Abstract
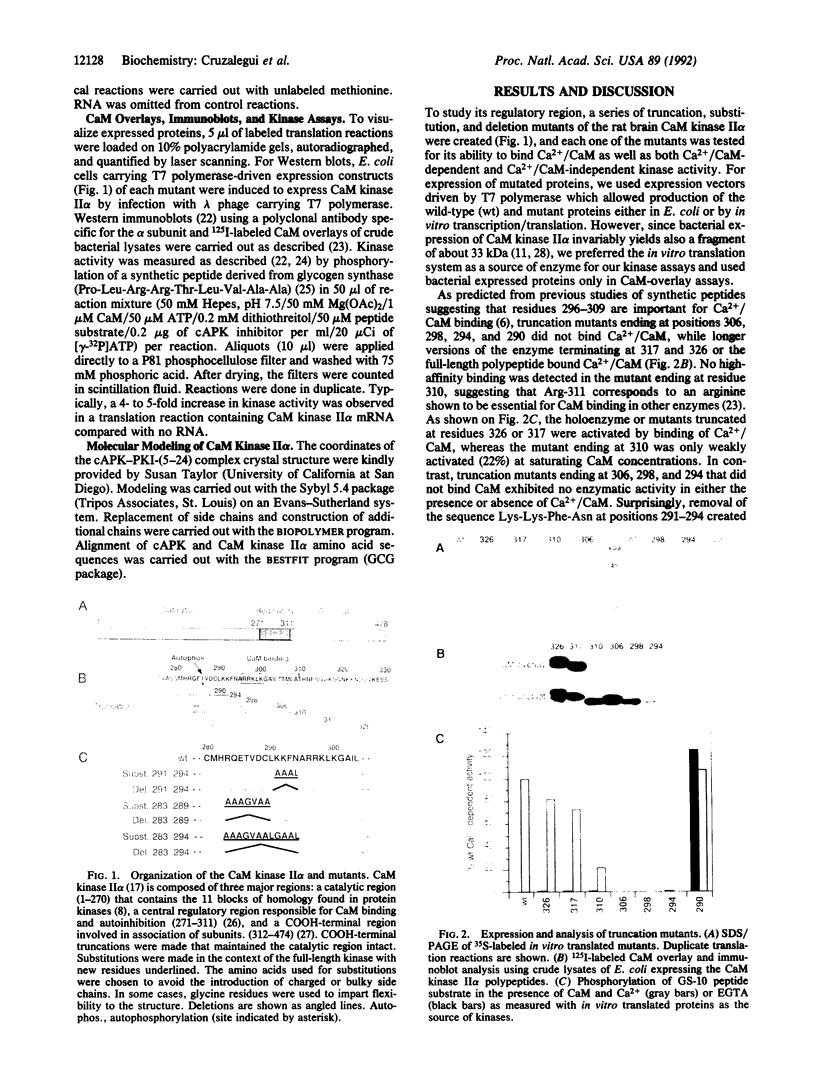
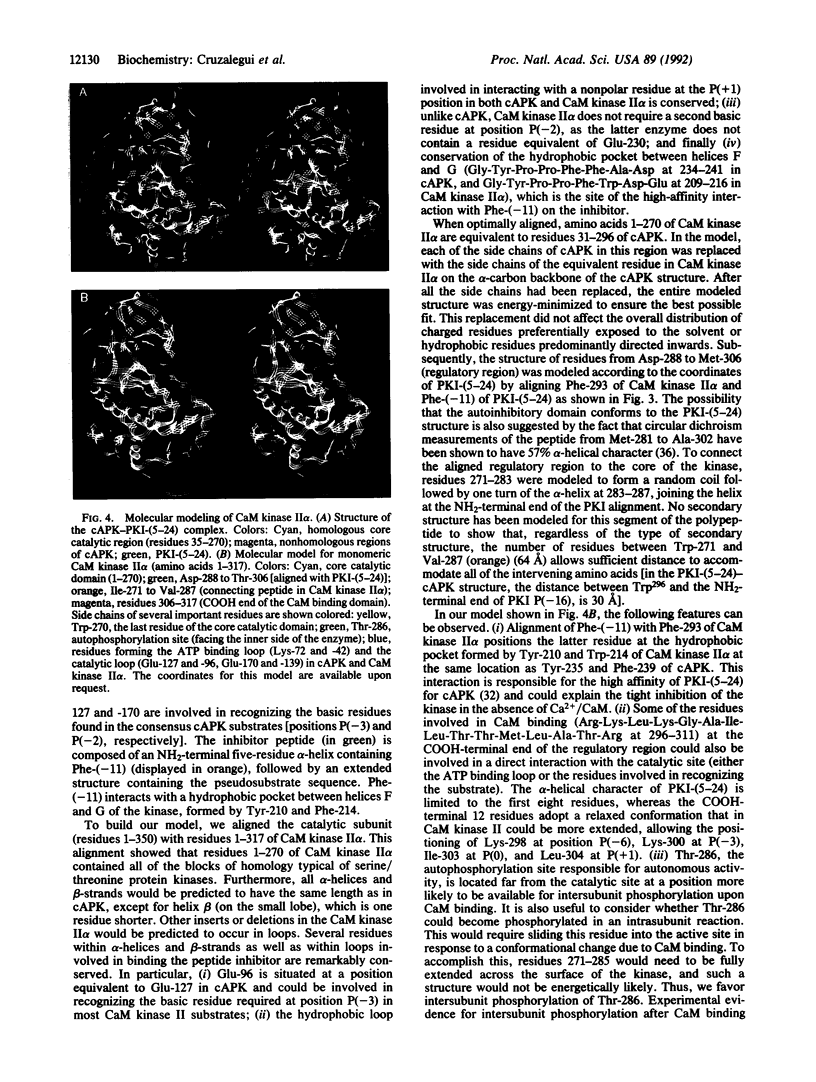
A regulatory region involved in both autoinhibition and calmodulin (CaM) binding has previously been identified in the multifunctional Ca2+/CaM-dependent protein kinase (CaM kinase II). We have tested the role of various segments of the regulatory region in autoinhibition by the analysis of a series of truncation, substitution, and deletion mutants of the CaM kinase II alpha subunit (CaM kinase II alpha). Unexpectedly, the sequence Lys-Lys-Phe-Asn at positions 291-294, adjacent to the CaM binding domain, was found to be sufficient to maintain an inhibited state in a truncated form of the kinase. However, these residues are not essential in the context of the full-length protein, indicating the importance of additional residues from the overlapping CaM binding domain. We propose here a molecular model for CaM kinase II alpha based on the three-dimensional structure of the cAPK-PKI-(5-24) (protein kinase inhibitor fragment) complex. It is predicted from this model that autoinhibition is of the pseudosubstrate variety and that autophosphorylation of Thr-286 could occur by an intersubunit reaction in the holoenzyme complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi I. C., Huang Q. H., Means A. R. Identification of amino acids essential for calmodulin binding and activation of smooth muscle myosin light chain kinase. J Biol Chem. 1992 Feb 15;267(5):3024–3029. [PubMed] [Google Scholar]
- Bagchi I. C., Kemp B. E., Means A. R. Intrasteric regulation of myosin light chain kinase: the pseudosubstrate prototope binds to the active site. Mol Endocrinol. 1992 Apr;6(4):621–626. doi: 10.1210/mend.6.4.1584224. [DOI] [PubMed] [Google Scholar]
- Brickey D. A., Colbran R. J., Fong Y. L., Soderling T. R. Expression and characterization of the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II using the baculovirus expression system. Biochem Biophys Res Commun. 1990 Dec 14;173(2):578–584. doi: 10.1016/s0006-291x(05)80074-9. [DOI] [PubMed] [Google Scholar]
- Colbran R. J., Fong Y. L., Schworer C. M., Soderling T. R. Regulatory interactions of the calmodulin-binding, inhibitory, and autophosphorylation domains of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1988 Dec 5;263(34):18145–18151. [PubMed] [Google Scholar]
- Colbran R. J., Smith M. K., Schworer C. M., Fong Y. L., Soderling T. R. Regulatory domain of calcium/calmodulin-dependent protein kinase II. Mechanism of inhibition and regulation by phosphorylation. J Biol Chem. 1989 Mar 25;264(9):4800–4804. [PubMed] [Google Scholar]
- Fong Y. L., Soderling T. R. Studies on the regulatory domain of Ca2+/calmodulin-dependent protein kinase II. Functional analyses of arginine 283 using synthetic inhibitory peptides and site-directed mutagenesis of the alpha subunit. J Biol Chem. 1990 Jul 5;265(19):11091–11097. [PubMed] [Google Scholar]
- Fong Y. L., Taylor W. L., Means A. R., Soderling T. R. Studies of the regulatory mechanism of Ca2+/calmodulin-dependent protein kinase II. Mutation of threonine 286 to alanine and aspartate. J Biol Chem. 1989 Oct 5;264(28):16759–16763. [PubMed] [Google Scholar]
- Glass D. B., Cheng H. C., Mende-Mueller L., Reed J., Walsh D. A. Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein. J Biol Chem. 1989 May 25;264(15):8802–8810. [PubMed] [Google Scholar]
- Hagiwara T., Ohsako S., Yamauchi T. Studies on the regulatory domain of Ca2+/calmodulin-dependent protein kinase II by expression of mutated cDNAs in Escherichia coli. J Biol Chem. 1991 Sep 5;266(25):16401–16408. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanley R. M., Means A. R., Ono T., Kemp B. E., Burgin K. E., Waxham N., Kelly P. T. Functional analysis of a complementary DNA for the 50-kilodalton subunit of calmodulin kinase II. Science. 1987 Jul 17;237(4812):293–297. doi: 10.1126/science.3037704. [DOI] [PubMed] [Google Scholar]
- Hanson P. I., Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J Biol Chem. 1992 Aug 25;267(24):17216–17224. [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Ikura M., Clore G. M., Gronenborn A. M., Zhu G., Klee C. B., Bax A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science. 1992 May 1;256(5057):632–638. doi: 10.1126/science.1585175. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Weinberger R. P., Waxham M. N. Active site-directed inhibition of Ca2+/calmodulin-dependent protein kinase type II by a bifunctional calmodulin-binding peptide. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4991–4995. doi: 10.1073/pnas.85.14.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Intrasteric regulation of protein kinases and phosphatases. Biochim Biophys Acta. 1991 Aug 13;1094(1):67–76. doi: 10.1016/0167-4889(91)90027-u. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Edelman A. M., Blumenthal D. K., Krebs E. G. Rabbit skeletal muscle myosin light chain kinase. The calmodulin binding domain as a potential active site-directed inhibitory domain. J Biol Chem. 1987 Sep 5;262(25):11958–11963. [PubMed] [Google Scholar]
- Knighton D. R., Pearson R. B., Sowadski J. M., Means A. R., Ten Eyck L. F., Taylor S. S., Kemp B. E. Structural basis of the intrasteric regulation of myosin light chain kinases. Science. 1992 Oct 2;258(5079):130–135. doi: 10.1126/science.1439761. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Kapiloff M. S., Durgerian S., Tatemoto K., Russo A. F., Hanson P., Schulman H., Rosenfeld M. G. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Cruzalegui F., LeMagueresse B., Needleman D. S., Slaughter G. R., Ono T. A novel Ca2+/calmodulin-dependent protein kinase and a male germ cell-specific calmodulin-binding protein are derived from the same gene. Mol Cell Biol. 1991 Aug;11(8):3960–3971. doi: 10.1128/mcb.11.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Hanson P. I., Stryer L., Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992 May 22;256(5060):1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Meyer T., Hanson P. I., Stryer L., Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992 May 22;256(5060):1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Patton B. L., Kennedy M. B. Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2(+)-independent activity. Neuron. 1988 Sep;1(7):593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988 May 25;263(15):7190–7195. [PubMed] [Google Scholar]
- Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988 May 25;263(15):7190–7195. [PubMed] [Google Scholar]
- Pearson R. B., Woodgett J. R., Cohen P., Kemp B. E. Substrate specificity of a multifunctional calmodulin-dependent protein kinase. J Biol Chem. 1985 Nov 25;260(27):14471–14476. [PubMed] [Google Scholar]
- Planas-Silva M. D., Means A. R. Expression of a constitutive form of calcium/calmodulin dependent protein kinase II leads to arrest of the cell cycle in G2. EMBO J. 1992 Feb;11(2):507–517. doi: 10.1002/j.1460-2075.1992.tb05081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Lou L. L. Multifunctional Ca2+/calmodulin-dependent protein kinase: domain structure and regulation. Trends Biochem Sci. 1989 Feb;14(2):62–66. doi: 10.1016/0968-0004(89)90045-5. [DOI] [PubMed] [Google Scholar]
- Schulman H. The multifunctional Ca2+/calmodulin-dependent protein kinase. Adv Second Messenger Phosphoprotein Res. 1988;22:39–112. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S. cAMP-dependent protein kinase. Model for an enzyme family. J Biol Chem. 1989 May 25;264(15):8443–8446. [PubMed] [Google Scholar]
- Waldmann R., Hanson P. I., Schulman H. Multifunctional Ca2+/calmodulin-dependent protein kinase made Ca2+ independent for functional studies. Biochemistry. 1990 Feb 20;29(7):1679–1684. doi: 10.1021/bi00459a002. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J., Kelly P. T. Functional analysis of Ca2+/calmodulin-dependent protein kinase II expressed in bacteria. J Biol Chem. 1989 May 5;264(13):7477–7482. [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J., Westgate S. A., Kelly P. T. Mutagenesis of Thr-286 in monomeric Ca2+/calmodulin-dependent protein kinase II eliminates Ca2+/calmodulin-independent activity. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1273–1277. doi: 10.1073/pnas.87.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y., Czernik A. J., Greengard P. Active catalytic fragment of Ca2+/calmodulin-dependent protein kinase II. Purification, characterization, and structural analysis. J Biol Chem. 1991 Aug 15;266(23):15391–15397. [PubMed] [Google Scholar]
- Yamauchi T., Ohsako S., Deguchi T. Expression and characterization of calmodulin-dependent protein kinase II from cloned cDNAs in Chinese hamster ovary cells. J Biol Chem. 1989 Nov 15;264(32):19108–19116. [PubMed] [Google Scholar]






