Abstract
α1B- adrenergic receptors contribute to vasoconstriction in humans. We tested the hypothesis that variation in the ADRA1B gene contributes to interindividual variability and ethnic differences in adrenergic vasoconstriction. We measured dorsal hand vein responses to increasing doses of phenylephrine in 64 Caucasians and 41 African-Americans and genotyped 34 ADRA1B variants. We validated findings in another model of catecholamine-induced vasoconstriction, the increase in mean arterial pressure (ΔMAP) during a cold pressor test (CPT). One ADRA1B variant, rs10070745, present in 14 African-American heterozygotes but not in Caucasians, was associated with a lower phenylephrine ED50 (geometric mean [95% CI], 144 [69–299] ng/ml) compared to 27 African-American non-carriers (208 [130–334] ng/ml; P=0.015) and contributed to the ethnic differences in ED50. The same variant was also associated with a greater ΔMAP during CPT (P=0.008). In conclusion, ADRA1B rs10070745 was significantly associated with vasoconstrictor responses after adrenergic stimulation and contributed to the ethnic difference in phenylephrine sensitivity.
Introduction
Alpha1A adrenergic receptors (α1A-ARs) are the prime mediators of vasoconstriction induced by activation of the sympathetic nervous system and thus play an important role in blood pressure regulation. Among the three α1-AR subtypes (α1A-, α1B-, and α1D-AR), the α1A-AR appears to be the principal mediator of physiological vasoconstriction,1,2 while the α1B- and α1D-AR also contribute, as demonstrated in various human and animal models.3,4
The association between genetic variants in the α1A-AR gene (ADRA1A) with outcomes such as vasoconstriction and blood pressure has been studied in a number of experimental models. In a human hand vein model, variation in ADRA1A did not explain the large interindividual variability or the ethnic differences in venoconstrictor responses to the infusion of the selective α1-AR agonist, phenylephrine.5–7 Also, ADRA1A variation was not associated with the rise in blood pressure after sympathetic stimulation in the setting of experimental stress.8–10
However, a recent study in 1614 Nigerians examining common variants in candidate genes of 28 different pathways implicated in the regulation of blood pressure found the strongest association between blood pressure and hypertension with variants in the alpha1-adrenergic pathway.11 Among the α1- AR- pathway genes, variants in the α1B-AR were most significantly associated with blood pressure and hypertension, while variants in the α1A-AR were not, suggesting that genetic variants in the α1B-AR, rather than in the α1A-AR subtype, may contribute to interindividual variability in regulation of vascular tone and blood pressure control.
α1B-ARs contribute to vasoconstriction in animal studies, possibly by mediating smooth muscle contraction directly and also by regulating the expression and function of other adrenergic receptors.3,12–14 Little is known about the effects of ADRA1B variation on vascular responses in humans. An early study found no association between phenylephrine-mediated vasoconstriction and four infrequent ADRA1B coding variants in 45 subjects with and without hypertension.15 Subsequently, the genetic architecture of ADRA1B was explored systematically in various ethnic groups, showing great interindividual and interethnic variability.16
We therefore set out to define the association between ADRA1B genotypes and vascular sensitivity to vasoconstriction induced by an α1-agonist using two experimental models: local venous responses to phenylephrine in the dorsal hand vein, and the increase in blood pressure during the cold pressor test (CPT), reflecting the systemic cardiovascular response to acute sympathetic activation. Previous studies showed ethnic differences in these responses, with African-Americans having a greater blood pressure increase after cold pressor stress and greater sensitivity to phenylephrine-induced vasoconstriction compared to Caucasians, suggesting that genetic factors may contribute to these responses.5,17,18 Thus, we tested the hypothesis that variation in ADRA1B contributes to interindividual and ethnic differences in agonist-mediated venoconstriction and in the stress-induced increase in blood pressure following a CPT.
METHODS
Subjects
We studied dorsal hand vein responses in 105 healthy normotensive Caucasians and African-Americans aged 18–45 years. Details of the study procedures and analyses of other genes were published previously.5,7 57 of the 105 subjects also participated in a second study that included a CPT. Pregnant females were excluded, and subjects took no medications for at least 2 weeks, abstained from alcohol and caffeine for at least 5 days, and received a diet containing 150 mmol/day of sodium, 70 mmol/day of potassium, and 600 mmol/day of calcium for at least 4 days prior to each study day. The Institutional Review Board of Vanderbilt University Medical Center approved the study protocols, and all subjects provided written informed consent.
Venous response to phenylephrine
Venous responses were measured in a dorsal hand vein with a linear variable differential transformer (LVDT) as previously described.5 In summary, a 24-gauge intravenous cannula was inserted into a suitable right dorsal hand vein and kept patent with saline solution infused at a flow rate of 0.4 mL/min. A LVDT (MHR 100; Shaevitz Engineering, Pennsaken, NJ) was mounted on the dorsum of the subject’s hand. A second intravenous cannula was inserted for blood sampling into the antecubital vein of the contralateral arm. After 30 minutes of saline infusion, a blood sample was taken for the determination of baseline plasma catecholamines and for genotyping. We determined the baseline vein diameter while a sphygmomanometer cuff around the upper arm was inflated to 50 mm Hg to induce venous filling. After 3 stable baseline measurements, we assessed vein constriction in response to increasing doses of phenylephrine, an α1-AR agonist. Phenylephrine (Elkins-Sinn, Cherry Hill, NJ) was infused through the cannula with a syringe infusion pump (Harvard Apparatus, Holliston, MA) at increasing dose rates (range, 12–12,000 ng/min). The infusion at each dose rate lasted 7 minutes, and the vein diameter was measured during the last two minutes of the infusion. The total flow rate through the vein was kept constant at 0.4 mL/min throughout the various phenylephrine dilutions. Heart rate and blood pressure were continuously monitored with a bedside cardiac monitor (Dinamap MPS; Johnson and Johnson Medical, Tampa, FL).
Analysis of hand vein response to phenylephrine
Venoconstriction was expressed as the percentage reduction in vein diameter from average baseline measurements, plotted against increasing doses of phenylephrine in individual semi-logarithm dose–response graphs and analyzed using a sigmoid dose–response model with variable slope (GraphPad Prism 4.03, GraphPad, La Jolla, CA). We determined the phenylephrine dose that produced 50% of maximal constriction (ED50, representing sensitivity to the drug) and also calculated the maximal venoconstriction (Emax, representing maximum response) for each subject. Analyses were performed by a single investigator unaware of the subject’s genotype.
Cold Pressor Test
Cold pressor tests were performed as previously described.18,19 All preparations for the CPT were performed only after the resting measurements had been obtained and after 30 minutes supine rest in order to minimize confounding through anticipation. With the subject in a supine position, the left foot was fully immersed up to the ankle for 2 minutes in a tub filled with a slurry of ice and water (4°C). Two readings of blood pressure and heart rate were taken with the semi-automated device (Dinamap MPS; Johnson and Johnson Medical, Tampa, FL), starting at approximately 15 and 45 seconds after foot immersion. At 1 minute, a blood sample (10 mL) was taken for determination of plasma catecholamine concentrations.
Determination of plasma catecholamine concentrations
Blood was collected into cooled heparinized tubes that were placed on ice until centrifuged at 4°C for 10 minutes at 3000 rpm. Plasma was separated and stored at −20°C in previously cooled tubes containing 40µL of reduced glutathione (6%) until assayed. Norepinephrine and epinephrine concentrations were measured by high-performance liquid chromatography using electrochemical detection with dihydroxybenzylamine as internal standard.20
Genotyping
We genotyped 34 ADRA1B SNPs listed in Supplementary Table S1. We selected 24 tagSNPs for ADRA1B (chromosome 5, position 159269 – 159343kb) from the Hapmap project using Haploview 4.2 software,21 using data based on Utah residents with Northern and Western ancestry (CEU) and on subjects of African ancestry in southwest USA (ASW). We excluded individuals from the Hapmap project with more than 5% missing genotypes and SNPs with minor allele frequencies (MAF) less than 5%. Two-marker haplotypes were used to tag SNPs in strong linkage disequilibrium (LD), defined as r2 >0.8. Furthermore, we included five previously published tag SNPs that were not captured in the Haploview tagging,16 and five additional SNPs previously associated with clinical outcomes (Supplementary Table S1). Genotyping was performed using the Sequenom platform (MassArray, San Diego, CA). For quality control, we included negative and positive controls with each genotyping run. Quality-control procedures included examination of marker and sample genotyping efficiency, allele-frequency calculations, and tests of Hardy-Weinberg equilibrium (HWE).
Statistical Analysis
The cohort was a convenience sample consisting of participants in previous studies, and the sample size was not based on a priori calculation.5,6,22 The primary outcome for the hand vein study was drug sensitivity (expressed as ED50), and drug efficacy (Emax) was the secondary outcome. ED50 values were not normally distributed and were therefore log transformed and expressed as geometric means with 95% confidence intervals (CIs). The primary outcome for the cold pressor test was the change in mean arterial blood pressure (ΔMAP).
We compared the outcomes among genotypes in a single marker analysis first in each ethnic group separately and then in the combined cohort. We then adjusted for potential confounders that were associated with the outcomes in our previous studies using the same endpoints.7 For the hand vein study, we adjusted for sex, BMI, resting norepinephrine concentrations and, for analyses of the combined cohort, ethnicity, using a multiple linear regression model. The secondary outcome, phenylephrine efficacy (Emax), was compared among genotypes using the non-parametric Kruskal-Wallis test. SNPs that were nominally significant in the hand vein study for either ED50 or Emax were then tested for association with blood pressure response during the CPT for validation. For the CPT, we adjusted for age, sex, BMI, and baseline mean arterial blood pressure, and additionally for ethnicity in analyses of the combined cohort.
For all genetic analyses, we assumed an additive genetic model, coding the genotypes according to the number of variant alleles (0–2). We used PLINK software (v. 1.07) to assess overall differences in the outcomes among the genotypes.23 Other statistical analyses were performed using SPSS software (v. 21, IBM® SPSS® Inc., Chicago, IL). All analyses were two-tailed, and a P-value < 0.05 was considered significant; permutation tests were performed to ensure that the empirical p-value of all SNPs was also significant at the 0.05 threshold.24
RESULTS
Genotyping
Minor allele frequencies for 34 ADRA1B SNPs in 105 subject were in the expected range, and all genotypes conformed to Hardy-Weinberg equilibrium (Supplemental Table S1). We did not identify any carriers of the rs10070745, rs7736470 and rs876529 variant in Caucasians.
Hand vein study
Subjects and outcomes
African-Americans (n=41) had a higher BMI (P = 0.044), diastolic blood pressure (P = 0.034) and a higher resting heart rate (P = 0.004) than Caucasians (n=64; Table 1). There was wide interindividual variability in response to phenylephrine, with the range of ED50 spanning three log units (11 to 5442 ng/min; geometric mean, 260 ng/min; 95% CI, 202 to 335 ng/min; Table 1), and the Emax ranging from 13.7% to 100% (median, 87%; IQR, 76% to 97%; Table 1). As previously reported in this cohort, African-Americans had a lower ED50 (i.e., greater sensitivity; adjusted P = 0.006) and a trend to a higher Emax compared to Caucasians (P = 0.079).7
Table 1.
Demographic and cardiovascular measurements in subjects who performed the hand vein study (n=105) and the subgroup that also participated in the cold pressor test (n=57).
| Characteristics | Hand vein study n = 105 |
Cold pressor test n = 57 |
|---|---|---|
| Age, years | 27.3± 7.2 | 26.4± 5.8 |
| Female sex, n (%) | 47 (44.8) | 23 (40) |
| Caucasians, n (%) | 64 (61) | 36 (63) |
| BMI, kg/m2* | 25.3 ± 4.5 | 24.8 ± 4.1 |
| Resting SBP, mmHg | 111.6 ± 11.5 | 115.5 ± 10.6 |
| Resting DBP, mmHg | 62.4 ± 8.3 | 64.5± 6.3 |
| Resting MAP, mmHg | 72.1 ± 6.6 | 81.5 ± 6.8 |
| Resting HR, bpm | 59.9 ± 8.3 | 64.0 ± 7.1 |
| Baseline Plasma norepinephrine, pg/ml | 167.0 ± 64.5 | 193.6 ± 111.8 |
| Baseline Plasma epinephrine, pg/ml | 19.5 ± 13.8 | 19.7± 13.3 |
| Phenylephrine ED50, ng/min, Geometric Mean (95%CI) | 260 (202 – 335) |
306 (213 – 440) |
| Phenylephrine Emax, %, Median (IQR) | 87 (76 – 97) |
82 (72 – 95) |
| ΔMAP after CPT, mmHg | 17.9± 9.1 | |
| ΔHR after CPT, bpm | 14.9± 13.4 |
BMI - body mass index; SBP – Systolic Blood Pressure; DBP – Diastolic Blood Pressure; MAP – Mean Arterial Pressure; HR – Heart rate; bpm -- beats per minute
ADRA1B variants and phenylephrine response
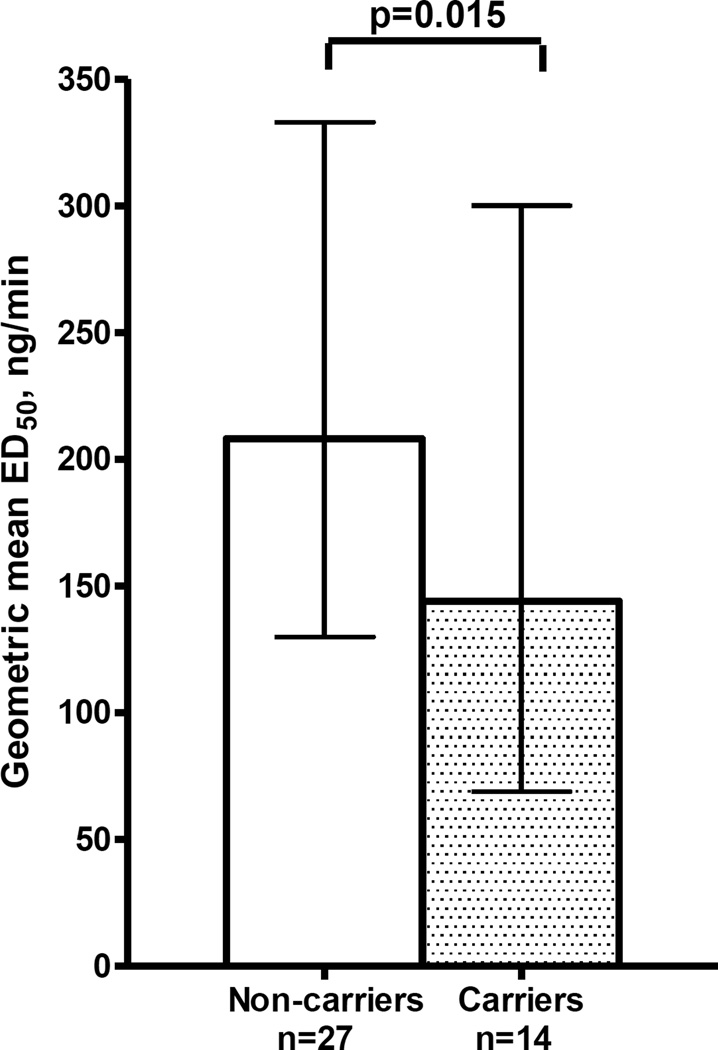
Among the 34 ADRA1B variants, one variant (rs10070745) was associated with the primary outcome, phenylephrine sensitivity (Table 2). The rs10070745 variant, present in 14 African-American heterozygotes but not in Caucasians, was associated with lower ED50 (β-coefficient=−0.47; 95% CI, −0.84 to −0.10; adjusted P = 0.015, Figure 1). This variant also contributed to the ethnic differences in ED50: the effect of ethnicity on ED50 (β = −0.29; 95% CI, −0.59 to −0.10, P = 0.006)]) was weakened and no longer statistically significant after adding the rs10070745 genotype to the adjusted model in the combined cohort (β = −0.20; 95% CI, −0.54 to 0.05, P = 0.11).
Table 2.
Genetic variants in ADRA1B associated with sensitivity to phenylephrine (ED50)
| SNP | Caucasians | African-Americans | |||||
|---|---|---|---|---|---|---|---|
| ED50, ng/min, geometric mean (95% CI) |
ED50, ng/min, geometric mean (95% CI) |
P-value* | |||||
| Number of variant alleles | Number of variant alleles | ||||||
| 0 | 1 | 2 | 0 | 1 | 2 | ||
| rs10070745 | 325 (234–452) n=64 |
n=0 | n=0 | 208 (130–334) n=27 |
144 (69–299) n=14 |
n=0 | 0.015 |
P value adjusted for sex, BMI and Baseline Norepinephrine
Figure 1.
ADRA1B rs10070745 association with sensitivity to phenylephrine (ED50) in African-Americans
The columns show geometric means, the error bars the 95% confidence intervals. Carriers of the variant allele had a significantly lower geometric mean ED50 (P=0.015).
The secondary outcome, phenylephrine Emax, was marginally associated with three variants (Table 3). The rs952037 variant was associated with higher Emax (higher efficacy) in the combined group (P = 0.041). The rs7737796 variant showed a non-significant trend to decreased Emax in both ethnic groups, which was statistically significant in the combined group (P=0.018, Table 3). The, rs17057303 showed a borderline association with lower Emax in African-Americans (P=0.044, Table 3).
Table 3.
Genetic variants of ADRA1B associated with maximum response to phenylephrine (Emax).
| SNP | Caucasians | African Americans | All | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Emax, % Median (IQR) |
P-value | Emax, %, Median (IQR) |
P-value | P-value | |||||
| Number of variant alleles | Number of variant alleles | ||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | ||||
| rs952037 | 82 (66 – 93) n= 29 |
85 (75 – 97) n=31 |
92 (72 – 100) n=4 |
0.39 | 87 (73 – 96) n=11 |
90 (82–98) n=19 |
94 (87–100) n=11 |
0.24 | 0.041 |
| rs7737796 | 92 (82–98) n=24 |
79 (64–88) n=32 |
82 (66–99) n =8 |
0.053 | 95 (86–98) n=10 |
86 (76–94) n=24 |
98 (87–100) n=7 |
0.10 | 0.018 |
| rs17057303 | 85 (75 – 96) n=63 |
61 n=1 |
n=0 | 0.20 | 92 (82 – 99) n=34 |
85 (64 – 89) n=7 |
n=0 | 0.044 | 0.11 |
Cold Pressor Test (CPT)
Subjects and outcomes
Of the 105 subjects that completed the hand vein study, 57 also participated in the CPT study. The baseline demographics of this subgroup were similar to those of the whole group (Table 1). Following CPT, there was a significant increase in systolic blood pressure (ΔSBP), diastolic blood pressure (ΔDBP) and mean arterial pressure (ΔMAP; all P < 0.001), without ethnic differences in these responses (all P >0.22).
ADRA1B variants and blood pressure response to Cold Pressor Test
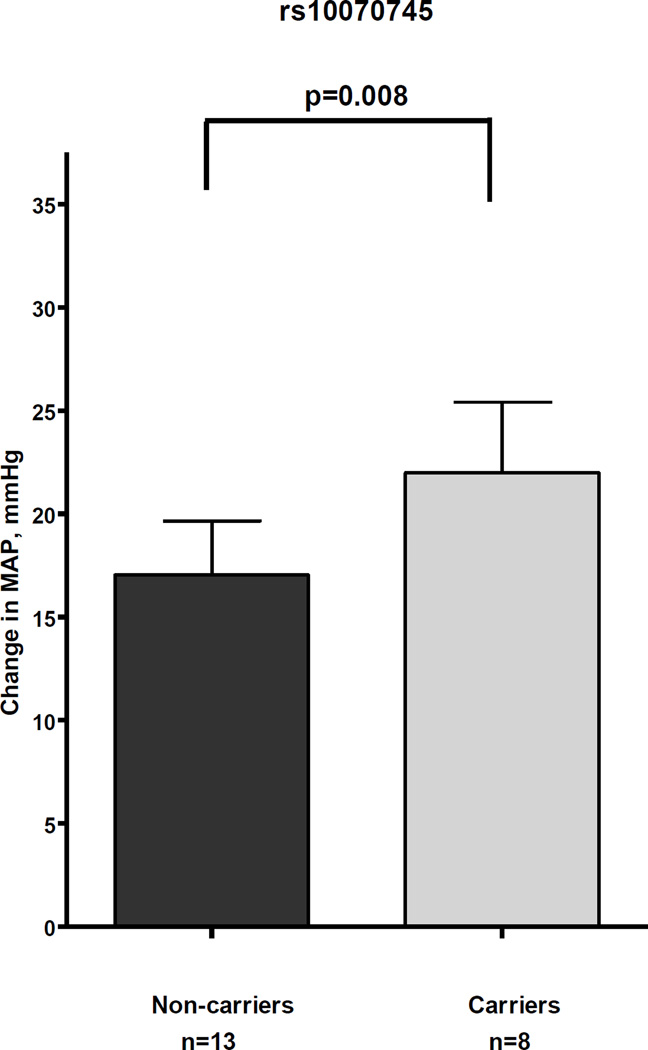
Of the four SNPs that were significantly associated with outcomes in the hand vein test, two SNPs (rs10070745 and rs17057303) were also associated with ΔMAP during the CPT (Table 4). The eight African-American subjects carrying the rs10070745 minor allele, the only variant associated with the primary outcome in the hand vein model (greater sensitivity to phenylephrine), had a 29% greater mean ΔMAP (22 mmHg; 95% CI, 14 to 30 mmHg) compared to the 13 African-American non-carriers (mean ΔMAP, 17 mmHg; 95% CI, 11 to 23 mmHg; P = 0.008, Table 4, Figure 2).
Table 4.
Mean arterial blood pressure change (ΔMAP) during the cold pressor test for four ADRA1B variants.
| SNP | Caucasians | African Americans | All | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔMAP, mmHg, Mean (95% CI) |
P- value* |
ΔMAP, mmHg, Mean (95% CI) |
P- value* |
P-value* | |||||
| Number of variant alleles | Number of variant alleles | ||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | ||||
| rs10070745 | 17 (14 – 20) n=36 |
n=0 | n=0 | NA | 17 (11 – 23) n=13 |
22 (14 –30) n=8 |
n=0 |
0.008 |
NA |
| rs17057303 | 18 (14–21) n= 35 |
8 n=1 |
n=0 | 0.38 | 20 (15 – 25) n=18 |
11 (−4 – 26) n=3 |
n=0 | 0.12 | 0.050 |
| rs952037 | 14 (10 – 18) n=20 |
22 (18 – 27) n =14 |
13 (−57 – 83) n=2 |
0.16 | 17 (12 – 22) n=5 |
19 (11 – 28) n=11 |
20 (13 – 28) n = 5 |
0.75 | 0.21 |
| rs7737796 | 15 (9 – 21) n=12 |
17 (13 – 22) n=19 |
23 (16 – 30) n=5 |
0.13 | 19 (14 – 25) n=5 |
18 (12 – 24) n=13 |
22 (−23 – 67) n=3 |
0.47 | 0.078 |
P value adjusted for age, sex, BMI, baseline mean arterial pressure and ethnicity for analysis of the combined group.
NA=not assessed.
Figure 2.
Association between rs10070745 and increase in mean arterial pressure (ΔMAP) during the cold pressor test.
The columns show the mean, the error bars the standard error of mean. Carriers of the variant alleles had significantly higher mean ΔMAP after CPT (P=0.008).
Furthermore, rs17057303, which was associated with a lower Emax in the hand vein study among African-Americans, also showed a trend to a lower ΔMAP following the CPT among African-Americans and in the combined cohort (Table 4). In the combined cohort, the four heterozygotes had a 44% lower mean ΔMAP (10 mmHg; 95% CI, 2 to 19 mmHg) compared to the 53 non-carriers (18 mmHg; 95% CI, 16 to 21 mmHg; P = 0.050).
DISCUSSION
The major new finding of the study is that genetic variation in ADRA1B affects phenylephrine-mediated venoconstriction and blood pressure changes following CPT. One variant, rs10070745, present only in African-Americans, was associated with both phenylephrine-mediated venoconstriction and blood pressure increase during the CPT.
Vascular α1B-ARs are expressed in arterial and venous beds.1,4,25 The α1B-AR appears to mediate vasoconstriction by directly activating the Gq signaling pathway, leading to increased intracellular calcium through generation of the second messengers, inositol (1,4,5)-triphosphate and diacylglycerol, and by regulating the expression and function of other adrenergic receptors, especially α1D-ARs.3,26,27 Animal studies using α1B-AR knockout mice revealed that the blood pressure response to phenylephrine was decreased by 45% compared to the wild-type, suggesting that α1B-ARs play an important role in vascular smooth muscle contraction and blood pressure changes in response to an α1-AR agonist.12
ADRA1B consists of two exons separated by a 20-kb intron and is located in a locus containing important candidate genes for blood pressure regulation.28 Genome-wide analyses have generally not found an association between ADRA1B and hypertension or resting blood pressure measurements.11,29 On the other hand, a recent candidate gene study in a Nigerian population found the strongest association between both blood pressure and hypertension with variants in ADRA1B.11 However, resting blood pressure is a phenotype affected by many factors. Therefore, to more specifically address the functional effects of ADRA1B variants, we used two models of adrenergically mediated vasoconstriction, the dorsal hand vein response to phenylephrine and the increase in blood pressure in response to sympathetic activation induced by a cold stimulus.
Vascular studies performed in the human dorsal hand vein provide several advantages. Most important, low doses of agonist that have minimal systemic effects are infused, and thus measures of vascular sensitivity can be obtained in vivo without the reflex responses that accompany systemic infusions of vasoactive drugs. Many investigators reported great inter-individual variation in phenylephrine-mediated venoconstriction; much of this variability is thought to be genetic.30,31 However, the genetic determinants of variability in α1-AR-mediated vascular responses have not been elucidated.
We previously reported increased sensitivity (lower ED50) for venous and arterial α1-AR-mediated vasoconstriction in African-Americans compared to Caucasians5,7. However, α2-AR mediated venoconstriction was similar in the two groups, suggesting that ethnic differences in α1-ARs and their proximal signal transduction pathway (e.g. coupling, phospholipase C activation, calcium release from sarcoplasmatic reticulum) could explain ethnic differences in α1-AR-mediated vasoconstriction.32 Based on this premise, we previously studied ADRA1A variants and found that they did not explain the ethnic differences in phenylephrine sensitivity, although two ADRA1A variants explained some of the inter-individual variation.7 In the present study the ADRA1B variant, rs10070745, was associated with phenylephrine sensitivity and also contributed to the difference observed among the two ethnic groups.
Dorsal hand vein responses represent a challenging phenotype that makes replication in a second cohort unfeasible. Thus, we examined associations of candidate variants identified in the hand vein model in a second vascular phenotype in which α1-AR-mediated vasoconstriction plays a role. The CPT causes substantial sympathetic activation and vasoconstriction leading to an increase in blood pressure. Concordant with increased sensitivity to phenylephrine in the dorsal hand vein, subjects with rs10070745 had a greater rise in mean arterial pressure following the CPT, suggesting greater vasoconstriction. The finding that the same variant is associated with two related vasoconstrictor phenotypes supports the validity of the findings.
In keeping with published data, the intronic rs10070745 variant was present in 17% of African Americans but not in Caucasians. We did not find a report of an association of this variant with any biological function. However, in the candidate gene study in a large Nigerian population that implicated α1B-AR pathway variants with blood pressure phenotype, ADRA1B rs10070745 was one of the variants associated with hypertension (Personal communication with the author, Nicholas Reder)). This variant is in linkage disequilibrium with several other intronic SNPs, and the mechanism of its association with enhanced vascular responses is unclear.
There are several limitations to our study. Phenylephrine is not selective for the α1B-AR subtype, but acts as an agonist also at α1A-ARs and α1D-ARs. However, there is no selective α1B-AR agonist available for use in humans, and phenylephrine is not known to have affinity for other adrenergic receptors in the human vascular beds. Our findings were derived using the dorsal hand vein model and may therefore not automatically be extrapolated to other venous or arterial vascular beds. However, we previously found similar ethnic differences in α1-AR-mediated vasoconstriction in both venous and arterial vascular beds, suggesting that responsiveness in the dorsal hand vein model may also reflect that in arterial vascular beds. It will be interesting to study the effects of the ADRA1B variants on arterial vasoconstriction. However, these studies are invasive and therefore difficult to conduct. Furthermore, although our sample size for the dorsal hand vein model study was fairly large for a translational study, some genotype groups were small, and we did not account for the multiple comparisons required by the large number of ADRA1B variants in the first study. Finally, our sample was not large enough to include homozygotes for the rs10070745 variant. Thus, our findings regarding the association of the rs10070745 variant with responsiveness to phenylephrine and cold pressor test are hypothesis-generating and need to be validated in other populations.
In conclusion, we found that the ADRA1B rs10070745 variant, present in African-Americans only, was associated with increased sensitivity to phenylephrine-mediated venoconstriction, contributed to the ethnic differences in hand vein response, and was associated with higher blood pressure responses to a CPT. Further studies exploring the association of this variant with blood pressure phenotypes and hemodynamic responsiveness in black populations, and in particular in subjects homozygous for the variant, will be of interest.
Supplementary Material
Acknowledgments
This study was supported by Vanderbilt CTSA grant 1 UL1 TR000445 from the National Center for Research Resources and P01 HL56693 from the National Institutes of Health. Dr. A. Adefurin’s research reported in this publication was supported by the National Institute of General Medical Science of the National Institutes of Health under award number T32 GM007569. Dr. Stein is the recipient of the Dan May Chair in Medicine.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, et al. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation. 1999;100(23):2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- 2.Leech CJ, Faber JE. Different alpha-adrenoceptor subtypes mediate constriction of arterioles and venules. Am J Physiol. 1996;270:H710–H722. doi: 10.1152/ajpheart.1996.270.2.H710. [DOI] [PubMed] [Google Scholar]
- 3.Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci. 2010;67:405–417. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan M, Sun J, Bird PI, Liu DL, Grigg M, Lim YL. Alpha1A- and alpha1B-adrenoceptors are the major subtypes in human saphenous vein. Life Sci. 2001;68:1191–1198. doi: 10.1016/s0024-3205(00)01027-4. [DOI] [PubMed] [Google Scholar]
- 5.Adefurin A, Ghimire LV, Kohli U, Muszkat M, Sofowora GG, Paranjape SY, et al. Blacks have a greater sensitivity to alpha1-adrenoceptor-mediated venoconstriction compared with Whites. Hypertension. 2013;61(4):915–920. doi: 10.1161/HYPERTENSIONAHA.111.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sofowora GG, Dishy V, Landau R, Xie HG, Prasad HC, Byrne DW, et al. Alpha 1A-adrenergic receptor polymorphism and vascular response. Clin Pharmacol Ther. 2004;75(6):539–545. doi: 10.1016/j.clpt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Adefurin A, Ghimire LV, Kohli U, Muszkat M, Sofowora GG, Li C, et al. Genetic variation in the alpha-adrenergic receptor and phenylephrine-mediated venoconstriction. Pharmacogenomics J. 2014 doi: 10.1038/tpj.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelsey RM, Alpert BS, Dahmer MK, Krushkal J, Quasney MW. Alpha-adrenergic receptor gene polymorphisms and cardiovascular reactivity to stress in Black adolescents and young adults. Psychophysiology. 2012;49:401–412. doi: 10.1111/j.1469-8986.2011.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Gu D, He J, Hixson JE, Rao DC, Lu F, et al. Genome-wide linkage and regional association study of blood pressure response to the cold pressor test in Han Chinese: the genetic epidemiology network of salt sensitivity study. Circ Cardiovasc Genet. 2014;7(4):521–528. doi: 10.1161/CIRCGENETICS.113.000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Kelly TN, Zhao Q, Li H, Huang J, Wang L, et al. Genome-wide association study identifies 8 novel loci associated with blood pressure responses to interventions in Han Chinese. Circ Cardiovasc Genet. 2013;6(6):598–607. doi: 10.1161/CIRCGENETICS.113.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reder NP, Tayo BO, Salako B, Ogunniyi A, Adeyemo A, Rotimi C, et al. Adrenergic alpha-1 pathway is associated with hypertension among Nigerians in a pathway-focused analysis. PLoS One. 2012;7(5):e37145. doi: 10.1371/journal.pone.0037145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, et al. Decreased blood pressure response in mice deficient of the alpha1b-adrenergic receptor. Proc Natl Acad Sci U S A. 1997;94(21):11589–11594. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanoue A, Koshimizu TA, Tsujimoto G. Transgenic studies of alpha(1)-adrenergic receptor subtype function. Life Sci. 2002;71:2207–2215. doi: 10.1016/s0024-3205(02)02012-x. [DOI] [PubMed] [Google Scholar]
- 14.Smith KM, Macmillan JB, McGrath JC. Investigation of alpha1-adrenoceptor subtypes mediating vasoconstriction in rabbit cutaneous resistance arteries. Br J Pharmacol. 1997;122:825–832. doi: 10.1038/sj.bjp.0701451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buscher R, Herrmann V, Ring KM, Kailasam MT, O'Connor DT, Parmer RJ, et al. Variability in phenylephrine response and essential hypertension: a search for human alpha(1B)-adrenergic receptor polymorphisms. J Pharmacol Exp Ther. 1999;291(2):793–798. [PubMed] [Google Scholar]
- 16.Buzas B, Belfer I, Hipp H, Lorincz I, Evans C, Phillips G, et al. Haplotype block and superblock structures of the alpha1-adrenergic receptor genes reveal echoes from the chromosomal past. Mol Genet Genomics. 2004;272(5):519–529. doi: 10.1007/s00438-004-1074-9. [DOI] [PubMed] [Google Scholar]
- 17.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36:945–951. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 18.Kurnik D, Friedman EA, Muszkat M, Sofowora GG, Xie HG, Dupont WD, et al. Genetic variants in the alpha2C-adrenoceptor and G-protein contribute to ethnic differences in cardiovascular stress responses. Pharmacogenet Genomics. 2008;18(9):743–750. doi: 10.1097/FPC.0b013e3282fee5a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohli U, Muszkat M, Sofowora GG, Harris PA, Friedman EA, Dupont WD, et al. Effects of variation in the human alpha2A- and alpha2C-adrenoceptor genes on cognitive tasks and pain perception. Eur J Pain. 2010;14(2):154–159. doi: 10.1016/j.ejpain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He HB, Deegan RJ, Wood M, Wood AJ. Optimization of high-performance liquid chromatographic assay for catecholamines. Determination of optimal mobile phase composition and elimination of species-dependent differences in extraction recovery of 3,4-dihydroxybenzylamine. J Chromatogr. 1992;574:213–218. [PubMed] [Google Scholar]
- 21.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 22.Muszkat M, Kurnik D, Sofowora GG, Wood AJ, Stein CM. Independent regulation of alpha1 and alpha2 adrenergic receptor-mediated vasoconstriction in vivo. J Hypertens. 2011;29:251–256. doi: 10.1097/HJH.0b013e3283407ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purcell SNB, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sham PC, Purcell SM. Statistical power and significance testing in large-scale genetic studies. Nat Rev Genet. 2014;15:335–346. doi: 10.1038/nrg3706. [DOI] [PubMed] [Google Scholar]
- 25.Giessler C, Wangemann T, Silber RE, Dhein S, Brodde OE. Noradrenaline-induced contraction of human saphenous vein and human internal mammary artery: involvement of different alpha-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:104–109. doi: 10.1007/s00210-002-0582-6. [DOI] [PubMed] [Google Scholar]
- 26.Theroux TL, Esbenshade TA, Peavy RD, Minneman KP. Coupling efficiencies of human alpha 1-adrenergic receptor subtypes: titration of receptor density and responsiveness with inducible and repressible expression vectors. Mol Pharmacol. 1996;50:1376–1387. [PubMed] [Google Scholar]
- 27.Hosoda C, Koshimizu TA, Tanoue A, Nasa Y, Oikawa R, Tomabechi T, et al. Two alpha1-adrenergic receptor subtypes regulating the vasopressor response have differential roles in blood pressure regulation. Mol Pharmacol. 2005;67(3):912–922. doi: 10.1124/mol.104.007500. [DOI] [PubMed] [Google Scholar]
- 28.Flordellis C, Paris H, Karabinis A, Lymperopoulos A. Pharmacogenomics of adrenoceptors. Pharmacogenomics. 2004;5:803–817. doi: 10.1517/14622416.5.7.803. [DOI] [PubMed] [Google Scholar]
- 29.Krushkal J, Xiong M, Ferrell R, Sing CF, Turner ST, Boerwinkle E. Linkage and association of adrenergic and dopamine receptor genes in the distal portion of the long arm of chromosome 5 with systolic blood pressure variation. Hum Mol Genet. 1998;7:1379–1383. doi: 10.1093/hmg/7.9.1379. [DOI] [PubMed] [Google Scholar]
- 30.Gupta A, Carruthers SG. Familial studies of heritability of alpha1-adrenergic receptor responsiveness in superficial veins. Clin Pharmacol Ther. 1997;62:322–326. doi: 10.1016/S0009-9236(97)90035-7. [DOI] [PubMed] [Google Scholar]
- 31.Luthra A, Borkowski KR, Carruthers SG. Genetic aspects of variability in superficial vein responsiveness to norepinephrine. Clin Pharmacol Ther. 1991;49:355–361. doi: 10.1038/clpt.1991.41. [DOI] [PubMed] [Google Scholar]
- 32.Muszkat M, Sofowora GG, Wood AJ, Stein CM. Alpha2-adrenergic receptor-induced vascular constriction in blacks and whites. Hypertension. 2004;43:31–35. doi: 10.1161/01.HYP.0000103694.30164.C7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.