Abstract
Prominent skin involvement is a defining characteristic of autoinflammatory disorders caused by abnormal IL-1 signaling. However, the pathways and cell types that drive cutaneous autoinflammatory features remain poorly understood. We sought to address this issue by investigating the pathogenesis of pustular psoriasis, a model of autoinflammatory disorders with predominant cutaneous manifestations. We specifically characterized the impact of mutations affecting AP1S3, a disease gene previously identified by our group and validated here in a newly ascertained patient resource. We first showed that AP1S3 expression is distinctively elevated in keratinocytes. Because AP1S3 encodes a protein implicated in autophagosome formation, we next investigated the effects of gene silencing on this pathway. We found that AP1S3 knockout disrupts keratinocyte autophagy, causing abnormal accumulation of p62, an adaptor protein mediating NF-κB activation. We showed that as a consequence, AP1S3-deficient cells up-regulate IL-1 signaling and overexpress IL-36α, a cytokine that is emerging as an important mediator of skin inflammation. These abnormal immune profiles were recapitulated by pharmacological inhibition of autophagy and verified in patient keratinocytes, where they were reversed by IL-36 blockade. These findings show that keratinocytes play a key role in skin autoinflammation and identify autophagy modulation of IL-36 signaling as a therapeutic target.
Abbreviations: 3-MA, 3-methyladenine; AID, autoinflammatory disorder; CRISPR, clustered regularly-interspaced short palindromic repeats; Cas9, CRISPR-associated endonuclease 9; GFP, green fluorescent protein; MALP-2, macrophage-activating lipopeptide 2; siRNA, small interfering RNA; TLR-2/6, Toll-like receptor 2/6
Introduction
Autoinflammatory disorders (AIDs) are a group of inherited conditions caused by abnormal activation of the innate immune system. AIDs typically present with recurrent and seemingly unprovoked episodes of systemic upset, which are almost invariably accompanied by joint and skin inflammation (Aksentijevich and Kastner, 2011). The latter can manifest with urticarial, pustular, or ulcerative eruptions, which are considered important markers of disease activity (Beer et al., 2014).
In the last 15 years, genetic studies have identified more than 30 AID genes, illuminating fundamental innate immune pathways and highlighting pathogenic mechanisms (most notably, abnormal IL-1 production) that have been successfully targeted by therapeutic interventions (de Jesus et al., 2015).
Despite these successes, the basis of organ-specific disease manifestations is still unclear. This is particularly true of skin pathology, because the nature of the cells and molecular mechanisms that mediate cutaneous inflammation in AID remain poorly defined (Beer et al., 2014).
We sought to address this issue by investigating the pathogenesis of pustular psoriasis, a severe AID manifesting with repeated eruptions of painful skin pustules. These can be localized to the palms and soles (palmar plantar pustulosis), toes and fingertips (acrodermatitis continua of Hallopeau) or affect most of the body surface (generalized pustular psoriasis). Although the lesions can be accompanied by arthritis and systemic upset, cutaneous involvement is the most prominent clinical feature of the disease (Griffiths and Barker, 2010). This makes pustular psoriasis an ideal model for investigating the molecular mechanisms that drive skin inflammation in AID.
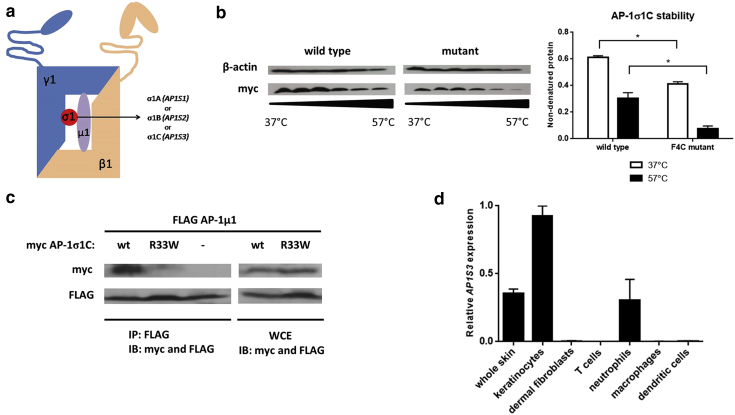
We specifically investigated the pathogenic role of AP1S3, a gene that we found to be mutated in all forms of pustular psoriasis (Setta-Kaffetzi et al., 2014). AP1S3 encodes a subunit of AP-1, a heterotetramer that mediates membrane trafficking between the post-Golgi network and the endosome (Robinson, 2004). The complex is composed of two large (AP-1γ1 and AP-1β1), one medium (AP-1μ1) and one small subunit (AP-1σ1). The latter exists in three alternative forms (AP-1σ1A, AP-1σ1B and AP-1σ1C), encoded by paralogous genes (AP1S1, AP1S2, AP1S3), so that the AP1S3 product is AP-1σ1C (Figure 1a). The σ1 subunit confers stability to AP-1 tetramers, so that mutations in AP1S genes are expected to disrupt the entire complex (Robinson, 2004).
Figure 1.
AP1S3 loss-of-function mutations are most likely to affect skin keratinocytes. (a) Schematic representation of AP-1 structure. (b) HEK293 cells were transfected with wild-type and mutant AP1S3 constructs. Lysates were subjected to the indicated temperature gradient, and soluble (nondenatured) proteins were analyzed by Western blotting. The densitometry shows the fraction of nondenatured protein (mean ± standard error of the mean of the results obtained in two experiments). (c) HEK293 cells were transfected with myc-tagged AP1S3 and FLAG-tagged AP1M1 constructs. Lysates were subjected to immune precipitation (IP) and immune blotting (IB) as indicated. The image is representative of results obtained in two experiments. (d) Real-time PCR analysis showing abundant AP1S3 expression in keratinocytes. The data show the mean ± standard error of the mean of measurements obtained in two donors. ∗P ≤ 0.05. IB, immune blotting; IP, immune precipitation; WCE, whole cell extracts; wt, wild type.
The AP-1 complex has also been implicated in the formation of autophagosomes (Guo et al., 2012). These are specialized vesicles that mediate the degradation of cellular components by autophagy, a catabolic process that can be activated by nutrient stress (e.g., starvation). Given that autophagy modulates cytokine production downstream of various pattern recognition receptors (Netea-Maier et al., 2016), we hypothesized that AP1S3 mutations would disturb autophagic activity, causing innate immune dysregulation. We then validated our pathogenic model in a variety of in vitro experimental systems and in patient cells.
Results
Validation of AP1S3 as a pustular psoriasis gene
Although we previously reported that two AP1S3 mutations (p.Phe4Cys and p.Arg33Trp) account for approximately 10% of European pustular psoriasis patients (Setta-Kaffetzi et al., 2014), the rarity of the disease has hindered the replication of this finding. To address this issue, we screened the AP1S3 coding region in 85 newly ascertained patients (53 European and 32 non-European subjects) (see Supplementary Table S1 online), recruited across Europe and East Asia. This uncovered p.Phe4Cys and p.Arg33Trp alleles in five unrelated individuals (n = 3 generalized pustular psoriasis and n = 2 palmar plantar pustulosis patients) (Table 1). All were of European descent, confirming the limited geographic distribution of the two mutations. Two of the three generalized pustular psoriasis patients carried the AP1S3 mutation in conjunction with a deleterious change in IL36RN, a pustular psoriasis gene encoding the IL-36 receptor antagonist (Marrakchi et al., 2011, Onoufriadis et al., 2011). One of these individuals exhibited a particularly severe, recalcitrant phenotype and had a sister with a milder form of the disease, who only carried the IL36RN mutation (see Supplementary Table S2 online).
Table 1.
Clinical phenotype of affected individuals bearing AP1S3 disease alleles
| Patient ID | Sex | Ethnicity | Diagnosis | Concurrent PV | Age of Onset, years | IL36RN Genotype | AP1S3 Genotype |
|---|---|---|---|---|---|---|---|
| T010091 | F | European | GPP | U | 68 | p.Ser113Leu/– | p.Phe4Cys/– |
| T030865 | F | European | GPP | N | <1 | p.Ser113Leu/– | p.Phe4Cys/– |
| T016713 | F | European | PPP | N | 55 | –/– | p.Arg33Trp/– |
| T026517 | F | European | PPP | N | 50 | –/– | p.Arg33Trp/– |
| T028754 | F | European | PPP | N | 49 | –/– | p.Arg33Trp/– |
Abbreviations: F, female; GPP, generalized pustular psoriasis; ID, identifier; N, no; PPP, palmar plantar pustulosis; PV, psoriasis vulgaris; U, unknown.
Taken together, these observations validate the involvement of AP1S3 in pustular psoriasis and suggest the possibility of epistasis between IL36RN and AP1S3 alleles.
AP1S3 mutations disrupt protein function in keratinocytes
Structural homology modeling indicates that the p.Phe4Cys change maps to a β-sheet required for protein folding, whereas the p.Arg33Trp substitution is expected to disrupt the interaction between AP-1σ1C and AP-1μ1A (Setta-Kaffetzi et al., 2014). This strongly suggests that both mutations are loss-of-function alleles.
To validate these predictions, we first examined the effect of p.Phe4Cys on the thermal stability of AP-1σ1C. After transfection of wild-type and mutant AP1S3 constructs into HEK293 cultures, we subjected cell lysates to a temperature gradient and monitored AP-1σ1C levels by western blotting. We found that p.Phe4Cys proteins were denatured significantly more quickly than their wild-type counterparts (Figure 1b), confirming that the mutation disrupts AP-1σ1C stability.
To investigate the impact of the p.Arg33Trp allele, we carried out co-immunoprecipitation experiments, using FLAG-AP1M1 and myc-AP1S3 constructs transfected into HEK293 cells. As expected, we found that wild-type myc-AP1σ1C co-precipitated with FLAG-AP1μ1A. This interaction, however, was disrupted when FLAG-AP1M1 was co-transfected with a p.Arg33Trp myc-AP1S3 cDNA (Figure 1c). Similar results were obtained in immunofluorescence experiments, showing that wild-type myc-AP1σ1C co-localized with FLAG-AP1μ1A, whereas the mutant p.Arg33Trp protein did not (see Supplementary Figure S1 online). Thus, we concluded that the p.Arg33Trp mutation disturbs the interaction between AP-1σ1C and AP-1μ1A, as predicted in-silico.
Having validated the loss-of-function nature of disease alleles, we sought to establish which cell types are most likely to be affected by AP1S3 deficiency. We therefore measured gene expression in biologically relevant cell populations. Although transcript levels were low in neutrophils and virtually undetectable in CD4+ T lymphocytes, we observed abundant gene expression in keratinocytes (Figure 1d). The impact of disease alleles was therefore modeled in this cell type.
AP1S3 deficiency causes impaired keratinocyte autophagy
Because autophagosome formation requires a functional AP-1 complex (Guo et al., 2012), we hypothesized that AP1S3 loss-of-function mutations may disrupt keratinocyte autophagy.
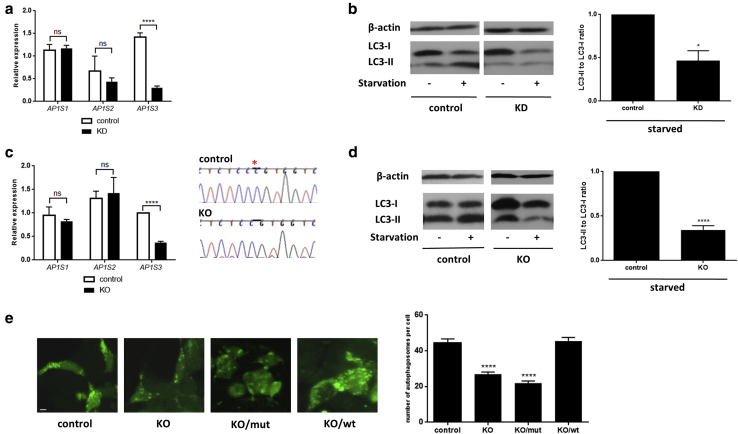
We first examined this possibility in a HaCaT keratinocyte cell line stably transduced with a silencing AP1S3 small hairpin RNA (Setta-Kaffetzi et al., 2014) (Figure 2a). After inducing autophagy by starvation, we monitored the conversion of the LC3-I protein into its modified form (LC3-II), which is a well-recognized autophagosome marker (Klionsky et al., 2012). We found that LC3-II levels were significantly reduced in AP1S3 knockdown versus control cell lines (Figure 2b).
Figure 2.
AP1S3 deficiency results in impaired autophagy. (a) After the generation of a HaCaT AP1S3 knockdown cell line, gene silencing was measured by real-time PCR, because of cross-reactivity of the anti-AP1σ1c antibody with the proteins encoded by AP1S1 and AP1S2. (b) Starvation-induced LC3-II accumulation was measured by Western blotting and densitometry. The data are presented as mean ± standard error of the mean of measurements obtained in four independent experiments. (c) HEK293 AP1S3 knockout cell lines harboring a c.124delC change (highlighted by a red asterisk in the chromatogram) were generated by CRISPR/Cas-9 editing. (d) Cells were starved to induce autophagy, and LC3-II accumulation was measured by Western blotting. The data are presented as described. (e) Control and AP1S3 KO HEK293 cells were transfected with GFP-LC3 and either an empty vector (control and KO panels) or a rescue construct (wild-type AP1S3 in KO/wt panel and p.Arg33Trp AP1S3 in KO/mut). Starvation-induced LC3 punctae were visualized by confocal fluorescence microscopy. The data are presented as mean ± standard error of the mean of measurements obtained in at least 15 cells per experiment. Scale bar = 5 μm. ∗P ≤ 0.05, ∗∗∗∗P ≤ 0.0001. Cas9, CRISPR-associated protein-9; CRISPR, clustered regularly interspaced short palindromic repeats; GFP, green fluorescent protein; KD, knockdown; KO, knockout; mut, mutated; ns, not significant; wt, wild-type.
We then repeated the experiment in a HEK293 AP1S3 knockout cell line, generated by clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated endonuclease-9 (Cas9) genome editing (Figure 2c). This confirmed that AP1S3 silencing causes a very significant decrease in starvation-induced LC3-II accumulation (Figure 2d).
To further validate our findings, we used fluorescence microscopy to visualize the expression of LC3-green fluorescent protein (GFP) constructs transfected into the HEK293 AP1S3 knockout cell line. We found that the number of autophagosomes that had incorporated LC3-GFP was significantly reduced in knockout versus control cells. This phenotype was rescued by the overexpression of wild-type but not mutant (p.Arg33Trp) AP1S3 constructs (Figure 2e).
Thus, AP1S3 deficiency disrupts autophagy induction in multiple experimental systems.
AP1S3 deficiency results in abnormal p62 accumulation and enhanced Toll-like receptor (TLR) 2/6 signaling
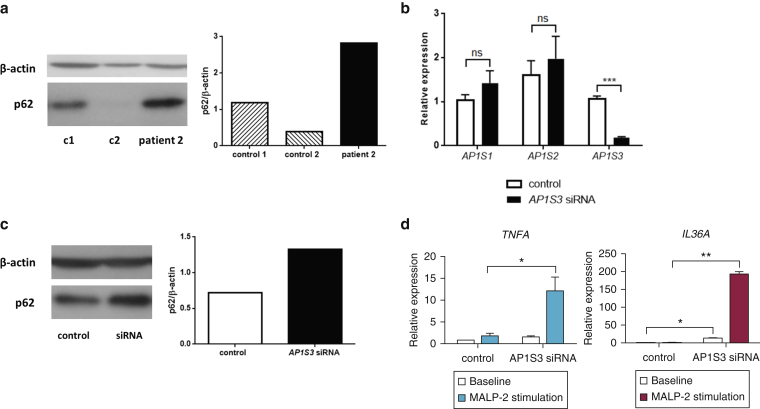
It has been shown that keratinocyte autophagy modulates NF-κB activation downstream of TLR-2/6 by regulating the degradation of the p62 adaptor protein (Lee et al., 2011). This led us to hypothesize that AP1S3 deficiency would cause an abnormal accumulation of p62, resulting in enhanced NF-κB signaling. We therefore measured p62 protein levels in keratinocytes cultured from the hair plucks of one affected individual (carrying the AP1S3 p.Arg33Trp mutation) and two healthy control subjects. We found that p62 expression was markedly increased in the patient’s cells (Figure 3a). A similar up-regulation was observed in normal primary keratinocytes transfected with AP1S3 small interfering RNAs (siRNAs) (Figure 3b and 3c) and in a HaCaT AP1S3 knockout cell line (see Supplementary Figure S2a and b online). We therefore concluded that the abnormal p62 accumulation observed in the patient was a result of AP1S3 deficiency.
Figure 3.
Abnormal p62 accumulation and enhanced TLR-2/6 signaling in AP1S3-deficient keratinocytes. (a) p62 levels were measured in patient and control subject keratinocytes by Western blotting and densitometry. (b) After the transfection of silencing (AP1S3 siRNA) and nonsilencing (control) siRNA pools into primary keratinocytes, (c) baseline p62 levels were measured by Western blotting and densitometry. (d) Alternatively, cells were stimulated with MALP-2 in triplicate, and the induction of TLR2/6-dependent genes was measured by real-time PCR. The data are representative of results obtained in at least two independent experiments and are presented as mean ± standard error of the mean of duplicate stimulations. ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001. c, control; ns, not significant; siRNA, small interfering RNA; TLR, Toll-like receptor.
To further explore these findings, we measured macrophage-activating lipopeptide 2 (MALP-2)–induced cytokine expression in primary keratinocytes transiently transfected with AP1S3 siRNAs (Figure 3d). Although there was no IL1B, IL6, or IL8 induction at the examined time point, we detected a marked increase in TNFA levels. We also observed a significant induction of IL36A (but not IL36B or IL36G), a cytokine that drives abnormal immune signaling in patients with IL36RN mutations (Onoufriadis et al., 2011). Importantly, the induction of TNFA and IL36A was significantly enhanced in AP1S3-deficient cells compared with control (Figure 3d).
We then repeated the MALP-2 stimulations in the HaCaT AP1S3 knockout cell line. This confirmed the abnormal induction of TNFA and IL36A in knockout cells (see Supplementary Figure S2c).
AP1S3 deficiency causes abnormal IL-1 signaling and up-regulates baseline IL-36 expression
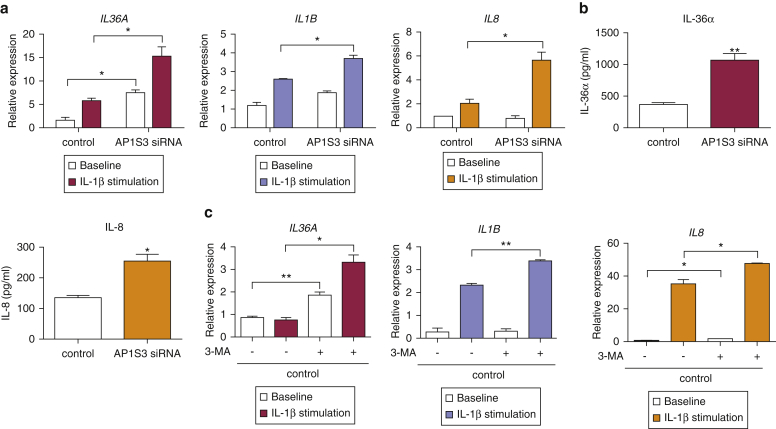
Autophagy-mediated degradation of p62 also regulates IL-1 signaling (Lee et al., 2012), a response that plays a major role in autoinflammation. To determine whether AP1S3 deficiency would also affect this pathway, we transfected primary keratinocytes with AP1S3 siRNA pools and measured cytokine levels after IL-1 stimulation. Although TNFA expression was unchanged at the examined time point, we observed a clear up-regulation of IL1B, IL8, and IL36A transcripts. The induction of all cytokines was markedly up-regulated in AP1S3-deficient cells compared with control (Figure 4a). These observations were replicated in HaCaT AP1S3-knockout cells (see Supplementary Figure S3a online), thus validating the effects of gene silencing on IL-1 signaling.
Figure 4.
AP1S3-deficient primary keratinocytes exhibit an abnormal immune profile, which can be recapitulated by autophagy inhibition. (a) After siRNA-mediated AP1S3 silencing, primary keratinocytes were stimulated with IL-1β, and gene expression was determined by real-time PCR. (b) Alternatively, cells were cultured for a further 48 hours in the absence of stimuli, and cytokine production was measured by ELISA. (c) Normal primary keratinocytes were cultured in the presence or absence of 3-MA and subsequently stimulated with IL-1β. Gene expression was determined by real-time PCR. All data are representative of results obtained in two independent experiments and are presented as mean ± standard error of the mean of (a) duplicate or (b, c) triplicate measurements. ∗P ≤ 0.05, ∗∗P ≤ 0.01. 3-MA, 3-methyladenine; siRNA, small interfering RNA.
Surprisingly, our experiments showed that baseline IL36A expression was markedly increased in AP1S3-deficient cells, both at the RNA and protein levels (Figures 4a and b, and see Supplementary Figure S2c). A similar, although less pronounced, effect was also observed for IL36B and IL36G mRNA expression (see Supplementary Figures S4a and S4b online) and IL-8 protein secretion (Figure 4b).
To determine whether this up-regulation was also a consequence of impaired autophagy, we cultured normal primary keratinocytes in medium supplemented with 3-methyladenine (3-MA), an agent that blocks the formation of autophagosomes (Klionsky et al., 2012). As expected, we found that 3-MA treatment caused an increase in IL-1–dependent cytokine expression. IL36A baseline expression was also up-regulated by 3-MA (Figure 4c). These observations, which were replicated in HaCaT keratinocytes (see Supplementary Figure S3b), show that the proinflammatory effects of AP1S3 deficiency are mediated by a disruption of keratinocyte autophagy.
Patients harboring AP1S3 mutations up-regulate IL-36 expression and IL-1 signaling
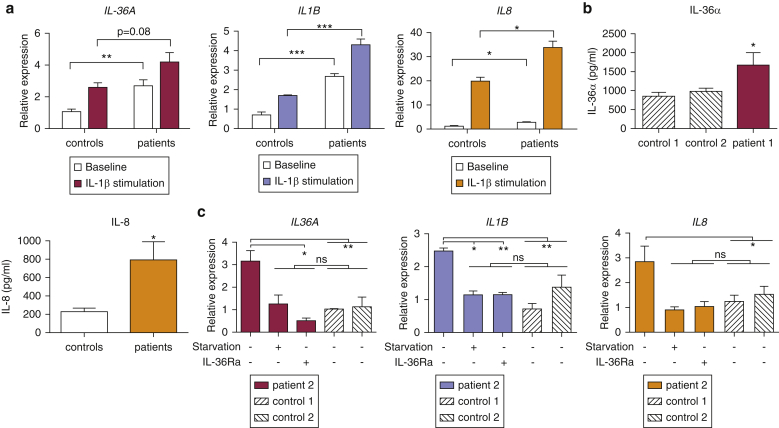
To validate the pathophysiological relevance of our findings, we cultured keratinocytes from the hair plucks of two affected individuals (each carrying an AP1S3 mutation and a wild-type IL36RN sequence) and two healthy control subjects. Although we observed only a weak response to MALP-2 stimulation, we found that cytokine levels were robustly up-regulated after IL-1 treatment. Importantly, the induction of IL1B, IL8, and IL36A transcripts was increased in the keratinocytes of patients compared with control (Figure 5a), replicating the results generated in AP1S3-knockdown cells.
Figure 5.
Abnormal cytokine expression in the keratinocytes of patients harboring AP1S3 mutations. (a) Primary keratinocytes were stimulated with IL-1β, and cytokine induction was measured by real-time PCR. The data are presented as mean ± standard error of the mean of duplicate stimulations carried out in the cells of two unrelated patients and two healthy control subjects. (b) IL-36α and IL-8 production was measured in culture supernatants by ELISA. Data are presented as mean ± standard error of the mean of triplicate measurements. (c) Primary keratinocytes were starved to induce autophagy or cultured in the presence of IL-36Ra. Gene expression was measured by real-time PCR. The data are presented as mean ± standard error of the mean of triplicate measurements, obtained in one patient and two healthy control subjects. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. ns, not significant.
The basal expression of IL-36 cytokines was also up-regulated in patient keratinocytes (Figure 5a and b and see Supplementary Figure S4c), further validating the data obtained in AP1S3-deficient cells. IL1B and IL8 baseline transcripts were also significantly overexpressed in the examined individuals (Figure 5a).
To further investigate the mechanisms underlying these observations, we measured transcript levels after autophagy induction by starvation, or blockade, of the IL-36 receptor with a recombinant antagonist (IL-36Ra). We found that both treatments could lower patient cytokine expression to the levels observed in healthy control subjects (Figure 5c, and see Supplementary Figure S4d). Although the experiment was carried out in a single patient, the results were also replicated in AP1S3-knockout cells (see Supplementary Figure S5 online), suggesting that impaired autophagy and enhanced IL-36 signaling both contribute to the abnormal immune profile associated with AP1S3 mutations.
Discussion
The aim of our study was to characterize the molecular mechanisms underlying the cutaneous features of AIDs. We therefore investigated the pathogenesis of pustular psoriasis, focusing our attention on AP1S3, a gene that is specifically mutated in this disease. We first validated the pathogenic involvement of this locus by demonstrating the presence of disease alleles in five of the 53 European patients (9.4%) who were included in our screening. We observed that AP1S3 mutations can be inherited in conjunction with IL36RN changes, modifying the phenotypic effect of the latter. This suggests that AP1S3 alleles may exacerbate the effects of IL36RN deficiency by disturbing IL-36 homeostasis, a notion that is borne out by the results of our functional studies.
First, our experiments showed that AP1S3 expression was low or undetectable in cells that do not respond to IL-36 stimulation (neutrophils and CD4+ T cells), whereas transcript levels were abundant in keratinocytes, where IL-36 signaling can be activated by TLR agonists (Gabay and Towne, 2015). The only other known gene for pustular psoriasis (CARD14) is also abundantly expressed in keratinocytes (Berki et al., 2015), suggesting that these cells play an important role in cutaneous autoinflammation. This is in keeping with the emerging view of keratinocytes as immune sentinels contributing to host defense through the engagement of innate receptors and the production of proinflammatory mediators (Di Meglio et al., 2011, Lowes et al., 2013).
The involvement of AP1S3 in IL-36 regulation is also supported by repeated observations of increased IL36A expression in AP1S3-deficient cells and in nonlesional keratinocytes, derived from patient hair plucks. Of note, stable AP1S3 knockout also led to constitutive up-regulation of IL1B and IL8 (see Supplementary Figure S3). Although this phenotype mirrored the expression profile observed in patients, it was not replicated in the transient gene-silencing experiments, where mRNA levels were measured shortly after knockdown initiation. Although IL36A was up-regulated at this early time point, the other two cytokines were not, suggesting that the overexpression of IL1B and IL8 may be secondary to IL-36 accumulation. Indeed, our experiments showed that IL-36 receptor blockade is sufficient to normalize IL1B and IL8 levels in patient keratinocytes.
Thus, our observations place IL-36 at the center of a proinflammatory loop that drives enhanced cytokine production in skin autoinflammation (see Supplementary Figure S6 online). This is in keeping with the results of recent studies, indicating that IL36A is markedly up-regulated in psoriatic skin and that this is unlikely to be a secondary consequence of inflammation (Swindell et al., 2016). Given that therapeutics blocking IL-36 are now under development (Wolf and Ferris, 2014), these discoveries have important translational implications.
Our experiments show that the effects of AP1S3 mutations on cytokine production are mediated by disruption of keratinocyte autophagy, causing abnormal p62 accumulation and enhanced NF-κB activation downstream of TLR-2/6 and IL-1R. Of note, p62 transcripts are up-regulated in psoriatic lesions, whereas the expression of molecules that contribute to skin inflammation is reduced in p62-deficient keratinocytes (Lee et al., 2011).
Here, IL-1 treatment of patient cells (which overexpress p62) caused a moderate (∼2-fold) induction of IL1B transcripts (Figure 5) but a substantial up-regulation of IL8 (>20-fold). Given that the latter cytokine plays a fundamental role in driving neutrophilic skin infiltration, this finding has a clear pathological relevance.
Autophagy can also modulate cytokine production at the posttranslational level, by degrading components of the inflammasome, the molecular complex that cleaves pro-IL1β into a bioactive molecule (Shi et al., 2012). Although this process has been chiefly investigated in mouse macrophages, it might also be active in human keratinocytes, where it could amplify the effects of AP1S3 mutations.
It is now widely accepted that perturbations of protein degradation play a pathogenic role in various AIDs with prominent dermatological features (Martinon and Aksentijevich, 2015). Evidence recently generated in animal models also indicates that therapeutic effects of anakinra (an IL-1 blocker widely used for the treatment of AIDs) are partly mediated by the rescue of defective autophagy (Iannitti et al., 2016). In the light of this evidence, our work warrants further studies of impaired keratinocyte autophagy as a pathogenic mechanism and therapeutic target in skin autoinflammation.
Methods
Participants
This study was performed in accordance with the declaration of Helsinki and was approved by the ethics committees of participating institutions. Written informed consent was obtained from all participants. Patients were ascertained by trained dermatologists (see Supplementary Table S3 online) on the basis of established diagnostic criteria (Griffiths and Barker, 2010). Patients 1 and 2 were described elsewhere as T002206 and T001882, respectively (Setta-Kaffetzi et al., 2014). Healthy volunteers were recruited within King’s College London. All affected individuals were screened for IL36RN and AP1S3 mutations as described (Onoufriadis et al., 2011, Setta-Kaffetzi et al., 2014).
Plasmids and constructs
The wild-type and mutant myc-tagged AP1S3 constructs are described elsewhere (Setta-Kaffetzi et al., 2014). The FLAG-AP1M1 construct was generated by cloning the gene coding sequence into a c-Flag pcDNA3 vector (Addgene #20011). CRISPR/Cas9 guide RNAs (see Supplementary Table S4 online) were designed with the CRISPR design tool (http://crispr.mit.edu/) and cloned into a pSpCas9BB-2A-GFP vector (Addgene #48138), as described elsewhere (Ran et al., 2013). All constructs were validated by Sanger sequencing.
Primary cell culture
Primary keratinocytes and dermal fibroblasts were isolated from healthy skin discarded after plastic surgery. The keratinocytes were maintained in Epilife keratinocyte medium supplemented with Supplement 7 and 1% penicillin-streptomycin, and the fibroblasts were grown in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (all reagents from Gibco, Waltham, MA).
Keratinocytes were derived from hair plucks as described elsewhere (Aasen and Izpisua Belmonte, 2010). Briefly, 12 hairs were plucked from the temporal scalp and placed in mTeSR1 medium (Stem Cell Technologies, Vancouver, Canada) containing 1% penicillin-streptomycin and 250 ng/ml amphotericin B (Sigma, St. Louis, MO). Once outgrowths were visible, mTeSR1 was replaced with Epilife keratinocyte medium containing Supplement 7 and 1% penicillin-streptomycin. After 14 days, cells were stimulated.
Thermal stability assay
HEK293 cells were transfected with the indicated constructs, using Lipofectamine 2000 (Life Technologies, Waltham, MA). Cell lysates were then incubated for 5 minutes across a 37–57 °C temperature gradient. Samples were centrifuged for 30 minutes at 13,000 rpm at 4 °C, and the soluble fraction (supernatant) was analyzed by Western blotting.
CRISPR/Cas9 genome editing
The protocol described by Ran et al. (2013) was used to edit HaCaT and HEK293 cells maintained in complete DMEM. Briefly, the guide RNA construct was transfected into the cells, using Lipofectamine 2000. After 48 hours, GFP-positive cells were isolated by flow cytometry and seeded for clonal expansion. The resulting cell lines were validated by Sanger sequencing of the target region, paralogous loci, and off-target sites predicted by the CRISPR design tool. The expression of AP1S1, AP1S2, and AP1S3 was also measured by real-time PCR. Control cells were transfected with an empty pSpCas9BB-2A-GFP vector.
Cell stimulation
For autophagy induction, cells were starved for 18 hours in Hank’s Balanced Salt solution (Gibco), and protein extracts were analyzed by Western blotting. For autophagy inhibition, cells were pretreated with 10mmol/L of 3-MA (Sigma) for 5 hours and then stimulated with 20ng/ml of IL-1β (Sigma) for 2 hours, in the presence 3-MA.
Alternatively, primary or immortalized keratinocytes were treated with 100ng/ml of MALP2 (Bio-techne, Minneapolis, MN) for 42 hours, 20ng/ml of IL-1β for 2 hours, 100ng/ml of IL-36Ra (Bio-techne) for 5 hours or were starved as described.
For transient gene-silencing experiments, cells were transfected for 48 hours with 33 nmol/L of AP1S3 ON-TARGET plus SMARTpool siRNA or ON-TARGETplus nontargeting siRNA (GE Dharmacon, Lafayette, CO) using Lipofectamine 2000 and stimulated as described above.
Real-time PCR and ELISA
RNAs isolated from skin, lymphocytes, in vitro derived macrophages/dendritic cells, and neutrophils were provided by Frank Nestle, Susan John, Leonie Taams (King’s College London), and Benjamin Fairfax (Wellcome Trust Centre for Human Genetics, Oxford), respectively. All remaining RNAs were isolated using the RNeasy Mini Plus kit (Qiagen, Hilden, Germany). Gene expression was assessed by real-time PCR by using the primers listed in Supplementary Table S4 online. Transcript levels were normalized to PPIA or B2M expression, measured with Applied Biosystems (Foster City, CA) TaqMan probes. IL-36α and IL-8 production was measured with the Human IL36A ELISA Kit (Sigma) and Human IL-8 ELISA Kit (Sigma).
Co-immunoprecipitation and Western blotting
A rabbit monoclonal anti-FLAG (1:50, Cell Signaling Technology, Danvers, MA) was used in co-immunoprecipitation experiments, whereas Western blots were probed with rabbit polyclonal anti-LC3 (Cell Signaling Technology), rabbit polyclonal anti-β actin (Cell Signaling Technology), rabbit polyclonal anti-p62 (Sigma), or mouse monoclonal anti-myc (Thermo Scientific, Waltham, MA) (all 1:1,000). Densitometry was undertaken with Image J software (Schneider et al., 2012).
Immunofluorescence microscopy
In the co-localization experiments, HEK293 cells were co-transfected with the indicated constructs, using Lipofectamine 2000. After 24 hours, cells were fixed and incubated with 1:500 mouse monoclonal anti-myc (Cell Signaling Technology) and 1:600 rabbit monoclonal anti-FLAG. Slides were imaged by using a C2 confocal microscope (Nikon, Tokyo, Japan), and z-stack images of at least 15 cells per slide were taken.
In autophagy induction experiments, HEK293 cells were transfected with a pEGFP-LC3 plasmid (Addgene #24920) and the indicated construct, using Lipofectamine 2000. After 24 hours, cells were starved for 18 hours in Hank’s Balanced Salt solution supplemented with 0.1 μmol/L of Bafilomycin A1 (Sigma). Cells were imaged as described above and autophagosomes were counted by using NIS-Elements Advanced Research software (Nikon).
Statistics
Means were compared with unpaired Student t tests. Error bars represent standard error of the mean.
ORCID
Francesca Capon: http://orcid.org/0000-0003-2432-5793
Alan Irvine: http://orcid.org/0000-0002-9048-2044
Conflict of Interest
Maja Mockenhaupt is the coordinator of the international RegiSCAR-project, which was/is funded (among others) by a consortium of pharmaceutical companies (Bayer Vital, Boehringer-lngelheim, Cephalon, GlaxoSmithKline, MSD Sharp and Dohme, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Servier, Tibotec, Grünenthal, Falk Pharma, UCB Biopharma, AB-Science). Maja Mockenhaupt is also a member of expert panels/advisory boards in the field of severe cutaneous adverse reaction coordinated by pharmaceutical companies (Boehringer Ingelheim, Merck, Sanofi). She has also been an expert in litigations concerning Stevens Johnson syndrome/toxic epidermal necrolysis. Helen Young is/has been a consultant or speaker to Abbott/Abbvie, Amgen, Janssen-Cilag, Leo-Pharma, Novartis, Lily, Stiefel, Teva Pharmaceuticals, and Wyeth/Pfizer.
Acknowledgments
This work was supported by the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s & St. Thomas’ National Health Service Foundation Trust in partnership with King’s College London, King’s College Hospital National Health Service Foundation Trust. FC, RCT, and JNB are supported by the Medical Research Council (award MR/L011808/1). ZB and MS were supported by Orszagos Tudomanyos Kutatasi Alap (National Scientific Research Fund) grants OTKA K105985 and OTKA K111885. MM and LV are part of The International Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) Consortium, funded by the European Commission (QLRT-2002-01738), GIS-Institut des Maladies Rares, and Institut National de la Santé et de la Reserche Médicale (INSERM) (4CH09G) in France and by a consortium of pharmaceutical companies. SKM is funded by a Medical Research Council Clinical Training Fellowship (MR/L001543/1). ST is supported by the King’s Bioscience Institute and the Guy’s and St Thomas’ Charity Prize PhD Programme in Biomedical and Translational Science. NSK received a British Skin Foundation PhD studentship (grant 3007s).
accepted manuscript published online 5 July 2016; corrected proof published online 12 August 2016
Footnotes
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.doi.org/10.1016/j.jid.2016.06.618.
Supplementary Material
References
- Aasen T., Izpisua Belmonte J.C. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc. 2010;5:371–382. doi: 10.1038/nprot.2009.241. [DOI] [PubMed] [Google Scholar]
- Aksentijevich I., Kastner D.L. Genetics of monogenic autoinflammatory diseases: past successes, future challenges. Nat Rev Rheumatol. 2011;7:469–478. doi: 10.1038/nrrheum.2011.94. [DOI] [PubMed] [Google Scholar]
- Beer H.D., Contassot E., French L.E. The inflammasomes in autoinflammatory diseases with skin involvement. J Invest Dermatol. 2014;134:1805–1810. doi: 10.1038/jid.2014.76. [DOI] [PubMed] [Google Scholar]
- Berki D.M., Liu L., Choon S.E., David Burden A., Griffiths C.E., Navarini A.A. Activating CARD14 mutations are associated with generalized pustular psoriasis but rarely account for familial recurrence in psoriasis vulgaris. J Invest Dermatol. 2015;135:2964–2970. doi: 10.1038/jid.2015.288. [DOI] [PubMed] [Google Scholar]
- de Jesus A.A., Canna S.W., Liu Y., Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015;33:823–874. doi: 10.1146/annurev-immunol-032414-112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio P., Perera G.K., Nestle F.O. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35:857–869. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Gabay C., Towne J.E. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J Leukocyte Biol. 2015;97:645–652. doi: 10.1189/jlb.3RI1014-495R. [DOI] [PubMed] [Google Scholar]
- Griffiths C.E.M., Barker J.N. Psoriasis. In: Burns T., Breathnach S., Cox N., Griffiths C.E.M., editors. Rook’s textbook of dermatology. Wiley-Blackwell; Chichester, UK: 2010. p.20.1–.60. [Google Scholar]
- Guo Y., Chang C., Huang R., Liu B., Bao L., Liu W. AP1 is essential for generation of autophagosomes from the trans-Golgi network. J Cell Sci. 2012;125:1706–1715. doi: 10.1242/jcs.093203. [DOI] [PubMed] [Google Scholar]
- Iannitti R.G., Napolioni V., Oikonomou V., De Luca A., Galosi C., Pariano M. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Com. 2016;7:10791. doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.M., Shin D.M., Yuk J.M., Shi G., Choi D.K., Lee S.H. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J Immunol. 2011;186:1248–1258. doi: 10.4049/jimmunol.1001954. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim H.R., Quinley C., Kim J., Gonzalez-Navajas J., Xavier R. Autophagy suppresses interleukin-1beta (IL-1beta) signaling by activation of p62 degradation via lysosomal and proteasomal pathways. J Biol Chem. 2012;287:4033–4040. doi: 10.1074/jbc.M111.280065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes M.A., Russell C.B., Martin D.A., Towne J.E., Krueger J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013;34:174–181. doi: 10.1016/j.it.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrakchi S., Guigue P., Renshaw B.R., Puel A., Pei X.Y., Fraitag S. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- Martinon F., Aksentijevich I. New players driving inflammation in monogenic autoinflammatory diseases. Nat Rev Rheumatol. 2015;11:11–20. doi: 10.1038/nrrheum.2014.158. [DOI] [PubMed] [Google Scholar]
- Netea-Maier R.T., Plantinga T.S., Van De Veerdonk F.L., Smit J.W., Netea M.G. Modulation of inflammation by autophagy: consequences for human disease. Autophagy. 2016;12:245–260. doi: 10.1080/15548627.2015.1071759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoufriadis A., Simpson M.A., Pink A.E., Di Meglio P., Smith C.H., Pullabhatla V. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432–437. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S. Adaptable adaptors for coated vesicles. Trend Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setta-Kaffetzi N., Simpson M.A., Navarini A.A., Patel V.M., Lu H.C., Allen M.H. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790–797. doi: 10.1016/j.ajhg.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.S., Shenderov K., Huang N.N., Kabat J., Abu-Asab M., Fitzgerald K.A. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell W.R., Sarkar M.K., Liang Y., Xing X., Gudjonsson J.E. Cross-disease transcriptomics: Unique IL-17A signaling in psoriasis lesions and an autoimmune PBMC signature. J Invest Dermatol. 2016;136:1820–1830. doi: 10.1016/j.jid.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J., Ferris L.K. Anti-IL-36R antibodies, potentially useful for the treatment of psoriasis: a patent evaluation of WO2013074569. Expert Opin Ther Pat. 2014;24:477–479. doi: 10.1517/13543776.2014.881473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.