]


