Abstract
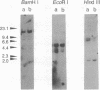
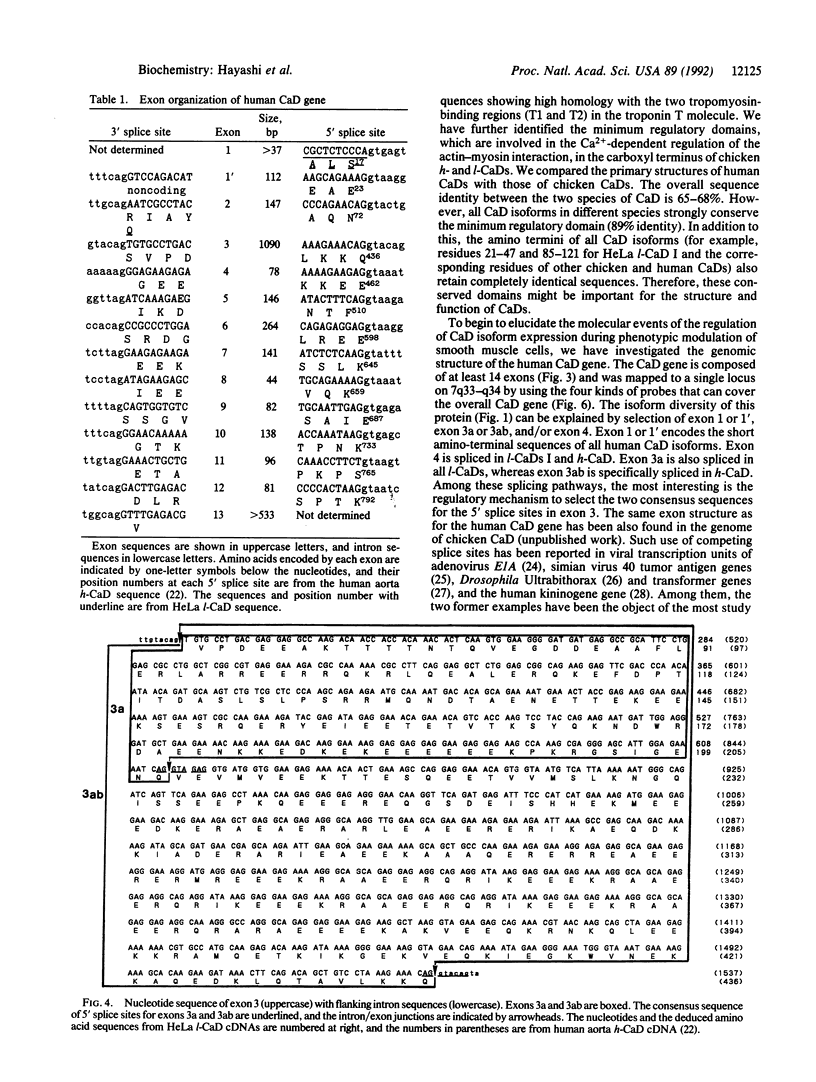
The high molecular weight caldesmon (h-CaD) is predominantly expressed in smooth muscles, whereas the low molecular weight caldesmon (l-CaD) is widely distributed in nonmuscle tissues and cells. The changes in CaD isoform expression are closely correlated with the phenotypic modulation of smooth muscle cells. During a search for isoform diversity of human CaDs, l-CaD cDNAs were cloned from HeLa S3 cells. HeLa l-CaD I is composed of 558 amino acids, whereas 26 amino acids (residues 202-227 for HeLa l-CaD I) are deleted in HeLa l-CaD II. The short amino-terminal sequence of HeLa l-CaDs is different from that of fibroblast (WI-38) l-CaD II and human aorta h-CaD. We have also identified WI-38 l-CaD I, which contains a 26-amino acid insertion relative to WI-38 l-CaD II. To reveal the molecular events of the expressional regulation of the CaD isoforms, the genomic structure of the human CaD gene was determined. The human CaD gene is composed of 14 exons and was mapped to a single locus, 7q33-q34. The 26-amino acid insertion is encoded in exon 4 and is specifically spliced in the mRNAs for both h-CaD and l-CaDs I. Exon 3 is the exon that encodes the central repeating domain specific to h-CaD (residues 208-436) together with the common domain in all CaD (residues 73-207 for h-CaD and WI-38 l-CaDs, and residues 68-201 for HeLa l-CaDs). The regulation of h- and l-CaD expression is thought to depend on selection of the two 5' splice sites within exon 3. Thus, the change in expression between l-CaD and h-CaD might be caused by this splicing pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy P. A., Helfand S. L., Hogness D. S. Segmental distribution of bithorax complex proteins during Drosophila development. Nature. 1985 Feb 14;313(6003):545–551. doi: 10.1038/313545a0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Bryan J., Lee R. Sequence of an avian non-muscle caldesmon. J Muscle Res Cell Motil. 1991 Aug;12(4):372–375. doi: 10.1007/BF01738592. [DOI] [PubMed] [Google Scholar]
- Dingus J., Hwo S., Bryan J. Identification by monoclonal antibodies and characterization of human platelet caldesmon. J Cell Biol. 1986 May;102(5):1748–1757. doi: 10.1083/jcb.102.5.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Frid M. G., Ornatsky O. I., Belkin A. M., Mukhin D. N., Orekhov A. N., Koteliansky V. E., Smirnov V. N. Modulation of human aorta smooth muscle cell phenotype: a study of muscle-specific variants of vinculin, caldesmon, and actin expression. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9542–9546. doi: 10.1073/pnas.85.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Fujio Y., Kato I., Sobue K. Structural and functional relationships between h- and l-caldesmons. J Biol Chem. 1991 Jan 5;266(1):355–361. [PubMed] [Google Scholar]
- Hayashi K., Kanda K., Kimizuka F., Kato I., Sobue K. Primary structure and functional expression of h-caldesmon complementary DNA. Biochem Biophys Res Commun. 1989 Oct 16;164(1):503–511. doi: 10.1016/0006-291x(89)91748-8. [DOI] [PubMed] [Google Scholar]
- Humphrey M. B., Herrera-Sosa H., Gonzalez G., Lee R., Bryan J. Cloning of cDNAs encoding human caldesmons. Gene. 1992 Mar 15;112(2):197–204. doi: 10.1016/0378-1119(92)90376-z. [DOI] [PubMed] [Google Scholar]
- Kalman M., Kalman E. T., Cashel M. Polymerase chain reaction (PCR) amplification with a single specific primer. Biochem Biophys Res Commun. 1990 Mar 16;167(2):504–506. doi: 10.1016/0006-291x(90)92052-2. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Kitagawa H., Fukushima D., Takagaki Y., Miyata T., Nakanishi S. Structural organization of the human kininogen gene and a model for its evolution. J Biol Chem. 1985 Jul 15;260(14):8610–8617. [PubMed] [Google Scholar]
- Krainer A. R., Conway G. C., Kozak D. Purification and characterization of pre-mRNA splicing factor SF2 from HeLa cells. Genes Dev. 1990 Jul;4(7):1158–1171. doi: 10.1101/gad.4.7.1158. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Conway G. C., Kozak D. The essential pre-mRNA splicing factor SF2 influences 5' splice site selection by activating proximal sites. Cell. 1990 Jul 13;62(1):35–42. doi: 10.1016/0092-8674(90)90237-9. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Mayeda A., Krainer A. R. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992 Jan 24;68(2):365–375. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy R. E., Lin J. L., Lin J. J. Characterization of cDNA clones encoding a human fibroblast caldesmon isoform and analysis of caldesmon expression in normal and transformed cells. J Biol Chem. 1991 Sep 5;266(25):16917–16924. [PubMed] [Google Scholar]
- Owada M. K., Hakura A., Iida K., Yahara I., Sobue K., Kakiuchi S. Occurrence of caldesmon (a calmodulin-binding protein) in cultured cells: comparison of normal and transformed cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3133–3137. doi: 10.1073/pnas.81.10.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shyamala V., Ames G. F. Genome walking by single-specific-primer polymerase chain reaction: SSP-PCR. Gene. 1989 Dec 7;84(1):1–8. doi: 10.1016/0378-1119(89)90132-7. [DOI] [PubMed] [Google Scholar]
- Sobue K., Kanda K., Tanaka T., Ueki N. Caldesmon: a common actin-linked regulatory protein in the smooth muscle and nonmuscle contractile system. J Cell Biochem. 1988 Jul;37(3):317–325. doi: 10.1002/jcb.240370306. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Sellers J. R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991 Jul 5;266(19):12115–12118. [PubMed] [Google Scholar]
- Sobue K., Tanaka T., Kanda K., Ashino N., Kakiuchi S. Purification and characterization of caldesmon77: a calmodulin-binding protein that interacts with actin filaments from bovine adrenal medulla. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5025–5029. doi: 10.1073/pnas.82.15.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi E., Hori T., O'Connell P., Leppert M., White R. R-banding and nonisotopic in situ hybridization: precise localization of the human type II collagen gene (COL2A1). Hum Genet. 1990 Nov;86(1):14–16. doi: 10.1007/BF00205165. [DOI] [PubMed] [Google Scholar]
- Takahashi E., Yamauchi M., Tsuji H., Hitomi A., Meuth M., Hori T. Chromosome mapping of the human cytidine-5'-triphosphate synthetase (CTPS) gene to band 1p34.1-p34.3 by fluorescence in situ hybridization. Hum Genet. 1991 Nov;88(1):119–121. doi: 10.1007/BF00204942. [DOI] [PubMed] [Google Scholar]
- Ueki N., Sobue K., Kanda K., Hada T., Higashino K. Expression of high and low molecular weight caldesmons during phenotypic modulation of smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9049–9053. doi: 10.1073/pnas.84.24.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. L., Wang L. W., Xu S. A., Lu R. C., Saavedra-Alanis V., Bryan J. Localization of the calmodulin- and the actin-binding sites of caldesmon. J Biol Chem. 1991 May 15;266(14):9166–9172. [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]