Abstract
A new species of entomopathogenic nematode (EPN), Steinernema biddulphi n. sp., was isolated from a maize field in Senekal, Free State Province of South Africa. Morphological and molecular studies indicated the distinctness of S. biddulphi n. sp. from other Steinernema species. Steinernema biddulphi n. sp. is characterized IJs with average body length of 663 μm (606–778 μm), lateral fields with six ridges in mid-body region forming the formula 2,6,2. Excretory pore located anterior to mid-pharynx (D% = 46). Hyaline layer occupies approximately half of tail length. Male spicules slightly to moderately curved, with a sharp tip and golden brown in color. The first generation of males lacking a mucron on the tail tip while the second generation males with a short filamentous mucron. Genital papillae with 11 pairs and one unpaired preanal papilla. The new species is further characterized by sequences of the internal transcribed spacer (ITS) and partial 28S regions (D2-D3) of the ribosomal DNA (rDNA). Phylogenetic data show that S. biddulphi n. sp. belongs to the “bicornutum” clade within the Steinernematidae family.
Keywords: D2-D3, entomopathogenic nematodes, ITS, new species, phylogeny, South Africa, Steinernema biddulphi, taxonomy
Entomopathogenic nematode species belonging to the family Steinernematidae (Rhabditida) are obligate and lethal endoparasites of insects that have a symbiotic relationship with specific bacteria of the genus Xenorhabdus (Poinar, 1990). Steinernematids have a worldwide distribution (Adams et al., 2006) and so far, more than 90 species have been described from all continents except Antarctica and this number is growing every year.
Entomopathogenic nematode species and isolates show variation in their host range, infectivity, environmental tolerances, and suitability for commercial production and formulation (Hazir et al., 2001; Koppenhofer and Fuzy, 2003). Therefore, the recovery of indigenous nematode strains and/or species may provide better results with regards to inundative release against local pests. This rationale has stimulated the coordination of many surveys in search for new species and strains (Mwaitulo et al., 2011; Kanga et al., 2012; Zadji et al., 2013).
In recent years, several surveys have been conducted in South Africa resulting in the recovery of eight new Steinernematid species, namely Steinernema khoisanae (Nguyen et al., 2006), S. citrae (Stokwe et al., 2011), S. sacchari (Nthenga et al., 2014), S. tophus (Cimen et al., 2014), S. innovationi (Cimen et al., 2015), S. jeffreyense (Malan et al., 2015), S. beitlechemi (Cimen et al., 2016), and S. fabii (Abate et al., 2016).
Surveys were conducted during mid-summer in the central regions of the Free State Province around the towns of Senekal and Winburg. Climatic conditions in this region can be characterized as cool, humid subtropical summer with rainfall (warmest month <22°C). The dominant vegetation type is grassland with crop-related agricultural activities including cultivation of maize (Zea mays L.), sunflower (Helianthus annuus L.), wheat (Triticum aestivum L.), and soya beans (Glycine max (L.) Merr.).
Among the isolated steinernematids, a nematode having two horn-like structures on the labial region of the IJ, belonging to the “bicornutum” group was recovered. Morphological and molecular analyses showed that this nematode differs from the previously described species S. riobrave (Cabanillas et al., 1994), S. bicornutum (Tallosi et al., 1995), S. ceratophorum (Jian et al., 1997), S. abbasi (Elawad et al., 1997), S. pakistanense (Shahina et al., 2001), S. bifurcatum (Shahina et al., 2014), S. yirgalemense (Nguyen et al., 2004), S. papillatum (San-Blas et al., 2015), and S. gowenii (San-Blas et al., 2016), as well as other steinernematid species. The new nematode species is described as Steinernema biddulphi n. sp. and named after the mountain “Biddulphsberg” near the town Senekal in South Africa.
Materials and Methods
Collection and examination of nematodes
Soil samples were collected from a maize field in Senekal, Free State, South Africa. Each sample consisted of 3 to 10 subsamples that were taken randomly from the surface to a depth of 20 cm. Five lastinstar Galleria mellonella (L.) (Lepidoptera: Pyralidae) larvae were placed in a 600-ml plastic container filled with moistened soil obtained from samples (Bedding and Akhurst, 1975). Containers were kept at room temperature (22°C ± 3°C). Mortality of G. mellonella larvae was checked daily and dead larvae were transferred to a modified White trap (White, 1927) until emergence of IJs (Kaya and Stock, 1997).
Steinernema biddulphi sp. n. was reared on last instar of G. mellonella as described by Kaya and Stock (1997). The first and second generation adults were obtained from the dissection of 3- and 5-d-old infected G. mellonella larvae, respectively. Infective juveniles were collected approximately 1 wk after emergence from the cadavers.
The IJs and the first and second generation adults were heat-killed in Ringer’s solution and fixed in triethanolamine formalin (Courtney et al., 1955). The nematode samples were subsequently processed in anhydrous glycerine for mounting (Seinhorst, 1959). Morphometric analysis of the specimens was conducted and photographs using a Zeiss Axioplan 2 microscope (Zeiss, Jena, Germany) equipped with an Olympus DP73 digital camera (Olympus, Hamburg, Germany).
Scanning electron microscopy
Nematode specimens were fixed in 4% formalin buffered with 0.1 M sodium cacodylate at pH 7.2 for 24 h at 4°C to 6°C. They were postfixed with a 2% osmium tetroxide (OsO4) solution for 12 h at 25°C, and then dehydrated in a graded ethanol series. The dehydrated specimens were critical point dried in liquid CO2, mounted on scanning electron microscopy (SEM) stubs, and coated with gold (Nguyen and Smart, 1995, 1997). The mounts were examined with a JEOL 7401 FE scanning electron microscope (JEOL, Eching, Germany) at 4-kV accelerating voltage.
Molecular characterization
DNA was extracted from single females. Each female was transferred into a sterile Eppendorf tube (500 μl) with 20 μl of extraction buffer (17.7 μl of ddH2O, 2 μl of 10 × PCR buffer, 0.2 μl of 1% tween, and 0.1 μl of proteinase K). Buffer and nematode were frozen at −20°C for 20 min and then immediately incubated at 65°C for 1 h, followed by 10 min at 95°C. The lysates were cooled on ice, then centrifuged (2 min, 9,000 g) and 2 μl of supernatant was used for PCR (Mráček et al., 2014).
A fragment of rDNA containing the ITS regions (ITS1, 5.8S, ITS2) was amplified using primers 18S: 5′-TTGATTACGTCCCTGCCCTTT-3′ (forward), and 28S: 5′-TTTCACTCGCCGTTACTAAGG-3′ (reverse) (Vrain et al., 1992). The other fragment containing D2-D3 expansion segments of the 28S rDNA was amplified using primers D2F: 5′-CCTTAGTAACGGCGAGTGAAA-3′ (forward) and 536: 5′-CAGCTATCCTGAGGAAAC-3′ (reverse) (Nguyen, 2007).
All PCR products were sequenced and for the ITS region, five sequence chromatograms were checked for the presence of intraindividual variability (Půža et al., 2015). The sequences were deposited in GenBank under accession numbers KT373857 (ITS sequence) and KT580950 (28S sequence). The sequences were edited and compared with those present in GenBank by means of a Basic Local Alignment Search Tool (BLAST) of the National Centre for Biotechnology Information. An alignment of our samples together with sequences of related steinernematid species were produced for ITS and 28S regions using default ClustalW parameters in MEGA 6.0 (Tamura et al., 2013) and optimized manually in BioEdit (Hall, 1999). Pairwise distances were computed using MEGA 6.0 (Tamura et al., 2013).
The phylogenetic trees of the ITS and 28S genes were obtained by the minimum evolution method (Rzhetsky and Nei, 1992) in MEGA 6.0 (Tamura et al., 2013). Steinernema nepalense and Steinernema scapterisci were used as outgroup. The minimum evolution tree was searched using the close-neighbor-interchange (CNI) algorithm (Nei and Kumar, 2000). The neighbor-joining algorithm (Saitou and Nei, 1987) was used to generate the initial tree. The evolutionary distances were computed using the p-distance method (Nei and Kumar, 2000) and are expressed as the number of base differences per site.
Bacterial symbiont
Bacterial DNA was extracted from a 2-d-old culture using DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. 16S RNA was amplified using primers 10F: 5′-AGTTTGATCATGGCTCAGATTG-3′ (forward) and 1507R: 5′-TACCTTGTTACGACTTCACCCCAG-3′ (reverse) (Sandström et al., 2001). Recombinase A gene (recA) was amplified using primers RecA1F: 5′-GCTATTGATGAAAATAAACA-3′ (forward) and RecA2R: 5′-RATTTTRTCWCCRTTRTAGCT-3′ (reverse) (Tailliez et al., 2010). Gyrase B gene (gyrB) was amplified using primers 8SF gyrB: 5′-TACACGAAGAAGAAGGTGTTTCAG-3′ (forward) and 9Rev gyrB: 5′-TACTCATCCATTGCTTCATCATCT-3′ (reverse) (Tailliez et al., 2010).
The PCR master mix consisted of ddH2O 7.2 µl, BSA 0.5 µl, 10× PCR buffer 1.25 µl, dNTPs 1 µl, 0.75 µl of each forward and reverse primers, polymerase 0.1 and 1 µl of DNA extract.
The PCR profiles were used as follows for 16S: 1 cycle at 94°C for 1 min followed by 33 cycles at 94°C for 60 s, 55°C for 60 s, 72°C for 2 min, and a final extension at 72 °C for 3 min, for RecA: 1 cycle at 94°C for 2 min followed by 35 cycles at 94°C for 30 s, 49.5°C for 35 s, 72°C for 60 s, and a final extension at 72°C for 2 min and for gyrB: 1 cycle at 94°C for 2 min followed by 35 cycles at 94°C for 30 s, 66°C for 35 s, 72°C for 60 s, and a final extension at 72°C for 2 min. All PCR products were sequenced and deposited in GenBank under the following accession numbers KX894736 (16S sequence), KX826946 (RecA sequence), and KX826947 (gyrB sequence).
Results and Discussion
Systematics
Steinernema biddulphi n. sp.
(Figs. 1–3; Table 1)
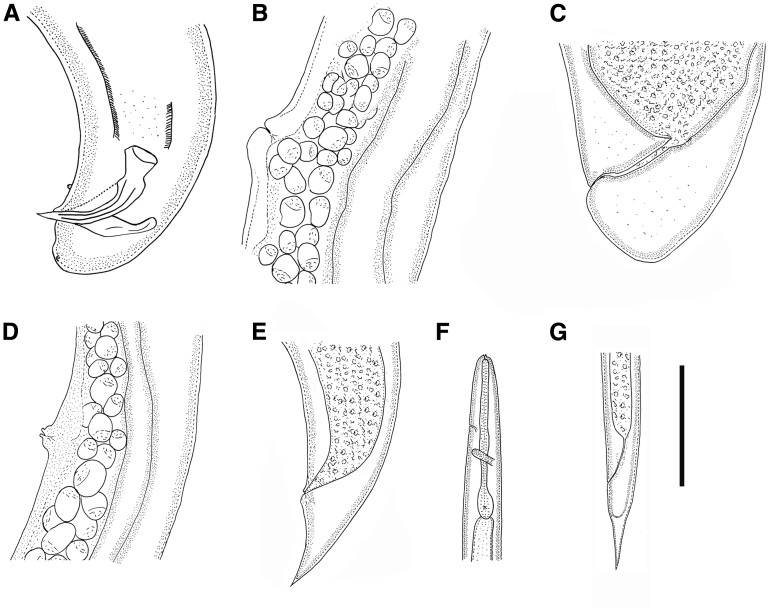
Fig. 1.
Steinernema biddulphi n. sp. A. First generation male, tail with spicules and gubernaculum, lateral. B. First generation female, vulval region. C. First generation female, tail. D. Second generation female, vulval region. E. Second generation female, conical tail. F, G. Infective juvenile. F. Anterior region showing excretory pore and nerve ring. G. Tail showing anus and hyaline region. (Scale bar in micrometers: A = 58; B = 143; C = 68; D = 71, E = 61; F = 91; G = 75).
Fig. 3.
Steinernema biddulphi n. sp. LM of infective juvenile (IJ), male and female. A, C. First generation female. A. Tail with postanal swelling. C. Vulval region. B, D. Second generation female. B. Tail with postanal swelling. D. Vulval region. E. First generation male, tail with spicules and gubernaculum. F. Second generation male, tail with spicules and gubernaculum. G, H. IJ. G. Anterior portion showing rounded head and excretory pore (arrow). H. Tail with anus and hyaline region.
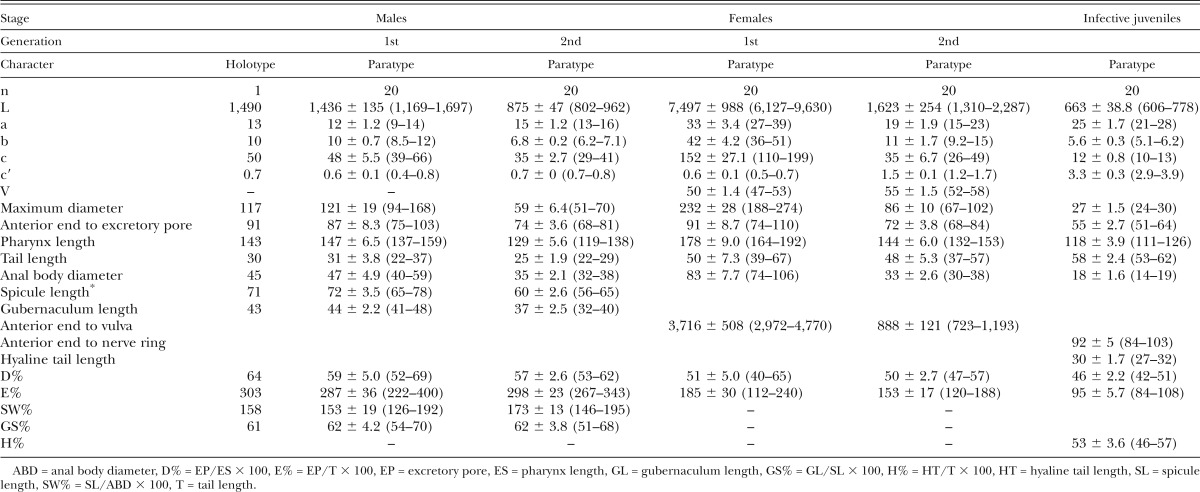
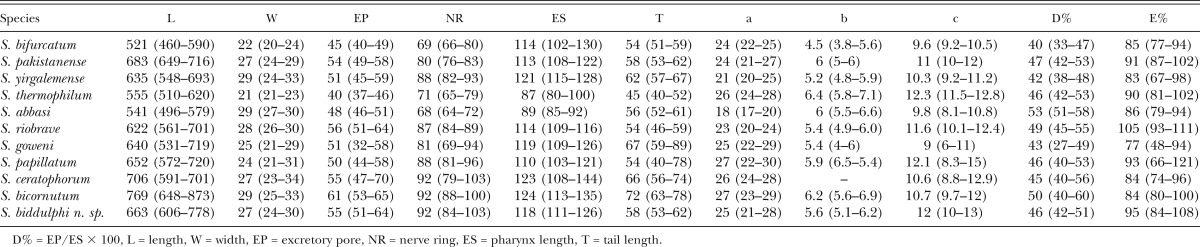
Table 1.
Morphometric characters (in μm) of Steinernema biddulphi n. sp. based on the holotype and 20 paratypes of each generations with mean ± SD and ranges given in brackets.
First generation male
Body curved ventrally posteriorly, J-shaped when heat-killed (Fig. 2H). Cuticle smooth under light microscopy, but with faint transverse striations visible under SEM. Stoma reduced, short, and wide. Excretory pore located anterior to nerve ring. Spicules paired, symmetrical, curved, with golden brown coloration, ca. 72 μm long, spicule tip sharp. Calomus distinct, but short. Lamina with two internal ribs, slightly curved. Velum extending from calomus to ca. 80% of lamina length. Gubernaculum boat shaped, manubrium of gubernaculum curved ventrally (Fig. 3F). Tail short, conoid, and terminus without mucron. Copulatory papillae totaling 23 in number, comprising 11 pairs and a single midventral papilla located anterior to anus. Paired papillae arranged as follows: six pairs subventral precloacal, one pair sublateral precloacal, one pair subventral adcloacal, one pair subdorsal postcloacal, and two subterminal postcloacal pairs.
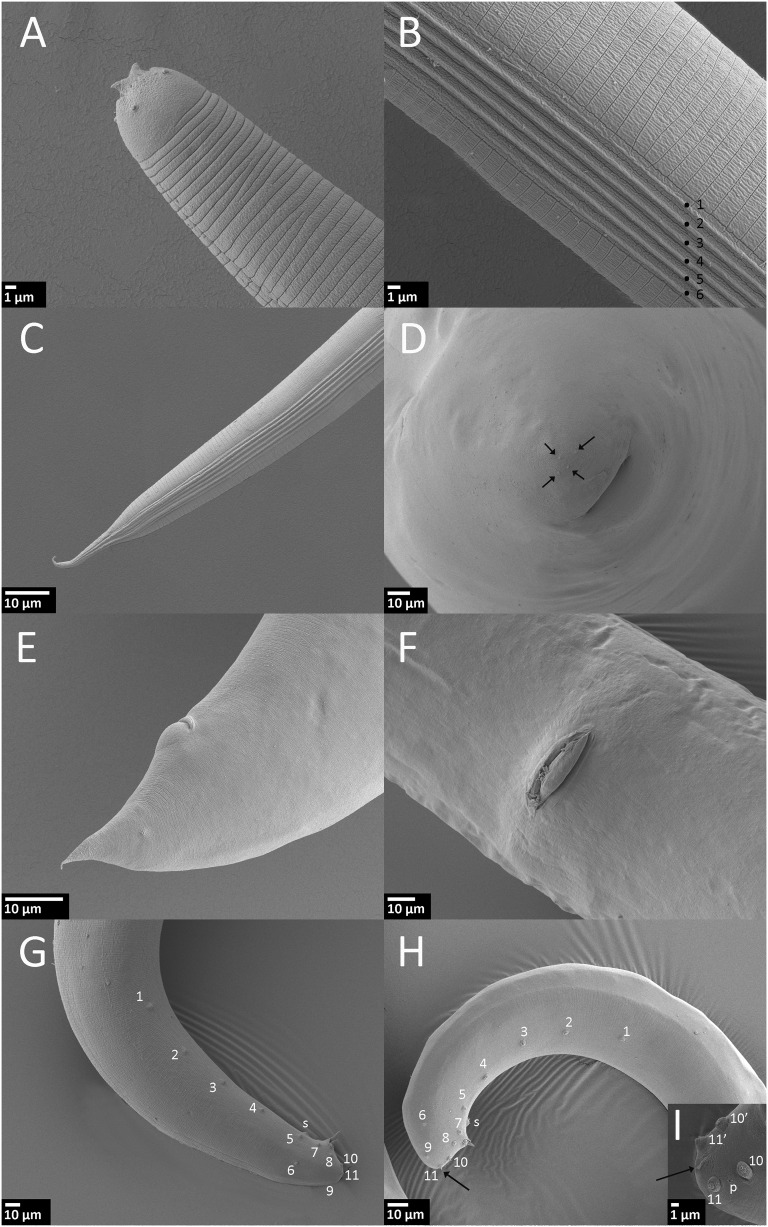
Fig. 2.
Steinernema biddulphi n. sp. Scanning electron microscopy of infective juvenile (IJ), male and female. A–C. IJ. A. Head region with horn-like structures. B. Lateral field in mid-body (ridges numbered 1 to 6). C. Lateral field in tail region. D. First generation female, tail, and four projections on tip of the tail (arrow). E, F. Second generation female. E. Tail with postanal swelling. F. Vulva. G. First generation male, tail with paired genital papillae (numbered) and single papilla (s), lateral. H, I. Second generation male. H. Tail with paired genital papillae, single papilla (s) and mucron (arrow). I. Tail with a part of paired genital papilae (numbered), mucron (arrow), and phasmid opening (p), ventro-lateral.
Second generation male
Similar to first generation, but with smaller body. Tail terminus with a short filamentous mucron that is only visible with SEM.
First generation female
Body usually C-shaped when heat-killed, variable in length, first generation females substantially larger (average 7,497 μm) than second generation females (average 1,623 μm). Head bluntly rounded, slightly tapering anteriorly, continuous with body, with six lips fused at base; each lip bears papilla. Outer circle of four cephalic papillae present. Lips distinct. Stoma ca. 8 μm long and 9 to 11 μm broad. Excretory pore located anterior to nerve ring and at level of midmetacorpus. Vulva opening at mid-body, slightly asymmetrical and in form of a transverse slit, protruding ca. 20 μm from body contour. Small epiptygma rarely observed. Vagina short, leading into paired uteri. Rectum narrow, anal opening distinct. Postanal swelling observed in most of the mature females. Tail of mature females obese, simply rounded, bearing four minute projections. Endotokia matricida occurred in more than half of the females.
Second generation female
Similar to the first generation in general morphology, but smaller. Vulva slightly protruding. Tail conical, longer than anal body diameter, with a pointed tip and without mucron. Postanal swelling distinct.
Infective juvenile
Body slender, tapering gradually from base of pharynx to anterior end and from anus to terminus (Fig. 1F,G). Average body length 663 μm, second stage cuticle sheath present after emergence from the host. Body almost straight or slightly bow shaped when heat-killed. Cuticle with a prominent striations (distinct under SEM) ca. 1.5 μm wide at mid-body. Lateral fields consisting of six ridges in mid-body region (i.e., seven lines). Lateral field beginning anteriorly with a cuticular depression (line) on the 1st annulus; at 17th annulus, two ridges appearing and changing to six ridges (seven lines) at excretory pore level. Close to anus, lateral field reducing to two ridges extending almost to tail tip. Formula of lateral field: 2,6,2. Cephalic region rounded, not offset from body contour. Four distinct cephalic papillae and a pair of pore-like amphidial apertures located laterally. Two horn-like structures present in labial region. Deirids not observed. Hemizonid visible, located just posterior to the nerve ring. Mouth closed, pharynx corpus slender, cylindrical, isthmus distinct, surrounded by nerve ring. Excretory pore located anterior to mid-pharynx (D% = 46). Cardia present. Rectum long, anus distinct. Tail conoid with pointed terminus. Hyaline layer occupying approximately half of tail length. Anus ca. 4 μm wide, sickle shaped. Phasmids distinct, located ca. 60% of tail length posterior to anus, with aperture anterior to the commencement of the hyaline portion.
Type host, locality, and habitat
Unknown in nature, from bait insect Galleria mellonella (L.). The type isolate, SGI-246, was recovered from a maize field in Senekal, Free State, South Africa (28°23′156″S, 27°30′406″E).
Type specimens
Holotype 1st generation male, paratype males (M1, 3 slides with 17 specimens; M2, 3 slides with 27 specimens), paratype females (F1, 6 slides with 33 specimens; F2, 3 slides with 26 specimens), and third stage juveniles (2 slides with 55 specimens) were deposited at the EPN collection in the Laboratory of Entomopathogenic Nematodes, Institute of Entomology, BC CAS, České Budějovice, Czech Republic. A total of 23 slides with paratype IJ (3 slides with 100 specimens), males and females (F1, 10 slides with 30 specimens; F2, 4 slide with 24 specimens; M1, 3 slides with 15 specimens; and M2, 3 slide with 20 specimens) were deposited at the Department of Biology, Faculty of Arts and Science, Adnan Menderes University, Turkey.
Etymology
The specific epithet derives from the mountain “Biddulphsberg” near the town Senekal where S. biddulphi n. sp. was isolated.
Symbiotic bacterium
Based on the BLAST search and phylogenetic analysis of the concatenated sequences, sequences of the 16S rDNA and recA and gyrB genes (data not shown), the symbiotic bacterium of S. biddulphi n. sp. (bacterial strain SGI-246) seems to be most closely related to Xenorhabdus indica (BLAST similarities 98% for 16S rDNA, and 97% for recA and gyrB genes). This bacterium was found in association with other nematodes from the “bicornutum ” group, namely Steinernema abbasi (Somvanshi et al., 2006), S. yirgalamense (Ferreira et al., 2016), and S. bifurcatum (Shahina et al., 2014).
Based on the molecular data, the strain SGI-246 could represent a new Xenorhabdus species, however, further research including DNA-DNA hybridization and phenotypic characters are necessary to draw any conclusion.
Life cycle
The life cycle of S. biddulphi n. sp. is similar to other Steinernema species. In an experimental infection of G. mellonella with a dose of 50 IJs per insect at a temperature of 22°C, the majority of insects were dead after 48 h, and fourth stage juveniles were present in the hosts. Two amphimictic generations occur inside the host, and the first and second generation stages could be observed after 2 and 8 d of initial infection, respectively. Infective juveniles appeared 10 d postinoculation.
Diagnostic
Steinernema biddulphi n. sp. is characterized by the following combination of morphological features: body length of IJs on average 663 μm (606–778 μm), lateral fields with six ridges in mid-body region forming the formula 2,6,2. Excretory pore located anterior to mid-pharynx (D% = 46). Hyaline layer occupies approximately half of tail length. Male spicules slightly to moderately curved, with a sharp tip, golden brown in color, manubrium elongate with a length to width ratio of 1.2:1. The first generation males lacking a mucron on the tail tip while the second generation males bearing a mucron on the tail tip. Genital papillae with 11 pairs and one unpaired preanal papilla. First generation females possess moderately protruding vulva, slightly protruding postanal swelling and peg-like tail tip without a mucron on the tip.
Relationships
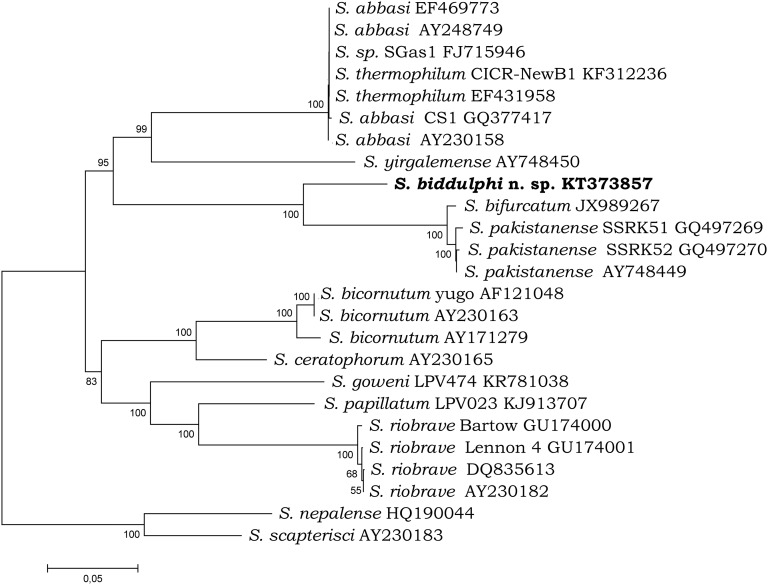
Steinernema biddulphi n. sp. can be distinguished from other Steinernema species by means of a combination of morphological and morphometric characteristics of males and IJs. Based on these data, Steinernema biddulphi n. sp. belongs to the “bicornutum” clade within the Steinernematidae family. Molecular data show that within this clade, Steinernema biddulphi n. sp. is sister to the pair of S. pakistanense and S. bifurcatum and this group is related to the pair of S. yirgalemense and S. abbasi.
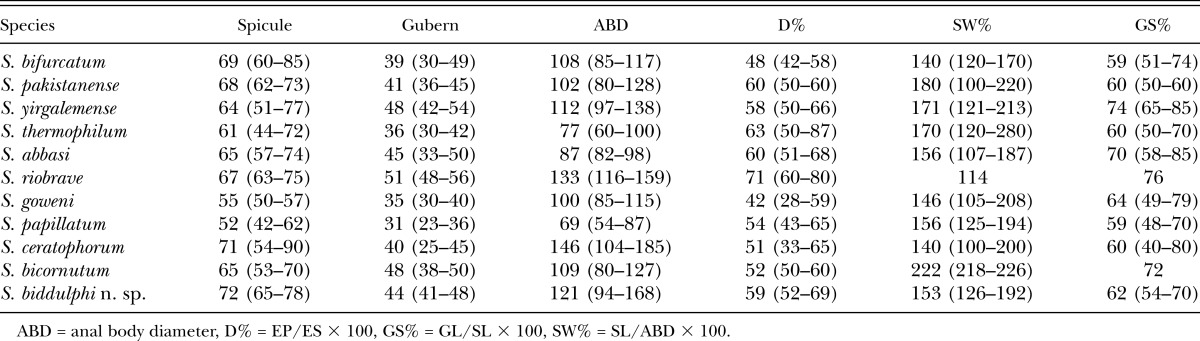
First generation males of Steinernema biddulphi n. sp. can be distinguished from the males of S. bifurcatum by a body length of 1,436 µm (1,169–1,697 μm) vs 1,192 µm (1,059–1,454 μm) (Table 2). The maximum body diameter of S. biddulphi n. sp. males (121 µm [94–168]) is substantially larger than in S. abbasi (87 µm [82–98]). The first generation males of S. biddulphi n. sp. also differ from S. yirgalemense in ratio GS% (62 [54–70] vs 74 [65–85]) (Table 2).
Table 2.
Comparative morphometrics (in μm) of first generation males of Steinernema biddulphi n. sp.; mean ± SD with ranges given in brackets.
The IJs of S. biddulphi n. sp. can be distinguished from S. abbasi, S. bifurcatum, and S. pakistanense by the distance from anterior end to nerve ring of 92 µm (84–103 μm) vs 68 µm (64–72 μm), 69 µm (66–80 μm), and 80 µm (76–83 μm), respectively (Table 3). Steinernema biddulphi n. sp. further differs from the two former species by the distance from anterior end to excretory pore of 55 µm (51–64 μm) vs 48 µm (46–51 μm) and 45 µm (40–49 μm), respectively. The ratio “a” of S. biddulphi n. sp. (25 [21–28]) is higher in comparison to S. abbasi (18 [17–20]) and S. yirgalemense (21 [20 to 25]). The IJs of S. biddulphi n. sp. are significantly longer in comparison to S. abbasi 663 µm (606–778) vs 541 µm (496–579) (Table 3). The lateral field pattern of the IJs of S. biddulphi n. sp. with six ridges at mid-body differs from all other species from the “bicornutum” group that have eight or seven ridges in total.
Table 3.
Comparative morphometrics (in μm) of infective juveniles of Steinernema biddulphi n. sp.; mean ± SD with ranges given in brackets.
Furthermore, the first generation females of Steinernema biddulphi n. sp. differ from the females of S. pakistanense and S. bifurcatum by having a postanal swelling.
The presence of four projections on the tail tip of the first generation females of S. biddulphi n. sp. is unique among steinernematid nematodes. Mráček et al. (2016) have shown the presence of three projections in females from “kraussei,” “affine,” “glaseri,” and “carpocapsae” groups, however, females from “bicornutum” were missing in the study cited above. Our study shows that four projections can occur in females from the “bicornutum” clade.
Molecular characterization and phylogenetic position
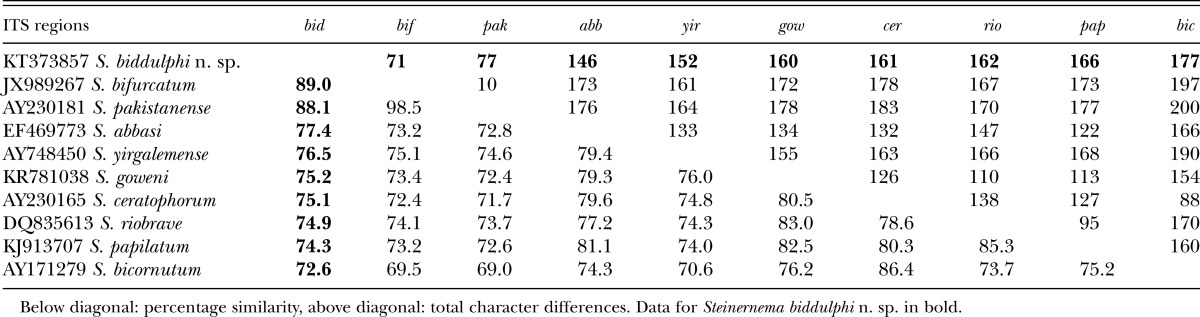
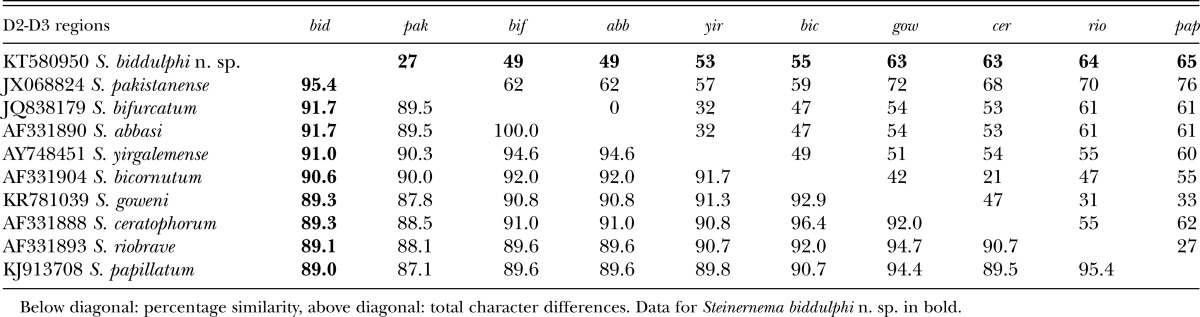
The ITS sequence of S. biddulphi n. sp. is separated from the other related species by 71 to 177 bp (Table 4). No sign of the intraindividual variability in the ITS sequence was observed. The D2 and D3 expansion fragments of the 28S rRNA gene, is separated by 27 to 65 bp from other related species (Table 5).
Table 4.
Pairwise distances of the ITS region between species of the “bicornutum” group.
Table 5.
Pairwise distances of the D2-D3 region between species of the “bicornutum” group.
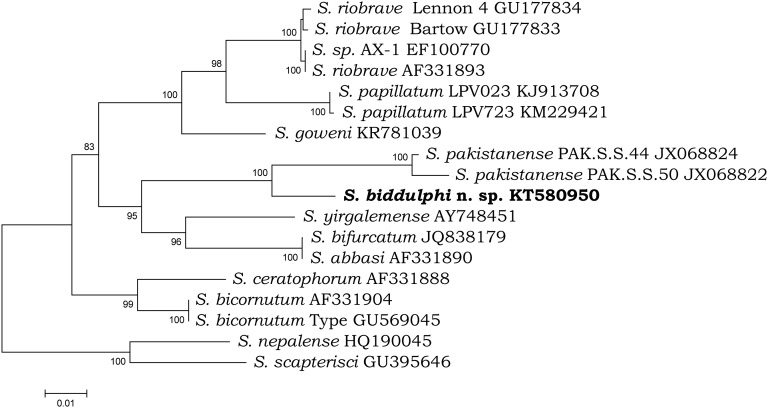
Both phylogenetic analyses demonstrated that S. biddulphi n. sp. is a sister species to the well-supported monophyletic clade containing S. pakistanense, S. bifurcatum, S. abbasi, and S. yirgalemense. Within this group, S. biddulphi n. sp. clusters with the Pakistani species, S. pakistanense (Figs. 4,5) and S. bifurcatum (Fig. 4). The position of the latter species in the 28S tree is different; however, the 28S rDNA sequence attributed to S. bifurcatum (JQ838179) obviously belongs to S. abbasi (Table 5; Fig. 5).
Fig. 4.
Phylogenetic relationships of the species from “bicornutum” group and other related species of Steinernema based on analysis of ITS rDNA regions. Steinernema nepalense and S. scapterisci was used as outgroup taxon. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (10,000 replicates) are shown next to the branches. Branch lengths indicate evolutionary distances and are expressed in the units of number of base differences per site.
Fig. 5.
Phylogenetic relationships of the species from “bicornutum” group and other related species of Steinernema based on analysis of D2-D3 expansion segments of the 28S rDNA. Steinernema nepalense and S. scapterisci was used as outgroup taxon. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (10,000 replicates) are shown next to the branches. Branch lengths indicate evolutionary distances and are expressed in the units of number of base differences per site.
In general, molecular data confirm the status of S. biddulphi n. sp. as a new species according to the phylogenetic and evolutionary species concept (Adams, 1998).
Literature Cited
- Abate BA, Malan AP, Tiedt LR, Wingfield MJ, Slippers B, Hurley BP. Steinernema fabii n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology. 2016;18:235–255. [Google Scholar]
- Adams BJ. Species concept and the evolutionary paradigm in modern nematology. Journal of Nematology. 1998;30:1–21. [PMC free article] [PubMed] [Google Scholar]
- Adams BJ, Fodor A, Koppenhöfer HS, Stackenbrandt E, Stock SP, Klein MG. Biodiversity and systematic of nematode–bacterium entomopathogens. Biological Control. 2006;38:4–21. [Google Scholar]
- Bedding RA, Akhurst RJ. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–110. [Google Scholar]
- Cabanillas HE, Poinar GO, Jr, Raulston JR. Steinernema riobravis n. sp. (Rhabditida: Steinernematidae) from Texas. Fundamental and Applied Nematology. 1994;17:123–131. [Google Scholar]
- Cimen H, Lee MM, Hatting J, Hazir S, Stock SP. Steinernema tophus n. sp. (Nematoda: Steinernematidae), a new entomopathogenic nematode from South Africa. Zootaxa. 2014;3821:337–353. doi: 10.11646/zootaxa.3821.3.3. [DOI] [PubMed] [Google Scholar]
- Cimen H, Lee MM, Hatting J, Hazir S, Stock SP. Steinernema innovationi n. sp. (Panagrolaimomorpha: Steinernematidae), a new entomopathogenic nematode species from South Africa. Journal of Helminthology. 2015;89:415–427. doi: 10.1017/S0022149X14000182. [DOI] [PubMed] [Google Scholar]
- Cimen H, Půža V, Nermuť J, Hatting J, Hazir S, Ramakuwela T. Steinernema beitlechemi n. sp., a new entomopathogenic nematode (Nematoda: Steinernematidae) from South Africa. Nematology. 2016;18:439–453. doi: 10.21307/jofnem-2017-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney WD, Polley D, Miller VI. TAF, an improved fixative in nematode technique. Plant Disease Reporter. 1955;39:570–571. [Google Scholar]
- Elawad S, Ahmad W, Reid A. Steinernema abbasi sp. n. (Nematoda: Steinernematidae) from the Sultanate of Oman. Fundamental and Applied Nematology. 1997;20:433–442. [Google Scholar]
- Ferreira T, Van Reenen CA, Tailliez P, Pagès S, Malan AP, Dicks LMT. First report of the symbiotic bacterium Xenorhabdus indica associated with the entomopathogenic nematode Steinernema yirgalemense. Journal of helminthology. 2016;90(01):108–112. doi: 10.1017/S0022149X14000583. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hazir S, Stock SP, Kaya HK, Koppenhöfer AM, Keskin N. Developmental temperature effects on five geographic isolates of the entomopathogenic nematode Steinernema feltiae (Steinernematidae) Journal of Invertebrate Pathology. 2001;77:243–250. doi: 10.1006/jipa.2001.5029. [DOI] [PubMed] [Google Scholar]
- Jian H, Reid AP, Hunt DJ. Steinernema ceratophorum n. sp. (Nematoda: Steinernematidae), a new entomopathogenic nematode from north-east China. Systematic Parasitology. 1997;37:115–125. [Google Scholar]
- Kanga FN, Waeyenberge L, Hauser S, Moens M. Distribution of entomopathogenic nematodes in Southern Cameroon. Journal of Invertebrate Pathology. 2012;109:41–51. doi: 10.1016/j.jip.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Kaya HK, Stock SP. 1997. Techniques in insect nematology. Pp. 281–324 in L. A. Lacey, ed. Manual of techniques in insect pathology. San Diego, CA: Academic Press.
- Koppenhofer AM, Fuzy EM. Ecological characterization of Steinernema scarabaei, a scarab-adapted entomopathogenic nematode from New Jersey. Journal of Invertebrate Pathology. 2003;83:139–148. doi: 10.1016/s0022-2011(03)00056-9. [DOI] [PubMed] [Google Scholar]
- Malan AP, Knoetze R, Moore SD. Isolation and identification of entomopathogenic nematodes from citrus orchards in South Africa and their biocontrol potential against false codling moth. Journal of Invertebrate Pathology. 2011;108:115–125. doi: 10.1016/j.jip.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Malan AP, Knoetze R, Tiedt LR. Steinernema jeffreyense n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Journal of Helminthology. 2015;90:262–278. doi: 10.1017/S0022149X15000097. [DOI] [PubMed] [Google Scholar]
- Malan AP, Nguyen KB, Addison MF. Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from the southwestern parts of South Africa. African Plant Protection. 2006;12:65–69. [Google Scholar]
- Mráček Z, Půža V, Nermuť J. Steinernema poinari sp. n. (Nematoda: Steinernematidae) a new entomopathogenic nematode from Czech Republic. Zootaxa. 2014;3760:336–350. doi: 10.11646/zootaxa.3760.3.2. [DOI] [PubMed] [Google Scholar]
- Mráček Z, Půža V, Nermuť J. The significance of the tail projections in female entomopathogenic nematodes within the family Steinernematidae. Journal of Helminthology. 2016;90:245–251. doi: 10.1017/S0022149X14000935. [DOI] [PubMed] [Google Scholar]
- Mwaitulo S, Haukeland S, Saethre MG, Laudisoit A, Maerere AP. First report of entomopathogenic nematodes from Tanzania and their virulence against larvae and adults of the banana weevil Cosmopolites sordidus (Coleoptera: Curculionidae) International Journal of Tropical Insect Science. 2011;31:154–161. [Google Scholar]
- Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. Pp. 333. New York, NY: Oxford University Press.
- Nguyen KB, Malan AP, Gozel U. Steinernema khoisanae n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology. 2006;8:157–175. [Google Scholar]
- Nguyen KB, Smart GC. Scanning electron microscope studies of Steinernema glaseri (Nematoda: Steinernematidae) Nematologica. 1995;41:183–190. [Google Scholar]
- Nguyen KB, Smart GC. Scanning electron microscope studies of spicules and gubernacula of Steinernema spp. (Nemata: Steinernematidae) Nematologica. 1997;43:465–480. [Google Scholar]
- Nguyen KB, Tesfamariam M, Gozel U, Gaugler R, Adams BJ. Steinernema yirgalemense n. sp. (Rhabditida: Steinernematidae) from Ethiopia. Nematology. 2004;6:839–856. [Google Scholar]
- Nguyen KB. 2007. Methodology, morphology and identification. Pp. 59–119 in K. B. Nguyen, and D. J. Hunt, eds. Entomopathogenic nematodes: Systematics, phylogeny and bacterial symbionts. Nematology Monographs and Perspectives, vol. 5. (Series editors: Hunt, D. J., and Perry, R. N.). Leiden, The Netherlands: Brill.
- Nthenga I, Knoetze R, Berry S, Tiedt LR, Malan AP. Steinernema sacchari n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology. 2014;16:475–494. [Google Scholar]
- Poinar GO., Jr 1990. Biology and taxonomy of Steinernematidae and Heterorhabditidae. Pp. 23–62 in R. Gaugler,and H. K. Kaya, eds. Entomopathogenic nematodes in biological control. Boca Raton, FL: CRC press.
- Půža V, Chundelová D, Nermuť J, Žurovcová M, Mráček Z. Intra-individual variability of ITS region in entomopathogenic nematodes (Steinernematidae: Nematoda): Implications for their taxonomy. Biocontrol. 2015;60:547–554. [Google Scholar]
- Rzhetsky A, Nei M. A simple method for estimating and testing minimum evolution trees. Molecular Biology and Evolution. 1992;9:945–967. [Google Scholar]
- San-Blas E, Portillo E, Nermut’ J, Půža V, Morales-Montero P. Steinernema papillatum n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Venezuela. Nematology. 2015;17:1081–1097. doi: 10.11646/zootaxa.4067.2.5. [DOI] [PubMed] [Google Scholar]
- San-Blas E, Morales-Montero P, Portillo E, Nermut’ J, Půža V. Steinernema goweni n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Zulia State, Venezuela. Zootaxa. 2016;4067(2):200–214. doi: 10.11646/zootaxa.4067.2.5. [DOI] [PubMed] [Google Scholar]
- Sandström JP, Russel JA, White JP, Moran NA. Independent origins and horizontal transfer of bacterial symbionts of aphids. Molecular Ecology. 2001;10:217–228. doi: 10.1046/j.1365-294x.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Seinhorst JW. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;4:67–69. [Google Scholar]
- Shahina F, Anis M, Reid AP, Rowe J, Maqbool MA. Steinernema pakistanense sp. n. (Rhabditida: Steinernematidae) from Pakistan. Journal of Nematology. 2001;11:124–133. [Google Scholar]
- Shahina F, Yan X, Qiu L, Han R, Gulsher M, Khanum TA, Javed S. A new entomopathogenic nematode, Steinernema bifurcatum n. sp. (Rhabditida: Steinernematidae) from Punjab, Pakistan. Nematology. 2014;16:821–836. [Google Scholar]
- Somvanshi VS, Lang E, Ganguly S, Swiderski J, Saxena AK, Stackebrandt E. A novel species of Xenorhabdus, family Enterobacteriaceae: Xenorhabdus indica sp. nov., symbiotically associated with entomopathogenic nematode Steinernema thermophilum Ganguly and Singh, 2000. Systematic and applied microbiology. 2006;29(7):519–525. doi: 10.1016/j.syapm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Stokwe NF, Malan AP, Nguyen KB, Knoetze R, Tiedt L. Steinernema citrae n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology. 2011;13:569–587. [Google Scholar]
- Tailliez P, Laroui C, Ginibre N, Paule A, Pagès S, Boemare N. Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp. nov., P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. International Journal of Systematic and Evolutionary Microbiology. 2010;60:1921–1937. doi: 10.1099/ijs.0.014308-0. [DOI] [PubMed] [Google Scholar]
- Tallosi B, Peters A, Ehlers RU. Steinernema bicornutum sp. n. (Rhabditida: Steinernematidae) from Vojvodina, Yugoslavia. Russian Journal of Nematology. 1995;3:71–80. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrain TC, Wakarchuk DA, Levesque AC, Hamilton RI. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundamental and Applied Nematology. 1992;15:563–73. [Google Scholar]
- White GF. A method for obtaining infective juvenile nematode larvae from cultures. Science. 1927;66:302–303. doi: 10.1126/science.66.1709.302-a. [DOI] [PubMed] [Google Scholar]
- Zadji L, Baimey H, Afouda L, Houssou FG, Waeyenberge L, De Sutter N, Moens M, Decraemer W. First record on the distribution of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Southern Benin. Russian Journal of Nematology. 2013;21:117–130. [Google Scholar]