Abstract
Spiral nematode species of the genus Rotylenchus have been reported on olive (Olea europaea L.) in several Mediterranean countries (Castillo et al., 2010; Ali et al., 2014). Nematological surveys for plant-parasitic nematodes on olive trees were carried out in Tunisia between 2013 and 2014, and two nematode species of Rotylenchus were collected from the rhizosphere of olive cv. Chemlali in several localities of Tunisia (Tables 1,2
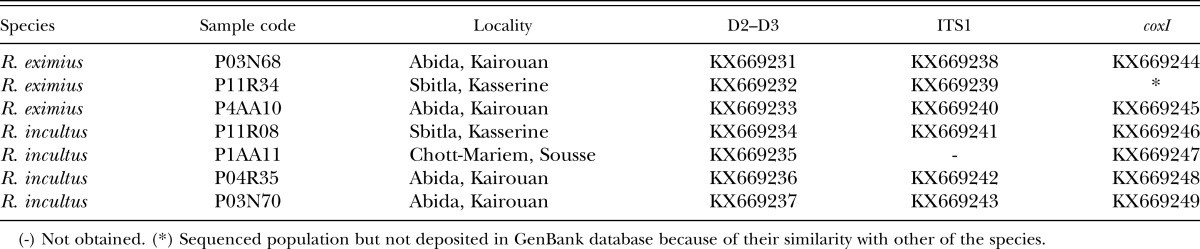
Table 1.
Rotylenchus eximius Siddiqi, 1964 and R. incultus Sher, 1965 sampled in olive cv. Chemlali in Tunisia and sequences used in this study.
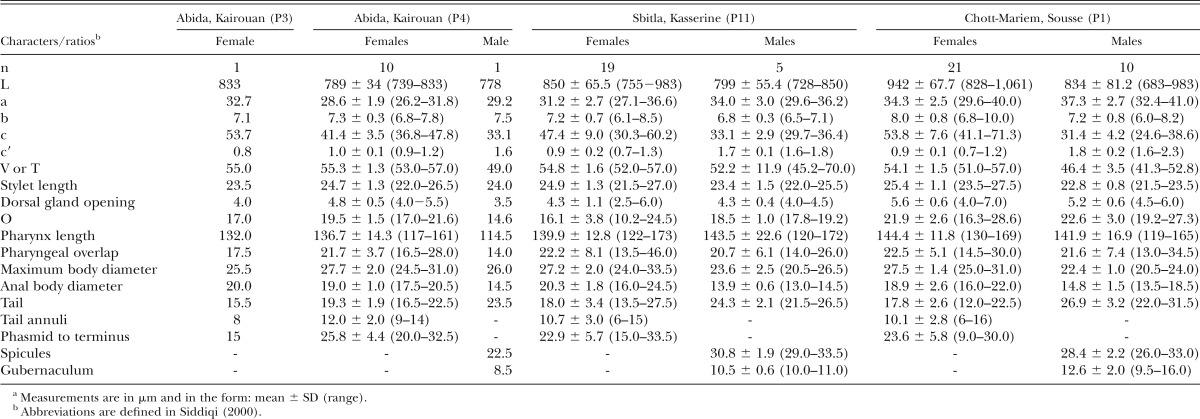
Table 2.
Morphometrics of Rotylenchus incultus Sher, 1965 from olive cv. Chemlali in Tunisia.a
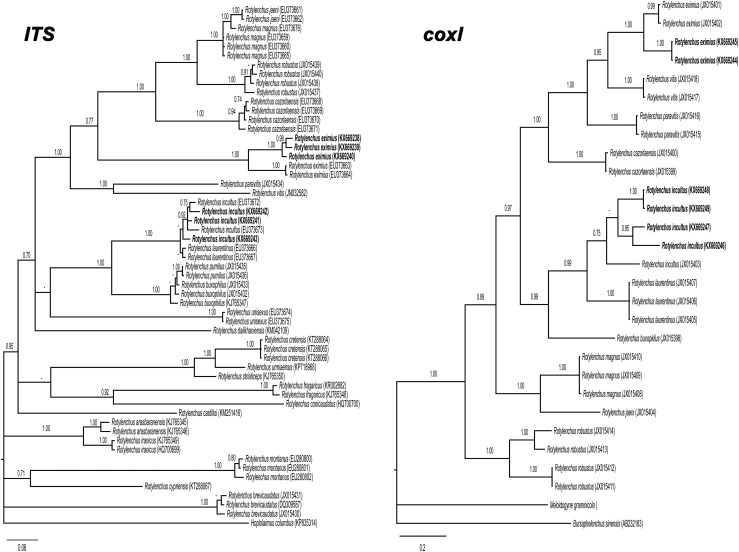
). Twenty-two soil samples of 3 to 4 kg were collected with a shovel from the upper 50 cm of soil from arbitrarily chosen olive trees. Nematodes were extracted from 500 cm3 of soil by centrifugal flotation method (Coolen, 1979). Specimens were heat killed by adding hot 4% formaldehyde solution and processed to pure glycerin using the De Grisse’s (1969) method. Measurements were done using a drawing tube attached to a Zeiss III compound microscope. Nematode DNA was extracted from single individuals and PCR assays were conducted as described by Castillo et al. (2003). Moderate-to-low soil populations of these spiral nematodes were detected (5.5–11.5, 1.5–5.0 individuals/500 cm3 of soil, respectively). This prompted us to undertake a detailed morphological and molecular comparative study with previous reported data. Morphological and molecular analyses of females identified these species as Rotylenchus eximius Siddiqi, 1964, and Rotylenchus incultus Sher, 1965. The morphology of R. eximius females (five specimens studied) was characterized by having a hemispherical lip region clearly off set, with four to five annuli, body without longitudinal striations, lateral fields areolated in the pharyngeal region only, stylet 32 to 36 μm long, and broadly rounded tail. The morphology of R. incultus females (51 females and 16 males; Table 2) was characterized by a hemispherical lip region with the basal annulus subdivided by irregular longitudinal striations, with three, rarely four annuli; stylet 21.5 to 27.5 μm long, female tail hemispherical with terminus regularly annulated; phasmids anterior to anus level (3–6 annuli above). The morphology of the isolated nematodes agreed with previous descriptions of R. eximius (Siddiqi, 1964; Castillo and Vovlas, 2005) and R. incultus (Sher, 1965; Castillo and Vovlas, 2005; Vovlas et al., 2008), respectively. A single individual was used for DNA extraction. Primers and PCR conditions used in this research were specified in Cantalapiedra-Navarrete et al. (2013), and a single amplicon of 800, 1,100, and 450 bp was obtained and sequenced for D2 to D3, ITS1, and cytochrome c oxidase subunit 1 (coxI), respectively. Sequence alignments for D2 to D3 (KX669231–KX669233), ITS1 (KX669238–KX669240), and coxI (KX669244–KX669245) from R. eximius, showed 99% to 97%, 98% to 94%, 93% similarity to other sequences of R. eximius deposited in GenBank (EU280794–DQ328741, EU373663–EU373664, JX015401–JX015402, respectively). Similarly, D2 to D3 (KX669234–KX669237), ITS1 (KX669241–KX669243), and coxI (KX669246–KX669249) sequence alignments from R. incultus, showed 99%, 99% to 95%, 99% to 90% similarity, respectively, to other sequences of R. incultus deposited in GenBank (EU280797, EU373672–EU373673, JX015403, respectively). The best fitted model of DNA evolution was obtained using jModelTest v. 2.1.7 (Darriba et al. 2012) with the Akaike information criterion. BI analyses were performed under the general time reversible (GTR) with invariable sites and a gamma-shaped distribution of substitution rates (GTR + I + G) model for ITS1 and coxI. Phylogenetic analyses of ITS1 and coxI using Bayesian inference (BI) placed R. eximius and R. incultus from Tunisia in subclades that included all R. eximius and R. incultus sequences deposited in GenBank (Fig. 1
Fig. 1.
Phylogenetic relationships within Rotylenchus species found in Tunisia and other species from GenBank. Bayesian 50% majority rule consensus trees as inferred from ITS1 and coxI sequences alignments under the GTR + I + G model. Posterior probabilities more than 0.70 are given for appropriate clades. Newly obtained sequences in this study are in bold. Scale bar = expected changes per site.
), which agrees with previous results (Cantalapiedra-Navarrete et al., 2013). Morphology, morphometry, and molecular and phylogenetic data obtained from these samples were consistent with R. eximius and R. incultus identification. To our knowledge, this is the first report of R. incultus in Tunisia. Consequently, all these data suggest that spiral nematode species of the genus Rotylenchus are predominant in olive as previously reported in other Mediterranean areas (Ali et al., 2014).
Keywords: Bayesian inference, detection, new geographic record, phylogeny, spiral nematodes
Literature Cited
- Ali N, Chapuis E, Tavoillot J, Mateille T. Plant-parasitic nematodes associated with olive tree (Olea europaea L.) with a focus on the Mediterranean Basin: A review. Comptes Rendus Biologies. 2014;337:423–442. doi: 10.1016/j.crvi.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Cantalapiedra-Navarrete C, Navas-Cortés JA, Liébanas G, Vovlas N, Subbotin SA, Palomares-Rius JE, Castillo P. Comparative molecular and morphological characterisations in the nematode genus Rotylenchus: Rotylenchus paravitis n. sp., an example of cryptic speciation. Zoologischer Anzeiger. 2013;252:246–268. [Google Scholar]
- Castillo P, Nico A, Navas-Cortés JA, Landa BB, Jiménez-Díaz RM, Vovlas N. Plant-parasitic nematodes attacking olive trees and their management. Plant Disease. 2010;94:148–162. doi: 10.1094/PDIS-94-2-0148. [DOI] [PubMed] [Google Scholar]
- Castillo P, Vovlas N. 2005. Bionomics and identification of the genus Rotylenchus (Nematoda: Hoplolaimidae). Leiden, Netherlands: Brill Academic Publishers, pp. 377.
- Castillo P, Vovlas N, Subbotin S, Troccoli A. A new root-knot nematode, Meloidogyne baetica n. sp. (Nematoda: Heteroderidae), parasitizing wild olive in Southern Spain. Phytopathology. 2003;93:1093–1102. doi: 10.1094/PHYTO.2003.93.9.1093. [DOI] [PubMed] [Google Scholar]
- Coolen WA. 1979. Methods for extraction of Meloidogyne spp. and other nematodes from roots and soil. Pp. 317–329 in F. Lamberti and C. E. Taylor, eds. Root-knot nematodes (Meloidogyne species). Systematics, biology, and control. New York: Academic Press.
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nature Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grisse AT. Redescription ou modifications de quelques techniques utilisées dans l’étude des nématodes phytoparasitaires. Mededelingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent. 1969;34:351–369. [Google Scholar]
- Sher SA. Revision of the Hoplolaiminae (Nematoda) V. Rotylenchus Filipjev, 19361) Nematologica. 1965;11:173–198. [Google Scholar]
- Siddiqi MR. Rotylenchus eximius n. sp. (Nematoda: Hoplolaiminae) found around almond roots in Tunisia. Nematologica. 1964;10:101–104. [Google Scholar]
- Vovlas N, Subbotin SA, Troccoli A, Liébanas G, Castillo P. Molecular phylogeny of the genus Rotylenchus (Nematoda, Tylenchida) and description of a new species. Zoologica Scripta. 2008;37:521–537. [Google Scholar]