Abstract
IMPORTANCE
Over ⅔ of U.S. women are overweight or obese, placing them at increased risk for postmenopausal breast cancer.
OBJECTIVE
To investigate the associations of overweight and obesity with risk of postmenopausal invasive breast cancer after extended follow-up in the Women’s Health Initiative (WHI) Clinical Trial.
DESIGN
The WHI protocol incorporated measured height and weight, baseline and annual or biennial mammography, and adjudicated breast cancer endpoints.
SETTING
40 U.S. clinical centers.
PARTICIPANTS
n=67,142 postmenopausal women aged 50–79 years were enrolled from 1993–1998 with a median of 13 years of follow-up through 2010; 3388 invasive breast cancers were observed.
MAIN OUTCOMES AND MEASURES
Height and weight were measured at baseline and weight was measured annually thereafter. Data were collected on demographic characteristics, personal and family medical history and personal habits (smoking, physical activity). Women underwent annual or biennial mammograms. Breast cancers were verified by medical records reviewed by physician adjudicators.
RESULTS
Women who were overweight and obese had an increased invasive breast cancer risk vs. normal weight women. Risk was greatest for obesity grades 2+3 (BMI>35.0 kg/m2) (hazard ratio [HR] for invasive breast cancer =1.58, 95% CI 1.40–1.79). BMI ≥ 35.0 kg/m2 was strongly associated with risk for ER+/PR+ breast cancers (HR=1.86 95% CI 1.60–2.17), but was not associated with ER− cancers. Obesity grade 2+3 was also associated with advanced disease including larger tumor size (HR=2.12 95%CI 1.67–2.69). (P=0.02), positive lymph nodes (HR=1.89 95%CI 1.46–2.45), (P=0.06), regional/distant stage (HR=1.94, 95%CI 1.52–2.47) (P=0.05) and deaths after breast cancer (HR=2.11 95%CI 1.57–2.84) (P<0.001). Women with baseline BMI<25.0 kg/m2 who gained >5% of bodyweight over the follow-up period had an increased breast cancer risk (HR=1.36 95% CI 1.1–1.65), but among women already overweight or obese we found no association of weight change (gain or loss) with breast cancer during follow-up. There was no effect modification of the BMI-breast cancer relationship by postmenopausal hormone therapy (HT) and the direction of association across BMI categories was similar for never, past and current HT use.
CONCLUSIONS/RELEVANCE
Obesity is associated with increased invasive breast cancer risk in postmenopausal women. These clinically meaningful findings should motivate programs for obesity prevention.
Keywords: breast cancer, obesity, postmenopausal women, Women’s Health Initiative
INTRODUCTION
Obesity is a major public health problem in the United States. Recent data demonstrate that the age-adjusted obesity (BMI ≥ 30.0 kg/m2) prevalence is 34.9% among all adults age 20 years and older while that for overweight plus obesity (BMI ≥ 25.0 kg/m2) is 68.5%.1 Obesity has been associated with breast cancer risk in observational studies,2,3 systematic reviews and meta-analyses.3–5 More recently, the 2012 Annual Report to the Nation on Cancer6 concluded that overweight and obese women have a relative risk for postmenopausal breast cancer of 1.13 and 1.25, respectively vs. normal weight women.
Despite relatively strong and consistent evidence that obesity may increase postmenopausal breast cancer risk, questions remain, including whether obesity is associated with breast cancer characteristics, such as tumor hormone receptor status and stage at diagnosis or whether use of postmenopausal hormone therapy (HT) modifies the obesity-breast cancer association, since both obesity and HT alter a woman’s hormone profile. Questions also remain regarding any interaction of race/ethnicity and obesity and breast cancer risk. Black women in the United States have higher rates of obesity1 and lower breast cancer rates, but higher mortality, than non-Hispanic white women.4 Here we examine the associations of overweight and obesity with postmenopausal breast cancer risk in the Women’s Health Initiative Clinical Trials (WHI CT)7,8 where the protocol requirements specified baseline and annual or semi-annual mammograms and measured weights.
METHODS
Design details of the three overlapping WHI CTs have been published.7 Briefly, women aged 50–79 years were recruited at 40 U.S. clinical centers from 1993–1998. Women could be randomized to one, two or all three CTs (one of two hormone trials and trials of dietary modification and calcium and vitamin D supplementation). Eligibility criteria included being postmenopausal and anticipated three years survival. Exclusions included prior breast cancer, other prior cancer (except non-melanoma skin cancer) within 10 years, and conditions related to adherence and safety. Trial protocols were reviewed and approved by the Institutional Review Boards at each clinical center and the Clinical Coordinating Center. All women signed informed consent. Re-consents were required to continue follow-up through the post-trial WHI Extension periods (2005–10 and 2011–16).
For the HT trials, women with an intact uterus (n=16,608) were randomized to oral conjugated equine estrogen (CEE) (0.625 mg/d) plus medroxyprogesterone acetate (MPA) (Prempro®) (2.5 mg/d) or placebo. Women with a prior hysterectomy (n=10,739) were randomized to oral CEE (0.625 mg/d) (Premarin®) or placebo. Dietary modification (DM) trial participants were randomized to an intervention (n=19,541) to reduce fat intake and increase fruit, vegetable and grain consumption or a comparison group (n=29,294). After one year, women could participate in the calcium plus vitamin D (CaD) trial, with randomization to a daily dose of vitamin D3 (400 IU) and calcium (1000 mg) or placebo.
Height, weight, waist circumference and hip circumference were measured at baseline and weight was measured at annual visits. Body mass index (BMI) was computed as weight(kg)/height(meters)2 and further defined as normal weight (BMI<25.0 kg/m2), overweight (25–<30 kg/m2), obese-grade I (30–<35 kg/m2) and obese-grades 2+3 (≥ 35 kg/m2).1 Weight change (%) was defined as [(annual visit weight – baseline weight)]/baseline weight × 100]. Baseline data were collected on demographic characteristics, smoking, alcohol, physical activity, medical history and family history of breast cancer. Mammograms and clinical breast exams were required at baseline and annually for women in the HT trials and baseline and biennially in the DM trial. Baseline serum sex hormone levels were available on 200 randomly selected HT participants.9
Details of outcomes data collection, adjudication and primary trial results have been published.10–15 Women were queried about new medical events every six months during the intervention and annually thereafter. Breast cancers and breast cancer characteristics (tumor hormone receptor status, histology, stage, grade, tumor size, nodal involvement) were verified by medical records and pathology report review by physician adjudicators using the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) coding system. Vital status was collected through follow-up of participants and proxies and periodic searches of the National Death Index. Cause of death was determined by medical record and death certificate review.
Statistical Analysis
Associations between obesity and breast cancer incidence and mortality are presented as hazard ratios [HR] and 95% confidence intervals [CI] from Cox models using event times measured as time from randomization. The proportional hazards assumption for the primary analysis was verified by Schoenfeld residuals (p > 0.38), and by visual inspection of linear time-varying coefficients. All analyses were stratified by baseline 5-year age groups, WHI randomization assignment(s), hysterectomy status, and study phase (intervention vs. post-intervention) and adjusted for age (continuous), race/ethnicity, education, parity, age at first birth, bilateral oophorectomy, family history of breast cancer, prior estrogen use and duration, prior estrogen plus progestin use and duration, smoking, diabetes, and alcohol consumption. Since mammography use was required by the WHI protocol and compliance was good,7,8 no additional adjustment for mammography use was applied. Breast cancer mortality data were collected as deaths attributed to breast cancer and as all deaths after breast cancer. Trend tests were computed using BMI categories as a continuous variable. When examining different breast cancer characteristics,16 heterogeneity in BMI trends was tested using competing risk methods. Graphical representation of the shape of the relative risk relationship across BMI categories was created by fitting nonparametric splines to the multivariable adjusted hazard ratios in R, version 2.15.3 (R Core Team, 2013, R Foundation, Vienna Austria).
Associations of weight change with breast cancer risk were examined with similar Cox regression models stratified by baseline BMI category and using a time-dependent weight change variable updated with annual weight measurements and displayed in five categories: weight stable (± 2% of baseline weight), 2%–5% weight gain, >5% weight gain, 2%–5% weight loss, or >5% weight loss. The trend test was based on these weight change categories and the test for heterogeneity in trends between baseline BMI category was based on interaction tests.
The relationship between BMI and breast cancer incidence within HT use subgroups was examined using similar approaches and the P-values were based on interaction tests. HT subgroups were determined compositely by baseline self-report of HT and randomization into the WHI HT trials. Specifically, participants randomized to HT were categorized as “current”; participants with no prior HT use were categorized as “never”; and all others were categorized as “past.” Lastly, participants not randomized in the HT trial were categorized per their baseline HT use. In exploratory analyses, nonparametric fits (spline) of the multivariable association between invasive breast cancer risk and BMI were examined; smoothing parameter was chosen objectively via Akaike information criteria (AIC). Similar analyses also examined the nonparametric risk of weight and included height as a covariate. Unless otherwise noted, all analyses were conducted in SAS version 9.3 (Cary, NC) and were not adjusted for multiple testing. Women with baseline weight (> 135 or < 35 kg) or BMI (> 50.0 or < 18.5 kg/m2) measurements were excluded; 67,142 of 68,132 participants and 3388 breast cancers were included in this study. See also eMethods in the Supplement.
RESULTS
Participant characteristics differed by baseline BMI category (Table 1). Obese women were likely to be younger, non-White, less educated, have had a hysterectomy or bilateral oophorectomy, been treated for diabetes, less likely to have used HT and report less recreational physical activity compared to normal weight women.
Table 1.
Baseline Characteristics of Women’s Health Initiative Clinical Trial Participants by baseline BMI (kg/m2) (n=67142)
| Normal < 25 kg/m2 |
Overweight 25 – < 30 kg/m2 |
Obese (grade 1) 30 – < 35 kg/m2 |
Obese (grade 2+3) ≥ 35 kg/m2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | % | n | % | n | % | n | % | P1 | |
|
| |||||||||
| Age at screening | <0.001 | ||||||||
| 50–59 | 6485 | 35.5 | 7954 | 32.8 | 5265 | 34.7 | 3742 | 39.4 | |
| 60–69 | 7949 | 43.6 | 11323 | 46.8 | 7153 | 47.2 | 4538 | 47.7 | |
| 70–79 | 3814 | 20.9 | 4941 | 20.4 | 2749 | 18.1 | 1229 | 12.9 | |
| Race/ethnicity | <0.001 | ||||||||
| White | 15813 | 86.7 | 20040 | 82.7 | 11930 | 78.7 | 7011 | 73.7 | |
| Black | 863 | 4.7 | 2162 | 8.9 | 1999 | 13.2 | 1794 | 18.9 | |
| Hispanic | 565 | 3.1 | 1081 | 4.5 | 750 | 4.9 | 437 | 4.6 | |
| American Indian | 55 | 0.3 | 92 | 0.4 | 79 | 0.5 | 62 | 0.7 | |
| Asian/Pacific Islander | 726 | 4.0 | 517 | 2.1 | 171 | 1.1 | 68 | 0.7 | |
| Unknown | 226 | 1.2 | 326 | 1.3 | 238 | 1.6 | 137 | 1.4 | |
| Education | <0.001 | ||||||||
| ≤ High school/GED or less | 3557 | 19.6 | 5668 | 23.6 | 4143 | 27.5 | 2704 | 28.6 | |
| School after high school | 6731 | 37.1 | 9626 | 40.0 | 6224 | 41.3 | 3985 | 42.2 | |
| College degree or higher | 7847 | 43.3 | 8754 | 36.4 | 4703 | 31.2 | 2756 | 29.2 | |
| Hysterectomy at randomization | 6487 | 35.6 | 10245 | 42.3 | 6885 | 45.4 | 4541 | 47.8 | <0.001 |
| Number of term pregnancies | <0.001 | ||||||||
| Never been pregnant/No term pregnancy | 2133 | 11.8 | 2474 | 10.3 | 1490 | 9.9 | 992 | 10.5 | |
| 1 | 1612 | 8.9 | 2001 | 8.3 | 1211 | 8.0 | 790 | 8.3 | |
| 2 | 4750 | 26.2 | 5610 | 23.3 | 3281 | 21.7 | 1983 | 21.0 | |
| 3 | 4597 | 25.3 | 5884 | 24.4 | 3567 | 23.6 | 2177 | 23.0 | |
| 4+ | 5057 | 27.9 | 8142 | 33.8 | 5540 | 36.7 | 3521 | 37.2 | |
| Age at first birth | <0.001 | ||||||||
| Never pregnant/No term pregnancy | 2133 | 12.7 | 2474 | 11.3 | 1490 | 10.9 | 992 | 11.6 | |
| <20 | 1993 | 11.9 | 3359 | 15.3 | 2610 | 19.0 | 1977 | 23.2 | |
| 20 – 29 | 11239 | 66.8 | 14442 | 65.8 | 8613 | 62.8 | 5003 | 58.6 | |
| 30+ | 1452 | 8.6 | 1669 | 7.6 | 1002 | 7.3 | 563 | 6.6 | |
| Family history of female relative with breast cancer | 3106 | 18.0 | 3966 | 17.3 | 2498 | 17.4 | 1567 | 17.5 | 0.30 |
| Bilateral oophorectomy | 2986 | 16.7 | 4487 | 19.0 | 3038 | 20.6 | 1974 | 21.5 | <0.001 |
| Treated diabetes (pills or injections) | 278 | 1.5 | 836 | 3.5 | 1022 | 6.7 | 1059 | 11.1 | <0.001 |
| Smoking status | <0.001 | ||||||||
| Never | 9156 | 50.7 | 12236 | 51.1 | 7788 | 52.0 | 4786 | 50.9 | |
| Past | 7057 | 39.1 | 9777 | 40.8 | 6211 | 41.4 | 4130 | 44.0 | |
| Current | 1834 | 10.2 | 1935 | 8.1 | 991 | 6.6 | 481 | 5.1 | |
| Duration of unopposed estrogen use | <0.001 | ||||||||
| None | 12160 | 66.7 | 15779 | 65.2 | 10144 | 66.9 | 6520 | 68.6 | |
| Past User | 2406 | 13.2 | 3403 | 14.1 | 2147 | 14.2 | 1363 | 14.3 | |
| Current User | 3664 | 20.1 | 5027 | 20.8 | 2864 | 18.9 | 1619 | 17.0 | |
| < 5 Years (Duration; corresponds to past or current use) | 2384 | 13.1 | 3322 | 13.7 | 2179 | 14.4 | 1440 | 15.1 | <0.001 |
| 5 – <10 Years | 1168 | 6.4 | 1666 | 6.9 | 986 | 6.5 | 577 | 6.1 | |
| 10+ Years | 2559 | 14.0 | 3463 | 14.3 | 1864 | 12.3 | 975 | 10.3 | |
| Duration of estrogen + progesterone use | <0.001 | ||||||||
| None | 12883 | 70.6 | 18478 | 76.3 | 12238 | 80.7 | 8021 | 84.4 | |
| Past User | 1756 | 9.6 | 2147 | 8.9 | 1164 | 7.7 | 629 | 6.6 | |
| Current User | 3604 | 19.8 | 3588 | 14.8 | 1761 | 11.6 | 856 | 9.0 | |
| < 5 Years (Duration; corresponds to past or current use) | 2807 | 15.4 | 3034 | 12.5 | 1696 | 11.2 | 887 | 9.3 | <0.001 |
| 5 – <10 Years | 1475 | 8.1 | 1507 | 6.2 | 732 | 4.8 | 382 | 4.0 | |
| 10+ Years | 1083 | 5.9 | 1199 | 5.0 | 499 | 3.3 | 218 | 2.3 | |
| HT randomization group | <0.001 | ||||||||
| CEE active | 1090 | 6.0 | 1798 | 7.4 | 1351 | 8.9 | 987 | 10.4 | |
| CEE placebo | 1076 | 5.9 | 1915 | 7.9 | 1367 | 9.0 | 975 | 10.3 | |
| CEE + MPA active | 2521 | 13.8 | 2992 | 12.4 | 1816 | 12.0 | 1047 | 11.0 | |
| CEE + MPA placebo | 2419 | 13.3 | 2835 | 11.7 | 1651 | 10.9 | 1052 | 11.1 | |
| Not randomized | 11142 | 61.1 | 14678 | 60.6 | 8982 | 59.2 | 5448 | 57.3 | |
| DM randomization group | 0.84 | ||||||||
| Intervention | 5005 | 27.4 | 6944 | 28.7 | 4451 | 29.3 | 2863 | 30.1 | |
| Comparison group | 7500 | 41.1 | 10452 | 43.2 | 6748 | 44.5 | 4226 | 44.4 | |
| Not randomized | 5743 | 31.5 | 6822 | 28.2 | 3968 | 26.2 | 2420 | 25.4 | |
|
| |||||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | P | |
|
| |||||||||
| Total energy expenditure/wk from physical activity (MET-hrs) | 13.9 | 14.1 | 11.1 | 12.4 | 8.5 | 11.0 | 6.4 | 9.5 | <0.001 |
| Height (cm) | 162.5 | 6.4 | 161.9 | 6.3 | 161.6 | 6.2 | 161.3 | 6.4 | <0.001 |
| Weight (kg) | 60.2 | 6.2 | 71.9 | 6.6 | 84.3 | 7.4 | 101.1 | 11.1 | <0.001 |
| Waist circumference (cm) | 75.4 | 6.7 | 86.1 | 7.6 | 96.5 | 8.3 | 108.3 | 10.0 | <0.001 |
| Hip circumference (cm) | 97.1 | 5.8 | 105.5 | 6.1 | 114.3 | 7.1 | 127.0 | 9.7 | <0.001 |
P-value is adjusted for age, race/ethnicity, education, and hysterectomy.
Women who were overweight, obese-grade 1 and obese-grades 2+3 had an increased invasive breast cancer risk relative to normal weight women (Table 2). The hazard ratios increased as BMI increased and displayed a dose-response effect with the greatest risk for women with grades 2+3 obesity (HR= 1.58 95%CI 1.40–1.79, P-trend <0.001). Tests of heterogeneity suggested that the association between BMI and breast cancer risk differed by hormone receptor status (P< 0.001). BMI was associated with an increased risk of ER+PR+ breast cancer and the hazard ratios increased at each BMI level suggesting a dose-response relationship (HR = 1.86, 95% CI 1.60–2.17 for BMI ≥ 35 kg/m2). In exploratory analyses, measures of central adiposity (waist circumference and waist-to-hip ratio) were added to the multivariable adjusted model of weight. Neither measure of central adiposity conferred any additional information (P > 0.40) beyond what was already explained by weight (data not shown).
Table 2.
Overall and tumor specific incidence of invasive breast cancer and other breast cancer outcomes (n, annualized %) and multivariable1 adjusted hazard ratios by baseline BMI in the WHI Clinical Trial
| Normal <25 kg/m2 |
Overweight 25– <30 kg/m2 |
Obese (grade I) 30– <35 kg/m2 |
Obese (grade 2+3) ≥35 kg/m2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| N | % | N | % | HR | CI | N | % | HR | CI | N | % | HR | CI | P-Value2 | |
|
| |||||||||||||||
| All Invasive Breast Cancer | 823 | 0.37 | 1183 | 0.41 | 1.17 | (1.06, 1.29) | 828 | 0.47 | 1.37 | (1.23, 1.53) | 554 | 0.51 | 1.58 | (1.40, 1.79) | <0.001 |
| Receptor Status | <0.001 | ||||||||||||||
| ER+ / PR+ | 489 | 0.22 | 734 | 0.25 | 1.21 | (1.07, 1.37) | 544 | 0.31 | 1.52 | (1.33, 1.74) | 376 | 0.35 | 1.86 | (1.60, 2.17) | |
| ER+ / PR− | 125 | 0.06 | 169 | 0.06 | 1.07 | (0.83, 1.38) | 97 | 0.05 | 1.09 | (0.82, 1.45) | 61 | 0.06 | 1.01 | (0.71, 1.44) | |
| ER− / PR− | 112 | 0.05 | 156 | 0.05 | 1.22 | (0.93, 1.60) | 93 | 0.05 | 1.15 | (0.84, 1.57) | 54 | 0.05 | 1.15 | (0.79, 1.67) | |
| HER2 | 0.52 | ||||||||||||||
| Positive | 95 | 0.04 | 143 | 0.05 | 1.31 | (0.99, 1.74) | 103 | 0.06 | 1.59 | (1.17, 2.17) | 57 | 0.05 | 1.37 | (0.94, 2.00) | |
| Negative | 494 | 0.22 | 715 | 0.25 | 1.17 | (1.04, 1.33) | 493 | 0.28 | 1.36 | (1.18, 1.56) | 347 | 0.32 | 1.72 | (1.47, 2.01) | |
| Triple Negative | 0.12 | ||||||||||||||
| Yes | 60 | 0.03 | 84 | 0.03 | 1.23 | (0.86, 1.77) | 40 | 0.02 | 0.90 | (0.57, 1.41) | 33 | 0.03 | 1.42 | (0.89, 2.28) | |
| No | 517 | 0.23 | 753 | 0.26 | 1.19 | (1.05, 1.34) | 542 | 0.30 | 1.45 | (1.27, 1.66) | 365 | 0.34 | 1.71 | (1.46, 1.99) | |
| Tumor Size (cm) | 0.02 | ||||||||||||||
| < 1 | 232 | 0.10 | 321 | 0.11 | 1.20 | (1.00, 1.45) | 219 | 0.12 | 1.35 | (1.10, 1.66) | 145 | 0.13 | 1.58 | (1.25, 1.99) | |
| 1 – < 2 | 333 | 0.15 | 461 | 0.16 | 1.09 | (0.93, 1.26) | 328 | 0.18 | 1.32 | (1.12, 1.56) | 189 | 0.17 | 1.29 | (1.06, 1.58) | |
| ≥ 2 | 187 | 0.08 | 303 | 0.10 | 1.34 | (1.09, 1.63) | 206 | 0.12 | 1.55 | (1.25, 1.93) | 163 | 0.15 | 2.12 | (1.67, 2.69) | |
| Positive Lymph Node | 0.06 | ||||||||||||||
| Yes | 168 | 0.08 | 245 | 0.08 | 1.22 | (0.98, 1.52) | 184 | 0.10 | 1.50 | (1.19, 1.89) | 138 | 0.13 | 1.89 | (1.46, 2.45) | |
| No | 579 | 0.26 | 825 | 0.28 | 1.16 | (1.03, 1.30) | 547 | 0.31 | 1.31 | (1.15, 1.49) | 345 | 0.32 | 1.45 | (1.25, 1.68) | |
| Histology | 0.92 | ||||||||||||||
| Ductal | 521 | 0.23 | 759 | 0.26 | 1.20 | (1.06, 1.35) | 554 | 0.31 | 1.48 | (1.29, 1.69) | 349 | 0.32 | 1.56 | (1.34, 1.83) | |
| Lobular | 69 | 0.03 | 130 | 0.04 | 1.41 | (1.03, 1.93) | 63 | 0.04 | 1.07 | (0.73, 1.57) | 62 | 0.06 | 1.90 | (1.29, 2.80) | |
| Ductal & Lobular | 126 | 0.06 | 139 | 0.05 | 0.95 | (0.73, 1.23) | 109 | 0.06 | 1.28 | (0.97, 1.70) | 66 | 0.06 | 1.35 | (0.97, 1.88) | |
| Other | 102 | 0.05 | 153 | 0.05 | 1.17 | (0.89, 1.54) | 95 | 0.05 | 1.17 | (0.86, 1.59) | 70 | 0.06 | 1.72 | (1.24, 2.40) | |
| Grade | 0.14 | ||||||||||||||
| Well Differentiated | 237 | 0.11 | 302 | 0.10 | 1.02 | (0.85, 1.23) | 192 | 0.11 | 1.12 | (0.91, 1.38) | 127 | 0.12 | 1.30 | (1.03, 1.65) | |
| Moderately Differentiated | 312 | 0.14 | 457 | 0.16 | 1.21 | (1.03, 1.42) | 337 | 0.19 | 1.50 | (1.27, 1.79) | 214 | 0.20 | 1.66 | (1.33, 2.02) | |
| Poorly Differentiated | 216 | 0.10 | 299 | 0.10 | 1.13 | (0.93, 1.36) | 204 | 0.11 | 1.29 | (1.04, 1.59) | 142 | 0.13 | 1.58 | (1.25, 2.00) | |
| Stage | 0.05 | ||||||||||||||
| Local | 622 | 0.28 | 902 | 0.31 | 1.17 | (1.05, 1.31) | 602 | 0.34 | 1.33 | (1.17, 1.51) | 383 | 0.35 | 1.48 | (1.28, 1.72) | |
| Regional/Distant | 187 | 0.08 | 271 | 0.09 | 1.21 | (0.99, 1.49) | 206 | 0.12 | 1.51 | (1.21, 1.89) | 156 | 0.14 | 1.94 | (1.52, 2.47) | |
|
| |||||||||||||||
| Other Cancer Outcomes | |||||||||||||||
| In Situ Breast Cancer | 230 | 0.10 | 305 | 0.10 | 1.00 | (0.83, 1.21) | 178 | 0.10 | 0.96 | (0.77, 1.19) | 129 | 0.12 | 1.32 | (1.03, 1.68) | 0.12 |
| Total Breast Cancer | 1038 | 0.47 | 1471 | 0.51 | 1.13 | (1.04, 1.24) | 996 | 0.56 | 1.29 | (1.17, 1.42) | 671 | 0.62 | 1.52 | (1.36, 1.70) | <0.001 |
| Breast Cancer Deaths | 64 | 0.03 | 82 | 0.03 | 0.93 | (0.65, 1.34) | 61 | 0.03 | 1.08 | (0.72, 1.62) | 67 | 0.05 | 2.25 | (1.51, 3.36) | <0.001 |
| Deaths after Breast Cancer | 137 | 0.06 | 185 | 0.06 | 1.12 | (0.87, 1.44) | 147 | 0.07 | 1.37 | (1.04, 1.79) | 120 | 0.10 | 2.11 | (1.57, 2.84) | <0.001 |
Analyses were adjusted for age, race/ethnicity, education, parity, age at first birth, bilateral oophorectomy, family history of breast cancer, E-alone use and duration, E+P use and duration, smoking status, diabetes, alcohol consumption, and stratified by baseline age group, HT trial randomization group, dietary trial randomization group, hysterectomy status, CaD trial randomization group (time-dependent) and extended follow-up (time-dependent).
Corresponds to a trend test for the main effect of BMI on invasive breast cancer or other breast cancer endpoints, or a test of heterogeneity for trends between BMI and invasive breast cancer subtypes.
Obesity was associated with more advanced disease including larger tumor size (P=0.02), positive lymph nodes (P = 0.06) and regional/distant stage at diagnosis (P= 0.05) (Table 2 and eFigure 1 in the Supplement). BMI was strongly associated with breast cancer mortality only for obesity grades 2+3 (HR=2.25, 95% CI 1.51–3.36) (P<0.001) and mortality after invasive breast cancer for all obesity grades (grade 1 HR = 1.35 95% CI 1.04–1.79 and grades 2+3 HR=2.11 95% CI 1.57–2.84) (P<0.001).
Women who gained > 5% of their baseline weight during follow-up had a modest increased risk (HR=1.12 95% CI 1.00–1.25, P-trend = 0.08) compared to weight stable women, but there was no change in risk for women who lost weight (Table 3). Subgroup analyses suggested that associations between weight change and breast cancer risk was modified by baseline BMI (P-interaction = 0.05). Women with normal BMI who gained > 5% of their body weight during follow-up increased their breast cancer risk, relative to weight stable women (HR=1.36 85% CI 1.11–1.65), but neither weight gain nor loss further changed risk for overweight and obese women.
Table 3.
Invasive breast cancer incidence (N, annualized %) and multivariable2 adjusted HR (95%CI) associated with weight change3 by baseline BMI subgroups in the WHI CT.
| Body Mass Index (kg/m2) | |||||
|---|---|---|---|---|---|
| Main Effect | Normal (<25) |
Overweight (25 – <30) |
Obese-grade I (30 – <35) |
Obese-grade 2+3 (≥ 35) |
|
| > 5% Weight Loss | |||||
| N | 628 | 106 | 221 | 161 | 140 |
| % | 0.44 | 0.36 | 0.43 | 0.44 | 0.52 |
| HR | 1.00 | 1.03 | 1.05 | 0.92 | 0.99 |
| 95%CI | (0.89, 1.12) | (0.81, 1.32) | (0.87, 1.27) | (0.74, 1.14) | (0.77, 1.27) |
| 2 to 5% Weight Loss | |||||
| N | 460 | 104 | 168 | 107 | 81 |
| % | 0.44 | 0.37 | 0.44 | 0.45 | 0.56 |
| HR | 1.07 | 1.02 | 1.17 | 1.01 | 1.03 |
| 95%CI | (0.95, 1.21) | (0.80, 1.31) | (0.96, 1.43) | (0.79, 1.29) | (0.76, 1.40) |
| Weight Stable* (within +/− 2%) | |||||
| N | 1065 | 259 | 367 | 265 | 174 |
| % | 0.38 | 0.32 | 0.37 | 0.43 | 0.45 |
| 2% to 5% Weight Gain | |||||
| N | 546 | 136 | 194 | 135 | 81 |
| % | 0.46 | 0.38 | 0.44 | 0.54 | 0.61 |
| HR | 1.09 | 1.04 | 1.09 | 1.15 | 1.15 |
| 95%CI | (0.97, 1.23) | (0.82, 1.30) | (0.90, 1.32) | (0.91, 1.45) | (0.85, 1.55) |
| > 5% Weight Gain | |||||
| N | 689 | 218 | 233 | 160 | 78 |
| % | 0.46 | 0.46 | 0.41 | 0.52 | 0.50 |
| HR | 1.12 | 1.36 | 0.98 | 1.14 | 1.00 |
| 95%CI | (1.00, 1.25) | (1.11, 1.65) | (0.81, 1.18) | (0.92, 1.42) | (0.74, 1.34) |
| P-Value1 | 0.08 | 0.05 | |||
Referent group
P-values correspond to the statistical significance of a 1-df test of trend for the main effect of weight change, or a 3-df test of the interaction between weight change and BMI subgroup.
Analyses were adjusted for age, race/ethnicity, education, parity, age at first birth, bilateral oophorectomy, family history of breast cancer, E-alone use and duration, E+P use and duration, smoking status, diabetes, alcohol consumption, baseline BMI group, and stratified by baseline age group, HT trial randomization group, dietary trial randomization group, hysterectomy status, CaD trial randomization group (time-dependent) and extended follow-up (time-dependent).
Percent change from baseline, (annual visit minus baseline) divided by baseline × 100, and included as a time-dependent variable.
A priori subgroup analyses investigated whether associations of BMI with invasive breast cancer risk varied by age, race/ethnicity and HT (Table 4 and eFigure 2). Baseline age modified the association of BMI with cancer risk such that the associations appeared slightly weaker among the youngest women (P-interaction=0.05), but the overall obesity-breast cancer risk relationship remained strong. There was no evidence of effect modification of the BMI-invasive breast cancer relationship by race/ethnicity (P-interaction =0.34). Among women with an intact uterus, use of E+P did not modify the association of BMI with cancer risk as the data support a similar trend between BMI and breast cancer risk across the E+P use categories (P-interaction = 0.78). Among women with a prior hysterectomy, data were suggestive, but not conclusive, of an interaction between E-alone and BMI in relation to breast cancer risk (P-interaction=0.11). In particular, a low incidence rate for the referent normal weight group (annualized percentage = 0.23%) among women who never used E-alone was associated with linear, dose-response risk estimates for overweight (HR=1.66, 95% CI 1.06–2.60), obesity-grade I (HR=2.16 95% CI 1.38–3.39) and obesity-grades 2+3 (HR=2.63, 95% CI 1.32–2.00). For the subgroup defined as “current use” of E-alone the BMI-associated risk was increased only for current E-alone users who were obese-grade I (HR=1.35 95% CI 1.07–1.71) or obese-grades 2+3 (HR = 1.47 95% CI 1.12–1.92). A post-hoc analysis that contrasted subgroups defined by never used E-alone and ever used E-alone (past or current) was more suggestive of effect modification; HR(95%CI) of 1.01 (0.83, 1.22), 1.28 (1.04, 1.58), 1.44 (1.14, 1.83) among women who ever used E-alone for overweight, obese-grade I, and obese-grades 2+3, respectively (P-interaction=0.04). In a sensitivity analysis differentiating between prior E+P or E-alone use among the post-hysterectomy group, a similar association was observed between BMI and breast cancer among women who never used E-alone or E+P. Specifically, HRs (95%CI) were 1.65 (1.02, 2.68), 2.30 (1.42, 3.73), and 2.80 (1.70, 4.60) for overweight, obese-grade 1 and obese grades 2+3, respectively.
Table 4.
Invasive breast cancer incidence (N, annualized %) and multivariable adjusted HR (95%CI) associated with baseline BMI by select baseline age, race/ethnicity And postmenopausal hormone use in the Women’s Health Initiative Clinical Trial.
| Normal <25 kg/m2 |
Overweight 25 – <30 kg/m2 |
Obese-Grade 1 30 – <35 kg/m2 |
Obese –Grade 2+3 ≥ 35 kg/m2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| N | % | N | % | HR | CI | N | % | HR | CI | N | % | HR | CI | P1 | |
|
| |||||||||||||||
| Main Effect | 823 | 0.37 | 1183 | 0.41 | 1.17 | (1.06, 1.29) | 828 | 0.47 | 1.37 | (1.23, 1.53) | 554 | 0.51 | 1.58 | (1.40, 1.79) | <0.001 |
|
| |||||||||||||||
| Age at screening | 0.05 | ||||||||||||||
| 50–59 | 312 | 0.37 | 375 | 0.37 | 1.02 | (0.87, 1.20) | 265 | 0.41 | 1.24 | (1.04, 1.48) | 191 | 0.43 | 1.33 | (1.09, 1.62) | |
| 60–69 | 353 | 0.37 | 566 | 0.42 | 1.26 | (1.09, 1.45) | 401 | 0.48 | 1.41 | (1.20, 1.66) | 280 | 0.55 | 1.73 | (1.45, 2.07) | |
| 70–79 | 158 | 0.37 | 242 | 0.45 | 1.29 | (1.03, 1.62) | 162 | 0.55 | 1.64 | (1.28, 2.10) | 83 | 0.65 | 1.75 | (1.27, 2.42) | |
| Race/ethnicity | 0.34 | ||||||||||||||
| White | 743 | 0.38 | 1025 | 0.42 | 1.18 | (1.06, 1.31) | 704 | 0.50 | 1.39 | (1.24, 1.56) | 435 | 0.53 | 1.56 | (1.36, 1.78) | |
| Black | 34 | 0.34 | 87 | 0.35 | 0.87 | (0.56, 1.36) | 81 | 0.36 | 0.98 | (0.63, 1.53) | 82 | 0.41 | 1.15 | (0.74, 1.80) | |
| Other | 46 | 0.26 | 71 | 0.31 | 1.15 | (0.76, 1.73) | 43 | 0.32 | 1.23 | (0.77, 1.97) | 37 | 0.50 | 2.20 | (1.36, 3.55) | |
|
| |||||||||||||||
| With Uterus2 | 576 | 0.40 | 776 | 0.46 | 1.20 | (1.07, 1.35) | 487 | 0.50 | 1.35 | (1.18, 1.54) | 308 | 0.54 | 1.52 | (1.30, 1.77) | <0.001 |
| Never used E+P | 227 | 0.37 | 326 | 0.40 | 1.14 | (0.95, 1.37) | 221 | 0.44 | 1.29 | (1.05, 1.59) | 164 | 0.51 | 1.46 | (1.17, 1.83) | 0.78 |
| Past E+P use | 33 | 0.25 | 54 | 0.38 | 1.57 | (0.98, 2.51) | 33 | 0.44 | 1.64 | (0.97, 2.78) | 20 | 0.49 | 1.84 | (0.97, 3.48) | |
| Current E+P use | 316 | 0.46 | 396 | 0.54 | 1.21 | (1.03, 1.42) | 233 | 0.59 | 1.36 | (1.13, 1.64) | 124 | 0.60 | 1.53 | (1.22, 1.91) | |
|
| |||||||||||||||
| Without Uterus3 | 247 | 0.31 | 407 | 0.33 | 1.09 | (0.91, 1.30) | 341 | 0.42 | 1.40 | (1.16, 1.69) | 246 | 0.48 | 1.62 | (1.32, 2.00) | <0.001 |
| Never used E-alone | 36 | 0.23 | 97 | 0.34 | 1.66 | (1.06, 2.60) | 99 | 0.45 | 2.16 | (1.38, 3.39) | 84 | 0.54 | 2.63 | (1.65, 4.18) | 0.11 |
| Past E-alone use | 39 | 0.33 | 62 | 0.31 | 0.85 | (0.55, 1.31) | 52 | 0.39 | 1.05 | (0.67, 1.64) | 41 | 0.46 | 1.32 | (0.82, 2.12) | |
| Current E-alone | 171 | 0.33 | 248 | 0.34 | 1.05 | (0.84, 1.30) | 190 | 0.42 | 1.35 | (1.07, 1.71) | 121 | 0.45 | 1.47 | (1.12, 1.92) | |
P-values (bold) correspond to the statistical significance of a 1-df test of trend for the main effect of BMI on invasive breast cancer risk in the full cohort, or by cohorts defined by baseline hysterectomy status. The remaining p-values correspond to tests of interaction within their respective cohorts.
Includes only participants that reported not having had a hysterectomy and were randomized to any WHI clinical trial (n= 38981).
Includes only participants that reported having had a hysterectomy and were randomized to any WHI clinical trial (n= 28158).
We next examined whether the interpretation of results varied by the type of obesity measure used: BMI or, weight including height as a covariate. The multivariable-adjusted risk for the BMI-invasive breast cancer association was mostly linear for the vast majority (middle 90%) of the distribution (eFigure 3a) and plateaued near 40 kg/m2; the 5th and 95th percentiles were 21.3 kg/m2 and 39.3 kg/m2, respectively. However, the multivariable-adjusted risk associated with weight (kg) was non-linear (eFigure 3b) even among the middle 90% of participants; the 5th and 95th percentiles were 54.5 kg and 104.5 kg, respectively.
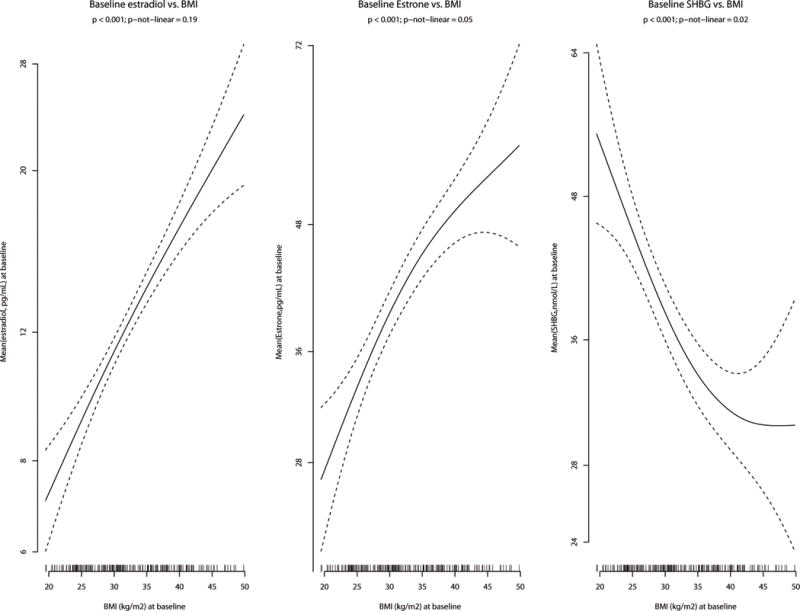
To better understand the shapes of the curves for the BMI and weight models where the breast cancer rates increase with both measures, but attenuated at the highest BMI levels (eFigures 3a, 3b), we explored the relationship between the sex hormones and BMI. Smoothed estimates of baseline mean estradiol, estrone and SHBG in the available subset of participants (n=200) were plotted against BMI (Figure 1). Estradiol had a linear relationship with BMI, but the association between estrone and BMI dampens for grades 2+3 obesity. Lastly, the sharp decrease observed between mean serum SHBG concentrations and increasing BMI levels-off for grades 2+3 obesity.
Figure 1.
DISCUSSION
The Women’s Health Initiative Clinical Trial examined the association of overweight and obesity with invasive breast cancer risk in postmenopausal women. Unlike many observational studies, weight, height and body circumferences were measured at baseline and annually using a standardized protocol throughout the trial, annual or biennial mammography was a required trial protocol element thus minimizing ascertainment bias, and breast cancer outcomes (including details on breast cancer characteristics: tumor hormone receptor status, histology, nodal involvement, tumor grade and disease stage) were adjudicated by physician adjudicators. In this context, BMI was positively associated with increased risk of invasive breast cancer (P<0.001). We observed a strong linear trend where the risk progressively increased across the BMI categories. The strongest associations were observed for women with a BMI >35 kg/m2; these women had a 58% increased risk of invasive breast cancer compared to women with BMI <25.0 kg/m2. Breast cancer deaths were also more than two-fold higher among grade 2+3 obesity compared to normal BMI.
Obesity was associated with breast cancer characteristics including tumor size, lymph node positivity and regional/distant stage at diagnosis. In addition, women with ER+/PR+ tumors who were obese-grade I or obese-grades 2+3 had 52% and 86% increased risk of breast cancer, respectively, compared to women of normal BMI. The growth of ER+ tumors are under estrogen influence17,18 and estrogen levels are higher in overweight and obese postmenopausal women due to the aromatization of androstendione and testosterone to estrogens in adipose tissue.19,20 Further, obese individuals have larger and more abundant adipose tissue cells than normal weight individuals and these women typically have greater endogenous synthesis of estrogens in their adipose tissue. Leptin may also increase estrogen levels21 and while we have no available leptin data, leptin is higher in overweight and obese individuals than in normal weight individuals.22,23 These biological relationships of BMI and altered hormone and cytokine profiles and the potential causal relationships with breast cancer risk are supported by our data showing a strong linear relationship between baseline BMI and both estradiol and estrone and are consistent with a previous report on the role of serum hormone and breast carcinogenesis.24
The WHI CT results differ from findings in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial (NSABP P-1) and the Study of Tamoxifen and Raloxifene (STAR).25,26–28 In contrast to the findings reported here in the WHI CT, the P-1 and STAR results showed a modest, but non-significant, increased risk for postmenopausal breast cancer (RR= 1.14, 95% CI 0.94–1.38) for women with a BMI ≥ 30.0 kg/m2 compared to women with a BMI < 25.0 kg/m2.25 Similar to the WHI CT, the NSABP trials had baseline breast cancer risk assessment, baseline and serial mammography, and adjudicated breast cancer outcomes. However, the NSABP results are not directly comparable to those reported here because nearly 75% of NSABP participants were randomized to tamoxifen or raloxifene, agents that decrease breast cancer incidence by almost 50%.26–28 As a result, there were fewer than 3,200 postmenopausal women who were randomized to placebo where findings could reasonably be compared to those in the WHI CT. The HRs for breast cancer risk in obese-grade I and obese-grades 2+3 NSABP postmenopausal-placebo participants were 1.77 and 1.28, respectively, P=0.36. However, the limited sample size precludes reliable generation of information regarding BMI influence on breast cancer risk in women not receiving these effective chemoprevention agents.
Several observational studies have reported that the relationship between obesity and breast cancer risk is modified by postmenopausal HT use.29–32 Huang found that higher vs. lower BMI was associated with an increased postmenopausal breast cancer risk (RR=1.59 95% CI 1.09–2.32, P-trend <0.001), except among current and past HT users.30 Subsequent observational studies from the Carolina Breast Cancer Study,31 a follow-up analysis from the Nurses’ Health Study,29 the Breast Cancer Surveillance Consortium,32 the WHI Observational Study33 and others34–37 have similarly reported apparent effect modification of the obesity-breast cancer relationship by HT use. Many investigators reporting interactions of HT and obesity in relation to breast cancer risk have posited that HT use obscures the effects of obesity, particularly in relation to their effects on circulating hormone levels. To our knowledge a biological mechanism to explain these associations has not been identified nor have results been confirmed with evidence from randomized clinical trials. Of note, two previous reports from the WHI clinical trials38,39 did not find an interaction between BMI and CEE-alone or CEE+MPA and in this report we found no effect modification and similar directions of associations were observed across BMI categories for never, past and current HT use. While we did find attenuations of the risk estimates for ever-users of estrogen-alone among women with a prior hysterectomy, the association between obesity and breast cancer remained. Differences in findings may be due to observational studies’ reliance on self-reported height and weight, self-reported HT, and may be subject to mammography screening and ascertainment bias when outcomes are collected by self-report. Notably, there are higher rates of routine screening mammograms for women receiving postmenopausal HT; the larger detection rates from screening mammograms could introduce bias in the observational studies if obese women underwent screening mammography at a different rate than normal weight women.40
The WHI findings of consistent dose-response risks across the BMI categories regardless of postmenopausal HT use have clinical implications. One report32 suggested that since the obesity-breast cancer risk was attenuated or not observed among HT users, obese women may benefit from HT use as they observed no excess breast cancer risk for these women. However, the preponderance of evidence suggests that postmenopausal HT is not beneficial for multiple health outcomes, including breast cancer, and the risks outweigh the benefits.41
One intriguing finding was that WHI women who began the study at BMI<25.0 kg/m2 and gained >5% of body weight over the follow-up period had a breast cancer HR=1.36 (95%CI 1.1, 1.65) compared to weight stable women. After menopause the breast tissue evolves toward a higher adipose content. Breast tissue adipocytes serve as a source of inflammatory cytokines as well as local estrogen production.19,20 It is possible that a weight gain-induced sudden and steep rise in breast adipocytes and exposure to cytokines and estrogens could explain why normal weight women who gain >5% bodyweight had an increased risk for breast cancer compared to weight stable women. These results suggest that prevention of weight gain may be an important public health strategy for reducing breast cancer risk.
In contrast, women who were overweight or obese at baseline had no change in risk by weight gain or loss during follow-up relative to weight stability. It is important to note that the WHI CT was not a weight loss trial and the weight change data we present may reflect both intentional and unintentional weight loss. Well-designed clinical trials are needed to definitively test whether weight loss and body composition changes in overweight and obese women or obesity prevention in normal weight women will reduce breast cancer risk. In addition, it is not clear at what stage in life excess weight confers the greatest risk. For example, during adolescence and pregnancy, breast epithelial cells undergo rapid division and differentiation. It is possible that obesity superimposed on this rapid cell growth may set the stage for aberrant cell growth and biological susceptibility to breast cancer.5,42 Another susceptible timepoint may be the menopause when breast tissue is undergoing further changes.
Strengths of this WHI-CT report include the large sample size, standardized data collection, adjudicated breast cancers, protocol-required mammography and limited loss to follow-up. Limitations include fewer race/ethnic minority participants, lack of data on tumor molecular characteristics,43 and fewer data on longer term weight and body composition changes and inability to distinguish from unintentional weight loss. Death from breast cancer was not common, so the elevated mortality risk for women with grade 2+3 obesity should be viewed with caution. Finally, we had insufficient power to examine risk for distant stage only due to very few cases presenting with distant stage at diagnosis.
In conclusion, obesity is associated with a dose-response increased postmenopausal breast cancer risk, particularly for ER+/PR+ disease, but risk does not vary by HT use or race/ethnicity. These clinically meaningful findings support the need for trials clinical trials evaluating the role of obesity prevention and treatment on breast cancer risk.
Supplementary Material
Acknowledgments
Short list of WHI investigators:
Program Office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD, USA).
Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA, USA).
Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC, USA); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA, USA); Rebecca Jackson (The Ohio State University, Columbus, OH, USA); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ, USA); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY, USA); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL, USA); Robert Wallace (University of Iowa, Iowa City/Davenport, IA, USA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA, USA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC, USA).
Role of Sponsor: The Women’s Health Initiative (WHI) project office at the National Heart, Lung and Blood Institute (NHLBI), which was the sponsor of WHI had a role in the overall design and conduct of the WHI, but no direct role in this manuscript with regards to design, interpretation of the data, review and approval of the manuscript and decision to submit the manuscript for publication. Decisions concerning the above, as well as overall data collection, management and analysis resided with committees composed of WHI investigators and included NHLBI representatives.
Dr. Neuhouser and Mr. Aragaki had full access to the data and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Mr. Aragaki, Dr. Neuhouser, Dr. Anderson and Dr. Prentice (all at Fred Hutchinson Cancer Research Center) are responsible for the data analysis.
Funding Sources: The WHI programs are funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts, HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Trial registration: clinicaltrials.gov Identifier: NCT00000611
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014 Feb 26;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund. American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 3.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status–a meta-analysis. Int J Cancer. 2009;124(3):698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 5.Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat. 2010;123(3):641–649. doi: 10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- 6.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 9.Edlefsen KL, Jackson RD, Prentice RL, et al. The effects of postmenopausal hormone therapy on serum estrogen, progesterone, and sex hormone-binding globulin levels in healthy postmenopausal women. Menopause. 2010;17(3):622–629. doi: 10.1097/gme.0b013e3181cb49e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 11.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 13.Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 14.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 15.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 16.Hefti MM, Hu R, Knoblauch NW, et al. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Can Res Treat. 2013;15(4):R68. doi: 10.1186/bcr3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nature Reviews. Cancer. 2011;11(12):886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 18.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–1568. [PubMed] [Google Scholar]
- 19.Goodwin PJ. Obesity and endocrine therapy: host factors and breast cancer outcome. Breast. 2013;22(Suppl 2):S44–47. doi: 10.1016/j.breast.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4(7):1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geisler J, Haynes B, Ekse D, Dowsett M, Lonning PE. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels. Journal of Steroid Biochem and Molec Biol. 2007;104(1–2):27–34. doi: 10.1016/j.jsbmb.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Harris RBS. Leptin-much more than a satiety signal. Anl Rev of Nutr. 2000;20:45–75. doi: 10.1146/annurev.nutr.20.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Hursting SD, Digiovanni J, Dannenberg AJ, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res. 2012;5(11):1260–1272. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao S, Chlebowski RT, Anderson GL, et al. Sex hormone associations with breast cancer risk and the mediation of randomized trial postmenopausal hormone therapy effects. Breast Cancer Research. 2014;16(2):R30. doi: 10.1186/bcr3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cecchini RS, Costantino JP, Cauley JA, et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res. 2012;5(4):583–592. doi: 10.1158/1940-6207.CAPR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 27.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 28.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 29.Chen WY, Hankinson SE, Schnitt SJ, Rosner BA, Holmes MD, Colditz GA. Association of hormone replacement therapy to estrogen and progesterone receptor status in invasive breast carcinoma. Cancer. 2004;101(7):1490–1500. doi: 10.1002/cncr.20499. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278(17):1407–1411. [PubMed] [Google Scholar]
- 31.Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG. Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol. 2000;151(7):703–714. doi: 10.1093/oxfordjournals.aje.a010265. [DOI] [PubMed] [Google Scholar]
- 32.Hou N, Hong S, Wang W, Olopade OI, Dignam JJ, Huo D. Hormone replacement therapy and breast cancer: heterogeneous risks by race, weight, and breast density. J Natl Cancer Inst. 2013;105(18):1365–1372. doi: 10.1093/jnci/djt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States) Cancer Causes Control. 2002;13(8):741–751. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 34.Li CI, Malone KE, Daling JR. Interactions between body mass index and hormone therapy and postmenopausal breast cancer risk (United States) Cancer Causes Control. 2006;17(5):695–703. doi: 10.1007/s10552-005-0001-7. [DOI] [PubMed] [Google Scholar]
- 35.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36(1):114–136. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinton LA, Richesson D, Leitzmann MF, et al. Menopausal hormone therapy and breast cancer risk in the NIH-AARP Diet and Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3150–3160. doi: 10.1158/1055-9965.EPI-08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson GL, Neuhouser ML. Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res. 2012;5(4):515–521. doi: 10.1158/1940-6207.CAPR-12-0091. [DOI] [PubMed] [Google Scholar]
- 41.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE. Weight gain, body mass index, hormone replacement therapy, and postmenopausal breast cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2004;13(2):220–224. doi: 10.1158/1055-9965.epi-03-0301. [DOI] [PubMed] [Google Scholar]
- 43.Caan BJ, Sweeney C, Habel LA, et al. Intrinsic subtypes from the PAM50 gene expression assay in a population-based breast cancer survivor cohort: prognostication of short- and long-term outcomes. Cancer Epidemiol Biomarkers Prev. 2014;23(5):725–734. doi: 10.1158/1055-9965.EPI-13-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


