Abstract
Leaders gathered at the US National Institutes of Health in November 2014 to discuss recent advances and emerging research areas in aspects of maternal-fetal immunity that may affect fetal development and pregnancy success.
Pregnancy is a unique situation in which the mother and the hemiallogeneic fetus peacefully coexist. Numerous fetal, maternal and placental mechanisms work in concert to protect the fetus from immunological recognition and rejection. During human placentation, several changes occur in the uterus. First, the stromal compartment of the endometrium differentiates into the decidua. Second, placental villous trophoblasts (PVTs) of fetal origin traverse the uterine epithelium and invade the decidua as well as the inner third of the myometrium. Third, the trophoblast differentiates, and fetal extravillous trophoblasts (EVTs) penetrate and extensively remodel uterine arteries. During this process they replace the endothelium and (partially) the muscle layer of these vessels. To a much lesser extent, the same process occurs in uterine veins. A successful pregnancy involves complex interactions between fetal trophoblasts and maternal decidual immune cells, which allow the embryo and then fetus to develop in the uterus while the mother’s immune system remains largely intact. Uterine natural killer (uNK) cells, immature dendritic cells (iDCs), T cells and macrophages contribute to modulating the uterine environment to sustain a successful pregnancy.
The placenta mediates hormonal, nutritional and oxygen support of the fetus while also playing an important immunomodulatory role. Fetal syncytiotrophoblasts, which form the surface of the chorionic villi and are bathed by maternal blood, release various-sized vesicles of numerous functions. For example, placenta-derived exosomes impair T cell signaling, downregulate the NK cell receptor NKG2D, stimulate apoptosis by means of the Fas ligand (FasL)- and TNF-related apoptosis-inducing ligand (TRAIL)-mediated pathways, and promote an immunosuppressive environment via the cytokine TGF-β and co-stimulatory molecule PD-L1, which primes regulatory T cells1. Gaining a better understanding of the unique immunological features and their dynamic changes during normal pregnancy will provide insights into pregnancy-associated diseases with an immune component, such as preterm labor, and lay the foundation for new strategies to improve perinatal outcomes.
Every day, approximately 800 women die from preventable causes related to pregnancy and childbirth2. Every year, about 15 million babies are born prematurely, and prematurity (less than 37 weeks’ gestation) is the leading cause of newborn deaths3. Accordingly, one of the United Nations Millennium Development Goals is to reduce preterm birth and the mortality of babies born preterm. As outlined in the Global Action Report on Preterm Birth (2012)4, discovery science, understanding causes, developing new tools and increasing research capacity are areas that are critical to achieving this global agenda. Therefore, the National Institute of Allergy and Infectious Diseases (NIAID) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health organized a workshop to bring together experts in the fields of Reproductive Biology and Immunology to assess the state of the science regarding maternal-fetal immunity and to discuss current knowledge gaps, identify challenges and outline future research directions. This Commentary discusses the seminal outcomes and recommendations from the workshop.
Regulators of early pregnancy
Establishment of pregnancy in humans requires continuous reciprocal communication between the conceptus (embryo and later fetus and associated extraembryonic or placental membranes) and the uterus of the mother. By 5–6 days after fertilization, after entry into the uterus, the blastocyst must develop a trophoblast covering that produces chorionic gonadotropin to signal pregnancy recognition and maintain progesterone production by the ovary. Chorionic gonadotropin also acts locally on uterine glandular epithelial cells, stromal fibroblasts and the vasculature to modulate immune cell functions, promote stromal cell decidualization, and enhance angiogenesis and vascular development. In turn, the uterine glandular epithelial cells, stromal decidual cells and immune cells, particularly uNK cells, regulate the growth and development of the conceptus. Appropriate development of the uterine decidua and embryonic placenta during the first trimester is critical to the maintenance of pregnancy. Emerging evidence strongly supports the tenet that defects in uterine gland secretory functions, stromal cell decidualization and/ or uNK cell development and function are causative factors in pregnancy failure and pregnancy complications such as preeclampsia and fetal growth restriction. Moreover, women with endometriosis have higher rates of implantation failure that are associated with defects in stromal cell decidualization and phenotypic alterations of uNK cells5,6. An increased understanding of early pregnancy biology is fundamentally important for the diagnosis, prevention and treatment of fertility and pregnancy problems as well as fertility-associated diseases in women. A key gap in our understanding of the human uterus and placenta is in early pregnancy, the time when the majority of pregnancies are lost. Therefore, it is essential to obtain and infer information from animal models on uterine and placental function during this critical early window, given the ethical limitations associated with obtaining samples from women.
All mammalian uteri contain endometrial glands that synthesize or transport and secrete substances essential for survival and development of the conceptus7. Uterine glands and their secretions have biological roles in blastocyst and embryo survival and implantation, uterine receptivity and stromal cell decidualization in humans and animal models. Tom Spencer (Washington State University, Pullman) and colleagues found that infertility and recurrent pregnancy loss observed in ovine and mouse uterine gland–deficient models unequivocally supports a primary role for uterine glands and, by inference, their secretions present in uterine luminal fluid in the survival and development of the embryo. The development and function of the uterine glands within the implantation site is regulated by trophoblast, decidual and NK cell factors8.
During the establishment of pregnancy, decidualization in primates and rodents is a multifaceted process. Notch, an evolutionarily conserved arbiter of cell fate, regulates diverse functions including cell survival, proliferation and differentiation. In mammalian species, the role of Notch has been studied extensively in the context of cancer biology, and Notch pathway inhibitors are being developed that target cancer stem cells. Notch1, one of four Notch receptors, mediates stromal cell survival during decidualization, and the absence of Notch1 results in decreased cell proliferation, increased apoptosis and impaired decidualization in the context of endometriosis5. To further evaluate the role of all Notch receptors in the context of endometrial biology, Asgerally Fazleabas and co-workers at Michigan State University inhibited all canonical Notch signaling by generating a uterine-specific deletion of Rbpj, the nuclear transducer of Notch signaling, in mice. The results included impaired decidualization and embryo implantation as a consequence of decreased progesterone receptor expression. In addition, Rbpj-deficient mice had delayed postpartum repair as a consequence of chronic inflammation and immunosuppression. Thus, Notch receptors play important physiological roles during the establishment of pregnancy as well as postpartum repair through modulation of the immune environment.
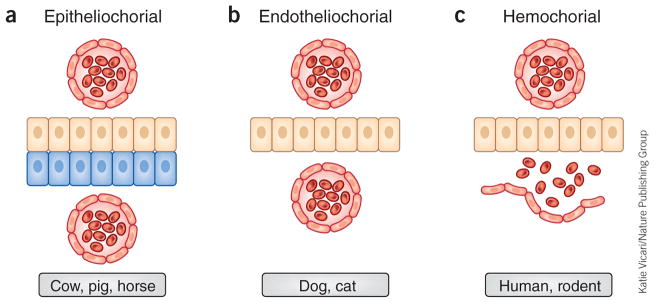
From the distribution of placental types, it is clear that no other mammal has a placenta identical to that of the human; although morphologically similar, the placentas of some nonhuman primates demonstrate less trophoblast invasiveness than is observed in humans. Placentas are classified in several ways. One type of classification is based on the tissues that exist between the chorion and maternal blood (Fig. 1). The major function of the placenta is to allow diffusion of nutrients and oxygen from the maternal blood to the fetal blood through the chorion and of waste products from the fetus back to the maternal blood. This exchange occurs through the permeable membrane of the placenta. This membrane may be composed of maternal blood vessel endothelium, connective tissue, uterine luminal epithelium and chorion (epitheliochorial, Fig. 1a), maternal blood vessel endothelium and chorion (endotheliochorial, Fig. 1b), or chorion (hemochorial, Fig. 1c).
Figure 1.
Classification of placentas based on histological assessment of the maternal-chorion interface. (a) Epitheliochorial placenta as seen in cow, pig and sheep: the barrier between maternal blood and the chorion (tan cells) consists of the maternal vascular endothelium and uterine epithelium (blue cells). Red cells are vascular endothelium. (b) Endotheliochorial placenta as observed in dog and cat: the barrier between maternal blood and the chorion consists of the maternal vascular endothelium. (c) Hemochorial placenta as seen in human and small rodents (such as rat and mouse): maternal blood directly bathes the chorionic villi.
Nonetheless, many features of the human placenta are widely shared, and the disc-like, hemochorial placental type of humans represents ancient mammalian character states that emerged well before the origin of primates. Whereas rodent placentas share hemochorial and disc-like features with the human placentas, differences occur in interdigitation, whereby fetal tissues branch to form villi that are either bathed in maternal blood or covered in maternal tissue. These differences result in variations in the degree of contact between maternal and fetal tissues at the area where exchange of maternal resources and nutrients occurs. Moreover, some emerging studies demonstrate marked differences in placental structure between genetic strains of rat, and we have yet to recognize and/or exploit similar differences within the widely diverse strains of genetic mouse models. Thus, appropriate animal models can be found, but they should be chosen on the basis of the specific scientific question being addressed. Furthermore, study of a variety of animal models can, as in the case of placental lactogens, reveal key features conserved across taxa and their importance in placental function. It is possible for researchers to obtain human term placentas after delivery and, in some instances, to obtain placentas from elective terminations of pregnancy in the first trimester. However, the period of development between embryo implantation and 8 weeks after fertilization is inaccessible, and embryo-maternal interactions during this early time frame of pregnancy differ substantially from those later in pregnancy8. It is during this early period that the major placental cell types differentiate and placental structures form; accordingly, this is a critical window of time that needs to be studied in order to understand the fundamental biology that contributes to placental dysfunction in humans. Laura Schulz and R. Michael Roberts (University of Missouri, Columbia) have found that human embryonic stem cells (hESCs) and induced pluripotent cells (iPSCs) provide an opportunity for addressing this gap. When treated with bone morphogenetic protein 4 (BMP4), alone or in combination with inhibitors of activin and mitogen-activated protein kinase (MAPK) signaling, these stem cells differentiate into placental trophoblast cells. By using iPSCs generated from pregnancies complicated by preeclampsia or other pregnancy-associated complications, it may be possible to identify the initial potential source of the dysfunction in these pregnancies9.
Immune homeostasis in the uterus
Mouse models have been critical for our understanding of reproductive biology, pregnancy and maternal-fetal interactions, including immune homeostasis. Cytokines, growth factors and hormonal signaling pathways play important roles during implantation and parturition. Sudhansu Dey (Cincinnati Children’s Hospital) demonstrated that early events of pregnancy, such as the timing of implantation and decidualization, can profoundly influence later events of pregnancy. For example, muscle segment homeobox (Msx) transcription factors and the noncanonical Wnt5a-Ror Ror-family receptor tyrosine kinase signaling pathway are critical to on-time implantation and success of the pregnancy. Many genes are specifically regulated in the uterine epithelium and/or stroma during the window of implantation (for example, Msx1), and alterations in these genes influence the timing and success of the pregnancy10. Furthermore, premature decidual senescence leads to aberrantly high amounts of cyclooxygenase-2 and prostaglandin F synthase, generating increased concentrations of uterine prostaglandin F2α and thereby triggering myometrial contractility in a paracrine manner to induce preterm birth11.
Martin Matzuk (Baylor College of Medicine) presented another example of the importance of the timing of implantation. Conditional deletion of the BMP type I receptor ALK2 in mice led to delayed embryo invasion into the uterine epithelium and stroma, a failure of uterine decidualization, embryonic loss and subsequent sterility12. In humans, ‘inferior’ decidualization (i.e., poor stromal cell proliferation and differentiation) could also lead to diseases of pregnancy (such as preeclampsia) and other poor outcomes such as premature delivery. It is also reasonable to speculate that such implantation-initiated insults consequently affect the delivered offspring, just as hormonal or nutritional abnormalities of pregnancy are believed to influence the risks for adult diseases.
Studies from the Matzuk laboratory revealed that the conditional uterine-specific deletion of Bmpr2, which encodes BMP receptor type 2, using a progesterone receptor (PR)-Cre construct also caused pregnancy defects. Conditionally Bmpr2-deficient mice had midgestation abnormalities in decidualization that led to major defects in the formation of the spiral arteries; these mice also showed trophoblast defects and a deficiency of uNK cells. These defects resulted in severe hemorrhage of the placentation sites, leading to placental abruption, intrauterine growth restriction, complete loss of the pregnancy and resultant sterility13. Various signaling pathways have been implicated in pathophysiological processes, including diseases of pregnancy. Interestingly, polymorphisms in genes encoding either human adrenomedullin (AM) or proteins in the TGF-β signaling pathway are associated with preeclampsia. Studies are under way to uncover new and specific molecular regulators of these pathways from implantation through mid-gestation.
Another molecule that has an important role at the maternal-fetal interface is AM, a peptide involved in multiple processes including embryonic development, angiogenesis, cardioprotection and innate immunity. Several types of cardiovascular stress (including pregnancy, septic shock and myocardial infarction) result in a substantial increase in plasma AM concentrations, which has recently been identified as an effective prognostic and diagnostic marker in the clinic. During a normal pregnancy, maternal AM concentrations increase, and abnormally low concentrations are associated with pregnancy complications such as preeclampsia, fetal growth restriction, gestational diabetes and miscarriage. Therefore, the dosage and biological activity of AM in humans appears to be critical for maintaining a healthy pregnancy. Kathleen Caron’s laboratory (University of North Carolina, Chapel Hill) was the first to demonstrate that haploinsufficiency for maternal AM caused a multitude of reproductive defects associated with abnormal implantation and fetal growth restriction14. Studies from the Caron laboratory revealed that mice lacking AM had abnormal spiral arteries, containing a thick layer of vascular smooth muscle cells, at embryonic day 13.5. Similar findings have also been observed in women with preeclampsia. AM regulates trophoblast invasion into the uterine epithelium and into uterine spiral arteries. Placentas from AM-null mice also showed fewer uNK cells and less recruitment of uNK cells to decidua, than did placentas from WT mice, and Caron and co-workers have also found that AM activates uNK cells. These studies establish an important and previously unrecognized aspect of fetal-to-maternal communication during pregnancy (Fig. 2a). The Caron laboratory recently demonstrated that CXCR7, an atypical chemokine receptor of the seven-transmembrane-receptor family, acts as a decoy receptor by controlling the amount of AM in the host. Recent studies with mice lacking CXCR7 revealed cardiac hyperplasia, defects in lymphatic vascular development and elevated uNK cell abundance15. All of these phenotypes could be reversed by depletion of the gene encoding CXCR7’s ligand AM, demonstrating the importance of CXCR7 in controlling the dosage of growth factors within the developing embryo and placenta. It is likely that CXCR7, along with other atypical chemokine receptors, is critical in capturing chemokines and restricting their access to cognate receptors. Caron’s group is currently working on characterizing the roles of these atypical chemokine receptors in controlling the dosage of AM peptide and other modulators of the innate immune response, as described below.
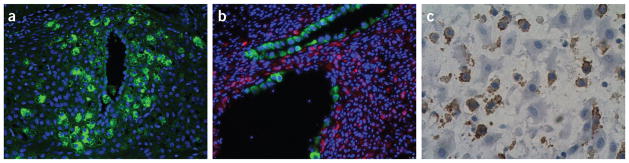
Figure 2.
Various immune cells interact with uterine and trophoblast cells during pregnancy. (a) Uterine spiral artery remodeling during pregnancy. uNK cells (green cells) surrounding a spiral artery in the mouse. Courtesy of P. Lenhart (University of North Carolina, Chapel Hill). (b) Trophoblast cells (green cells) are replacing the endothelial cells of one spiral artery (bottom) and have completely replaced endothelial cells in the other artery (above) in the rat. Courtesy of D. Chakraborty (University of Kansas Medical Center, Kansas City). (c) Human pregnant uterine immune cells: CD14+ decidual macrophages (brown) in close contact with extravillous trophoblast. Sample collected from a third-trimester decidua, cesarean section term not labor.
Inflammation and pregnancy
High amounts of the pro-inflammatory cytokines (such as interleukin 6 (IL-6), IL-15 and tumor necrosis factor (TNF)) and chemokines (such as CXCL10, CXCL8 and CCL2) characterize early implantation. These cytokines can be secreted by endometrial cells as well as by cells of the immune system that are recruited to the site of implantation. Indeed the utero-placental unit of both humans and mice is richly populated with hematopoietic cells16,17. Several lines of evidence point to a pivotal role of maternal cells in shaping the cytokine profile at the maternal-fetal interface. Gil Mor (Yale University School of Medicine) described how the uterine immune infiltrate plays a central role in the process of tissue renewal and differentiation but also participates in the development of a receptive endometrium. Studies have shown that depletion of uterine DCs (uDCs) results in a severe impairment of implantation and leads to embryo resorption18. However, this effect is not related to tolerance but rather to alterations in the process of implantation and decidualization. Immune cells may potentially regulate blastocyst binding to the epithelium through four mechanisms. These include rapid movement of stored adhesion molecules to the cell surface; inflammation-induced expression of new adhesion molecules; increased affinity of specific molecules after initial cell contact; and reorganization of adhesion molecules on the surface epithelium. Any of these possibilities or their combination could represent the response of the endometrial epithelium to immune cells recruited to the site of implantation.
Many of the studies associated with the uterine regulation of T cells have focused on characterizing the presence and the role of T regulatory (Treg) cells. The function of Treg cells, however, could not explain the control of T cell distribution in the pregnant uterus. Studies from Adrian Erlebacher and colleagues (New York University School of Medicine) in mice suggest that decreased chemoattraction of T cells to the decidua occurs to support fetomaternal tolerance. Interestingly, they noted that the regulation of chemokines at the decidua involves alterations in histone methylation patterns around chemoattractant-encoding genes such as Cxcl9 and Cxcl10. The fact that the inhibition of chemokine production in the decidua is associated with specific histone modifications suggests that epigenetic regulators contribute to controlling the capacity of the decidua to attract T cells19. These findings provide a new interpretation of the regulation of the maternal immune system by the pregnant uterus. Contrary to previous studies focused on mechanisms whereby the placenta (trophoblast cells) induces either cell death of T cells (according to the Fas-FasL hypothesis, for example) or deletion of T cells, studies from the Erlebacher laboratory suggest that the decidua has an active role in controlling the migration of maternal T cells through the implantation site.
A second area that still is poorly understood is the role of the placenta as an immune modulator. Pregnant women represent an immunologically unique population because their immune system is influenced by signals originating from the placenta. The presence of the fetus and placenta alters maternal immunity and physiology to sustain and protect the pregnancy. Studies from the Mor laboratory have shown that the placenta may function as an immune-modulatory organ that regulates the immune responses of cells present both at the implantation site and systemically. However, this modulation is not suppressive, but protective. In general, the maternal immune system is well prepared to control infections and ensure the survival of the fetus. Paradoxically, the placenta is also a target for viral infections. Recent studies from Elizabeth Bonney (University of Vermont, Burlington) and colleagues suggest that although the placenta can be infected by viruses, it has a unique capacity to prevent expansion of the virus and transmission to the fetus20. What remains unclear is the effect of viral infection on the normal homeostasis of the placenta and its interaction with the maternal immune system.
Immunity and pregnancy complications
Maternal innate immune cells are abundant in the rhesus macaque decidua and are involved in a variety of functions. These include pathogen immunosurveillance, orchestration of the local response to the presence of the conceptus, angiogenesis and vascular remodeling, decidual maturation and differentiation, and normal implantation and placental development. Ted Golos (University of Wisconsin–Madison) discussed the influence of decidual leukocytes and programming on pregnancy success in nonhuman primates. Though placental major histocompability complex (MHC) expression and morphology are highly variable among species, placental MHC expression in Old World primates closely resembles that in humans. By mechanisms that are yet unknown, anti-placental passive immunization restricts macaque placental development and alters decidual maturation. Studies have shown that there are placental MHC and maternal killer-cell immunoglobulin-like receptor (KIR) genotypes associated with adverse pregnancy outcomes in humans21. Moreover, the placenta has a tissue-specific microbiome that is distinct from that of the lower genital tract and akin to the oral microbiome22. Current gaps in our knowledge in this area include questions about whether macaque placental MHC expression functionally models human placental expression of HLA-G and/or HLA-C; how placental MHC–maternal immune system interactions are altered, activated or suppressed by reproductive-tract or systemic infection; the sequence of events preceding adverse pregnancy outcomes, including infection at the maternal-fetal interface; and the interactions (if any) that occur between the placental microbiome and the decidual immune environment. In terms of future research directions, points of discussion included that anti-placental passive immunization or experimental infection strategies might provide opportunities to develop predictive human biomarkers for placentation and implantation defects; that it will be important to determine whether the interaction between specific decidual leukocyte subsets and placental MHC in an infection setting is beneficial or deleterious to pregnancy outcome; that further work needs be done on the possibility that pathogens express reproductive tract–specific virulence factors or have reproductive tract tropism; and that CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 genomic editing offers a fresh opportunity to interrogate the decidual-placental dialog by modifying specific placental targets.
Pregnancy represents a time of extreme vulnerability for the mother and fetus during which complications often have devastating consequences for the health of both individuals. No cures exist for widely prevalent complications in human reproduction, including preeclampsia, prematurity and stillbirth. Many of these idiopathic human pregnancy complications are linked with disruptions in fetal tolerance. Pregnancy confers unique susceptibility to infection; and microbes are increasingly implicated in triggering pregnancy complications and infection-induced disruptions in fetal tolerance. Sing Sing Way (Cincinnati Children’s Hospital) discussed studies conducted in his laboratory using Listeria monocytogenes to investigate why pregnancy confers infection susceptibility across mammalian species. Evidence from mouse studies revealed an expansion of immunesuppressive maternal Treg cells that is required for maintaining fetal tolerance but also causes susceptibility to prenatal infection23. Maternal infection during pregnancy disrupts fetal tolerance, resulting in pregnancy complications such as those that occur after the depletion of maternal Treg cells. Over-riding fetal tolerance may play an important role in the immune pathogenesis of pregnancy complications triggered by both infections and noninfectious etiologies.
The mechanisms by which pregnancy confers susceptibility to infection with some microbes but not others; how prenatal infection causes pregnancy complications; whether placental-fetal microbial invasion is necessary for disrupting fetal tolerance and triggering pregnancy complications; and whether there is overlap in immune pathogenesis of pregnancy complications triggered by various microbes that cause prenatal infection and non-infectious disruptions in fetal tolerance all have yet to be elucidated. Future research directions to pursue include discrimination between the immune suppression that sustains fetal tolerance and that which predisposes the mother to infection during pregnancy; the relationship between fetal tolerance and protection against infection (and non-infection)-induced pregnancy complications; and attempts to improve rodent pregnancy models to make them more amenable to the analysis of human pregnancy complications.
Mike McCune (San Francisco General Hospital and University of California, San Francisco) discussed fetal human immune ontogeny and the implications for maintenance of the maternal-fetal interface. He described how the human fetus appears to be relatively protected from mother-to-child transmission of HIV in utero; that such protection is associated with an immune system that emanates from a fetal-specific multilineage hematopoietic stem/progenitor cell (HSPC)24; and that the T cell lineage derived from the fetal HSPC is primarily tolerogenic in nature24, and fetal classical monocytes are functionally distinct from their adult counterparts26. Areas requiring further study include the processes by which the fetal immune system confers relative resistance to mother-to-child transmission of HIV; the signals that trigger conversion of the fetal immune system to the more immunoreactive adult immune system; and the possibility that interindividual variation in immune layering might be predictive and possibly causal of susceptibility to the development of atopic disease and/or of poor responses to childhood vaccines. Future experiments might test whether it is possible to create antigen-specific tolerance to defined epitopes by immunization in utero and/or by oral feeding at birth; whether the same immune mechanisms that confer relative resistance to mother-to-child transmission of HIV in utero confer resistance to infection by some but not other infectious agents encountered during pregnancy; whether the fetal HSPC can be turned on during adulthood so that tolerance to given antigens could arise; and whether it might be possible to accelerate development of the adult immune system in the fetus, thereby preventing atopic disease and augmenting responses to childhood vaccines.
Tolerance and pregnancy establishment
A striking feature of the maternal-fetal interface is the accumulation of NK cells, which account for more than half of the maternal immune cells in the human decidua during pregnancy. Susan Fisher’s group (University of California, San Francisco) demonstrated that maternal decidual macrophages inhibit NK cell killing of cytotrophoblasts27. As background, it is commonly thought that human decidual NK cells do not react to these fetal cells with which they coexist within the uterine wall. This conclusion is based largely on the fact that decidual leukocytes, a mixture of NK cells and macrophages, are anergic in killing assays. However, the Fisher group recently showed that, upon purification, decidual NK cells can kill K562 targets. The same was true for primary cytotrophoblast targets, even when they were isolated from the same pregnancy as the NK cells. These results suggested that an inhibitory mechanism exists, and the group asked whether this involved macrophages. This theory was confirmed, as removal of macrophages enabled NK cell killing of targets, which was again inhibited when they were added back. With regard to the molecular underpinnings of this phenomenon, decidual macrophages produced significant amounts of TGF-β1, and removal of TGF-β1 increased decidual leukocyte killing of primary cytotrophoblast targets. Thus, the group proposed that this newly identified mechanism and other yet-to-be-discovered pathways normally induce maternal NK cell quiescence in the pregnant uterus; this raises the possibility that this situation could change in pathological pregnancies, a point that they are currently investigating.
Michael Soares (University of Kansas Medical Center, Kansas City) addressed the cooperative efforts of NK cells and trophoblasts in the establishment of pregnancy. Specifically, his group is investigating the role of these cells using the rat as a model system. The approaches include genetic and immunologic rat models of NK cell depletion and placental insufficiency. First, they uncovered temporal coordination, with NK cells arriving and acting first, followed by EVTs. Each cell type had similar and different roles. Both NK cells and EVTs target the extracellular matrix and smooth muscle cells of the uterine spiral arteries, altering vaso-regulation and facilitating nutrient delivery (Fig. 2b). EVTs go even further by supplanting the endothelium of uterine arteries. Subsequently, they assume a phenotype resembling that of endothelium, a process termed pseudovascularization. NK cells restrict the EVT program, thereby protecting the mother while preventing precocious and excessive invasion and restructuring of the uterine spiral arteries. NK and trophoblast cells communicate directly through cell-cell and extracellular mediators and indirectly via their actions on uterine spiral arteries and modulation of oxygen delivery. In normal pregnancy, these cell populations direct formation of the maternal-fetal interface in real time, balancing the needs of the mother and fetus. Aberrations in NK and/or EVT functions, which are the hallmark of many pregnancy-related diseases, disrupt nutrient delivery to the developing placenta, jeopardizing the health of the mother and fetus with potential long-term, negative impacts on postnatal health.
Jack Strominger and Tamara Tilburgs (Harvard University) focused on the human trophoblast nonclassical MHC molecule HLA-G, an immune molecule of paramount importance in trophoblast biology and the maintenance of pregnancy. They reported on progress in three areas. The first is the unique mechanism that leads to expression of this molecule in fetal EVTs that reside within the uterine wall. The essential participation in transcription of HLA-G of a remote enhancer is particularly notable. Many interesting mechanistic details of its transcriptional regulation remain to be revealed. Second, they developed a protocol for the preparation of EVTs, villous trophoblasts and each of the major subsets of leukocytes found in the decidua (NK cells, macrophages and T cells) from a single first-trimester human placental sample. In contrast to the results of published studies using surrogate cells, the coculture of EVTs with each of the three types of leukocytes did not induce EVT-specific cytokine response in any of them. However, incubation of EVTs with CD4+ T cells increased the frequency of Foxp3+ Treg cells. Third, they found that the role of HLA-G in pregnancy involves trogocytosis of HLA-G from EVTs by decidual NK cells followed by its endocytosis and signaling from endosomes. The consequences of the intracellular HLA-G signaling need to be further defined. Studies on primary EVTs are crucial for understanding fetal-maternal tolerance and the role of EVTs in the development of pregnancy complications such as preterm delivery and pre-eclampsia.
Conclusion and future directions
This workshop was held with the goal of understanding immune-regulatory mechanisms at the maternal-fetal interface. Workshop participants identified several aspects of these mechanisms that require a better understanding relative to a successful pregnancy, which are highlighted in Box 1. Some of the areas included interactions between specific decidual leukocyte subsets and placental MHC molecules during normal and adverse pregnancy outcomes; mechanisms that distinguish between immune suppression that sustains fetal tolerance and that which predisposes the mother to infection; the signals that trigger the conversion of the fetal immune system to the immunoreactive system of the adult; the role of dendritic cells as well as uterine macrophages and NK cells throughout pregnancy (Fig. 2c); cytokine regulation of intrauterine functions; and dysregulated function of decidual T cells in pregnancy complications. Animal and in vitro cell models also are critical for understanding the basic tenets of the reproductive biology and immunology of pregnancy establishment and maintenance, particularly since sampling during human pregnancy is difficult or impossible to conduct for research purposes.
Box 1. Areas of maternal-fetal immunity requiring further study.
Nature of key intrinsic and extrinsic factors for establishment of pregnancy
Mechanisms regulating uterine receptivity
Mechanisms regulating uterine gland differentiation and development
Genes regulating immune cells during pregnancy
Regulators of growth-factor signaling pathways during and after implantation
Roles and functions of atypical chemokine receptors governing maternal-fetal communication
Signaling pathways between the embryo and the uterus
Regulation and trafficking of uterine dendritic cells, natural killer cells, T cells and macrophages during normal and adverse pregnancy outcomes
Epigenetic control of decidual inflammation
Interaction of the microbiome and immune cells at the maternal-fetal interface
Immune cells and infection models during pregnancy
Genetic approaches (such as, CRISPR-Cas9) for placental manipulation beyond the mouse
Cross-disciplinary collaborations
Mechanistic basis for the way that pregnancy primes immunological shifts for immune components with self-specificity
Using hESCs and iPSCs to study embryo-maternal interactions during the period of development between embryo implantation and 8 weeks after fertilization
Uterine mediators for spiral artery formation and uNK cell migration and differentiation
Additional fetal-derived factors that influence maternal vascular adaptation to pregnancy
Pathways that induce maternal NK cell quiescence in the pregnant uterus
Improved animal models, including determination and exploitation of strain variation in placental biology
Factors that can be used as clinical diagnostic and/or therapeutic tools
In 1953 Medawar28 described two aspects of the evolution of viviparity, namely the endocrinological and the immunological. Immunologists have tended to consider the enigma of pregnancy through the latter lens. During this workshop it became clear that some aspects of pregnancy, including decidual function and spiral artery remodeling, are now being considered from both the endocrinological and physiological and the immunological points of view. Moreover, the immunological paradox of pregnancy has evoked interest in examining the mechanisms that contribute to pregnancy success in contrast to those that provoke rejection in the context of allogeneic transplants. This workshop demonstrated that tolerance is only one aspect of immune interactions during pregnancy and that the immune system has important roles in several critical active functions, as summarized in Figure 3. In the future, more effort should be placed on such interdisciplinary approaches to understanding the delicate balance of cellular and molecular interactions occurring at the embryonic/fetal–maternal interface. Information obtained from studies exploring the relationship of altered immune cell functions to placentation defects are likely to have an impact on addressing some of the goals of the Human Placenta Project29, which aims to revolutionize our understanding of human placental formation and function in real time. Studies during normal pregnancy and during pregnancy complications with an immune etiology are warranted and likely to be informative.
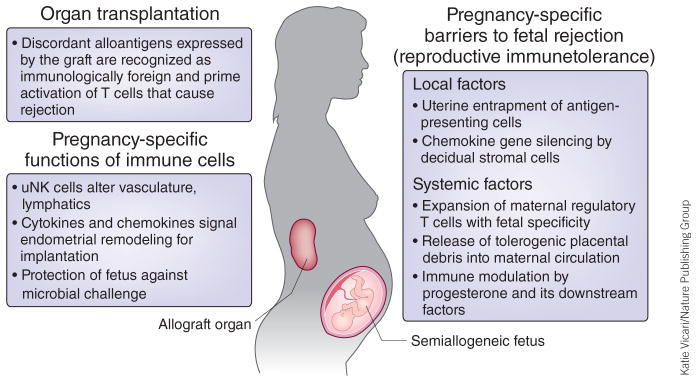
Figure 3.
Tolerance to the semiallogeneic fetus compared with organ transplantation. Fetal tissues expressing paternal antigens during pregnancy and allografts after transplantation are each recognized as immunologically foreign. However, in contrast to transplanted organ allografts, which require exogenous immune suppression to prevent rejection, an increasingly wide variety of local and systemic immunological shifts occur during pregnancy that maintain fetal tolerance and prevent ‘rejection’ of the semiallogeneic fetus.
NIAID and the Eunice Kennedy Shriver NICHD remain committed to supporting these interdisciplinary efforts, which have the potential to provide new tools and therapeutic approaches for promoting pregnancy maintenance and fetal survival and thus for promoting the health of women and infants worldwide.
Acknowledgments
The authors thank P. Lenhart and D. Chakraborty for their time and effort in providing immunohistochemical sections for this article.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Mincheva-Nilsson L, Baranov V. Am J Reprod Immunol. 2014;72:440–457. doi: 10.1111/aji.12311. [DOI] [PubMed] [Google Scholar]
- 2.Global Health Observatory. World Health Observatory (GHO data)—Maternal and reproductive health. World Health Organization; 2013. http://www.who.int/gho/maternal_health/en/ [Google Scholar]
- 3.The Partnership for Maternal, Newborn and Child Health. PMNCH Progress Report 2012. World Health Organization; 2012. http://www.who.int/pmnch/knowledge/publications/pmnch_2012_report/en/ [Google Scholar]
- 4.Born too soon: The Global Action Report on Preterm Birth. World Health Organization; Geneva: 2012. http://www.who.int/maternal_child_adolescent/documents/born_too_soon/en/ [Google Scholar]
- 5.Su RW, et al. J Clin Endocrinol Metab. 2014;100:E433–E442. doi: 10.1210/jc.2014-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT. Am J Reprod Immunol. 2014;72:262–269. doi: 10.1111/aji.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer TE. Semin Reprod Med. 2014;32:346–357. doi: 10.1055/s-0034-1376354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton GJ, Scioscia M, Rademacher TW. J Reprod Immunol. 2011;89:118–125. doi: 10.1016/j.jri.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Amita M, et al. Proc Natl Acad Sci USA. 2013;110:E1212–E1221. doi: 10.1073/pnas.1303094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daikoku T, et al. Dev Cell. 2011;21:1014–1025. doi: 10.1016/j.devcel.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirota Y, et al. J Clin Invest. 2010;120:803–815. doi: 10.1172/JCI40051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clementi C, et al. PLoS Genet. 2013;9:e1003863. doi: 10.1371/journal.pgen.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagashima T, et al. J Clin Invest. 2013;123:2539–2550. doi: 10.1172/JCI65710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Wu Y, Caron K. Biol Reprod. 2008;79:1169–1175. doi: 10.1095/biolreprod.108.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein KR, et al. Dev Cell. 2014;30:528–540. doi: 10.1016/j.devcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racicot K, Kwon J, Aldo P, Silasi M, Mor G. Am J Reprod Immunol. 2014;72:107–116. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlebacher A. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 18.Plaks V, et al. J Clin Invest. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nancy P, et al. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raj RS, Bonney E, Phillippe M. Reprod Sci. 2014;21:1434–1451. doi: 10.1177/1933719114537720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiby SE, et al. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aagaard K, et al. Sci Transl Med. 2014;6:237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin L, et al. J Immunol. 2014;192:2970–2974. doi: 10.4049/jimmunol.1302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mold JE, et al. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mold JE, et al. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krow-Lucal ER, Kim C, Burt T, McCune J. Blood. 2014;123:1897–1904. doi: 10.1182/blood-2013-11-536094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Co EC, et al. Biol Reprod. 2013;88:155. doi: 10.1095/biolreprod.112.099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medawar PB. Symp Soc Exp Biol. 1953;7:320–338. [Google Scholar]
- 29.Guttmacher AE, Maddox Y, Spong C. Placenta. 2014;35:303–304. doi: 10.1016/j.placenta.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]