Abstract
Product sharing among participants can impact on adherence and compromise the outcome in clinical trials. We describe incidents of product sharing at the Durban clinical research sites conducting the VOICE trial. The Durban sites enrolled 2750 women with 1103 and 1647 participants randomized to the vaginal gel and oral tablet arms respectively. Monthly pill and applicator counts including product assessments were conducted by pharmacists. Discrepancies with product counts prompted discussions with participants. Thirty-two cases of product sharing were identified. Vaginal gels were more commonly shared than oral tablets. Product sharing between study participants and their female friends or relatives living in the same household was identified as the most common source of product sharing in this analysis. Study product counts and pharmacist-driven discussions with participants may help to identify reasons for product sharing and inform the development of strategies for PrEP implementation outside of the research setting.
Keywords: HIV prevention, Pill count, Product sharing, Pre-exposure prophylaxis, VOICE
Introduction
Antiretrovirals (ARVs), used in the treatment of HIV, have been tested or are currently being tested as oral, topical or long acting injectable pre-exposure prophylaxis (PrEP) in several HIV prevention studies [1–13]. Placebo controlled efficacy trials are important to demonstrate safety and effectiveness of any intervention. A randomized controlled trial (RCT) ensures that treatment allocation occurs by a chance mechanism [14]. Blinding in a RCT also affects trial outcomes and is important in reducing bias [15].
A major challenge impacting on the outcome of PrEP trials is adherence to study product [16–19]. Sharing of study product in clinical trials is considered to be a possible factor affecting adherence [16, 20, 21] and has been identified as a potential source of efficacy dilution in clinical trials [22]. If participants are using products that they have not been assigned, then differences between the treatment arms may not be detected [23]. Product sharing has also been recognized as a factor which could negatively affect the use of pill counts in a clinical trial [20].
While prescription drug sharing in the general public has been widely studied [24–27], there is currently limited data on product sharing in clinical trials. Product sharing has previously occurred in clinical trials where the intervention has been shared with other participants and persons not participating in the clinical trial [20, 21, 28]. In these trials, participants admitted to product sharing however the overall incidence of observed product sharing was low [20, 21, 28, 29]. The impact of product sharing in PrEP trials is however not fully understood, thus collecting data on product sharing will be useful in better assessing its impact in HIV prevention trials [30].
The Vaginal and Oral Interventions to Control the Epidemic (VOICE) trial (ClinicalTrials.gov number: NCT00705679) was a randomized controlled trial to test the safety and effectiveness of a 1 % tenofovir vaginal gel, oral tenofovir (TDF) and oral Truvada (TDF/FTC) [7]. This paper describes the product sharing incidents identified by study pharmacists in the VOICE trial conducted at the South African Medical Research Council (SAMRC) sites in Durban, KwaZulu-Natal, South Africa; and the approaches used by pharmacists to address product sharing incidents. In this manuscript the term “sharing” refers to instances where participants intentionally shared study products; where there was suspected product sharing, and situations when other women acquired study products by accident, or without the participant’s knowledge.
Methods
The VOICE Trial
The VOICE trial was a five-arm, double-blinded study in which women were randomized to receive either vaginal gel or oral tablets as PrEP, and within each group, randomly assigned to either 1 % tenofovir vaginal gel or placebo gel; or to oral TDF, oral TDF/FTC or oral placebo. Participants were randomized to the five study arms in a 1:1:1:1:1 ratio. A total of 5029 women from 15 sites based in South Africa, Uganda and Zimbabwe were enrolled in the study. The seven SAMRC Durban clinical research sites (CRSs) enrolled a total of 2750 women, from November 2009 to June 2011, with 1103 and 1647 participants randomized to the vaginal gel and oral tablet arms respectively. Ethics approval for the SAMRC Durban sites was obtained from the SAMRC Ethics Committee (reference number: EC08-011) prior to commencement of the trial. Written informed consent was obtained from all participants enrolled in the trial.
Dispensing of Study Product
Study drug randomization and subsequent accurate dispensing by pharmacists were critical processes to ensure that participants received the correct product. TDF and TDF/FTC tablet specifications (colour, shape) could not be matched hence a matched placebo was used for each drug. Thus participants received two bottles of tablets at each monthly visit. At enrolment, participants randomized to the oral arm were dispensed with one bottle of TDF or placebo (30 tablets) and one bottle of TDF/FTC or placebo (30 tablets) to be taken orally once daily. Participants randomized to the gel arm were dispensed with three cartons of 1 % tenofovir vaginal gel applicators or placebo (10 applicators in each carton; 30 applicators in total) to be inserted intravaginally once daily. At each scheduled monthly visit, participants who were eligible for product use were provided with a 30 day supply which would last until their next scheduled visit. Participants were counselled by study nurses and pharmacists to return unused gel applicators and remaining tablets to the CRS pharmacy at every follow up visit. In cases where participants were travelling or could not attend their next scheduled visit, they were dispensed sufficient product to last until their next visit. The Division of AIDS (DAIDS) medical officer was consulted if more than a 60 day supply of study product was to be dispensed to participants. A documented quality control process involving a second pharmacist was done at every visit to prevent dispensing errors.
Study Product Assessments
Product use assessment, pill and applicator counts were used as tools by pharmacists to assess product returns. The assessment included a reconciliation of study products dispensed to and returned by participants using a product returns worksheet. Product labels bearing participant unique identifiers and pharmacy specific product codes were checked against pharmacy records to verify that participants returned the correct product. Whilst these tools were used to monitor adherence, the pharmacy procedures also helped to identify return of study product not dispensed to these participants. If a code did not match the pharmacy records, this implied that either the wrong product was dispensed or the participant returned another participant’s product. Investigations were also conducted by pharmacists to check for dispensing errors which could have contributed to incorrect product returns.
Discrepancies with product returns prompted discussions with the participants, pharmacists and clinic staff. These discussions were initiated by pharmacists to determine reasons for discrepant counts. Pharmacists ensured that blinding was always maintained during these discussions by not divulging product specific information. During these discussions, some participants disclosed the reasons why they returned products not previously dispensed to them. Data on all discussions and possible product sharing incidents were collected from November 2009 to August 2012. Ethical and study protocol considerations were important factors that impacted on the manner in which discussions were conducted with participants.
All cases of product sharing identified by pharmacists were reported to DAIDS and the SAMRC Ethics Committee. The Ethics Committee acknowledged reporting of these incidents.
Adherence and Product Use Counselling
The pharmacist-driven adherence counselling sessions were directed mainly by the reconciled product counts conducted in pharmacy and from the self-reported adherence by participants. Upon identification of potential product sharing, pharmacists provided counselling to participants individually. Tablet and gel use instructions were reinforced and product sharing was discouraged. These counselling sessions were conducted in a private counselling room. Discussions with participants included: using product specifically issued to them and not sharing with other individuals; recognising their unique participant identification numbers (PTID) on product labels; verifying the PTID on product labels prior to use; safe keeping of study products; separate storage of study products if residing with other study participants in the same household; and the importance of contacting the clinic in the event of requiring more study product. All individual counselling sessions were documented.
Several measures were implemented by pharmacists to address product sharing. These measures were implemented in participants identified as potentially sharing product. Pharmacists labelled individual gel applicators and tablet bottles with unique coloured stickers to aid participants in identifying study product as their own. The aim of this approach was to prevent the participant from accidentally mixing her products with those of another participant that she was living with. The stickers were also placed in pharmacy records so that the same coloured stickers were used for all follow up visits and alerted pharmacists to possible product sharing.
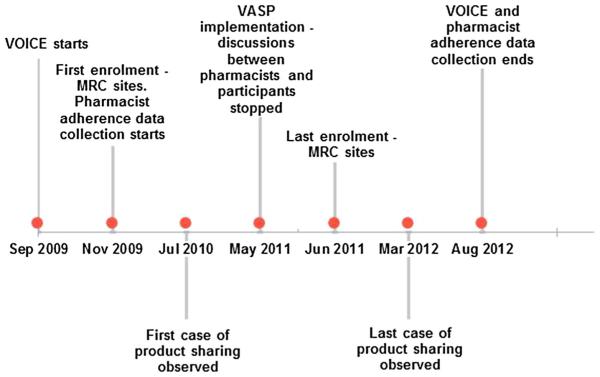
VOICE Adherence Strengthening Program (VASP) was an adherence support intervention introduced in May 2011 by the VOICE protocol team [31]. Given that the focus of VASP counselling was not on perfect adherence, it did not take into consideration product counts and self-reported product use [31]. The focus was rather on the participant’s experiences with using product with regard to what made product use easier and barriers to product use. Although product counts were still carried out by pharmacists, no discussions were held with participants when pharmacists noted product count discrepancies or incorrect product was returned. Nurses, counsellors and pharmacists continued to reinforce monthly product use counselling, without reference to product counts. Product sharing messages were included as general topics for discussion with participants when providing product use instructions. VASP implementation and data collection dates are described in Fig. 1.
Fig. 1.
Timeline showing VOICE data collection and VASP implementation (not to scale)
Results
The median age of the women described in this analysis was 22 years. The majority of women were in the 20–24 years age group (52 %). All of the women were unmarried, 94 % did not live with their primary sex partner and 42 % completed secondary schooling. Majority (74 %) lived in homes either owned by themselves or family members. The median number of rooms per house was four.
A total of 33,660 monthly visits were attended by 2750 participants and 32 incidents of product sharing or possible product sharing were identified by the pharmacists. On average, participants attended 12.2 monthly visits at which study product was dispensed. A summary of these incidents are presented in Table 1. Amongst women enrolled at the Durban sites, there were 18 incidents where participants returned study products not assigned to them. A further 14 possible product sharing incidents were identified by pharmacists based on product count discrepancies and discussions with participants. No dispensing errors were reported by pharmacists as confirmed by internal audits of pharmacy documentation. From the 32 participants identified in product sharing incidents, pharmacokinetic (PK) analysis results were available for 15 participants. Of the 15 participants, 12 were randomized to the gel arm and three to the oral arm. Eleven of the 15 participants were on the active arm and four on the placebo arm. The results showed that study drug was detected in four of these participants. Of these four participants, there was one participant on the placebo vaginal gel arm who had drug detected during PK testing. Of the 32 participants suspected of sharing study product, 23 were randomized to the gel group and nine to the oral group. There were three cases of intentional sharing reported. Incidents of product sharing occurred at random throughout the study from July 2010 to March 2012. Of the 32 cases reported, there were 13 cases of incorrect product returned after implementation of VASP. There were no safety concerns and no incidence of social harm reported by participants during discussions with pharmacists.
Table 1.
Summary of product sharing incidents (Nov 2009 to Aug 2012)
| Reasons for sharinga | Total number of incidents | No. of participants on gel arm | No. of participants on oral arm |
|---|---|---|---|
| Living together (friends and family) | 20 | 12 | 8 |
| Friends visiting | 4 | 3 | 1 |
| Mix-up with co-worker | 1 | 1 | 0 |
| Mix-up during travelling | 1 | 1 | 0 |
| Altruistic reasons | 1 | 1 | 0 |
| Unknown | 5 | 5 | 0 |
| Totals | 32 | 23 | 9 |
As per participant report
Discussions with participants showed that the majority of product sharing incidents occurred within the same household where participants were living with other women. This comprised 20/32 incidents [62.5 %]. In these discussions participants reported that their sisters, cousins and friends had access to their study product. Ten participants on the gel arm reported that they lived in the same household as other women who were enrolled in VOICE and were also using study gel. In some cases where product sharing occurred between two enrolled participants, both participants were counselled by the nurse or pharmacist. One participant who returned the incorrect study gels reported that she was using study gels every day and did not miss doses. When informed of the gel mix-up, she revealed that she had a cousin also enrolled in the VOICE study and using study gel. She reported that her cousin stored her product in a separate cupboard but could not explain how the mix-up occurred. Another case involved a participant on the vaginal gel arm who reported that her friend, also on study gel, visited for a few days resulting in their product getting mixed up. Other causes of possible product sharing as reported by participants included friends who visited participants’ homes and had access to study products (four incidents), altruistic reasons (one incident), mix-up of study products with a co-worker (one incident) and a mix-up of study product whilst travelling with another participant (one incident). There was an incident reported from one CRS where a participant reported giving two gel applicators to a friend to increase sexual pleasure for altruistic reasons. The majority of participants who reported product sharing did not report the reasons why sharing had occurred. There were five incidents of sharing where the reasons for sharing study product could not be identified. There were no repeat incidents of product sharing involving the same participant.
Discussion
Majority (62.5 %) of reported cases of product sharing involved friends and family who had access to study product. Participants reported that family members and friends may have taken their study product without their knowledge and in some cases participants could not explain how incorrect study product was in their possession. From these discussions, it could not always be confirmed whether friends and family members were enrolled in the trial or not.
Preliminary results from VOICE D, an ancillary study to explore participant’s adherence challenges during the VOICE trial, corroborated these findings of product sharing [32]. Participants in VOICE D reported sharing with their family or friends who wanted to use the study product. Product sharing was also reported in the FEM-PrEP follow up study where some participants reported giving pills to other people, some of whom were HIV positive [29]. This is consistent with other studies which showed that sharing of medication including ARV’s is common practice among friends, neighbours and relatives [21, 33–35].
To our knowledge, no studies have reported possible product sharing identified by CRS pharmacists in clinical trials. Although counselling and product use instructions were provided at every study visit, it is a possibility that participants did not pay attention or chose to ignore product use instructions [19]. The findings from this study showed that vaginal gel was shared more commonly than oral tablets. This was more likely due to tablet bottles being hidden away from view due to the perception that tablets contain ARV’s whereas gel applicators were not easily recognisable and therefore not stored securely [36]. Gel sharing was more common possibly due to perceived sexual benefits gained from its use [19, 36]. Only one participant identified by our pharmacists as suspected of sharing product disclosed that she gave gel applicators to her friend for use to increase sexual pleasure.
The discussions that were conducted with participants gave valuable insight into participants living conditions. In some cases, pharmacists found that participants shared study product storage space with other women in the same household. When questioned about how incorrect product came into their possession, participants could only supply limited information e.g. living with a sister or cousin. PK results showed that one participant randomized to the placebo gel arm had detectable TDF drug levels. Dispensing errors were investigated and ruled out thus pointing to sharing between participants. This participant reported living with a cousin who was also on the gel arm and enrolled at a different site. They had shared storage space and applicators could have been mixed up as per participant report.
The utility of returned product counts in identifying incidents of suspected product sharing is limited by reliance on the participant to return unused study product at each study visit. Participants may count and dispose of surplus unused study product, or may not return any unused study product if they were intentionally sharing study product, in order to appear adherent [32]. As a result the exact number of product sharing incidents at the SAMRC Durban sites is not known and could be much higher than estimated by pharmacists. It is therefore difficult to assess whether product sharing had an impact on the VOICE trial due to the few incidents of product sharing identified by site pharmacists. Another limitation of pill counts is the inability to identify possible cross group sharing i.e. a participant on the gel arm may have used a friend’s oral study product and vice versa. The pill counts were designed to reconcile tablets only or gel only. Although dispensing errors did not occur for the participants identified in the analysis, ruling out errors could only identify potential cases of product sharing.
Conclusion
In order to minimize the impact of product sharing in PrEP trials, early identification is critical. Physical verification of study product returns by pharmacists can play a valuable role in identifying possible incidents of study product sharing. Pharmacist-driven discussions with participants should be conducted to ascertain reasons for product sharing. Clear messaging and reinforcement on the correct use and return of unused study products must be consistently provided to participants. Findings from this study may inform the design of adherence monitoring strategies for PrEP implementation outside of the research setting.
Acknowledgments
The authors would like to thank all the women who participated in the VOICE Trial, the VOICE study teams at the South African Medical Research Council, HIV Prevention Research Unit (Durban); Leanne Vallabhjee for assisting with the analysis of data, and Renee Street and Nathlee Abbai for their valuable comments and review of the manuscript.
The Microbicide Trials Network is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health.
Footnotes
Author Contribution Jeeva Moodley developed the concept, conducted the analysis and drafted the manuscript. Sarita Naidoo, Jayajothi Moodley and Gita Ramjee reviewed the manuscript and provided input.
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
ClinicalTrials.gov number: NCT00705679
Contributor Information
Jeeva Moodley, Email: jeeva.moodley@mrc.ac.za.
Sarita Naidoo, Email: sarita.naidoo@mrc.ac.za.
Jayajothi Moodley, Email: jothi.moodley@mrc.ac.za.
Gita Ramjee, Email: gita.ramjee@mrc.ac.za.
References
- 1.Karim QA, Karim SSA, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten J, Celum C. Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women: the Partners PrEP Study; 6th IAS Conference on HIV pathogenesis, treatment and prevention; Rome. 2011. [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok tenofovir study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 7.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rees H, Delany-Moretlwe S, Baron D, et al. FACTS 001 phase III trial of pericoital tenofovir 1% gel for HIV prevention in women; Conference on retroviruses and opportunistic infections (CROI); Seattle. 2015. [Google Scholar]
- 9.Molina JM, Capitant C, Spire B, et al. On demand PrEP with oral TDF-FTC in MSM: results of the ANRS Ipergay trial; Conference on retroviruses and opportunistic infections; Seattle. 2015. [Google Scholar]
- 10.McCormack S, Dunn D. Pragmatic open-label randomised trial of Preexposure prophylaxis: The PROUD study; Conference on retroviruses and opportunistic infections (CROI); Seattle. 2015. [Google Scholar]
- 11.Devlin B, Nuttall J, Wilder S, Woodsong C, Rosenberg Z. Development of dapivirine vaginal ring for HIV prevention. Antiviral Res. 2013;100:S3–8. doi: 10.1016/j.antiviral.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Spreen B, Rinehart A, Smith K, Margolis D, Ford S, Piscitelli S. HIV PrEP dose rationale for cabotegravir (GSK1265744) long-acting injectable nanosuspension. AIDS Res Human Retrovir. 2014;30(S1):A12. [Google Scholar]
- 13.Jackson A, McGowan I. Long-acting rilpivirine for HIV prevention. Curr Opin HIV AIDS. 2015;10(4):253–7. doi: 10.1097/COH.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 14.Stanley K. Design of randomized controlled trials. Circulation. 2007;115(9):1164–9. doi: 10.1161/CIRCULATIONAHA.105.594945. [DOI] [PubMed] [Google Scholar]
- 15.Viera AJ, Bangdiwala SI. Eliminating bias in randomized controlled trials: importance of allocation concealment and masking. Fam Med. 2007;39(2):132. [PubMed] [Google Scholar]
- 16.Ambia J, Agot K. Barriers and facilitators of adherence in userdependent HIV prevention trials, a systematic review. Int STD Res Rev. 2013;1:12–29. [Google Scholar]
- 17.Koenig LJ, Lyles C, Smith DK. Adherence to antiretroviral medications for HIV pre-exposure prophylaxis. Am J Prev Med. 2013;1(44):S91–8. doi: 10.1016/j.amepre.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 18.Underhill K, Operario D, Skeer M, Mimiaga M, Mayer K. Packaging PrEP to prevent HIV: an integrated framework to plan for pre-exposure prophylaxis implementation in clinical practice. J Acquired Immune Deficiency Syndromes. 2010;55(1):8. doi: 10.1097/qai.0b013e3181e8efe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodsong C, MacQueen K, Amico KR, et al. Microbicide clinical trial adherence: insights for introduction. J Int AIDS Soc. 2013;16(1) doi: 10.7448/IAS.16.1.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob ST, Baeten JM, Hughes JP, et al. A post-trial assessment of factors influencing study drug adherence in a randomized biomedical HIV-1 prevention trial. AIDS Behav. 2011;15(5):897–904. doi: 10.1007/s10461-010-9853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Wijgert J, Jones H, Pistorius A, et al. Phase III microbicide trial methodology: opinions of experienced expanded safety trial participants in South Africa. SAHARA J. 2006;2(3):311–9. doi: 10.1080/17290376.2005.9724856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mâsse BR, Boily M-C, Dimitrov D, Desai K. Efficacy dilution in randomized placebo-controlled vaginal microbicide trials. Emerg themes Epidemiol. 2009;6(1):5. doi: 10.1186/1742-7622-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lader EW, Cannon CP, Ohman EM, et al. The clinician as investigator participating in clinical trials in the practice setting. Circulation. 2004;109(21):2672–9. doi: 10.1161/01.CIR.0000128702.16441.75. [DOI] [PubMed] [Google Scholar]
- 24.Goldsworthy RC, Schwartz NC, Mayhorn CB. Beyond abuse and exposure: framing the impact of prescription-medication sharing. Am J Public Health. 2008;98(6):1115. doi: 10.2105/AJPH.2007.123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel KL, Honein MA, Moore CA. Sharing prescription medication among teenage girls: potential danger to unplanned/undiagnosed pregnancies. Pediatrics. 2003;111(Supplement 1):1167–70. [PubMed] [Google Scholar]
- 26.Ellis J, Mullan J. Prescription medication borrowing and sharing: risk factors and management. Aust Fam Physician. 2009;38(10):816. [PubMed] [Google Scholar]
- 27.Beyene KA, Sheridan J, Aspden T. Prescription medication sharing: a systematic review of the literature. Am J Public Health. 2014;104(4):e15–26. doi: 10.2105/AJPH.2013.301823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pool R, Montgomery CM, Morar NS, et al. Assessing the accuracy of adherence and sexual behaviour data in the MDP301 vaginal microbicides trial using a mixed methods and triangulation model. PLoS ONE. 2010;5(7):e11632. doi: 10.1371/journal.pone.0011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corneli AL, McKenna K, Perry B, et al. The science of being a study participant: FEM-PrEP participants’ explanations for overreporting adherence to the study pills and for the where-abouts of unused pills. J AIDS. 2015;68(5):578–84. doi: 10.1097/QAI.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 30.Dimitrov D, Boily M-C, Mâsse BR, Brown ER. Impact of pill sharing on drug resistance due to a wide-scale oral prep intervention in generalized epidemics. J AIDS Clin Res. 2012;(4) doi: 10.4172/2155-6113.s5-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Straten A, Mayo A, Brown ER, et al. Perceptions and experiences with the VOICE adherence strengthening program (VASP) in the MTN-003 trial. AIDS Behav. 2014;19(5):770–83. doi: 10.1007/s10461-014-0945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Straten A, Musara P, Etima J, et al. Disclosure of pharmacokinetic (PK) drug results promotes open discourse on non-adherence among women in VOICE. AIDS Res Hum Retrovir. 2014;30(S1):A42–3. [Google Scholar]
- 33.El-Khatib Z, Richter M. (ARV-) free state? the moratorium’s threat to patients’ adherence and the development of drug-resistant HIV. SAMJ. 2009;99(6):412–4. [PubMed] [Google Scholar]
- 34.Kurtz SP, Buttram ME, Surratt HL. Vulnerable infected populations and street markets for ARVs: potential implications for PrEP rollout in the USA. AIDS care. 2014;26(4):411–5. doi: 10.1080/09540121.2013.837139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal E, Piroth L, Cua E, et al. Preexposure prophylaxis (PrEP) of HIV infection in France: a nationwide cross-sectional study (PREVIC study) AIDS Care. 2014;26(2):176–85. doi: 10.1080/09540121.2013.803014. [DOI] [PubMed] [Google Scholar]
- 36.van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS ONE. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]