Summary
Bruton’s tyrosine kinase (BTK) mediates B cell signaling and is also present in innate immune cells but not T cells. BTK propagates B cell receptor (BCR) responses to antigen-engagement as well as to stimulation via CD40, toll-like receptors (TLRs), Fc receptors (FCRs) and chemokine receptors. Importantly, BTK can modulate signaling, acting as a “rheostat” rather than an “on-off” switch; thus, overexpression leads to autoimmunity while decreased levels improve autoimmune disease outcomes. Autoreactive B cells depend upon BTK for survival to a greater degree than normal B cells, reflected as loss of autoantibodies with maintenance of total antibody levels when BTK is absent. This review describes contributions of BTK to immune tolerance, including studies testing BTK-inhibitors for treatment of autoimmune diseases.
Keywords: Bruton’s tyrosine kinase, autoimmunity, B lymphocyte signaling, autoimmune inflammatory arthritis, type 1 diabetes, systemic lupus erythematosus, BTK inhibitors
Introduction
The primary contribution of Bruton’s tyrosine kinase (BTK) to human health is to support humoral immunity, as demonstrated by its first mention in the literature, a description of a boy who was highly susceptible to infection with encapsulated bacteria (1). This report, by Colonel Ogden C. Bruton, described a disease that came to be known as X-linked agammaglobulinemia (XLA). Patients with XLA lack most antibodies, yet they can do relatively well with treatment, consisting of regular administration of replacement immunoglobulins from pooled human serum, and antibiotics (2, 3). The protein defective in XLA was discovered in 1993, both in humans and in the similar X-linked immunodeficiency (xid) mouse model, and is now known as Bruton’s tyrosine kinase (4–8). That same year, xid mice were reported to be protected against collagen-induced arthritis (CIA), the first report to specifically link Btk with autoimmune inflammatory arthritis (9). Since that time, BTK’s structure and function have been painstakingly delineated and a profusion of small molecular BTK-inhibitors has been developed for use in lymphoma and autoimmune disease(10–22). There is evidence from mouse models that Btk has a special role in governing immune tolerance in B cells (23–26). Thus, unlike other methods of targeting B lymphocytes, BTK inhibitors hold promise for improving B cell-related autoimmunity without inducing the degree of immunodeficiency seen in XLA. This review describes the known features of BTK pertinent to immune tolerance and its potential as a therapeutic target in autoimmunity.
B cell contributions to autoimmunity
B cell signaling is critical to B cell tolerance, and BTK plays a central role. Autoantibodies are often considered to be a readout of autoreactive T cell help, but this approach ignores contributions of B cell intrinsic tolerance mechanisms, which begin in the bone marrow, prior to T cell interactions. Approximately 70–80% of developing B cells are autoreactive, but most are culled at the immature stage via a process known as receptor editing or by apoptosis (27). In genetic backgrounds that favor autoimmunity this selection process is flawed, and there are increased numbers of naïve autoreactive B cells available to interact with T cells (28–30). These B cells act as antigen-presenting cells (APCs), specialized to concentrate autoantigen, and can be the exclusive APC that drives T cell mediated autoimmunity (31–33). B cells also produce cytokines and have regulatory functions (34). B cells in inflamed tissue may have specialized roles, as their removal can prevent autoimmune disease, even when T cells remain (35, 36). B cell responses in germinal centers that form in these inflamed tissues may lead to autoantibodies and autoreactive memory B cells. Therefore, understanding how B cell signaling mediates B cell tolerance is a key to preventing and treating autoimmune disease (23–26, 37, 38).
BTK-mediated signaling
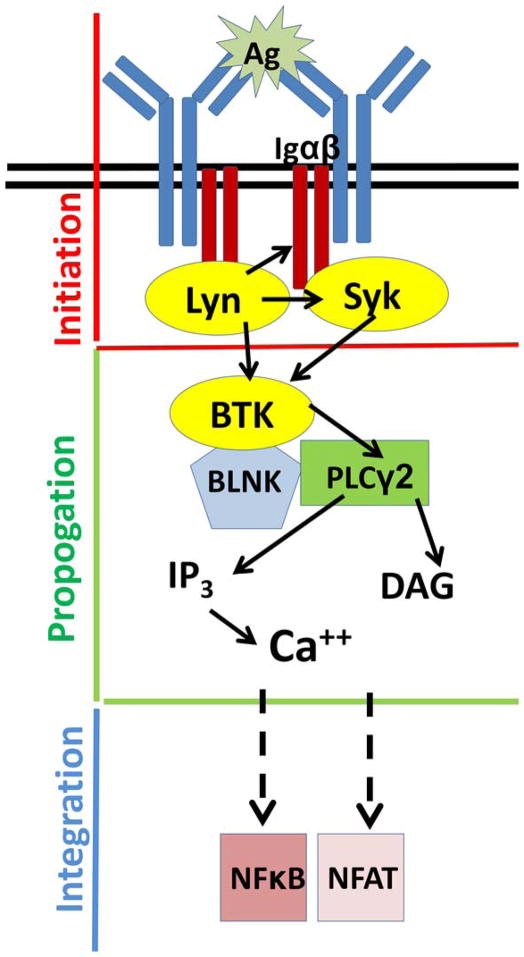
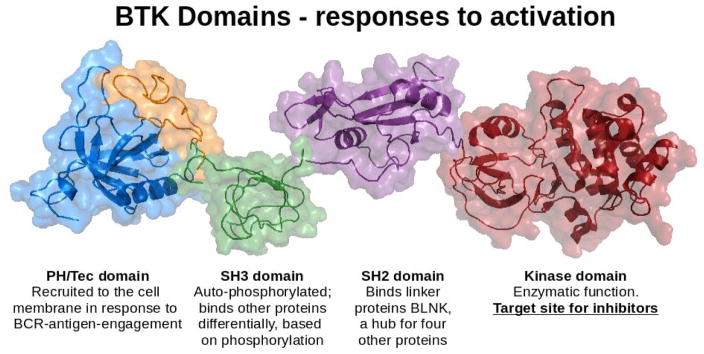
The B cell receptor (BCR) is the primary sensor that initiates signaling (Figure 1). There are two primary components of the BCR: Membrane-bound antibody, and Igα/Igβ heterodimers that provide the cytosolic signaling function. Each B cell expresses 2 × 105 identical BCRs, and antigen-engagement triggers the signaling cascade, prompting phosphorylation by SRC-family kinases of immunoreceptor tyrosine-based activation motifs (ITAMs) on Igα and Igβ (39). Dual phosphorylation of these ITAMs allows spleen tyrosine kinase SYK to dock and become activated(40). These proximal, or initiating, signaling components are critical to the survival of B cells, and loss of any of these components results in severe depletion of the B cell compartment. Initiation of the signaling cascade affects multiple components that interact to propagate the signal. BTK is a 659 amino acid protein, arranged in five domains that enable multiple functions (Figure 2 and (41)). The N-terminal pleckstrin homology (PH) domain binds phosphatidylinositol 3,4,5-triphosphate (PIP3) generated by phosphoinositide 3-kinase (PI3K) in response to BCR signaling, resulting in recruitment of BTK from the cytosol to the cell membrane. The PH domain of Btk is critical, as xid mice that have a mutation (R28C) in this component have a phenotype that is almost identical to that of Btk-deficient mice in which the protein is absent (6–8, 10, 42, 43). BTK’s SRC homology 2 (SH2) domain binds phosphorylated tyrosines, which facilitates docking to the adaptor protein BLNK, a signalosome hub that anchors multiple proteins in close proximity for signaling interactions. BTK is activated by tyrosine phosphorylation at Y551, classically accomplished by LYN, as well as by SYK, which requires BLNK docking to facilitate this interaction (17, 44, 45). BTK’s SRC homology 3 (SH3) binds various other proteins, in some cases dependent on its own autoregulated phosphorylation status (17, 41, 46). Thus, BTK has docking functions, as well as enzymatic activity, and there is evidence that it may make important contributions as an adaptor molecule, independent of its catalytic function (11, 12, 22). This has potentially important implications for predicting and understanding the effects of small molecular inhibitors that exclusively target the kinase function. The primary action of BTK’s kinase domain is to phosphorylate phospholipase C gamma 2 (PLCγ2), which then cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) to generate two second messengers, inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) (41, 47). IP3 binds its receptor on the endoplasmic reticulum to initiate calcium flux, leading to nuclear transport of the transcription factor nuclear factor of activated T cells (NFAT). DAG activates Protein Kinase C β (PKCβ) with downstream activation of the NFκB pathway, as well as several mitogen-activated protein kinase (MAPK) pathways(15, 48–50). Of note, BTK also mediates signaling via CD40, Fc Receptors, chemokine receptors and TLRs, in myeloid cells as well as in B cells, although many of those pathways are less well-defined (10, 14, 15, 37, 49–53).
Figure 1. Simplified schematic of BTK’s position in the signaling cascade.
Antigen-BCR binding triggers a signaling pathway in which BTK is recruited to the cell membrane from the cytosol, docks with the linker protein BLNK and phosphorylates PLCγ2, with downstream calcium flux and cellular activation via nuclear translocation of transcription factors NFκB and NFAT. Yellow=kinase, blue=adaptor, pink=transcription factor. Dashed arrows=multiple proteins involved. Inspired by Dal Porto and Cambier (129).
Figure 2. Structural rendering of subunits of BTK.
PH/Tec domain, blue and orange (130) (residues 2–170, 1BTK.pdb). SH3 domain, green (131)(residues 212–275, 1AWW.pdb). SH2 domain, purple (132) (residues 270–386, 2GE9.pdb). Kinase domain, red (133) (residues 397–659, 1K2P.pdb). The arrangement of the structures is for context, and is not intended to imply relative position or lack of inter-domain motion. PH=pleckstrin homology; SH=SRC homology. Image made with the PyMOL Molecular Graphics System, Version 1.6.0.0 (Schrödinger, LLC).
BTK regulation of B cell signaling responses relevant to autoimmunity
B cells are highly sensitive to the loss of proximal signaling proteins such as IgM or Syk, which cause B cell immunodeficiency(54–56). However, proteins involved in signal propagation can modulate signal responsiveness, rather than turning it on or off. Because tolerance induction and maintenance require nuanced responses to signaling, these proteins are good targets for fine-tuning and improving tolerance, and Btk regulation in mouse models has been shown to be important for B cell tolerance. Transgenic Btk overexpression causes a systemic lupus erythematosus-like disease, propagated by spontaneous germinal centers and autoantibody formation (25), and a model featuring constitutively activated Btk results in autoreactive IgM plasma cells (57). Conversely, Btk expression at 25% normal levels abrogates autoantibody production and an autoimmune syndrome produced in lyn-deficient mice (23). Autoreactive-prone B1a cells and anergic (An1) B cells both rely on Btk, and our lab has shown that Btk-deficiency eliminates 95% of anti-insulin B cells and protects against Type 1 diabetes (T1D) in nonobese diabetic (NOD) mice (10, 26, 37). Btk may also affect cytokine contributions to autoimmune disease, by supporting IL10 production by B cells(38). We showed that this B cell cytokine alteration may have downstream effects on T cells from Btk−/−/NOD mice, which also produce lower levels of IL10 in response to stimulation with anti-CD3/anti-CD28. This was specific to IL10, as IFNγ and IL17 production were normal (37). Importantly, IL10 has been shown to drive autoantibody production by human B cells from patients with systemic lupus erythematosus (SLE, lupus) and IL10 blockade has provided protection against lupus in the NZB x NZW model (58, 59).
Functional outcomes of BTK deficiency in humans
XLA is caused by more than 600 different mutations in the BTK gene, and is characterized by near-complete absence of B cells in humans, due to developmental arrest at the pre-B cell stage. These patients have fewer than 1% normal B cell levels, undetectable plasma cells, and very low serum immunoglobulin levels (1, 60–62). They are highly susceptible to infections with encapsulated bacteria that cause pneumonia, otitis media and sinusitis, requiring lifelong immunoglobulin replacement purified from donor pools. This treatment generally allows patients to live otherwise healthy lives, implying that BTK’s primary importance in humans resides in humoral immunity(2, 3). Patients with XLA are not generally thought to develop autoimmune disease, despite the fact that their few remaining B cells have an immature, high-IgM, phenotype, and are enriched for polyreactive, autoreactive-prone specificities (62, 63). There has been only one report of a patient developing T1D and a few reports of juvenile arthritis (64–66). Of note, however, a recent survey of XLA patients showed a majority had some self-reported symptoms consistent with inflammation or autoimmunity, although few had been formally diagnosed with autoimmune disease(67). The authors of that study noted that so little antibody is present that it is unlikely to cause these findings, but hypothesized that myeloid cell defects might contribute. Indeed, macrophages, neutrophils, dendritic cells and mast cells also express BTK (51, 68–71), although its role is not well-defined in those cell types. Some TLR responses are aberrant in the absence of BTK, which could contribute to susceptibility to infectious diseases (72, 73). In addition, overproduction of inflammatory cytokines in response to TLR signaling has been reported, which could contribute to inflammation in XLA patients (74, 75). In mouse models, Btk-deficient bone marrow-derived dendritic cells (BMDCs) exhibited increased levels of CD86 in response to lipopolysaccharide (LPS), and became antigen-presenting MHC class-IIhigh cells at a higher rate than their Btk-sufficient counterparts. This inflammatory phenotype was linked to a decreased ability of these BMDCs to produce IL-10, impairing the regulation of these cells (71).
BTK-deficiency as models for BTK-inhibitors
It is important to recognize that BTK-deficiency in human patients does not predict BTK inhibitor effects, for at least two reasons. First, BTK is a complex molecule with multiple functions, as discussed above. (Fig 2 and (11)). BTK-inhibitors target only the kinase domain, leaving other BTK activities intact, and in fact, ibrutinib does not recapitulate the XLA phenotype, as it does not reduce total IgG levels(19). Second, patients suffering from autoimmunity have a full repertoire of mature B cells, including those with normal and autoreactive specificities, cell populations not available for study in XLA patients. Thus there is no “natural experiment” in humans that allows in vivo evaluation of BTK function in mature B cells, or to differentiate their effects on autoreactive versus normal B cells. Btk-deficient murine B cells offer an advantage for these studies. Although they show slight delay in developmental progression at the pre-B cell stage, in addition to decreased Vκ transcription, this is offset by increased IL-7-driven proliferation that allows development of mature peripheral B cells for study of BTK function (76, 77) Btk-deficient murine B cells in the periphery have a developmental block in maturation at the transitional stage, but have 50–80% of normal B cell numbers, and contain some B cells in all subsets except for peritoneal B1a cells (8, 10, 37). These Btk-deficient B cells are suboptimally activated in response to stimulation of BCR, CD40, and TLRs (10, 37). While deficient in T-independent humoral responses, they are nevertheless able to respond to T-dependent immunizations. This suggests that B cells that have matured beyond the bone marrow stage may not rely heavily on Btk to respond to T cell-dependent vaccines (41). Therefore using small molecular inhibitors to target BTK in humans with mature B cells may not cause major humoral immunodeficiency, in contrast to XLA, in which B cells lack BTK from birth.
BTK contributions to pre-clinical models of autoimmune disease
Studies identifying a role for Btk in autoimmunity began using the xid mouse model more than a decade before the protein itself was discovered (Table I). Most of these early studies tested this x-linked antibody defect in a variety of murine models that depend upon autoantibodies, such as SLE and hemolytic anemia. The first of these used F1 males of New Zealand Black (NZB) mice crossed with CBA/N mice that carried the xid mutation during studies to understand gender differences in autoimmunity and showed data suggesting reduced autoantibody (anti-erythrocyte) production (78). The same group next found that NZB mice with the xid mutation did not develop anti-DNA antibodies, while aged (16 month old) MRL/1 mice with xid still did (79). The authors noted that NZB autoantibodies are mostly IgM and likely to arise from the T-independent B1 subset that is absent in xid mice, while MRL/1 autoantibodies were mostly IgG, associated with T cell driven lymphoproliferation. These findings implied that different B cell subsets and immune processes were responsible for the autoantibodies in the different models. Of note, a second group found that anti-erythrocyte and anti-DNA autoantibodies did occur in some NZB mice with xid, implying heterogeneity in this model (80). Next, congenic NZB mice with only the xid component of the CBA/N strain were developed (81). These mice were protected against autoantibody production, splenomegaly, hemolytic anemia, and early death, despite retaining the T cell abnormalities associated with NZB mice. Further work with this model showed that aging, or polyclonal stimulation with TLR ligands, could overcome disease protection(82, 83). Additional murine models of SLE, including NZBxNZW, C3H.gld/gld, and MRL.lpr/lpr have also been crossed with xid mice with similar results (Table I and references (84–86)). The first published work to use Btk-deficiency in a model of autoimmune arthritis was performed as part of a study of x-linked genes in 1993, just prior to identification of the protein responsible for the defect. The xid locus from CBA/N mice was crossed onto DBA/1 mice. DBA/1-xid offspring proved resistant to induction of collagen-induced arthritis, and failed to develop autoantibodies to type II collagen (9). Fourteen mice were used in this experiment, and the mechanism of disease protection was not elucidated at that time, although the findings were considered surprising, since CIA depends on T cells, and T cell-dependent B cell responses, not thought to be affected by xid. Our lab recently used the K/BxN spontaneous and serum transfer models to further investigate the role of Btk in arthritis, and discovered that its primary contribution is in the B cell compartment, especially germinal center development and function. Interestingly, autoantibodies were severely reduced while total IgG remained at near normal levels in Btk-deficient K/BxN mice, and spontaneous autoimmune arthritis was strikingly reduced (87). However, Btk-deficient recipients of K/BxN serum transfer developed arthritis at the same rate as Btk-sufficient littermates, indicating that innate contributions to arthritis are not affected by loss of Btk. This differs from a number of studies using BTK inhibitors that have shown efficacy in both innate and adaptively driven forms of autoimmune arthritis (88–90). These findings suggest that off-target effects of the inhibitors may contribute to their disease outcomes, and highlight the importance of including genetic approaches to define functional effects of Btk, rather than relying exclusively on inhibitors. We have also shown that Type 1 diabetes (T1D), considered to be T cell-mediated, is prevented by Btk-deficiency in the nonobese diabetic (NOD) mouse model of this disease, despite the absence of this protein in T cells, reinforcing the evidence that the primary function of B cells in this disease is to present autoantigens to T cells (31–33, 37). Anti-insulin B cells and autoantibodies were severely reduced, while total B cell numbers and total IgG levels were not (26, 37). We also showed that Btk supports naturally occurring autoreactive-prone anergic (An1) and autoreactive marginal zone (MZ) NOD B cells (26, 37, 91), which may also have implications for arthritis, as this subset was recently shown to be a primary source of anti-collagen B cells in collagen-induced arthritis (92).
Table I.
BTK and autoimmunity in murine models
| Autoimmune disease studies | Model used | Immune outcomes | Disease outcomes |
|---|---|---|---|
| Hemolytic anemia Ref: (78) 1979 |
CBA/N (xid) x NZB | Reduced anti-erythrocyte antibodies | N/D |
| Lupus Ref: (79) 1980 |
CBA/N (xid) x NZB and CBA/N (xid) x MRL/1 | Reduced anti-DNA antibodies in NZB (mostly IgM) but not aged MRL/1 (mostly IgG). | N/D |
| Hemolytic anemia and Lupus Ref: (80) 1980 |
CBA/N (xid) x NZB | Found that some offspring did have anti-erythrocyte and anti-DNA antibodies, suggesting heterogeneity in the model. | N/D |
| Lupus Refs: (81–83) 1981, 1982, 1987 |
NZB.xid | Loss of anti-DNA autoantibodies. Loss of splenomegaly. T cell abnormalities of NZB were not reversed. |
Protection from fatal renal disease. Disease could be restored by age or chronic stimulation with TLR ligands. |
| Lupus Ref: (84) 1982 |
NZBxNZW.xid/xid | Loss of anti-DNA autoantibodies, but not normal antibodies. Even immunization to DNA could not induce autoantibody formation. | Protection from fatal renal disease. |
| Lupus Refs:(85, 86) 1983, 1987 |
MRL.lpr/lpr xid and C3H.gld/gld xid | Decreased autoantibody production, despite persistence of T cell abnormalities and lymphadenopathy. | Reduced renal damage and death from autoimmunity. |
| Collagen-induced arthritis (CIA) Ref: (9) (1993) |
Xid | Lack of antibodies against type II collagen | Protection against arthritis. |
| Lupus-like autoimmune disease induced by Lyn deficiency Ref:(121, 122) 1998 |
Lyn-deficient xid and Btk−/−/ Lyn−/− | Severe reduction of B cell numbers and impaired B cell function; Protection against autoantibodies seen in Lyn−/− |
Protection against glomerulonephritis and/or splenomegaly seen in Lyn−/− |
| Lupus-like autoimmune disease induced by Lyn deficiency Ref: (23) 2003 |
Btklo/ Lyn−/− | Restoration of B cell numbers and function compared with Btk−/−/ Lyn−/−; Protection against autoantibodies seen in Lyn−/− |
Protection against splenomegaly seen in Lyn−/− |
| Experimental autoimmune encephalomyelitis (EAE) Ref: (123) 2002 |
Myelin oligodendrocyte glycoprotein-induced (MOG)- EAE DBA/1-xid | Reduced anti-MOG antibodies; increased MOG-specific T cell responses without change in IFNγ | Reduced severity of EAE |
| EAE, Dextran sulfate sodium (DSS)-induced colitis, carrageenan-induced edema Ref: (124) 2004 |
Xid mice | Poor macrophage survival and production of reactive oxygen intermediates (ROI) in response to lipopolysaccharide (LPS); normal phagocytosis, normal CD80/CD86 regulation, and near-normal motility of macrophages. | Reduced EAE, colitis and foot-pad edema |
| Lupus Ref: (38) 2008 |
56R.Btk−/− and Btklo/56R.Btk−/− | Loss of anti-DNA antibodies seen in 56R model; Btklo restored anti-DNA IgM but not IgG |
ND |
| Lupus-like autoimmune disease Ref: (25) 2012 |
B cell-specific BTK overexpression | Increased B cell activation and survival, spontaneous germinal centers, increased plasma cells and autoantibodies | Lupus-like autoimmune disease in lungs, kidneys and salivary glands |
| Type 1 diabetes Refs: (26, 37, 91) 2014, 2009, 2015 |
Btk−/−/NOD | Loss of most anti-insulin Tg B cells, An1 B cells, a subset of MZ B cells, and anti-insulin antibody. Preservation of total IgG levels. | Protection against T1D in NOD mice. Disease restored by presence of residual Btk−/− anti-insulin Tg B cells |
BTK inhibitors
The recognition of BTK as an important B cell target has generated a race to produce BTK-specific kinase inhibitors. The first inhibitor was described in 1999, and now dozens of recent publications report the outcomes of pre-clinical and clinical trials for these drugs, including ibrutinib, CC-292, RN486 and CGI1746 (Table II and (18, 21, 53, 88–90, 93–101). Many BTK-inhibitors are dosed orally and appear to be fairly well tolerated (18). Ibrutinib was recently the first-in-class to be FDA approved for treatment of mantle cell lymphoma and Waldenstrom macroglobulinemia (101, 102). This orally dosed daily drug is an irreversible inhibitor that binds BTK Cys481 (103). The irreversibility allows it to bind BTK in cells for 24 hours, even though the serum half-life is 2–3 hours, a feature which is thought to reduce side effects. Known off-target binding includes EGFR, HER2, HER4, BMX, JAK3, TEC and BLK (21). Ibrutinib also binds interleukin-2-inducible T cell kinase (ITK), shifting T cells away from TH2 toward TH1 cytokine profiles (103). Ibrutinib was shown to prevent both collagen induced arthritis (CIA) and collagen autoantibody induced arthritis (CAIA), and to reduce inflammatory cytokine release by macrophages and monocytes (89). CGI1746 stabilizes BTK in an inactive nonphosphorylated enzyme conformation, and was found to decrease FcγR mediated inflammation induced by immune complexes, and to prevent autoantibody production and arthritis in the CIA, CAIA, and K/BxN serum transfer models (88). CC-292 (formerly AVL-292) also binds Cys481 irreversibly and prevented CIA as well as improving arthritis outcomes when used therapeutically after disease onset in that model (96). Of note, a search of ClinicalTrials.gov reveals an on-going study of CC-292 versus placebo as co-therapy with methotrexate in active rheumatoid arthritis (NCT01975610). RN486 is a reversible inhibitor shown to prevent and reverse autoimmune and inflammatory arthritis in CIA, CAIA, and rat adjuvant induced arthritis (AIA) (90, 104). An early, non-covalent, reversible, BTK-inhibitor, LFM-A13 was shown to affect BTK responses and to effectively prevent graft-versus-host disease in murine allogenic bone marrow transplant studies (93, 105). However, it also has off-target effects as well as a high IC 50 (17.2 μM), requiring high doses for effect, and use of this drug in pre-clinical trials has become less common recent years (99). This explosion in the number of therapeutic trials of BTK-inhibitors highlights the importance of fully understanding their mechanisms of action. Our data suggest that low, intermittent dosing might be used to eliminate autoreactive B cells without inducing global B cell immunodeficiency. Lower dosing may be particularly useful for improving specificity of drug action, as specific kinase inhibition is difficult due to similarities in the kinase domains of different proteins. These drugs are designed very carefully for exclusive binding of BTK, yet even the best are known to have off-target effects, which may affect outcomes (103). Of note, the fact that other kinase inhibitors have had difficulty translating to clinical use for autoimmune disease has raised some skepticism, leading some experts to express only guarded optimism about this class of drugs (106).
Table II.
Pre-clinical studies of BTK-inhibitors for autoimmunity.
| Autoimmune disease studies | Models | Drug | Immune outcomes | Disease outcomes |
|---|---|---|---|---|
| Murine autoimmune arthritis Ref: (100) 2007 |
Collagen induced arthritis (CIA) | “Compound 4” by Celera | ND | Improved arthritis scores |
| Murine Lupus Ref: (125) 2010 |
MRL/Fas | Ibrutinib | Decreased autoantibodies | Improved renal impairment |
| Murine autoimmune arthritis Ref:(88) 2011 |
CIA, serum transfer models: collagen autoantibody induced arthritis (CAIA) and K/BxN | CGI1746 | Decreased FcγR mediated inflammation induced by immune complexes; prevented autoantibody production | Prevented autoimmune (CIA) and inflammatory arthritis (CAIA and K/BxN) |
| Rat autoimmune inflammatory arthritis Ref: (126) 2011 |
Rat Collagen induced arthritis | GDC-0834 | ND | Reduced ankle swelling |
| Murine autoimmune and inflammatory arthritis Ref: (89) 2011 |
CIA and CAIA | Ibrutinib | Reduced inflammatory cytokine release by macrophages and monocytes | Prevented arthritis in both CIA and CAIA. |
| Rodent arthritis Refs: (90, 104) 2012, 2015 |
mouse CIA, CAIA, and rat adjuvant-induced arthritis (AIA) | RN486 | Reduced tumor necrosis factor α (TNFα) production by monocytes stimulated with IgG-coated beads, reduced B cell activation, reduced autoantibodies and inflammatory markers in serum | Prevented arthritis in CIA and CAIA models and reduced arthritis when used therapeutically in CIA; reduced splenomegaly and AIA in rats |
| Glomerular nephritis (Lupus) Ref: (127) 2012 |
B6.sle1 and B6.Sle1.Sle3 | PCI-32675 (Ibrutinib), given for 56 days | Reduced autoantibodies, B cells, DCs, macrophages, neutrophils and activated T cells; normal numbers of naïve T cells | Reduced splenomegaly and glomerular nephritis, Reduced B cells in the kidneys despite no decrease in cellularity overall |
| Murine autoimmune arthritis Ref: (96) 2013 |
CIA | CC-292 | ND | Prevented and treated CIA |
| Glomerular nephritis (Lupus) Ref: (20) 2013 |
NZB x NZW | RN486 given for 8 weeks beginning at age 32 weeks | Reduced IgG autoantibodies, autoreactive B cells, B cell activation and splenic plasma cells, with preserved total IgG | Reduced proteinuria and glomerulosclerosis, IgG, IgM and C3 deposition, reduced macrophage infiltrations |
| Glomerular nephritis (Lupus) Ref: (128) 2013 |
NZBxW_F1 and Anti-glomerular basement membrane (GBM) antibody induced nephritis | PF-06250112, given for 12 weeks beginning at age 26 weeks | Reduced autoantibodies compared with vehicle-treated, reduced naïve B cells at high doses, reduced germinal center (GC) B cells and splenic plasma cells at all doses, reduced activated T cells with normal numbers of naïve T cells, preserved total IgG and IgA levels. | Reduced proteinuria, glomerular injury, cellular inflammatory infiltrates, IgG and C3 in the spontaneous model and prevention of proteinuria in the antibody-induced model. |
| Type 1 diabetes Ref: (26) 2014 |
Non-obese diabetic mice | Ibrutinib | Eliminated transgenic anti-insulin B cells in vitro | ND |
| Human rheumatoid arthritis and psoriatic arthritis Ref: (53) 2014 |
In vitro assays using synovial explants and human macrophages | RN486 | BTK was present in B cells, macrophages, monocytes and neutrophils in tissues. RN486 significantly inhibited IL6 production by stimulated macrophages, but only trended toward TNFα reduction. | ND |
Early studies of B cell-related outcomes in patients on BTK-inhibitors
The effect of BTK inhibitors on normal or autoreactive human B cells is not yet known. Some recent studies of patients with B cell malignancies treated with ibrutinib show that immunoglobulin levels are not reduced, indicating that targeting BTK with inhibitors need not replicate the typical immunodeficient phenotype associated with XLA(19, 107, 108). For example, patients with chronic lymphocytic leukemia (CLL) treated with ibrutinub showed no reduction in IgG for the first 6 months, followed by a decrease of 23% at 24 months, then stabilizing after that. IgM increased transiently and IgA levels showed substantial and sustained improvement, doubling at 24 months. Patients with the best IgA recovery also had fewer infections and infection rate correlated with IgA, not IgG levels. Polyreactive IgM and IgA, but not IgG, antibodies also increased, thought to be concurrent with renewed B cell populations, but also possibly similar to polyreactive antibodies seen in XLA patients (63). The number of normal B cells increased but did not recover entirely, remaining abnormally low even at 24 months, even in patients with otherwise normal lymphocyte counts. B cell precursors were present in about half of bone marrow samples (109). This evidence of improvement from an impaired baseline after treatment needs to be replicated in patients who do not start treatment in a state of humoral immunodeficiency however. There is also early evidence from patients undergoing treatment for leukemia that BTK inhibitors may have a beneficial effect on autoimmunity. One retrospective study that assessed autoimmune cytopenias in CLL showed a much lower than expected incidence in patients treated with ibrutinib (110). This was not a randomized controlled trial, and also does not address autoimmunity in other disease processes, but is early evidence suggestive that BTK-inhibitors may be useful for targeting autoreactive B cells in humans.
Conclusions
BTK structure, and function in murine B cells, has been well-defined since its discovery more than two decades ago. BTK contributions to human immunity are best-known by the defects conferred when it is absent, including loss of B lymphocytes and antibodies, while its role in shaping mature and autoreactive human B cell repertoires is not clear. BTK-inhibitors target only the kinase domain and do not necessarily impair the linker function. Their use in humans so far does not appear to recapitulate the XLA phenotype, although published studies at this time are limited to studies of patients with B cell related lymphomas. Furthermore, off-target effects of these drugs limit the interpretation of the role of BTK. Nevertheless, these drugs appear to be well-tolerated, and work in preclinical models suggests that they may prove useful for targeting autoreactive B cells in patients with autoimmune disease.
Expert Commentary
After more than two decades of careful work to understand BTK contributions to immunity and autoimmunity, we now stand at the verge of applying this knowledge to human health. Small molecular inhibitors are in the pipeline and the first is now in use in patients with B cell related lymphoma. Pre-clinical trials for autoimmunity are underway. Questions that must be answered include how these inhibitors affect patients, including normal and autoreactive B cells and antibodies as well as autoimmune disease outcomes, and potential for immunodeficiency. Given the fact that XLA patients have B cells that tend to be autoreactive it is also important to maintain vigilance for a possible increase in autoimmunity. B cell repertoire studies currently applied to patients with autoimmune disease would be valuable in understanding the impact of BTK inhibitors on patient repertoire (28, 63, 111). Also, BTK has been reported to have a role in B cell migration and adhesion (112), and in clinical trials with Ibrutinib-treated mantle cell lymphoma (MCL), patients exhibited egress of malignant cells from lymphatic tissue into peripheral blood (113). The impact of this mechanism of action may vary by autoimmune disease, as disruption of lymphoid structures by CXCL13 blockade has been shown in mouse models to ameliorate CIA and EAE (114, 115), but not T1D (116). Increased study of how lymphocytosis may affect autoimmune pathogenesis may be important in the future. In addition, the role of BTK in inflammatory responses mediated by innate cell populations is very poorly understood. Detailed studies of innate signaling responses such as those governed by TLRs have mostly been performed in B cells and there is evidence that BTK-mediated myeloid cell responses to the same TLRs may differ. BTK inhibitors that recently became commercially available for research are currently commonly used in these investigations and results of those studies are often cited as evidence of BTK’s role in various signaling pathways. However, their off-target effects may confound some of those outcomes and other approaches using specific genetic targeting are also needed. Understanding which drug effects can be attributed to BTK signaling, and which may be due to off-target kinase binding, is important for these studies, as well as for best practices in drug design and dosing. Finally, some chronic lymphocytic leukemia (CLL) patients have been reported to develop resistance to Ibrutinib, through mutations affecting binding to BTK or by hyper induction of PLCγ2 (117–120). Though autoreactive B cells lack the oncogenes that drive B cell lymphomas to continuously proliferate, the possibility of acquired resistance should not be ignored when considering treatment with BTK inhibitors.
Five-year view
The rapid advances in the development of BTK-inhibitors, including their current use in cancer and clinical trials for autoimmunity, suggests that these drugs will be in clinical use for autoimmune disease within five years. It may be hoped that careful dosing would blunt autoimmunity without inducing immunodeficiency, which would represent an advance over other anti-B cell drugs, such as rituximab, which eliminates all B cells. Caveats, however, are the unknown outcomes cited above, including the possible paradoxical increases in autoreactive B cells due to altered selection during development or in germinal centers, as well as the potential for immunodeficiency. Careful analysis of these outcomes during clinical trials will help refine the transition of this promising class of drugs into useful treatments for patients with autoimmune disease.
Key Issues.
Autoreactive B cells depend more upon B cell signaling than normal B cells do, as indicated by loss of autoreactive prone B cells including B1a, An1, transgenic anti-insulin B cells, marginal zone B cells in the nonobese-diabetic mouse model of autoimmune diabetes.
Total IgG is normal or near-normal while autoantibodies are significantly reduced in Btk-deficient mouse models.
Btk-deficient mice can still make T-dependent immune responses, suggesting that precise BTK targeting may have an advantage over global B cell elimination for treatment of autoimmunity without induction of humoral immunodeficiency.
X-linked aggamaglobulinemia cannot predict the potential effect of BTK-inhibitors on mature human B cells, as cells do not develop beyond the pre-B cell stage.
BTK is a multi-component signaling protein with adaptor function as well as kinase function, and most kinase inhibitors target only the kinase component.
Several BTK-inhibitors have proven effective in multiple pre-clinical models of autoimmune and inflammatory arthritis.
Comparison of BTK-deficiency with effects of BTK-inhibition suggest that some proportion of their disease protective effects may be due to off-target binding.
BTK-signaling in innate cells requires further investigation.#
Footnotes
Financial and competing interests disclosure
P Kendall holds a patent for the use of BTK inhibitors for type-1 diabetes. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1**.Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–8. First report describing humoral immunodeficiency in a male child with a disease later termed x-linked agammablobulinemia (XLA) and attributed to Bruton’s tyrosine kinase difficiency. [PubMed] [Google Scholar]
- 2.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, Conley ME, Cunningham-Rundles C, Ochs HD. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 3.Howard V, Greene JM, Pahwa S, Winkelstein JA, Boyle JM, Kocak M, Conley ME. The health status and quality of life of adults with X-linked agammaglobulinemia. Clinical immunology. 2006;118:201–8. doi: 10.1016/j.clim.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4**.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. doi: 10.1038/361226a0. The first of two seminal papers identifying the B cell tyrosine kinase deficient in XLA, later named Bruton’s tyrosine kinase. [DOI] [PubMed] [Google Scholar]
- 5**.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T, Quan S, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–90. doi: 10.1016/0092-8674(93)90667-f. One of two seminal papers identifying B cell tyrosine kinase deficient in XLA, later named Bruton’s tyrosine kinase. [DOI] [PubMed] [Google Scholar]
- 6**.Thomas JD, Sideras P, Smith CI, Vorechovsky I, Chapman V, Paul WE. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993;261:355–8. doi: 10.1126/science.8332900. Discovery of the mutation in BTK leading to humoral immunodeficiency in the xid mouse model (published concurrently with #7) [DOI] [PubMed] [Google Scholar]
- 7**.Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–61. doi: 10.1126/science.8332901. Discovery of the mutation in BTK leading to humoral immunodeficiency in the xid mouse model (published concurrently with #6) [DOI] [PubMed] [Google Scholar]
- 8.Khan WN, Sideras P, Rosen FS, Alt FW. The role of Bruton’s tyrosine kinase in B-cell development and function in mice and man. Ann NY Acad Sci. 1995;764:27–38. doi: 10.1111/j.1749-6632.1995.tb55802.x. [DOI] [PubMed] [Google Scholar]
- 9*.Jansson L, Holmdahl R. Genes on the X chromosome affect development of collagen-induced arthritis in mice. Clin Exp Immunol. 1993;94:459–65. doi: 10.1111/j.1365-2249.1993.tb08218.x. First study showing murine collagen-induced arthritis outcomes to be improved in xid mice, annotated with acknowledgement of the news that BTK had been discovered. This work paved the way for the first human trials of BTK-inhibitors in autoimmune disease, now underway for rheumatoid arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Muller S, Kantor AB, Herzenberg LA, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–99. doi: 10.1016/1074-7613(95)90114-0. Detailed study showing that mice with BTK-deficiency have the same phenotype as xid mice with a pleckstrin homology mutation, and further defining its function. [DOI] [PubMed] [Google Scholar]
- 11.Middendorp S, Dingjan GM, Maas A, Dahlenborg K, Hendriks RW. Function of Bruton’s tyrosine kinase during B cell development is partially independent of its catalytic activity. J Immunol. 2003;171:5988–96. doi: 10.4049/jimmunol.171.11.5988. [DOI] [PubMed] [Google Scholar]
- 12.Middendorp S, Zijlstra AJ, Kersseboom R, Dingjan GM, Jumaa H, Hendriks RW. Tumor suppressor function of Bruton tyrosine kinase is independent of its catalytic activity. Blood. 2005;105:259–65. doi: 10.1182/blood-2004-07-2708. [DOI] [PubMed] [Google Scholar]
- 13.Sideras P, Muller S, Shiels H, Jin H, Khan WN, Nilsson L, Parkinson E, Thomas JD, Branden L, Larsson I, et al. Genomic organization of mouse and human Bruton’s agammaglobulinemia tyrosine kinase (Btk) loci. Journal of Immunology. 1994;153:5607–17. [PubMed] [Google Scholar]
- 14.Petro JB, Rahman SM, Ballard DW, Khan WN. Bruton’s tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J Exp Med. 2000;191:1745–54. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petro JB, Khan WN. Phospholipase C-gamma 2 couples Bruton’s tyrosine kinase to the NF-kappaB signaling pathway in B lymphocytes. J Biol Chem. 2001;276:1715–9. doi: 10.1074/jbc.M009137200. [DOI] [PubMed] [Google Scholar]
- 16.Saffran DC, Parolini O, Fitch-Hilgenberg ME, Rawlings DJ, Afar DE, Witte ON, Conley ME. Brief report: a point mutation in the SH2 domain of Bruton’s tyrosine kinase in atypical X-linked agammaglobulinemia. The New England journal of medicine. 1994;330:1488–91. doi: 10.1056/NEJM199405263302104. [DOI] [PubMed] [Google Scholar]
- 17.Rawlings DJ, Scharenberg AM, Park H, Wahl MI, Lin S, Kato RM, Fluckiger AC, Witte ON, Kinet JP. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–5. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 18.Kelly V, Genovese M. Novel small molecule therapeutics in rheumatoid arthritis. Rheumatology. 2013;52:1155–62. doi: 10.1093/rheumatology/kes367. [DOI] [PubMed] [Google Scholar]
- 19.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A, Hedrick E, Fowler NH. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mina-Osorio P, LaStant J, Keirstead N, Whittard T, Ayala J, Stefanova S, Garrido R, Dimaano N, Hilton H, Giron M, Lau KY, Hang J, Postelnek J, Kim Y, Min S, Patel A, Woods J, Ramanujam M, DeMartino J, Narula S, Xu D. Suppression of glomerulonephritis in lupus-prone NZB x NZW mice by RN486, a selective inhibitor of Bruton’s tyrosine kinase. Arthritis and rheumatism. 2013;65:2380–91. doi: 10.1002/art.38047. [DOI] [PubMed] [Google Scholar]
- 21.Burger JA. Bruton’s tyrosine kinase (BTK) inhibitors in clinical trials. Curr Hematol Malig Rep. 2014;9:44–9. doi: 10.1007/s11899-013-0188-8. [DOI] [PubMed] [Google Scholar]
- 22.Saito K, Tolias KF, Saci A, Koon HB, Humphries LA, Scharenberg A, Rawlings DJ, Kinet JP, Carpenter CL. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–78. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- 23.Whyburn LR, Halcomb KE, Contreras CM, Lowell CA, Witte ON, Satterthwaite AB. Reduced dosage of Bruton’s tyrosine kinase uncouples B cell hyperresponsiveness from autoimmunity in lyn−/− mice. J Immunol. 2003;171:1850–8. doi: 10.4049/jimmunol.171.4.1850. [DOI] [PubMed] [Google Scholar]
- 24.Satterthwaite AB, Cheroutre H, Khan WN, Sideras P, Witte ON. Btk dosage determines sensitivity to B cell antigen receptor cross-linking. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13152–7. doi: 10.1073/pnas.94.24.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kil LP, de Bruijn MJ, van Nimwegen M, Corneth OB, van Hamburg JP, Dingjan GM, Thaiss F, Rimmelzwaan GF, Elewaut D, Delsing D, van Loo PF, Hendriks RW. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood. 2012;119:3744–56. doi: 10.1182/blood-2011-12-397919. [DOI] [PubMed] [Google Scholar]
- 26.Bonami RH, Sullivan AM, Case JB, Steinberg HE, Hoek KL, Khan WN, Kendall PL. Bruton’s tyrosine kinase promotes persistence of mature anti-insulin B cells. J Immunol. 2014;192:1459–70. doi: 10.4049/jimmunol.1300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 28.Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, Joly F, Rosenzwajg M, Sene D, Benech P, Musset L, Klatzmann D, Meffre E, Cacoub P. Expansion of autoreactive unresponsive CD21-/low B cells in Sjogren’s syndrome-associated lymphoproliferation. Arthritis and rheumatism. 2013;65:1085–96. doi: 10.1002/art.37828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnunen T, Chamberlain N, Morbach H, Cantaert T, Lynch M, Preston-Hurlburt P, Herold KC, Hafler DA, O’Connor KC, Meffre E. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest. 2013;123:2737–41. doi: 10.1172/JCI68775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quach TD, Manjarrez-Orduno N, Adlowitz DG, Silver L, Yang H, Wei C, Milner EC, Sanz I. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J Immunol. 2011;186:4640–8. doi: 10.4049/jimmunol.1001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–8. [PubMed] [Google Scholar]
- 32.Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SA, Schlachterman A, Song HK, Noto LE, Jevnikar AM, Barker CF, Naji A. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163:743–50. [PubMed] [Google Scholar]
- 33.Kendall PL, Case JB, Sullivan AM, Holderness JS, Wells KS, Liu E, Thomas JW. Tolerant anti-insulin B cells are effective APCs. J Immunol. 2013;190:2519–26. doi: 10.4049/jimmunol.1202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kendall PL, Woodward EJ, Hulbert C, Thomas JW. Peritoneal B cells govern the outcome of diabetes in non-obese diabetic mice. Eur J Immunol. 2004;34:2387–95. doi: 10.1002/eji.200324744. [DOI] [PubMed] [Google Scholar]
- 36.Kendall PL, Yu G, Woodward EJ, Thomas JW. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J Immunol. 2007;178:5643–51. doi: 10.4049/jimmunol.178.9.5643. [DOI] [PubMed] [Google Scholar]
- 37.Kendall PL, Moore DJ, Hulbert C, Hoek KL, Khan WN, Thomas JW. Reduced diabetes in btk-deficient nonobese diabetic mice and restoration of diabetes with provision of an anti-insulin IgH chain transgene. J Immunol. 2009;183:6403–12. doi: 10.4049/jimmunol.0900367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halcomb KE, Musuka S, Gutierrez T, Wright HL, Satterthwaite AB. Btk regulates localization, in vivo activation, and class switching of anti-DNA B cells. Molecular immunology. 2008;46:233–41. doi: 10.1016/j.molimm.2008.08.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flaswinkel H, Reth M. Dual role of the tyrosine activation motif of the Ig-alpha protein during signal transduction via the B cell antigen receptor. The EMBO journal. 1994;13:83–9. doi: 10.1002/j.1460-2075.1994.tb06237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowley RB, Burkhardt AL, Chao HG, Matsueda GR, Bolen JB. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J Biol Chem. 1995;270:11590–4. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 41.Corneth OB, Klein Wolterink RG, Hendriks RW. BTK Signaling in B Cell Differentiation and Autoimmunity. Current topics in microbiology and immunology. 2016;393:67–105. doi: 10.1007/82_2015_478. [DOI] [PubMed] [Google Scholar]
- 42.Hendriks RW, de Bruijn MF, Maas A, Dingjan GM, Karis A, Grosveld F. Inactivation of Btk by insertion of lacZ reveals defects in B cell development only past the pre-B cell stage. The EMBO journal. 1996;15:4862–72. [PMC free article] [PubMed] [Google Scholar]
- 43.Kerner JD, Appleby MW, Mohr RN, Chien S, Rawlings DJ, Maliszewski CR, Witte ON, Perlmutter RM. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity. 1995;3:301–12. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 44.Baba Y, Hashimoto S, Matsushita M, Watanabe D, Kishimoto T, Kurosaki T, Tsukada S. BLNK mediates Syk-dependent Btk activation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2582–6. doi: 10.1073/pnas.051626198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurosaki T, Kurosaki M. Transphosphorylation of Bruton’s tyrosine kinase on tyrosine 551 is critical for B cell antigen receptor function. J Biol Chem. 1997;272:15595–8. doi: 10.1074/jbc.272.25.15595. [DOI] [PubMed] [Google Scholar]
- 46.Morrogh LM, Hinshelwood S, Costello P, Cory GO, Kinnon C. The SH3 domain of Bruton’s tyrosine kinase displays altered ligand binding properties when auto-phosphorylated in vitro. European journal of immunology. 1999;29:2269–79. doi: 10.1002/(SICI)1521-4141(199907)29:07<2269::AID-IMMU2269>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Guo S, Ferl GZ, Deora R, Riedinger M, Yin S, Kerwin JL, Loo JA, Witte ON. A phosphorylation site in Bruton’s tyrosine kinase selectively regulates B cell calcium signaling efficiency by altering phospholipase C-gamma activation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14180–5. doi: 10.1073/pnas.0405878101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimoto A, Okada H, Jiang A, Kurosaki M, Greenberg S, Clark EA, Kurosaki T. Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J Exp Med. 1998;188:1287–95. doi: 10.1084/jem.188.7.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan WN. Regulation of B lymphocyte development and activation by Bruton’s tyrosine kinase. Immunol Res. 2001;23:147–56. doi: 10.1385/IR:23:2-3:147. [DOI] [PubMed] [Google Scholar]
- 50.Antony P, Petro JB, Carlesso G, Shinners NP, Lowe J, Khan WN. B cell receptor directs the activation of NFAT and NF-kappaB via distinct molecular mechanisms. Exp Cell Res. 2003;291:11–24. doi: 10.1016/s0014-4827(03)00338-0. [DOI] [PubMed] [Google Scholar]
- 51.Jongstra-Bilen J, Puig Cano A, Hasija M, Xiao H, Smith CI, Cybulsky MI. Dual functions of Bruton’s tyrosine kinase and Tec kinase during Fcgamma receptor-induced signaling and phagocytosis. J Immunol. 2008;181:288–98. doi: 10.4049/jimmunol.181.1.288. [DOI] [PubMed] [Google Scholar]
- 52.Paracha RZ, Ali A, Ahmad J, Hussain R, Niazi U, Muhammad SA. Structural evaluation of BTK and PKCdelta mediated phosphorylation of MAL at positions Tyr86 and Tyr106. Comput Biol Chem. 2014;51:22–35. doi: 10.1016/j.compbiolchem.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Hartkamp LM, Fine JS, van Es IE, Tang MW, Smith M, Woods J, Narula S, DeMartino J, Tak PP, Reedquist KA. Btk inhibition suppresses agonist-induced human macrophage activation and inflammatory gene expression in RA synovial tissue explants. Ann Rheum Dis. 2015;74:1603–11. doi: 10.1136/annrheumdis-2013-204143. [DOI] [PubMed] [Google Scholar]
- 54.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–83. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 55.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 56.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–6. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 57.Kersseboom R, Kil L, Flierman R, van der Zee M, Dingjan GM, Middendorp S, Maas A, Hendriks RW. Constitutive activation of Bruton’s tyrosine kinase induces the formation of autoreactive IgM plasma cells. European journal of immunology. 2010;40:2643–54. doi: 10.1002/eji.201040521. [DOI] [PubMed] [Google Scholar]
- 58.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P, Emilie D. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med. 1994;179:305–10. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valiaho J, Smith CI, Vihinen M. BTKbase: the mutation database for X-linked agammaglobulinemia. Human mutation. 2006;27:1209–17. doi: 10.1002/humu.20410. [DOI] [PubMed] [Google Scholar]
- 61.Pearl ER, Vogler LB, Okos AJ, Crist WM, Lawton AR, 3rd, Cooper MD. B lymphocyte precursors in human bone marrow: an analysis of normal individuals and patients with antibody-deficiency states. Journal of Immunology. 1978;120:1169–75. [PubMed] [Google Scholar]
- 62.Conley ME. B cells in patients with X-linked agammaglobulinemia. Journal of Immunology. 1985;134:3070–4. [PubMed] [Google Scholar]
- 63.Ng YS, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase is essential for human B cell tolerance. J Exp Med. 2004;200:927–34. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patiroglu T, Akar HH, Gunduz Z, Sisko S, Ng YY. X-linked agammaglobulinemia in two siblings with a novel mutation in the BTK gene who presented with polyarticular juvenile idiopathic arthritis. Scandinavian journal of rheumatology. 2015;44:168–70. doi: 10.3109/03009742.2014.995699. [DOI] [PubMed] [Google Scholar]
- 65.Martin S, Wolf-Eichbaum D, Duinkerken G, Scherbaum WA, Kolb H, Noordzij JG, Roep BO. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. The New England journal of medicine. 2001;345:1036–40. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]
- 66.Machado P, Santos A, Faria E, Silva J, Malcata A, Chieira C. Arthritis and X-linked agammaglobulinemia. Acta reumatologica portuguesa. 2008;33:464–7. [PubMed] [Google Scholar]
- 67.Hernandez-Trujillo VP, Scalchunes C, Cunningham-Rundles C, Ochs HD, Bonilla FA, Paris K, Yel L, Sullivan KE. Autoimmunity and inflammation in X-linked agammaglobulinemia. Journal of clinical immunology. 2014;34:627–32. doi: 10.1007/s10875-014-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith CI, Baskin B, Humire-Greiff P, Zhou JN, Olsson PG, Maniar HS, Kjellen P, Lambris JD, Christensson B, Hammarstrom L, et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol. 1994;152:557–65. [PubMed] [Google Scholar]
- 69.Kawakami Y, Yao L, Miura T, Tsukada S, Witte ON, Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon Fc epsilon RI cross-linking. Mol Cell Biol. 1994;14:5108–13. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lachance G, Levasseur S, Naccache PH. Chemotactic factor-induced recruitment and activation of Tec family kinases in human neutrophils. Implication of phosphatidynositol 3-kinases. J Biol Chem. 2002;277:21537–41. doi: 10.1074/jbc.M201903200. [DOI] [PubMed] [Google Scholar]
- 71.Kawakami Y, Inagaki N, Salek-Ardakani S, Kitaura J, Tanaka H, Nagao K, Xiao W, Nagai H, Croft M, Kawakami T. Regulation of dendritic cell maturation and function by Bruton’s tyrosine kinase via IL-10 and Stat3. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:153–8. doi: 10.1073/pnas.0509784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lougaris V, Baronio M, Vitali M, Tampella G, Cattalini M, Tassone L, Soresina A, Badolato R, Plebani A. Bruton tyrosine kinase mediates TLR9-dependent human dendritic cell activation. The Journal of allergy and clinical immunology. 2014;133:1644–50. e4. doi: 10.1016/j.jaci.2013.12.1085. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Lau KY, Jung J, Ravindran P, Barrat FJ. Bruton’s tyrosine kinase regulates TLR9 but not TLR7 signaling in human plasmacytoid dendritic cells. European journal of immunology. 2014;44:1130–6. doi: 10.1002/eji.201344030. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez-Serrano ME, Estrada-Garcia I, Mogica-Martinez D, Gonzalez-Garay A, Lopez-Herrera G, Berron-Ruiz L, Espinosa-Padilla SE, Yamazaki-Nakashimada MA, Vargas-Hernandez A, Santos-Argumedo L, Estrada-Parra SA, Espinosa-Rosales FJ. Increased pro-inflammatory cytokine production after lipopolysaccharide stimulation in patients with X-linked agammaglobulinemia. Journal of clinical immunology. 2012;32:967–74. doi: 10.1007/s10875-012-9706-z. [DOI] [PubMed] [Google Scholar]
- 75.Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, Yao H, Cao X. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nature immunology. 2011;12:416–24. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- 76.Middendorp S, Dingjan GM, Hendriks RW. Impaired precursor B cell differentiation in Bruton’s tyrosine kinase-deficient mice. J Immunol. 2002;168:2695–703. doi: 10.4049/jimmunol.168.6.2695. [DOI] [PubMed] [Google Scholar]
- 77.Stadhouders R, de Bruijn MJ, Rother MB, Yuvaraj S, Ribeiro de Almeida C, Kolovos P, Van Zelm MC, van Ijcken W, Grosveld F, Soler E, Hendriks RW. Pre-B cell receptor signaling induces immunoglobulin kappa locus accessibility by functional redistribution of enhancer-mediated chromatin interactions. PLoS Biol. 2014;12:e1001791. doi: 10.1371/journal.pbio.1001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78*.Taurog JD, Moutsopoulos HM, Rosenberg YJ, Chused TM, Steinberg AD. CBA/N X-linked B-cell defect prevents NZB B-cell hyperactivity in F1 mice. J Exp Med. 1979;150:31–43. doi: 10.1084/jem.150.1.31. First report linking BTK to autoimmune disease, prior to knowledge that BTK was the defect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cowdery JS, Jr, Taurog JD, Steinberg AD. Effect of CBA/N xid on spontaneous production of antibodies to DNA in MRL/1 and NZB backcross mice. Scandinavian journal of immunology. 1980;12:499–502. doi: 10.1111/j.1365-3083.1980.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 80.Romain PL, Cohen PL, Fish F, Ziff M, Vitetta ES. The specific B cell subset lacking in the CBA/N mouse is not required for the production of autoantibody in (CBA/N x NZB)F1 male mice. Journal of Immunology. 1980;125:246–51. [PubMed] [Google Scholar]
- 81.Taurog JD, Raveche ES, Smathers PA, Glimcher LH, Huston DP, Hansen CT, Steinberg AD. T cell abnormalities in NZB mice occur independently of autoantibody production. J Exp Med. 1981;153:221–34. doi: 10.1084/jem.153.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smathers PA, Steinberg BJ, Reeves JP, Steinberg AD. Effects of polyclonal immune stimulators upon NZB.xid congenic mice. Journal of Immunology. 1982;128:1414–9. [PubMed] [Google Scholar]
- 83.Waegell WO, Gershwin ME, Castles JJ. The use of congenital immunologic mutants to probe autoimmune disease in New Zealand mice. Progress in clinical and biological research. 1987;229:175–97. [PubMed] [Google Scholar]
- 84.Steinberg BJ, Smathers PA, Frederiksen K, Steinberg AD. Ability of the xid gene to prevent autoimmunity in (NZB X NZW)F1 mice during the course of their natural history, after polyclonal stimulation, or following immunization with DNA. J Clin Invest. 1982;70:587–97. doi: 10.1172/JCI110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steinberg EB, Santoro TJ, Chused TM, Smathers PA, Steinberg AD. Studies of congenic MRL-Ipr/Ipr.xid mice. Journal of Immunology. 1983;131:2789–95. [PubMed] [Google Scholar]
- 86.Seldin MF, Reeves JP, Scribner CL, Roths JB, Davidson WF, Morse HC, 3rd, Steinberg AD. Effect of xid on autoimmune C3H-gld/gld mice. Cellular immunology. 1987;107:249–55. doi: 10.1016/0008-8749(87)90284-x. [DOI] [PubMed] [Google Scholar]
- 87.Nyhoff LE, Barron B, Johnson EM, Bonami RH, Maseda D, Fensterheim BA, Han, Blackwell TS, Crofford LJ, Kendall PL. Bruton’s tyrosine kinase deficiency inhibits autoimmune arthritis but fails to block immune complex-mediated inflammatory arthritis. Arthritis & Rheumatology. 2016 doi: 10.1002/art.39657. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Paolo JA, Huang T, Balazs M, Barbosa J, Barck KH, Bravo BJ, Carano RA, Darrow J, Davies DR, DeForge LE, Diehl L, Ferrando R, Gallion SL, Giannetti AM, Gribling P, Hurez V, Hymowitz SG, Jones R, Kropf JE, Lee WP, Maciejewski PM, Mitchell SA, Rong H, Staker BL, Whitney JA, Yeh S, Young WB, Yu C, Zhang J, Reif K, Currie KS. Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat Chem Biol. 2011;7:41–50. doi: 10.1038/nchembio.481. [DOI] [PubMed] [Google Scholar]
- 89.Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P, Robinson WH, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther. 2011;13:R115. doi: 10.1186/ar3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu D, Kim Y, Postelnek J, Vu MD, Hu DQ, Liao C, Bradshaw M, Hsu J, Zhang J, Pashine A, Srinivasan D, Woods J, Levin A, O’Mahony A, Owens TD, Lou Y, Hill RJ, Narula S, DeMartino J, Fine JS. RN486, a selective Bruton’s tyrosine kinase inhibitor, abrogates immune hypersensitivity responses and arthritis in rodents. J Pharmacol Exp Ther. 2012;341:90–103. doi: 10.1124/jpet.111.187740. [DOI] [PubMed] [Google Scholar]
- 91.Case JB, Bonami RH, Nyhoff LE, Steinberg HE, Sullivan AM, Kendall PL. Bruton’s Tyrosine Kinase Synergizes with Notch2 To Govern Marginal Zone B Cells in Nonobese Diabetic Mice. J Immunol. 2015;195:61–70. doi: 10.4049/jimmunol.1400803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palm AK, Friedrich HC, Mezger A, Salomonsson M, Myers LK, Kleinau S. Function and regulation of self-reactive marginal zone B cells in autoimmune arthritis. Cellular & molecular immunology. 2015;12:493–504. doi: 10.1038/cmi.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mahajan S, Ghosh S, Sudbeck EA, Zheng Y, Downs S, Hupke M, Uckun FM. Rational design and synthesis of a novel anti-leukemic agent targeting Bruton’s tyrosine kinase (BTK), LFM-A13 [alpha-cyano-beta-hydroxy-beta-methyl-N-(2, 5-dibromophenyl)propenamide] J Biol Chem. 1999;274:9587–99. doi: 10.1074/jbc.274.14.9587. [DOI] [PubMed] [Google Scholar]
- 94.Rushworth SA, MacEwan DJ, Bowles KM. Ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369:1277–8. doi: 10.1056/NEJMc1309710. [DOI] [PubMed] [Google Scholar]
- 95.Akinleye A, Chen Y, Mukhi N, Song Y, Liu D. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol. 2013;6:59. doi: 10.1186/1756-8722-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evans EK, Tester R, Aslanian S, Karp R, Sheets M, Labenski MT, Witowski SR, Lounsbury H, Chaturvedi P, Mazdiyasni H, Zhu Z, Nacht M, Freed MI, Petter RC, Dubrovskiy A, Singh J, Westlin WF. Inhibition of Btk with CC-292 provides early pharmacodynamic assessment of activity in mice and humans. J Pharmacol Exp Ther. 2013;346:219–28. doi: 10.1124/jpet.113.203489. [DOI] [PubMed] [Google Scholar]
- 97.Robak T, Robak E. Tyrosine kinase inhibitors as potential drugs for B-cell lymphoid malignancies and autoimmune disorders. Expert Opin Investig Drugs. 2012;21:921–47. doi: 10.1517/13543784.2012.685650. [DOI] [PubMed] [Google Scholar]
- 98.Dias AL, Jain D. Ibrutinib: a new frontier in the treatment of chronic lymphocytic leukemia by Bruton’s tyrosine kinase inhibition. Cardiovasc Hematol Agents Med Chem. 2013;11:265–71. doi: 10.2174/1871525712666140115143914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vargas L, Hamasy A, Nore BF, Smith CI. Inhibitors of BTK and ITK: state of the new drugs for cancer, autoimmunity and inflammatory diseases. Scandinavian journal of immunology. 2013;78:130–9. doi: 10.1111/sji.12069. [DOI] [PubMed] [Google Scholar]
- 100.Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC, Mendonca RV, Sweeney MD, Scott KC, Grothaus PG, Jeffery DA, Spoerke JM, Honigberg LA, Young PR, Dalrymple SA, Palmer JT. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2:58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 101**.Cameron F, Sanford M. Ibrutinib: first global approval. Drugs. 2014;74:263–71. doi: 10.1007/s40265-014-0178-8. Announcement of first FDA approval for use of a small molecular BTK inhibitor for human patients, specifically for lymphoma. [DOI] [PubMed] [Google Scholar]
- 102.Chakraborty R, Kapoor P, Ansell SM, Gertz MA. Ibrutinib for the treatment of Waldenstrom macroglobulinemia. Expert Rev Hematol. 2015;8:569–79. doi: 10.1586/17474086.2015.1061427. [DOI] [PubMed] [Google Scholar]
- 103.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu TM, Chang BY, Larkin KM, Stefanovski MR, Chappell DL, Frissora FW, Smith LL, Smucker KA, Flynn JM, Jones JA, Andritsos LA, Maddocks K, Lehman AM, Furman R, Sharman J, Mishra A, Caligiuri MA, Satoskar AR, Buggy JJ, Muthusamy N, Johnson AJ, Byrd JC. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lou Y, Han X, Kuglstatter A, Kondru RK, Sweeney ZK, Soth M, McIntosh J, Litman R, Suh J, Kocer B, Davis D, Park J, Frauchiger S, Dewdney N, Zecic H, Taygerly JP, Sarma K, Hong J, Hill RJ, Gabriel T, Goldstein DM, Owens TD. Structure-based drug design of RN486, a potent and selective Bruton’s tyrosine kinase (BTK) inhibitor, for the treatment of rheumatoid arthritis. Journal of medicinal chemistry. 2015;58:512–6. doi: 10.1021/jm500305p. [DOI] [PubMed] [Google Scholar]
- 105.Cetkovic-Cvrlje M, Uckun FM. Dual targeting of Bruton’s tyrosine kinase and Janus kinase 3 with rationally designed inhibitors prevents graft-versus-host disease (GVHD) in a murine allogeneic bone marrow transplantation model. British journal of haematology. 2004;126:821–7. doi: 10.1111/j.1365-2141.2004.05126.x. [DOI] [PubMed] [Google Scholar]
- 106.Ruderman EM, Pope RM. More than just B-cell inhibition. Arthritis Res Ther. 2011;13:125. doi: 10.1186/ar3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O’Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, Grant B, Richards DA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Izumi R, Hamdy A, Chang BY, Graef T, Clow F, Buggy JJ, James DF, Byrd JC. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun C, Tian X, Lee YS, Gunti S, Lipsky A, Herman SE, Salem D, Stetler-Stevenson M, Yuan C, Kardava L, Moir S, Maric I, Valdez J, Soto S, Marti GE, Farooqui MZ, Notkins AL, Wiestner A, Aue G. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126:2213–9. doi: 10.1182/blood-2015-04-639203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rogers KA, Ruppert AS, Bingman A, Andritsos LA, Awan FT, Blum KA, Flynn JM, Jaglowski SM, Lozanski G, Maddocks KJ, Byrd JC, Woyach JA, Jones JA. Incidence and description of autoimmune cytopenias during treatment with ibrutinib for chronic lymphocytic leukemia. Leukemia. 2016;30:346–50. doi: 10.1038/leu.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, Schieferl S, Hom J, Jenks S, Feldman RJ, Mehr R, Wei C, Lee FE, Cheung WC, Rosenberg AF, Sanz I. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nature immunology. 2015;16:755–65. doi: 10.1038/ni.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, van Gils JM, Hendriks RW, Pals ST, Spaargaren M. Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26:93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 113.Chang BY, Francesco M, De Rooij MF, Magadala P, Steggerda SM, Huang MM, Kuil A, Herman SE, Chang S, Pals ST, Wilson W, Wiestner A, Spaargaren M, Buggy JJ, Elias L. Egress of CD19(+)CD5(+) cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood. 2013;122:2412–24. doi: 10.1182/blood-2013-02-482125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Finch DK, Ettinger R, Karnell JL, Herbst R, Sleeman MA. Effects of CXCL13 inhibition on lymphoid follicles in models of autoimmune disease. Eur J Clin Invest. 2013;43:501–9. doi: 10.1111/eci.12063. [DOI] [PubMed] [Google Scholar]
- 115.Klimatcheva E, Pandina T, Reilly C, Torno S, Bussler H, Scrivens M, Jonason A, Mallow C, Doherty M, Paris M, Smith ES, Zauderer M. CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015;16:6. doi: 10.1186/s12865-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Henry RA, Kendall PL. CXCL13 blockade disrupts B lymphocyte organization in tertiary lymphoid structures without altering B cell receptor bias or preventing diabetes in nonobese diabetic mice. J Immunol. 2010;185:1460–5. doi: 10.4049/jimmunol.0903710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, Xue L, Li DH, Steggerda SM, Versele M, Dave SS, Zhang J, Yilmaz AS, Jaglowski SM, Blum KA, Lozanski A, Lozanski G, James DF, Barrientos JC, Lichter P, Stilgenbauer S, Buggy JJ, Chang BY, Johnson AJ, Byrd JC. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. The New England journal of medicine. 2014;370:2286–94. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Furman RR, Cheng S, Lu P, Setty M, Perez AR, Guo A, Racchumi J, Xu G, Wu H, Ma J, Steggerda SM, Coleman M, Leslie C, Wang YL. Ibrutinib resistance in chronic lymphocytic leukemia. The New England journal of medicine. 2014;370:2352–4. doi: 10.1056/NEJMc1402716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu TM, Woyach JA, Zhong Y, Lozanski A, Lozanski G, Dong S, Strattan E, Lehman A, Zhang X, Jones JA, Flynn J, Andritsos LA, Maddocks K, Jaglowski SM, Blum KA, Byrd JC, Dubovsky JA, Johnson AJ. Hypermorphic mutation of phospholipase C, gamma2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood. 2015;126:61–8. doi: 10.1182/blood-2015-02-626846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng S, Guo A, Lu P, Ma J, Coleman M, Wang YL. Functional characterization of BTK(C481S) mutation that confers ibrutinib resistance: exploration of alternative kinase inhibitors. Leukemia. 2015;29:895–900. doi: 10.1038/leu.2014.263. [DOI] [PubMed] [Google Scholar]
- 121.Satterthwaite AB, Lowell CA, Khan WN, Sideras P, Alt FW, Witte ON. Independent and opposing roles for Btk and lyn in B and myeloid signaling pathways. J Exp Med. 1998;188:833–44. doi: 10.1084/jem.188.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Takeshita H, Taniuchi I, Kato J, Watanabe T. Abrogation of autoimmune disease in Lyn-deficient mice by the mutation of the Btk gene. International immunology. 1998;10:435–44. doi: 10.1093/intimm/10.4.435. [DOI] [PubMed] [Google Scholar]
- 123.Svensson L, Abdul-Majid KB, Bauer J, Lassmann H, Harris RA, Holmdahl R. A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. European journal of immunology. 2002;32:1939–46. doi: 10.1002/1521-4141(200207)32:7<1939::AID-IMMU1939>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 124.Mangla A, Khare A, Vineeth V, Panday NN, Mukhopadhyay A, Ravindran B, Bal V, George A, Rath S. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood. 2004;104:1191–7. doi: 10.1182/blood-2004-01-0207. [DOI] [PubMed] [Google Scholar]
- 125.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu L, Di Paolo J, Barbosa J, Rong H, Reif K, Wong H. Antiarthritis effect of a novel Bruton’s tyrosine kinase (BTK) inhibitor in rat collagen-induced arthritis and mechanism-based pharmacokinetic/pharmacodynamic modeling: relationships between inhibition of BTK phosphorylation and efficacy. J Pharmacol Exp Ther. 2011;338:154–63. doi: 10.1124/jpet.111.181545. [DOI] [PubMed] [Google Scholar]
- 127.Hutcheson J, Vanarsa K, Bashmakov A, Grewal S, Sajitharan D, Chang BY, Buggy JJ, Zhou XJ, Du Y, Satterthwaite AB, Mohan C. Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus. Arthritis research & therapy. 2012;14:R243. doi: 10.1186/ar4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rankin AL, Seth N, Keegan S, Andreyeva T, Cook TA, Edmonds J, Mathialagan N, Benson MJ, Syed J, Zhan Y, Benoit SE, Miyashiro JS, Wood N, Mohan S, Peeva E, Ramaiah SK, Messing D, Homer BL, Dunussi-Joannopoulos K, Nickerson-Nutter CL, Schnute ME, Douhan J., 3rd Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. Journal of Immunology. 2013;191:4540–50. doi: 10.4049/jimmunol.1301553. [DOI] [PubMed] [Google Scholar]
- 129.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Molecular immunology. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 130.Hyvonen M, Saraste M. Structure of the PH domain and Btk motif from Bruton’s tyrosine kinase: molecular explanations for X-linked agammaglobulinaemia. The EMBO journal. 1997;16:3396–404. doi: 10.1093/emboj/16.12.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hansson H, Mattsson PT, Allard P, Haapaniemi P, Vihinen M, Smith CI, Hard T. Solution structure of the SH3 domain from Bruton’s tyrosine kinase. Biochemistry. 1998;37:2912–24. doi: 10.1021/bi972409f. [DOI] [PubMed] [Google Scholar]
- 132.Huang KC, Cheng HT, Pai MT, Tzeng SR, Cheng JW. Solution structure and phosphopeptide binding of the SH2 domain from the human Bruton’s tyrosine kinase. J Biomol NMR. 2006;36:73–8. doi: 10.1007/s10858-006-9064-3. [DOI] [PubMed] [Google Scholar]
- 133.Mao C, Zhou M, Uckun FM. Crystal structure of Bruton’s tyrosine kinase domain suggests a novel pathway for activation and provides insights into the molecular basis of X-linked agammaglobulinemia. The Journal of biological chemistry. 2001;276:41435–43. doi: 10.1074/jbc.M104828200. [DOI] [PubMed] [Google Scholar]