Summary
Introduction
Children with chronic respiratory failure and upper airway disorders may require tracheostomy placement and long-term mechanical ventilation, yet many improve to permit ventilator weaning and decannulation.
Methods
As a quality improvement project, we conducted a chart review of patients followed by our Ventilator Care Program who underwent evaluation for weaning nocturnal ventilation (NV) and/or decannulation from 2007–2014. We collected patient demographics and characterized location, monitoring techniques, and outcomes for patients undergoing weaning NV or decannulation. We attempted to implement end tidal carbon dioxide (ETCO2) monitoring and used linear regression to compare ETCO2 with morning pCO2.
Results
Weaning NV was successful in 20/21 patients. Decannulation was successful in 18/21 attempts. Once implemented, ETCO2 was piloted and successfully performed in 12 attempts (29%). Blood testing was performed in 24/42 trials (57%). When measured, the final ETCO2 result partially correlated with morning pCO2 (R2 = 0.53, P < 0.02). Neither blood testing nor ETCO2 was performed for the four patients with unsuccessful attempts.
Conclusions
Inpatient observation for weaning NV and decannulation is safe and, in most cases, successful. With close observation, weaning NV at home may also be safe. Blood testing and ETCO2 monitoring were frequently utilized, but rarely affected decision-making since signs of respiratory distress were observed clinically prior to testing. ETCO2 monitoring may provide reassurance without venipuncture. With our experience, we propose an algorithm for weaning NV and decannulation.
Keywords: mechanical ventilation, oxygen and therapy, tracheostomy, decannulation, ventilator weaning
INTRODUCTION
Children with chronic respiratory failure and airway conditions may require tracheostomy placement and long-term mechanical ventilation, yet many improve to permit weaning nocturnal ventilation (NV) and decannulation.1–11 Other centers have characterized their experiences and outcomes with long-term mechanical ventilation2,4,6,12 and decannulation,1,2,5–10 but strategies for weaning NV2 and tracheostomy decannulation have been rarely described.7–9,13 Some centers utilize polysomnography (PSG) to determine the safety of weaning NV.2 Others have described weaning mechanical ventilation in the post-acute care setting.3 In 2006, our hospital established a comprehensive and interdisciplinary Ventilator Care Program (VCP) to provide safe, consistent, and cost-effective care to children requiring long-term mechanical ventilation in both the inpatient and clinical settings. However, the management of patients with tracheostomies undergoing weaning NV and decannulation was inconsistent and at the discretion of individual physicians. The VCP developed a Pediatric Respiratory Care Unit (PRCU) as part of the inpatient pulmonary unit. In addition to an attending pulmonologist, advanced practice providers, and pediatric residents, our PRCU team now includes nurses and respiratory therapists with focused experience in tracheostomy care and long-term mechanical ventilation. The purpose of this quality improvement project was to describe how weaning NV and decannulation of patients in our VCP has been performed historically, trial implementation of end tidal carbon dioxide (ETCO2) monitoring, and develop a consistent approach to weaning NV and decannulation for use in our center’s PRCU.
METHODS
We performed a retrospective chart review of patients followed by our VCP with tracheostomy tubes who underwent evaluation for either weaning NV or decannulation from 2007 to 2014. The institutional review board’s quality improvement panel approved this study as a quality improvement project and informed consent was waived. Exclusion criteria included children with neuromuscular disease and those who underwent surgical airway reconstruction. We accessed the hospital electronic medical record (EMR) to collect patient demographics and characterize the indications for initiation of long-term mechanical ventilation. These indications were placed into broad diagnostic categories (chronic lung disease [CLD], upper airway obstruction [UAO], or both). Chronic lung disease included patients with bronchopulmonary dysplasia, congenital diaphragmatic hernia, and other conditions associated with alveolar simplification or parenchymal lung disease. We additionally collected information regarding the patient location where weaning was attempted, monitoring techniques (including laboratory studies, PSG, ETCO2 monitoring), any respiratory distress that precluded weaning NV or decannulation, and outcomes for patients undergoing weaning NV or decannulation. Patients who were admitted to the PRCU were evaluated routinely by nurses, respiratory therapists, and the on-call pulmonary clinician (i.e., a pediatric resident or advanced practice provider) as well as cardiorespiratory monitoring and pulse oximetry. Intermittent ETCO2 monitoring (BCI Capnocheck II, Smiths Medical, Dayton, OH) was implemented beginning in 2012. The last recorded overnight ETCO2 measurement and the morning pCO2 (by peripheral venous or capillary blood gas assessment) were compared by linear regression using the Prism software package (v. 6.0f, GraphPad, La Jolla, CA). A P-value of <0.05 was considered significant.
RESULTS
Weaning NV
Twenty-one patients underwent evaluation for weaning NV (Table 1; expanded clinical details in Supplemental Table S1) with an age range of 12–43 months. The majority of these patients (71%) were male. Most patients (67%) had CLD, and 29% had both CLD and UAO. Eleven patients (52%) had tracheomalacia and/or bronchomalacia. At the time of attempted weaning NV, 12 patients (57%) had documented evidence of one-way valve use and/or capping of the tracheostomy tube. Forty-eight percent of the patients were admitted to the PRCU to evaluate weaning NV; the remainder took place in the home setting. At the time of attempted weaning NV, all had been weaned from daytime ventilation while awake and all had uncuffed tracheostomy tubes. The median positive end expiratory pressure or continuous positive airway pressure was six (range 4–8). Nineteen percent had PSGs. ETCO2 monitoring outside of PSG was introduced programmatically in 2012 and was successful in six of 11 attempts. Only one patient was unable to successfully wean from NV, and this patient developed respiratory distress during a PSG. As the patient demonstrated clear clinical evidence of distress, no blood testing was performed. Two patients had respiratory concerns overnight, but reassuring laboratory monitoring permitted successful weaning NV. No patients were treated with non-invasive ventilation after weaning NV and there were no deaths.
TABLE 1.
Attempts to Wean Nocturnal Ventilation
| Subjects (n) | 21 |
|---|---|
| Age in months (median, range) | 29, 12–43 |
| Sex (male/female) | 15/6 |
| Diagnostic category | |
| CLD | 14 |
| UAO | 1 |
| CLD/UAO | 6 |
| Location of wean | |
| Inpatient | 10 |
| Outpatient | 11 |
| Polysomnogram | 4 |
| Blood analysis | |
| HCO3 | 10 |
| pH | 12 |
| pCO2 | 12 |
| ETCO2 monitored | 6 |
| Respiratory distress | 3 |
| Success (yes/no) | 20/1 |
CLD, chronic lung disease; UAO, upper airway obstruction; ETCO2, end-tidal carbon dioxide.
Decannulation
Decannulation was attempted 21 times in 18 patients with an age range of 7–90 months (Table 2, expanded clinical details in Supplemental Table S1). The majority (94%) of these patients were male. Most patients (61%) had CLD, 28% had UAO, and 11% had both CLD and UAO. Nine patients had tracheomalacia and/or bronchomalacia. Twelve of the decannulation attempts (57%) were preceded by documented downsizing of the tracheostomy tubes. Two of the three unsuccessful trials were not preceded by a documented downsizing of the tracheostomy tube. Prior to the decannulation attempt, all patients had uncuffed tracheostomy tubes and all had documented evidence of one-way valve use and/or capping of the tracheostomy tubes. All patients had airway evaluations ranging from 0 to 9 months prior to the decannulation attempt, either by a pulmonologist (flexible bronchoscopy; n = 5), an otolaryngologist (rigid bronchoscopy; n = 6), or both procedures (n = 10). Two of the patients who underwent both procedures occurred when flexible bronchoscopy identified airway granulomas that necessitated removal by subsequent rigid bronchoscopy. A total of six patients had granulomas, and five of these were removed. Two patients had adenotonsillectomies, one of which occurred after failed decannulation. Only three patients were noted to have persistent airway malacia at the time of the airway evaluation. Eleven patients had formal sizing of their airways reported. All patients underwent decannulation as an inpatient, with inpatient stays ranging from 1 to 5 days. The patient who stayed 5 days became acutely ill with a febrile illness during the stay, but successfully decannulated. Only one patient had a PSG. ETCO2 monitoring was performed successfully in 6 of 12 attempts. There were three patients who did not successfully decannulate; two patients suffered respiratory distress shortly after falling asleep. One patient was decannulated during a single night admission (without an overnight capping trial) and returned to the Emergency Department shortly after discharge with respiratory distress. Neither blood testing nor ETCO2 was performed in any of the three patients who did not successfully decannulate. The three patients who initially were unable to decannulate successfully subsequently decannulated 10, 12, and 22 months later. Aside from these three attempts, no patients required re-cannulation or died in the decannulation group.
TABLE 2.
Decannulation Attempts
| Subjects/attempts (n) | 18/21 |
|---|---|
| Age in months (median, range) | 31, 7–90 |
| Sex (male/female) | 17/1 |
| Diagnostic Category | |
| CLD | 11 |
| UAO | 5 |
| CLD/UAO | 2 |
| Location of decannulation | |
| Inpatient | 21 |
| Bronchoscopy | 21 |
| Length of stay (days; median, range) | 2, 1–5 |
| Polysomnogram | 1 |
| Blood analysis | |
| HCO3 | 9 |
| pH | 11 |
| pCO2 | 11 |
| ETCO2 monitored | 6 |
| Overnight concerns | 3 |
| Success (yes/no) | 18/3 |
CLD, chronic lung disease; UAO, upper airway obstruction; ETCO2, end-tidal carbon dioxide.
Both Weaning WN and Decannulation
Eleven patients underwent both weaning NV and decannulation. The mean time from weaning NV to decannulation was 211 days with a range of 35–784 days. The patient who waited 784 days underwent an unsuccessful attempt prior to successful decannulation.
ETCO2 Monitoring
In the patients with available pCO2 and ETCO2 results (n = 11), the last ETCO2 measurement partially correlated with the morning pCO2 (R2 = 0.53, P < 0.02; Fig. 1).
Fig. 1.
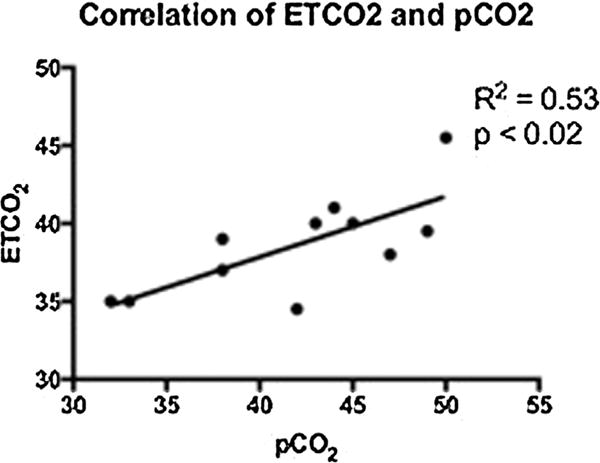
Correlation between ETCO2 and serum pCO2 among the 11 patients where both end-tidal and peripheral venous or capillary pCO2 were measured, the last ETCO2 measurement showed a partial correlation with the blood pCO2.
DISCUSSION
We present the approaches that our institution has adopted for weaning NV (Fig. 2) and decannulation (Fig. 3) as a result of this quality improvement project. In this set of patients known to our VCP, the majority (38/42) of attempts to wean NV or to decannulate were successful.
Fig. 2.
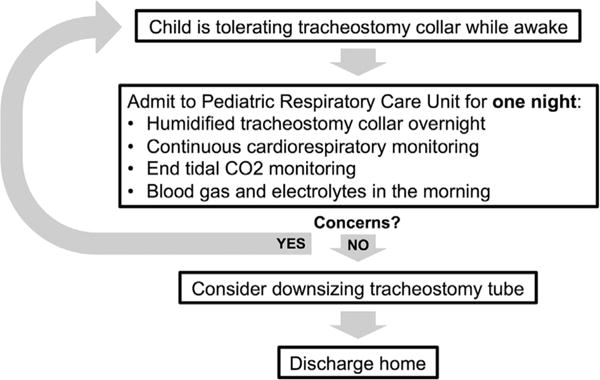
Proposed algorithm for weaning nocturnal ventilation.
Fig. 3.
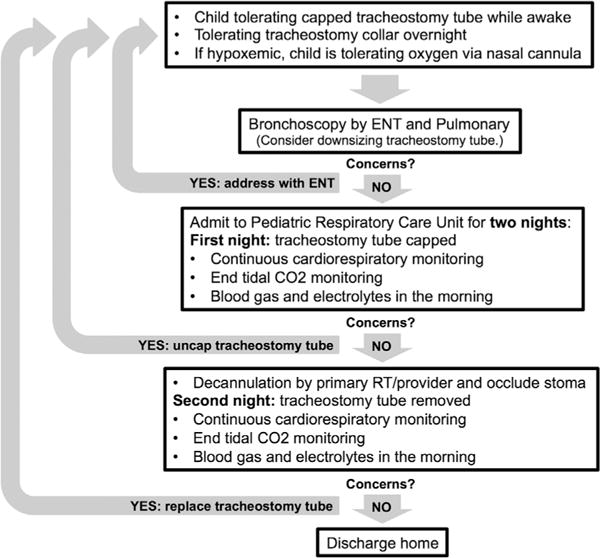
Proposed algorithm for decannulation.
Patients who underwent weaning NV at home suffered no adverse events. The patient who was unable to wean from NV did so during a PSG. All patients who attempted to wean NV during inpatient stays were successful. The ideal timing for weaning NV remains unclear, but our high rate of success suggests that some children could have weaned NV earlier.
A previous study suggested using PSG to help assess readiness for weaning NV.2 However, PSG may not be feasible due to lack of access, prolonged wait times, and high costs. Wait times for PSG range from 1 month to over a year at both pediatric and adult centers.14 Waiting a year to obtain PSG would significantly lengthen weaning times for patients. Additionally, we generally perform weaning NV during the summer (May–September) when the child is most likely to be healthy and not require intensification of care. With wait times of at least several months, planning for weaning NV using PSG would be challenging in our institution. Therefore, we have chosen to continue performing these attempts in our PRCU using the process described in Figure 1. Our results suggest weaning NV in the home setting may be safe, and therefore weaning NV at home can be considered in cases when alert and fully trained caregivers are able to closely monitor the child. We do not currently recommend weaning NV in the home setting; however, as we currently are unable to monitor the child’s ventilation status.
Three patients were unable to successfully decannulate; monitoring after decannulation is therefore important. However, the ideal duration of inpatient monitoring before discharge is unclear. Previous studies suggest 24–48 hr of observation after decannulation,7,8 and in the current study, length of stay was typically 1–3 nights. The patient who failed decannulation after one night’s observation and experienced respiratory distress in the home setting did not follow the proposed algorithm and therefore was not a failure of this approach. From the current study, observation for 24 hr after decannulation appeared to be adequate in most cases.
Other institutions have described their approach to decannulation. Sidman et al.8 described their approach to decannulation: when patients have not required mechanical ventilation for at least 2 months, they underwent bronchoscopy with subsequent decannulation and observation for at least 24 hr. de Trey et al.7 performed rigid bronchoscopy, downsized, and capped the tracheostomy tube for at least 24 hr with subsequent decannulation and observation for 48 hr. Bandyopadhyay et al.9 performed bronchoscopy and decannulation followed by PSG. The American Thoracic Society’s official statement describes both sequential downsizing and “one-stage” decannulation in the operating room after airway evaluation.15 We agree that an airway evaluation by a pulmonologist and/or otolaryngologist is crucial to ensure that there are no anatomic reasons precluding decannulation. Five patients (24%), in our study underwent excision of granulomas prior to decannulation. Joint procedures can minimize anesthesia as two patients initially only underwent flexible bronchoscopy, yet required subsequent rigid bronchoscopy. This assessment should include formal sizing of the airway using direct laryngoscopy and serial endotracheal tubes with assessment for leak in order to exclude subglottic stenosis that can contribute to unsuccessful decannulation attempts.15
PSG may also be utilized to determine readiness for decannulation.2 However, our VCP and sleep medicine providers discussed potential implementation of PSG and concluded that, in our institution, formal PSG would cause unnecessary delay, would require additional training of sleep lab staff, and would fail to utilize the existing expertise of our PRCU. Routine use of PSG may be an effective approach at other institutions.
Blood testing and ETCO2 monitoring demonstrated good correlation. Upon implementation, ETCO2 was challenging to obtain because of lack of device availability or staff training, but this improved over time with respiratory therapist education. Some patients did not tolerate the ETCO2 monitor, and therefore data were not available. Labs were often performed after the patient had been awake for several hours, while the patient was crying, or with difficulty accessing the sample. In these cases, the measured serum bicarbonate level may provide insight into interpretation of the blood gas results. We suggest that ETCO2 monitoring may provide reassurance without the stress and complications of venipuncture.
This study is limited by its retrospective design. We reviewed weaning NV and decannulation in children who were cared for by our VCP, and clinical decisions were made at the discretion of the provider. We propose a standardized approach to weaning NV and decannulation, but this was not formally evaluated during this study period. As a quality improvement project, we do not expect that these observations will be generalizable to other centers. However, in spite of this implicit limitation, there is benefit in sharing our institution’s approach to these understudied processes in medically complex children. Moving forward, we will continue to improve utilization of ETCO2 monitoring when evaluating patients undergoing weaning NV or decannulation. Further evaluation of these algorithms will occur through ongoing Plan-Do-Study-Act (PDSA) cycles.16 We are improving communication by developing order sets in the EMR for weaning NV and decannulation. Finally, we will formally investigate adverse outcomes to better understand the pitfalls and benefits of different approaches to weaning NV and decannulation.
CONCLUSION
In conclusion, we describe how weaning NV and decannulation are performed in our institution’s PRCU. Although our VCP has not adopted the practice, weaning NV may be safe in the home setting with close monitoring by trained caregivers. ETCO2 monitoring and peripheral venous/capillary pCO2 were correlated and may provide reassurance of clinical stability, but the patients’ respiratory status drove clinical decision-making. Prospective studies are needed to determine the optimal indications, timing, and approaches for weaning NV and decannulation in complex ventilator- and tracheostomy-dependent children.
Supplementary Material
Acknowledgments
Funding source: Children’s Hospital Colorado Patient Safety Grant, NIH K23 HL121090-01, NIH 5T32HL007670.
ABBREVIATIONS
- CLD
Chronic lung disease
- EMR
Electronic medical record
- ETCO2
End tidal carbon dioxide
- NV
Nocturnal ventilation
- PRCU
Pediatric respiratory care unit
- PSG
Polysomnogram
- UAO
Upper airway obstruction
- VCP
Ventilator care program
Footnotes
Conflict of interest: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Com G, Kuo DZ, Bauer ML, Lenker CV, Melguizo-Castro MM, Nick TG, Makris CM. Outcomes of children treated with tracheostomy and positive-pressure ventilation at home. Clin Pediatr (Phila) 2013;52:54–61. doi: 10.1177/0009922812465943. [DOI] [PubMed] [Google Scholar]
- 2.Cristea AI, Carroll AE, Davis SD, Swigonski NL, Ackerman VL. Outcomes of children with severe bronchopulmonary dysplasia who were ventilator dependent at home. Pediatrics. 2013;132:e727–e734. doi: 10.1542/peds.2012-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien JE, Birnkrant DJ, Dumas HM, Haley SM, Burke SA, Graham RJ, Kharasch VS. Weaning children from mechanical ventilation in a post-acute care setting. Dev Neurorehabil. 2006;9:365–372. doi: 10.1080/13638490500523192. [DOI] [PubMed] [Google Scholar]
- 4.Overman AE, Liu M, Kurachek SC, Shreve MR, Maynard RC, Mammel MC, Moore BM. Tracheostomy for infants requiring prolonged mechanical ventilation: 10 years’ experience. Pediatrics. 2013;131:e1491–e1496. doi: 10.1542/peds.2012-1943. [DOI] [PubMed] [Google Scholar]
- 5.Funamura JL, Durbin-Johnson B, Tollefson TT, Harrison J, Senders CW. Pediatric tracheotomy: indications and decannulation outcomes. Laryngoscope. 2014;124:1952–1958. doi: 10.1002/lary.24596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng J, Lioy J, Sobol S. Effect of tracheostomy timing in premature infants. Int J Pediatr Otorhinolaryngol. 2013;77:1873–1876. doi: 10.1016/j.ijporl.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 7.de Trey L, Niedermann E, Ghelfi D, Gerber A, Gysin C. Pediatric tracheotomy: a 30-year experience. J Pediatr Surg. 2013;48:1470–1475. doi: 10.1016/j.jpedsurg.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 8.Sidman JD, Jaguan A, Couser RJ. Tracheotomy and decannulation rates in a level 3 neonatal intensive care unit: a 12-year study. Laryngoscope. 2006;116:136–139. doi: 10.1097/01.mlg.0000189293.17376.0b. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay A, Cristea AI, Jalou HE, Slaven JE, Givan D, Ackerman VL, Daftary A. Clinical characteristics of children with tracheotomy who failed decannulation: a retrospective single center study. Am J Respir Crit Care Med. 2015;191:A1910. [Google Scholar]
- 10.Al-Samri M, Mitchell I, Drummond DS, Bjornson C. Tracheostomy in children: a population-based experience over 17 years. Pediatr Pulmonol. 2010;45:487–493. doi: 10.1002/ppul.21206. [DOI] [PubMed] [Google Scholar]
- 11.Berry JG, Graham DA, Graham RJ, Zhou J, Putney HL, O’Brien JE, Roberson DW, Goldmann DA. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124:563–572. doi: 10.1542/peds.2008-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien JE, Dumas HM, Haley SM, Ladenheim B, Mast J, Burke SA, Birnkrant DJ, Whitford K, Palazzo R, Neufeld JA, Kharasch VS. Ventilator weaning outcomes in chronic respiratory failure in children. Int J Rehabil Res. 2007;30:171–174. doi: 10.1097/MRR.0b013e32813a2e24. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell RB, Hussey HM, Setzen G, Jacobs IN, Nussenbaum B, Dawson C, Brown CA, 3rd, Brandt C, Deakins K, Hartnick C, Merati A. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148:6–20. doi: 10.1177/0194599812460376. [DOI] [PubMed] [Google Scholar]
- 14.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–672. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 15.Sherman JM, Davis S, Albamonte-Petrick S, Chatburn RL, Fitton C, Green C, Johnston J, Lyrene RK, Myer C, 3rd, Othersen HB, Wood R, Zach M, Zander J, Zinman R. Care of the child with a chronic tracheostomy. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:297–308. doi: 10.1164/ajrccm.161.1.ats1-00. [DOI] [PubMed] [Google Scholar]
- 16.Guinane CS, Sikes JI, Wilson RK. Using the PDSA cycle to standardize a quality assurance program in a quality improvement-driven environment. Jt Comm J Qual Improv. 1994;20:696–705. doi: 10.1016/s1070-3241(16)30118-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


