Abstract
Background
Extreme shyness in childhood arising from behavioral inhibition (BI) is among the strongest risk factors for developing social anxiety. Although no imaging studies of intrinsic brain networks in BI children have been reported, adults with a history of BI exhibit altered functioning of frontolimbic circuits and enhanced processing of salient, personally-relevant information. BI in childhood may be marked by increased coupling of salience (insula) and default (ventromedial prefrontal cortex) network hubs.
Methods
We tested this potential relation in 42 children ages 9 to 12, oversampled for high-BI. Participants provided resting-state functional magnetic resonance imaging. A novel topographical pattern analysis of salience network intrinsic functional connectivity was conducted, and the impact of salience-default coupling on the relation between BI and social anxiety symptoms was assessed via moderation analysis.
Results
High-BI children exhibit altered salience network topography, marked by reduced insula connectivity to dorsal anterior cingulate and increased insula connectivity to ventromedial prefrontal cortex. Whole-brain analyses revealed increased connectivity of salience, executive, and sensory networks with default network hubs in children higher in BI. Finally, the relation between insula-ventromedial prefrontal connectivity and social anxiety symptoms was strongest among the highest BI children.
Conclusions
BI is associated with an increase in connectivity to default network hubs that may bias processing toward personally-relevant information during development. These altered patterns of connectivity point to potential biomarkers of the neural profile of risk for anxiety in childhood.
Keywords: Temperament, Social anxiety, Development, Risk, fMRI
Introduction
Social anxiety disorders are rooted in early patterns of socioemotional development. Part of the difficulty in addressing social anxiety-linked maladaptation comes from the fact that the most devastating outcomes often manifest in adolescence or emerging adulthood, quite late in the course of developmental processes related to disorder. This may contribute to the lack of progress in identifying pathophysiological mechanisms or predictors of anxiety risk trajectories 1–3. Studying children with behaviorally inhibited (BI) temperament, or extreme shyness, provides a unique opportunity to uncover neural biomarkers of developmental risk for later anxiety 4,5, which may not fully overlap with markers of concurrent clinical 6,7 or trait 8,9 anxiety. Children with the BI risk phenotype are fearful in response to novelty and withdrawn in social situations and BI children exhibit heightened rates of social anxiety 10,11. BI is also one of the strongest childhood predictors of later social anxiety disorder, with approximately half of BI children going on to develop social anxiety 12–16. The BI-anxiety link is so robust it has been suggested that childhood BI may be a prodromal period in the emergency of social anxiety disorder, or that later diagnoses may simply reflect pre-existing disorders 17. To probe the patterns of brain organization associated with risk for anxiety due to BI, the present study examines intrinsic functional connectivity (iFC) in children varying in level of BI.
Prior research on young adults characterized as BI in childhood or adulthood has shown that BI is related to biases in the processing of salient information and systematic differences in underlying neural networks 18. Specifically, children high in BI display biased attention to threat 19 and psychophysiological and neuroendocrine indicators of limbic hyperactivation 20–22. Young adults characterized as BI exhibit altered frontolimbic functional connectivity 23–25 and heightened amygdala reactivity in response to novel or threatening stimuli 21,26,27. Furthermore, BI individuals with the most biased frontolimbic function may be at the highest risk for anxiety 22,24,28. In this way, neural processing biases may be present in BI children on a developmental trajectory toward anxiety 19,29.
BI-to-anxiety links are not isolated to the amygdala circuitry 30,31. BI is also related to heightened striatal processing of rewards 30,32–34, which points toward a more general enhancement in processing personally-salient cues 25. Indeed, the striatal response is particularly elevated in BI individuals when presented with self-relevant stimuli, such as feedback from peers they are interested in interacting with 33,35.
Taken together, a theme emerges that BI is associated with differences in functioning within core neural systems involved in processing personally salient information, which may reflect an early emerging alteration in the organization of intrinsic brain networks that coordinate such processing, such as the salience (SN) and default mode (DMN) networks 36–41. The SN (insula; dorsal anterior cingulate cortex, dACC) is involved in filtering salient information in support of goal-directed behavior 42, while the DMN (posterior cingulate cortex, PCC; ventromedial prefrontal cortex, vmPFC) is purported to support self-referential, internally-focused processing 36,43–45. Thus, a shift in the balance of insula connectivity—decreasing to SN and increasing to DMN regions—would suggest that BI is marked by an “internal shift,” consistent with studies showing BI individuals exhibit biases in information processing toward more personally-relevant information (e.g., threat, reward, and social feedback).
The current study is the first to examine iFC in children at risk for social anxiety in order to capture patterns of neural connectivity that may underlie the emergence and progression of socioemotional profiles. As part of a larger study, children were assessed for BI in middle childhood via parental report. While BI is often assessed in toddlerhood22, the stability of the trait lends itself to characterization later in life46,47. In addition, maternal report of BI is predictive of anxiety level over time10.
First, we examined BI-linked patterns of iFC with a novel topographical pattern analysis to probe for the hypothesized “internal shift.” Second, we conducted traditional whole-brain seed-based analyses in a priori (amygdala subregions, insula, and posterior cingulate), as well as data-derived networks, to determine whether our findings were stable across analytic approaches as well as to reveal broader connectivity patterns related to BI. Three predictions were made: (i) For SN topography, BI would relate to a shift in the topography of insula connectivity from dorsal to ventral medial PFC. (ii) For BI individuals, traditional whole-brain analyses would reveal that regions involved in processing salient information (amygdala, insula) would show increased iFC with the DMN (PCC and vmPFC), and reduced iFC with typical SN regions. (iii) Finally, high BI children showing the greatest shift in salience network iFC would be at greatest risk for anxiety (exhibit greatest symptoms), consistent with the notion that shifts in the organization of intrinsic brain networks are evident in children on a developmental trajectory toward social anxiety problems.
Materials and methods
Study procedures
Data are from the baseline session of a larger ongoing study. While undergoing functional magnetic resonance imaging (fMRI), participants were instructed to lay quietly as a neutral slideshow (nature photographs) was presented for passive viewing. The Pennsylvania State University Institutional Review Board approved study procedures. Procedures were explained to participants and a caregiver, who then provided written assent and consent prior to participation.
Participants
Participants included in the analyses were 42 children ages 9-12, oversampled for BI based on parental report on the Behavioral Inhibition Questionnaire (BIQ). The BIQ is useful for characterizing BI in later childhood, allowing researchers to study BI children outside the context of large, multi-method assessment studies that recruit children in the first few years of life 46,47. Children were excluded from participation if they had a severe psychiatric diagnosis (other than anxiety/depression; e.g., bipolar disorder), IQ below 70, or a current severe medical illness. Of the children who were screened for participation, 34 BI (BIQ Total score > 120 or BIQ Social novelty score > 60) and 45 non-inhibited (BN) children provided MRI data, with 17 BI and 25 BN children providing usable data included for analysis (details below; see Table 1 for participant characteristics; see Supplemental Materials for participant recruitment pipeline). This rate of usable data is typical of developmental psychiatric neuroimaging studies in this age range 48. The BI and BN participants did not significantly differ in movement in the scanner (Table 1). Categorical recruitment based on BIQ score allowed for oversampling of BI children to ensure needed representation at the high end of the BI spectrum. To understand the full BI-iFC relation, analyses were also conducted using continuous BI scores. Social anxiety symptoms were assessed by parent report on the computerized Diagnostic Interview Schedule for Children version 4 (C-DISC 4) 49 administered by a trained research assistant. Three participants (two BI and one BN) met criteria for any anxiety disorder; removing these individuals from the analyses reported below did not substantially alter the findings (see Supplemental Materials for details).
Table 1.
Participant characteristics for full sample (All) and participants recruited from high and low BI populations. Means (sd) for age, Behavioral Inhibition Questionnaire total scores (BIQ), and social phobia symptoms assessed by the DISC clinical interview. Block design, Vocabulary, and Full Scale IQ Estimate (FSIQ-E) 80 are from the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) 81. Mean framewise displacement in functional MRI scan (FD) and mean number of functional MRI volumes censored from analyses due to movement (FD>0.5mm) did not significantly differ between groups (p's > 0.3).
| Group | N | Sex | Age | BIQ* | Social Phobia* | Block Design | Vocab. | FSIQ-E | FD | Censored volumes |
|---|---|---|---|---|---|---|---|---|---|---|
| High BI | 17 | 6M/11F | 10.1 (0.8) | 125.5 (16.1) | 3.1 (0.5) | 10.2 (2.9) | 11.8 (2.9) | 105.9 (14.9) | 0.24 (0.13) | 24 (17) |
| Low BI | 25 | 12M/13F | 10.2 (0.9) | 75.6 (21.6) | 0.6 (0.5) | 11.2 (3.5) | 12.9 (2.8) | 111.4 (15.1) | 0.21 (0.12) | 19 (22) |
| All | 42 | 18M/24F | 10.1 (0.8) | 95.8 (32) | 1.6 (2.7) | 10.8 (3.3) | 12.5 (2.9) | 109.2 (15.1) | 0.22 (0.13) | 21 (20) |
p < 0.05 in BI versus BN group comparison.
Functional MRI Processing
Resting state fMRI (6 minutes; 180 volumes) and high resolution T1 scans were acquired on a Siemens Magnetom Trio 3T scanner, followed by standard preprocessing in SPM8 and connectivity analyses in the CONN toolbox (see Supplemental Materials) 50. Participants were excluded if they exceeded absolute thresholds for translation (3mm) or framewise displacement (FD; more than 1/3 of the time series exceeding 0.5mm FD) 9,51. Within the BI and BN groups, the included versus excluded participants did not significantly differ (p's > 0.20) in age, sex, BIQ scores (Total, Social novelty, or Situational novelty scales), social phobia symptoms, or IQ (block design, vocabulary, or full scale).
SN iFC topography
To assess how the topography of SN connectivity relates to BI, we extracted iFC between the right insula and ROIs spanning the anterior cingulate cortex (ACC) from dorsal to ventral. This was done separately for inferior and superior aspects of the ACC, and left and right hemispheres (Figure S1) 52. For BI versus BN children, the topographical gradient of insula-ACC iFC was examined by plotting the LOESS smoothed curve 53 of insula iFC from dorsal to ventral ACC ROIs for each group. To assess whether these patterns of iFC across ACC were specific to the insula, analyses were performed again using a control seed in the right frontal eye field region of the precentral sulcus (x=25, y=-13, z=50) instead of the insula 44. This region was selected since, like the anterior insula, it resides in the frontal cortex and is considered a core node in the task positive network 44.
A priori seeds
For whole-brain, seed-based analyses, a priori seeds (Figure S1) were placed in bilateral amygdala subregions (basolateral, centromedial, and superficial amygdala), insula, and posterior cingulate. Rather than examining connectivity of amygdala as a whole, subregions were examined separately as they have known anatomical and functional variation relevant to anxiety 23,54–56.
Global iFC seeds
We also used data-driven analyses to derive seed regions from anywhere in the brain where iFC related to continuous BI score. The a priori seeds limit analysis to regions where one expects that iFC will relate to BI; thus, a data-driven analysis is important to assess whether findings from a priori analyses are representative of underlying patterns in the data or are specific to the confirmatory a priori approach.
To derive data-driven seeds, first, global iFC (or “intrinsic connectivity contrast”) maps were computed for each participant. These maps contain, for each voxel, a measure of the mean absolute value of the strength of iFC between the voxel and all other voxels in the brain 57, providing an index of global iFC strength for the voxel (similar to the approach taken with the AFNI function 3dTcorrMap) 58. Second, data-derived seed regions were generated from a whole-brain analysis searching for clusters where global iFC significantly relates to BI controlling for age and mean FD.
iFC related to BI controlling for age and movement
For a priori and global iFC seeds, seed-to-voxel functional connectivity analyses were performed in the CONN toolbox yielding whole-brain maps of Fisher-Z transformed coefficients of correlation between the seed and voxel time courses for each participant. To reveal regions where connectivity with the seed region is related to BI, these maps were entered into whole-brain regression with continuous BI score as a regressor, controlling for age and mean FD (to ensure findings are not accounted for by between-subject variation in micromovement) 23. For comparison, additional analyses are reported in the Supplemental Materials showing patterns of iFC related to social anxiety symptoms.
Moderation analyses
To assess the hypothesis that higher BI individuals would show a stronger relation between anxiety symptoms and altered insula-ACC connectivity, we conducted moderation analyses in the PROCESS SPSS macro for extracted clusters in dACC and vmPFC, as these are regions where insula iFC showed the strongest differences as a function of BI 59. Social Phobia symptoms were used as the dependent variable, with iFC as the independent variable and continuous BI scores as the moderator, controlling for age (predictors mean centered).
Results
Insula-ACC iFC topography
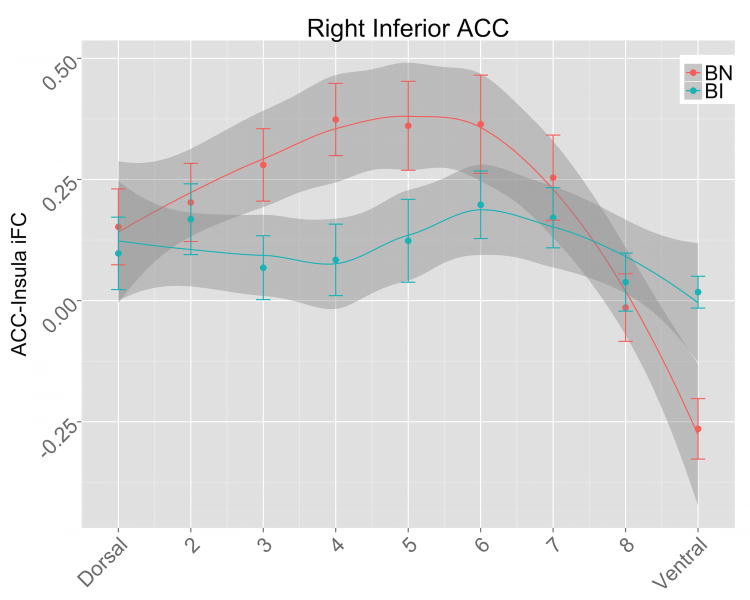
The LOESS curves revealed differences in insula-ACC iFC topography for BI versus BN participants, with BN children showing a curvilinear pattern of iFC, paralleling expected patterns of SN connectivity (positive to dACC and negative to vmPFC). This pattern is significantly diminished and flattened in BI children, particularly for right inferior ACC ROIs (Figure 1; Figure S2). Insula-ACC iFC topography was highly distinct from FEF-ACC iFC topography (Figure S3), for which LOESS modeling revealed a linear pattern among BN participants, and no significant differences between BI and BN groups.
Figure 1.
Insula-ACC iFC patterns. In BN children (blue), there is a curvilinear pattern of right inferior ACC connectivity to the insula—iFC increases to a peak in the mid-dorsal ACC, and then drops for ventral ACC (which extends to sub-genual ACC/vmPFC). The topographical pattern of iFC in inferior ACC is disrupted in BI children (red), who for both right and left inferior ACC show a more linear dorsal-ventral topography, lacking the mid-dorsal peak and negative vmPFC iFC that is prominent in the BN group (note non-overlapping 95% confidence intervals shaded in gray in dACC and vmPFC). Lines: smoothed (LOESS) curve of insula iFC for dorsal to ventral ROIs, for BI and BN groups, with 95% confidence intervals in gray shading. The curves were generated in the R Statistics software using the ggplot package with the stats_smooth function. LOESS modeling has the advantage of not assuming a specific function (e.g., linear, quadratic) in the topographic pattern of insula-anterior cingulate connectivity, which is unknown and may vary between groups. The resulting LOESS curve and 95% confidence intervals for each group reveal regions where the groups' pattern of iFC differs. Points: group mean insula iFC for each ACC ROI, with error bars for 1 standard error of the mean.
A priori networks
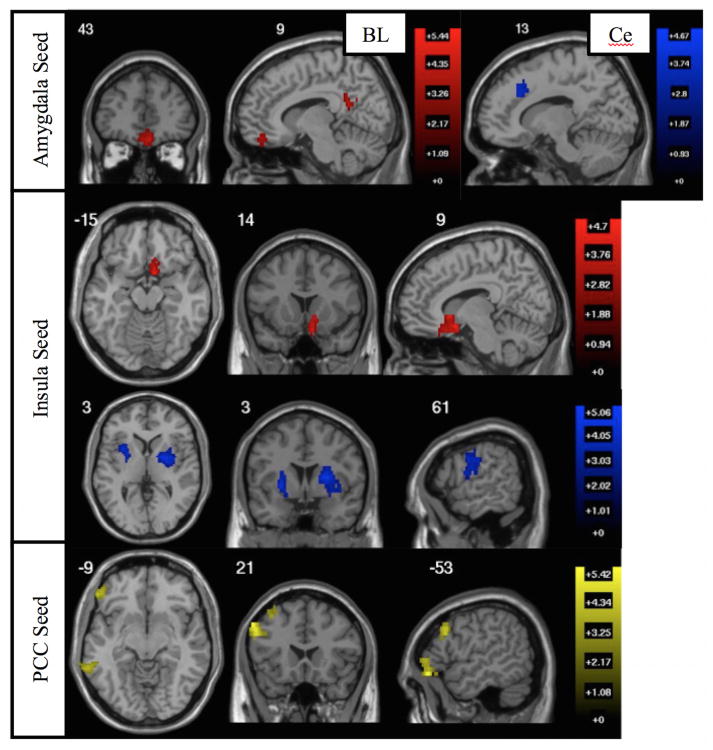
In whole-brain analyses of BI-associated connectivity, BI levels were linked to amygdala, insula, and PCC connectivity with vmPFC, anterior cingulate, lateral PFC, motor cortex, and striatum (see Table 2; Figure 2).
Table 2.
Seed-based iFC networks. Regions where connectivity with seed regions (bold) relates to BI (a priori seeds italicized, global iFC seeds non-italicized). Positive (+) or negative (-) cluster labels indicate direction of relation between BI and iFC.
| Seed | Cluster label | Voxels | X | Y | Z | |
|---|---|---|---|---|---|---|
| Amygdala BL L | ||||||
| + | PCC | 108 | 11 | -52 | 20 | |
| + | vmPFC | 99 | 4 | 44 | -20 | |
| Amygdala Ce L | ||||||
| - | dACC | 115 | 14 | 28 | 30 | |
| Insula R | ||||||
| + | vmPFC | 105 | 11 | 19 | -5 | |
| - | putamen R | 351 | 25 | -7 | 0 | |
| - | motor R | 162 | 64 | -11 | 30 | |
| - | putamen L | 138 | -25 | -2 | -5 | |
| PCC | ||||||
| + | dlPFC L | 140 | -60 | 21 | 40 | |
| + | SFG L | 125 | -14 | 30 | 60 | |
| + | MTG L | 120 | -69 | -41 | -20 | |
| + | vlPFC L | 101 | -51 | 39 | -15 | |
| + | MFG L | 98 | -34 | 14 | 45 | |
| SMS R | ||||||
| + | vmPFC | 206 | 7 | 32 | -30 | |
| - | insula/dACC R | 466 | 32 | 14 | 10 | |
| - | insula R | 233 | 43 | -2 | 20 | |
| - | inf. occ-temp L | 190 | -7 | -73 | -5 | |
| - | lateral occipital L | 169 | -51 | -85 | 5 | |
| - | IPL L | 103 | -57 | -30 | 50 | |
| - | dlPFC L | 101 | -41 | 46 | 15 | |
| Frontal Pole | ||||||
| - | dlPFC/insula L | 160 | -37 | 44 | 15 | |
| - | dlPFC R | 144 | 34 | 53 | 15 | |
| - | cerebellum L | 122 | -32 | -55 | -30 | |
| - | IFG/opercula R | 116 | 30 | 7 | 25 | |
| - | supramarginal L | 109 | -73 | -41 | 30 | |
| - | supramarginal R | 109 | 48 | -55 | 60 | |
| - | dACC L | 90 | -14 | 9 | 35 | |
| Occipital/Cereb. | ||||||
| + | temporal pole | 445 | -48 | 14 | -20 | |
| + | occipital pole | 146 | -7 | -89 | -20 | |
| + | frontal pole | 116 | -18 | 73 | 15 |
Figure 2.
A priori network iFC related to BI. Whole-brain seed-to-voxel analyses examining where connectivity with the seed region related to BI score, controlling for age and movement. All results corrected for multiple comparisons at clusterwise FDR p<0.05 (voxelwise p<0.005). Top: Regions where BI related to greater iFC with left basolateral amygdala (red) and reduced iFC with left centromedial amygdala (blue). No other amygdala subregions exhibited connectivity related to BI. Middle: Regions where BI related to greater insula iFC (red; single vmPFC cluster overlapping medial orbitofrontal cortex, ventral ACC, and nucleus accumbens) and reduced insula iFC (blue; right motor, bilateral putamen). Bottom: Regions where BI related to greater iFC with PCC.
Global iFC networks
In the whole-brain analysis to reveal seed regions where global iFC related to BI, BI level was linked to increased global iFC in frontal pole and a left occipital/cerebellar cluster, and diminished global iFC in right somatosensory cortex (SMS; Figure S4). In the whole-brain analysis of BI-associated connectivity with these global iFC seed regions, BI level was linked to increased vmPFC connectivity (with SMS seed) and reduced insula and dACC connectivity (with SMS and frontal pole seeds; Table 2 and Figure S5).
BI moderation of iFC-anxiety association
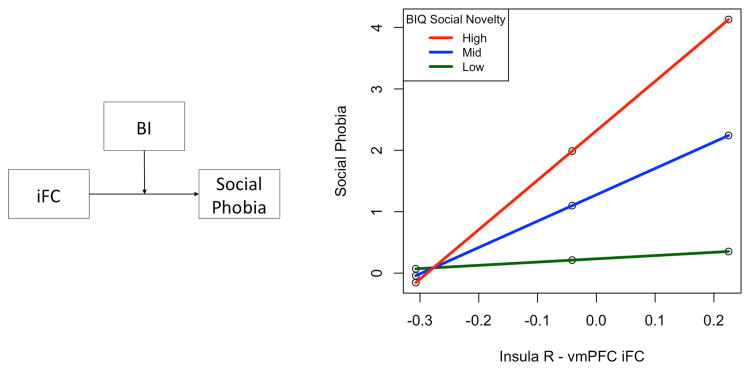
BI score moderated the relation between insula-vmPFC connectivity and anxiety symptoms, with higher BI associated with a stronger positive relation between insula-vmPFC connectivity and social phobia (F(1,37)=7.85, p=0.008, R2 change=0.12; Figure 3). There was no relation between insula-dACC iFC and anxiety symptoms, and no moderation by BI (p's > 0.30).
Figure 3.
BI moderation of iFC-anxiety relation. (a) Moderation model. (b) For children with higher social novelty BIQ scores, greater anxiety symptoms were related to increased iFC from insula to vmPFC.
Discussion
In this first examination of intrinsic brain organization patterns related to BI in childhood, we find across analytic approaches that risk for social anxiety is related to perturbations in intrinsic functional connectivity with limbic and prefrontal regions. Specifically, (1) the topographic pattern of salience network connectivity varied as a function of BI, (2) BI was related to increased connectivity between putative “salience processing” regions (amygdala and insula) and putative internal processing regions (vmPFC), and (3) insula-vmPFC connectivity was related to social anxiety symptoms only for children with the highest levels of BI. These findings suggest that increased coupling of intrinsic brain networks that support processing salient and internally relevant information is evident in children at risk for social anxiety. This is consistent with the notion that an “internal shift” in brain organization may influence the progression from childhood BI to later anxiety problems.
Salience Network Connectivity
Typically, the salience network is thought to filter information from the sensory milieu in support of goal-directed behavior 42,60–63. Insula fluctuations are typically anticorrelated with default mode fluctuations, an asynchrony that may function to minimize the intrusion of internal, default-network processes into goal-directed functioning 64. Here we found that individuals higher in BI have increased insula, amygdala, and somatosensory cortex connectivity with typical DMN regions of vmPFC and PCC, and diminished connectivity with typical SN regions of dACC and dorsal striatum. The topographical organization of insula connectivity across the medial prefrontal cortex captures this disruption in SN organization; BI children had a shift in SN topography, marked by a decrease in insula-dACC connectivity and increase in insula-vmPFC connectivity, relative to BN children. This can also been seen as a “flattening” of SN topography as the pattern of iFC across the medial PFC in BI children had significantly less differentiation, marked by a more linear pattern with minimal slope.
The shift in SN organization in BI children is consistent with research showing a disruption in filtering salient stimuli (e.g., threats and rewards), which seems to be biased toward internally-relevant information in socially inhibited individuals 54. Prior studies have shown that BI is related to biased attention and neural responses to threat, novelty, and reward 19,21,24,26,27,65–67, which are exacerbated by contexts in which stimuli have increased self-relevance (i.e., are contingent on the participant's behavior) 35. Blackford and colleagues 54 also reported increased connectivity of SN and DMN regions in socially inhibited adults. Following the insula model of anxiety 68, it seems that altered connectivity of the salience network, perhaps marked by an “internal shift,” has a central role in inhibited social behavior.
One interpretation of increased connectivity between salience processing and internal processing regions suggests that there is a heightened sensitivity to, or hypervigilance for, internally-relevant information such as threats, rewards, social feedback, or intrusive internal experiences (e.g., thoughts, emotions, or sensations) in BI. Typically hypervigilance is used in reference to behavioral analyses (e.g., attention bias to threat) 19, but may also apply at the neural level. In this case, the internal shift in SN connectivity in BI children may reduce thresholds for activation in “internal processing” regions not typically involved in SN function 40,69. The concomitant reduction in insula connectivity with regions involved in goal-directed motivation (dACC and dorsal striatum) 70 may further contribute to a disruption in processing salient information.
The notion of an internally hypervigilant pattern of SN organization in BI children is consistent with the approach-avoidance conflict model of developmental risk for anxiety 30 and the insula model of anxiety 68. While children typically show increased social approach motivation as they enter adolescence, the approach-avoidance model notes that BI children also exhibit enhanced sensitivity to self-relevant information, including feedback from peers and social signals of potential threat, leading to an approach-avoidance conflict 19,30,32. Our findings suggest a possible underlying neural mechanism for this conflict, which may arise and persist due to an internally hypervigilant pattern of intrinsic brain network organization 60,68.
It is worth noting that although it is not clear whether BI is a distinct phenomenon from pediatric or prodromal social anxiety disorder 17, findings from the present study are consistent with the overall network of frontolimbic regions that has been linked to clinical anxiety and developmental risk 71–74. However, our exploratory analyses of connectivity related to anxiety symptoms (see Supplemental Materials) finds limited overlap between neural correlates of BI and social phobia or total anxiety symptoms, suggesting that the present findings may be specific to BI. One exception was found in similar cross-network connectivity of default mode and executive regions for both the BI and social phobia symptoms analyses. These findings suggest that follow-up study is warranted to directly address this issue, which may reveal both similarities and differences in the neural correlates of childhood BI and specific forms of anxiety.
Further study will also be needed to determine the underlying nature of differences in findings across studies of clinical diagnoses of anxiety and anxiety risk (e.g., whether they are due to differences in samples and methods, or true differences in clinical versus at-risk populations), and whether the present findings of altered SN topography extend to clinical anxiety in children and adults. The present results point toward the need for longitudinal studies to determine the stability of the present findings across ages and methods of assessing BI, as well as to assess whether developmental trajectories of salience network connectivity can contribute to the prediction of anxiety disorder onset. Although much remains to be learned about neural systems underlying BI and risk for anxiety, the present findings of disruptions in the brain networks involved in processing salient information adds to previous data suggesting systemic perturbations in information processing across threat, reward, and social domains.
Dorsolateral Prefrontal Cortex Connectivity
Higher levels of BI were related to (1) increased intrinsic connectivity of the posterior cingulate with dorsolateral PFC (dlPFC) and ventrolateral PFC (vlPFC) regions, and (2) reduced connectivity of the frontal pole with putative central executive and attentional control network regions across lateral prefrontal and parietal cortex 62,63,75. Similar to the internal shift described in limbic and medial PFC regions of the salience network, these findings suggest the possibility that executive control networks may undergo a similar shift, marked by increased connectivity with DMN and reduced connectivity with typical control network regions.
Of particular interest is the relation between BI and increased vlPFC-PCC connectivity. Prior studies have found vlPFC is involved in executive control of attention responses to threat and shows increased response to threat in anxious individuals 76,77. The vlPFC is considered an important neural target for anxiety interventions such as attention bias modification training 78, and future studies should explore whether such interventions impact vlPFC-PCC connectivity.
BI Moderation of iFC-anxiety relation
Not all BI children go on to develop clinical anxiety; thus it is important to identify which children are at highest risk. Here we found that for children with the most extreme levels of BI, there were stronger relations between increased anxiety symptoms and greater SN-DMN connectivity. This suggests that those high BI children who also exhibit the strongest SN-DMN coupling might be at highest risk. In turn, the internal shift in SN connectivity may reflect a mechanism leading BI children on a developmental trajectory to later anxiety 19,29. Prior research has found that among adolescents with a history of BI, greater anxiety is related to elevated neural response to novelty 79 and error 28, and perturbed frontolimbic function 22,24. Current findings point to a potential developmental antecedent for these previously reported moderation relations.
Although the present study is limited by its cross-sectional examination of neurodevelopmental risk for anxiety, we have shown here that the patterns of brain network organization observed in high BI children are also related to increased anxiety symptoms in children highest in BI, and thus may be an important biomarker of the BI risk phenotype. However, further work will need to follow BI children over time to determine what role these patterns may play in psychosocial developmental trajectories, and whether they can aid in the early identification of at risk individuals who will go on to develop social anxiety. A second, associated limitation reflects our use of parent report in late childhood to characterize levels of BI and anxiety, and future studies should include observational measures. Specifically, while the BIQ has been useful in identifying BI children46,47, additional studies will be needed to determine whether our findings extend to children identified as BI via multi-method assessment (behavioral observation and parent reports) in the first few years of life.
Summary
Childhood BI was associated with altered patterns of intrinsic functional connectivity, marked by increased connectivity of salience, executive, and sensory networks with default mode network hubs. Children at temperamental risk for anxiety appear to have goal-directed salience and executive networks that are less goal-focused, and a resting state default network that is less restful. The blurring of internal (DMN) and goal-directed (SN and executive) networks in BI children may be a biomarker of early risk for anxiety, contributing to pathophysiological processes leading from shyness in early life to anxiety in adulthood. Further study of these patterns of brain organization and their development may provide an opportunity to develop intuitive neural biomarkers for identifying individuals at risk.
Supplementary Material
Acknowledgments
All authors contributed to the design, analysis, and interpretations of the study. BTT and KPE wrote the paper, with editing and comments from SM and FH. BTT had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data were collected with the help of the Pennsylvania State University Social, Life, and Engineering Sciences Imaging Center (SLEIC).
Funding: Funding for this study was provided by a grant from NIH (BRAINS Award R01 MH094633-01) to KPE.
Footnotes
Conflict of Interest Statement: All authors declare no conflicts of interest.
References
- 1.Taber-Thomas B, Pérez-Edgar K. In: The Oxford Handbook of Emerging Adulthood. Arnett JJ, editor. 2015. [Google Scholar]
- 2.Pine DS, Leibenluft E. BIomarkers with a mechanistic focus. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0498. [DOI] [PubMed] [Google Scholar]
- 3.Paulus MP. Pragmatism instead of mechanism: A call for impactful biological psychiatry. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0497. [DOI] [PubMed] [Google Scholar]
- 4.Lahat A, et al. Early childhood temperament predicts substance use in young adults. Transl Psychiatry. 2012;2:e157. doi: 10.1038/tp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clauss JA, Avery SN, Blackford JU. The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta-analysis. Prog Neurobiol. 2015;127–128:23–45. doi: 10.1016/j.pneurobio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao W, et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage. 2010;52:1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Pannekoek JN, et al. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol. 2013;23:186–195. doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin S, et al. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chronis-Tuscano A, et al. Stable Early Maternal Report of Behavioral Inhibition Predicts Lifetime Social Anxiety Disorder in Adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirshfeld-Becker DR, et al. Behavioral Inhibition in Preschool Children At Risk Is a Specific Predictor of Middle Childhood Social Anxiety: A Five-Year Follow-up. J Dev Behav Pediatr. 2007;28:225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- 12.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075.e1. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Dev Psychopathol. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- 14.Hirshfeld DR, et al. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Kagan J, Snidman N. Early childhood predictors of adult anxiety disorders. Biol Psychiatry. 1999;46:1536–1541. doi: 10.1016/s0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Edgar K, Fox N. Temperament and anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Edgar KE, Guyer AE. Behavioral Inhibition: Temperament or Prodrome? Curr Behav Neurosci Rep. 2014;1:182–190. doi: 10.1007/s40473-014-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson HA, Pine DS, Fox NA. Behavioral Inhibition and Developmental Risk: A Dual-Processing Perspective. Neuropsychopharmacology. 2015;40:207–224. doi: 10.1038/npp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Edgar KE, et al. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagan J, Fox NA. In: Handbook of Child Psychology. Damon W, Lerner RM, editors. Vol. 3. John Wiley & Sons, Inc.; 2007. pp. 167–225. [Google Scholar]
- 21.Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58:1459–1473. [PubMed] [Google Scholar]
- 22.Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- 23.Roy AK, et al. Alterations in amygdala functional connectivity reflect early temperament. Biol Psychol. 2014;103:248–254. doi: 10.1016/j.biopsycho.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardee JE, et al. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry. 2013;74:273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clauss JA, et al. Structural and functional bases of inhibited temperament. Soc Cogn Affect Neurosci. 2014;9:2049–2058. doi: 10.1093/scan/nsu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Edgar K, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants ‘grown up’: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 28.McDermott JM, et al. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pérez-Edgar K, Taber-Thomas B, Auday E, Morales S. In: Children and Emotion New Insights into Developmental Affective Sciences. Lagattuta KH, editor. Vol. 26. Karger; 2014. pp. 42–56. [Google Scholar]
- 30.Caouette JD, Guyer AE. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Dev Cogn Neurosci. 2014;8:65–76. doi: 10.1016/j.dcn.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miskovic V, Schmidt LA. Social fearfulness in the human brain. Neurosci Biobehav Rev. 2012;36:459–478. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Helfinstein SM, Fox NA, Pine DS. Approach–withdrawal and the role of the striatum in the temperament of behavioral inhibition. Dev Psychol. 2012;48:815–826. doi: 10.1037/a0026402. [DOI] [PubMed] [Google Scholar]
- 33.Guyer AE, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev Psychopathol. 2014;26:229–243. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyer AE, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bar-Haim Y, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckner RL, Krienen F, Yeo B. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 37.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 38.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 39.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 40.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 41.Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn Sci. 2012;16:181–188. doi: 10.1016/j.tics.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 44.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broeren S, Muris P. A Psychometric Evaluation of the Behavioral Inhibition Questionnaire in a Non-Clinical Sample of Dutch Children and Adolescents. Child Psychiatry Hum Dev. 2010;41:214–229. doi: 10.1007/s10578-009-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bishop G, Spence SH, Mcdonald C. Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Dev. 2003;74:1899–1917. doi: 10.1046/j.1467-8624.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- 48.O'Shaughnessy ES, Berl MM, Moore EN, Gaillard WD. Pediatric Functional Magnetic Resonance Imaging (fMRI): Issues and Applications. J Child Neurol. 2008;23:791–801. doi: 10.1177/0883073807313047. [DOI] [PubMed] [Google Scholar]
- 49.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. diagnostic interview schedule for children version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Whitfield-Gabrieli S, Nieto-Castanon A. CONN: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 51.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margulies DS, et al. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Cleveland WS, Devlin SJ. Locally weighted regression: An approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 54.Blackford JU, et al. Amygdala–cingulate intrinsic connectivity is associated with degree of social inhibition. Biol Psychol. 2014;99:15–25. doi: 10.1016/j.biopsycho.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roy AK, et al. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 57.Martuzzi R, et al. A Whole-Brain Voxel Based Measure of Intrinsic Connectivity Contrast Reveals Local Changes in Tissue Connectivity with Anesthetic without A Priori Assumptions on Thresholds or Regions of Interest. NeuroImage. 2011;58:1044–1050. doi: 10.1016/j.neuroimage.2011.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gotts SJ, et al. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135:2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. The Guilford Press; 2013. [Google Scholar]
- 60.Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 61.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 62.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 65.Hardin MG, et al. Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Personal Individ Differ. 2006;40:699–711. doi: 10.1016/j.paid.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helfinstein SM, et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49:479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barker TV, Troller-Renfree S, Pine DS, Fox NA. Individual differences in social anxiety affect the salience of errors in social contexts. Cogn Affect Behav Neurosci. 2015:1–13. doi: 10.3758/s13415-015-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paulus MP, Stein MB. An Insular View of Anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 69.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 71.Birn RM, et al. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry. 2014;19:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oathes DJ, Patenaude B, Schatzberg AF, Etkin A. Neurobiological Signatures of Anxiety and Depression in Resting-State Functional Magnetic Resonance Imaging. Biol Psychiatry. 2015;77:385–393. doi: 10.1016/j.biopsych.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roy AK, et al. Intrinsic Functional Connectivity of Amygdala-Based Networks in Adolescent Generalized Anxiety Disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–299.e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Monk CS, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 77.Telzer EH, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79:216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fox NA, Pine DS. Temperament and the Emergence of Anxiety Disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:125–128. doi: 10.1016/j.jaac.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reeb-Sutherland BC, et al. Attention to novelty in behaviorally inhibited adolescents moderates risk for anxiety. J Child Psychol Psychiatry. 2009;50:1365–1372. doi: 10.1111/j.1469-7610.2009.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sattler JM. Assessment of Children: WISC-III and WPPSI-R Supplement. Jerome M. Sattler, Publisher, Inc.; 1992. [Google Scholar]
- 81.Wechsler D. Wechsler intelligence scale for children–Fourth Edition (WISC-IV) Psychological Corporation; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.