Abstract
Background
Candida is a leading cause of infection in infants on extracorporeal membrane oxygenation (ECMO). Optimal micafungin dosing is unknown in this population because ECMO can alter drug pharmacokinetics (PK).
Methods
To characterize micafungin PK and safety in infants on ECMO, we conducted an open label PK trial. Infants on ECMO either received IV micafungin 4 mg/kg/day for invasive candidiasis prophylaxis, or 8 mg/kg/day when a fungal infection was suspected or confirmed. We collected plasma samples after single and multiple micafungin doses. We defined the therapeutic target as the adult exposure associated with efficacy in Phase III trials, and the prophylactic target as one-half of the therapeutic target.
Results
We enrolled 12 infants (124 samples) with a median age of 59 days. Using a 1-compartment model, median weight-normalized volume of distribution and clearance were 0.64 L/kg and 0.041 L/kg/h, respectively. Dose-exposure simulations revealed that doses of 2.5 and 5 mg/kg/day matched exposure targets for prophylaxis and treatment of invasive candidiasis, respectively. We did not observe any drug-related adverse events.
Conclusions
In infants on ECMO, micafungin volume of distribution was higher and clearance was in the upper range of previously published values for infants not on ECMO. Based on these data, we recommend dosing of 2.5 and 5 mg/kg/day for prophylaxis and treatment of invasive candidiasis, respectively, to match adult exposure proven effective against Candida spp.
Keywords: Extracorporeal membrane oxygenation, micafungin, antifungal, pharmacokinetics, Candida prophylaxis, Candida therapy, infants
Introduction
Extracorporeal membrane oxygenation (ECMO) provides respiratory and cardiac support in critically ill infants when conventional modes of life-support fail.1 Although potentially life-saving, ECMO support is associated with a high risk of nosocomial infections in children (8-16%).2 The most common cause of ECMO-related nosocomial infection in infants and children and the second most common cause in neonates is invasive candidiasis.2 Invasive candidiasis causes high rates of morbidity and mortality and is difficult to treat because of the organism’s ability to form biofilms on indwelling catheters.3 For this reason, recommended treatment of invasive candidiasis consists of both antifungal therapy and removal of indwelling catheters.4,5 However, catheter removal for children on ECMO is impossible; therefore, therapy relies upon optimal dosing of antifungal agents.
Micafungin is an attractive antifungal drug in infants on ECMO because it is fungicidal against a broad spectrum of Candida spp, and can penetrate biofilms.6-8 However, the optimal dosing of micafungin remains to be established in the setting of ECMO because ECMO support can alter drug pharmacokinetics (PK).9,10 In infants on ECMO, the volume of distribution (V) of drugs typically increases due to the large volume of blood required to prime the ECMO circuit, disease state (e.g., inflammation, anasarca), and drug adsorption by the ECMO circuit itself.9-13 Clearance (CL) of drugs can be affected by the organ dysfunction commonly observed in infants on ECMO, as well as non-specific drug extraction by the circuit itself. Micafungin may be more vulnerable to adsorption by the circuit because it is highly protein bound (>99%).14 Therefore, it is likely that current micafungin dosing recommendations for treatment (2-3 mg/kg every 24h) are inadequate for infants on ECMO.15 In this open-label PK trial, we determined micafungin PK in infants on ECMO and compared the resulting micafungin exposure to adult exposures known to be effective against invasive candidiasis.
Materials and Methods
Design and study population
This was a prospective, open-label, PK and safety study of micafungin conducted at Duke University Medical Center. We enrolled infants (0-2y) supported with ECMO, excluding those with a history of echinocandin allergy. Infants without fungal infection were enrolled in the prophylaxis arm and received IV micafungin per study protocol (4 mg/kg every 24h, infused over 1h). Infants with suspected or confirmed fungal infections who were prescribed micafungin per standard of care, were enrolled in the treatment arm and dosing was adjusted per study protocol to 8 mg/kg every 24h, infused over 1h. Duration of micafungin prophylaxis was up to 8 days, whereas duration of therapy in the treatment arm was determined by the treating physician. Because micafungin is light-sensitive, drug vials and infusion bags were protected from light.16 However, the ECMO circuit is not protected from light, so it is possible that micafungin in the blood was exposed to light while transiting the ECMO circuit. This trial was approved by the institutional review board of Duke University Medical Center, registered with clinicaltrials.gov (NCT01666769), and conducted under a Food and Drug Administration investigational new drug application (No.115255). Written informed consent was obtained from the legal guardian of each infant.
PK sampling
We collected up to 14 plasma PK samples (200 µL of whole blood per sample) around dose 1 and 4 via a peripheral arterial catheter. Sampling intervals were as follows (1 sample per interval): 0-4h before the start of infusion, and 0-30 min, 60-90 min, 2-4h, 8-10h, 12-16h, and 22-24h after the end of micafungin infusion. Samples were collected in ethylenediaminetetraacetic acid microcontainers and processed immediately or placed on ice (<30 minutes) until processing. Plasma was separated via centrifugation (3000g for 10 minutes at 4°C), manually aspirated and transferred to polypropylene tubes. Plasma samples were frozen at –20°C for a maximum of 24h, and stored at −80°C until analysis. PK samples were protected from light when stored in the freezer. No special precautions were undertaken during processing of samples because reconstituted micafungin was shown to be stable up to 24 hours at room temperature when exposed to light.16
ECMO Circuit Configuration
Two types of ECMO circuits were used; S3 and CardioHelp (see figure, Supplemental Digital Content 1). A hemofilter (Sorin DHFO.2, Sorin Group, Milan, Italy) was used in infants requiring hemofiltration. The prime volume for each circuit was 450mL and included packed red blood cells (350mL), fresh frozen plasma (50mL), Plasmalyte® crystalloid (50mL), sodium bicarbonate (25mEq), and heparin (100units).
PK analysis
Plasma micafungin concentrations were determined using a validated high performance liquid chromatography assay with fluorescent detection.17 The lower limit of quantification was 0.05 mg/L. Intraday precision ranged from 1.28% for the highest concentration of the standard curve (25.00 mg/L) to 17.90% for the lowest concentration (0.05 mg/L). Interday precision ranged from 3.27% to 14.41% within the concentration range of the standard curve (0.05–25.00 mg/L).
Primary outcomes were micafungin CL and V. PK data were analyzed by nonlinear regression with the most appropriate model using Winonlin v6.3 (Pharsight Co., St. Louis, MO). The model fit was evaluated using successful minimization, diagnostic plots, goodness of fit as assessed by the Akaike Information Criterion and precision of the parameter estimates. We assessed systemic exposure by estimating area under the concentration-time curve from 0 to 24h (AUC0–24) after the 1st and the 4th dose. AUC0–24 was computed by the linear up log down trapezoidal method using observed data. We assessed the relationship between PK parameters and covariates potentially affecting micafungin disposition (age, serum albumin, aspartate aminotransferase, total bilirubin, and duration of ECMO support) by visual inspection of scatter plots.18-20 Finally, we explored the relationship between PK parameters and the number of doses (single vs multiple doses), and the presence of the hemofilter in the ECMO circuit, using a Wilcoxon rank-sum test.
Assessment of dose-exposure relationship
For a target efficacy exposure, we used an AUC0-24 range of 75-139 mg*h/L as a surrogate pharmacodynamic (PD) end point.19,21 This endpoint matches the micafungin exposure achieved by adult participants who cleared their fungal infection in the large, phase III efficacy trial.22 There is no established target exposure for Candida prophylaxis, however, IV micafungin 50 mg (1 mg/kg in children) daily was proven effective in preventing fungal infections in 882 adults and children undergoing hematopoietic stem cell transplantation.23 Because micafungin PK is linear in this dosing range, the prophylactic exposure target was set to 50% of the therapeutic target (37.5-69.5 mg*h/L).19,21,24
In order to characterize micafungin exposure, we simulated a variety of dosing regimens and measured the proportion of infants in our population who achieved the therapy and prophylaxis exposure targets after the 1st and 4th micafungin dose. Simulations were performed by estimating the maximum (Cmax) and minimum (Cmin) micafungin concentrations for each individual for the first dosing interval and at steady state using the equation for an intermittent infusion with the individual parameter estimates from the final model. We then used the predicted Cmax and Cmin values to calculate the AUC for the dosing interval from 0 to 24h and at steady state using the equations for the linear-up log-down trapezoidal approach.
Safety
The secondary outcome was the proportion of infants experiencing adverse events (AEs). AEs were defined as any untoward medical occurrence whether or not considered drug-related during the conduct of the clinical trial. AEs were recorded while on study drug and for 7 days after the last study dose of micafungin. Liver toxicity and hypokalemia were the AEs of special interest because they were described previously in 3% and 2% of infants and children receiving micafungin, respectively.21 Levels of serum aspartate aminotransferase, alanine aminotransferase and potassium were therefore measured at baseline (within 72h prior to the first study dose), and at the end of the study (within 72h after the last study dose). Other laboratory determinations were recorded from consent through 72h after the last study dose, if performed per standard of care. Other laboratory determinations included albumin, blood urea nitrogen, serum creatinine, sodium, complete blood count and microbiology culture results. The safety data were summarized descriptively. We used STATA 13 (College Station, TX) to perform the statistical analyses.
Results
We enrolled 12 infants on ECMO support with a median (range) postnatal age of 59 days (0, 574) (Table 1). Two infants were born preterm (27 and 34 weeks of gestational age [GA]) and were 122 and 187 days postnatal age at time of enrollment, respectively. Infants were supported by ECMO for a median time of 4 days (2, 10) before the first micafungin dose. Of the 12 infants, 11 (92%) received prophylactic intravenous (IV) micafungin (4 mg/kg every 24h) for 4 (2, 8) days. One infant (ID # 2) was treated for presumed fungal infection with IV micafungin (8 mg/kg every 24h) for 6 days. Site of drug administration included central venous catheter (n=7) and ECMO circuit, post-oxygenator (n=1). Site of drug administration was not recorded for 4 infants. All 11 infants from the prophylactic group had PK samples collected around dose 1, and 5/11 (45%) of these infants remained on micafungin long enough to complete sampling around dose 4. The one infant in the therapeutic group had PK samples collected around doses 2 and 5. PK samples were collected from a peripheral arterial line in all infants but one for whom site of PK sampling was not recorded. Hemofiltration was used in 4/12 (33%) infants (Table 2).
Table 1.
Clinical Characteristics*
| N=12 | |
|---|---|
|
| |
| Age (days) | 59 (0.0, 574) |
|
| |
| Gestational age (weeks) | 39 (27, 39) |
|
| |
| Weight (kg) | 3.7 (2.9, 9.6) |
|
| |
| Birth weight (kg) | 3 (2.4, 4.3) |
|
| |
| Male, n (%) | 5 (42) |
|
| |
| Race, n (%) | |
| White | 6 (50) |
| African American | 6 (50) |
|
| |
| Diagnosis, n (%) | |
| Cardiomyopathy | 4 (34) |
| Pulmonary hypertension | 3 (25) |
| Congenital heart disease | 3 (25) |
| Congenital diaphragmatic hernia | 1 (8) |
| Respiratory failure | 1 (8) |
|
| |
| Serum creatinine (mg/dL)† | 0.5 (0.2, 1.6) |
|
| |
| ECMO support, n (%) | |
| Veno-arterial | 11 (92) |
| Veno-venous | 1 (8) |
|
| |
| ECMO circuit configuration, n (%) | |
| S3 | 8 (67) |
| Cardiohelp | 4 (33) |
|
| |
| Micafungin indication n (%) | |
| Prophylaxis | 11 (92) |
| Therapy | 1 (8) |
|
| |
| Micafungin dosing (mg/kg/day) | 4 (4, 8) |
|
| |
| Duration of therapy (days) | 4 (2, 8) |
|
| |
|
Duration of ECMO support at time of
first micafungin dosing (days) |
4 (2, 10) |
|
| |
| Number of PK samples per infant | 11 (6, 14) |
|
| |
| PK samples obtained around, n (%) | |
| Dose 1-2 | 12 (100) |
| Dose 4-5 | 6 (50) |
Results are expressed as median (range) unless otherwise specified
Median serum creatinine for each subject over the study period.
Table 2.
Pharmacokinetic Parameters
| ID | Age (days) |
Clearance (L/kg/h) |
Volume of distribution (L/kg) |
Half-life (h) |
Dose (mg/kg/day) |
AUC (mg*h/L) | Albumin*
(g/dL) |
|
|---|---|---|---|---|---|---|---|---|
| 0-24h | 72-96h | |||||||
| 1 | 0 | 0.036 | 0.98 | 19 | 4 | 69 | 117 | 3.1 |
| 2 | 1 | 0.040 | 0.76 | 13 | 8 | 213§ | 178† | 3.6 |
| 3 | 1 | 0.046 | 0.64 | 10 | 4 | 72 | - | 2.5 |
| 4† | 4 | 0.042 | 0.75 | 12 | 4 | 68 | - | 2.8 |
| 5 | 16 | 0.062 | 0.91 | 10 | 4 | 53 | - | 2.5 |
| 6 | 44 | 0.058 | 0.65 | 8 | 4 | 59 | - | 2.1 |
| 7† | 74 | 0.026 | 0.56 | 15 | 4 | 93 | 163 | 4.2 |
| 8† | 108 | 0.043 | 0.53 | 9 | 4 | 74 | 109 | 2.5 |
| 9 | 122 | 0.044 | 0.69 | 11 | 4 | 77 | 84 | 2.7 |
| 10 | 135 | 0.030 | 0.44 | 10 | 4 | 105 | - | 3.1 |
| 11 † | 187 | 0.040 | 0.46 | 8 | 4 | 84 | - | 3.8 |
| 12 | 574 | 0.028 | 0.61 | 15 | 4 | 89 | 187** | 3.7 |
|
Median
(range) |
59
(0, 574) |
0.041
(0.026, 0.062) |
0.64
(0.44, 0.98) |
11
(8, 19) |
4
(4, 8) |
74‡
(53, 106) |
117‡
(84, 187) |
3.0
(2.1, 4.2) |
Clearance and volume of distribution estimates were determined using a one-compartmental model fitting micafungin concentrations after both dose 1 and 4
AUC: area under the concentration-time curve
Hemofilter in line
Median serum albumin value over the study period (from 72h prior to first study dose through 72h after last study dose)
AUC 24-48h
AUC 96-120h
AUC 79-103h
Summary statistics do not include AUC values from subject #2
Pharmacokinetics
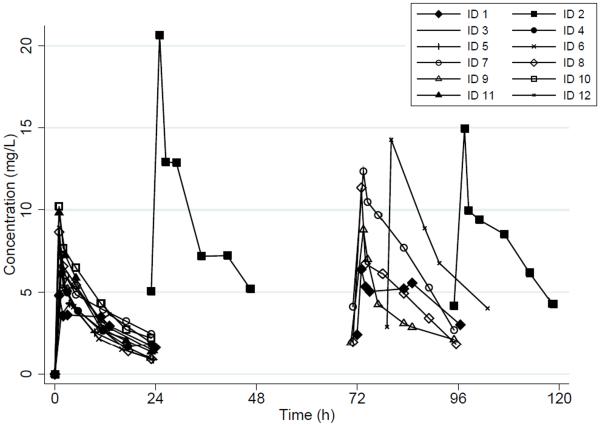
We collected a total of 124 plasma samples, with a median of 11 (6, 14) samples per infant. Plasma concentration-time profiles are shown in Figure 1. A 1-compartment model with zero-order infusion appropriately fit the data (Table 2). PK parameters were estimated with high precision as evidenced by median CL and V coefficient of variation of 6% (5, 14) and 11% (8, 14), respectively. Three/124 (2%) PK samples were excluded from the analysis; one concentration was below the lower limit of quantification (subject ID#5), and 2 plasma samples lacked confirmation of sampling time (Subject ID#12). PK parameter estimates did not change when these 3 concentrations were removed from the analysis. Median [95% confidence interval] CL decreased over the study period from 0.04 L/kg/h [0.03, 0.05], to 0.02 L/kg/h [0.02, 0.03] after single and multiple doses, respectively. Median [95% CI] V was 0.62 L/kg [0.53, 0.73) and 0.52 L/kg [0.31, 0.67] after a single and multiple doses, respectively.
Figure 1. Micafungin Concentration-Time Profiles*.
*All subjects received IV micafungin 4 mg/kg every 24h, except for subject ID #2 who received 8 mg/kg every 24h
Subject ID # 2: PK samples were collected around dose 2 and 5
Subject ID # 12: The fourth dose was given 79h after the first dose, instead of 72h.
We did not observe a significant difference in CL between infants with and without a hemofilter in the circuit (median [range] CL of 0.04 L/kg/h [0.03, 0.04] vs 0.04 L/kg/h [0.03, 0.06], respectively, p=0.50) nor did we observe a significant relationship between V and the presence of a hemofilter (0.54 L/kg [0.46, 0.75] and 0.67 L/kg [0.44, 0.98] in infants with and without a hemofilter, respectively; p=0.17). In evaluating other covariates of interest, we observed an inverse correlation between CL and age, with older infants demonstrating lower CL per kg of bodyweight (see figure, Supplemental Digital Content 2). The same relationship also was observed for V and age where older infants had lower V per kg of bodyweight (see figure, Supplemental Digital Content 2). An inverse relationship also was observed between CL and serum albumin; subjects with low serum albumin had higher CL (see figure, Supplemental Digital Content 2). However, no relationship was observed between V and serum albumin, or between CL and the following covariates: AST, serum creatinine, or total bilirubin (data not shown). Finally, no relationship was observed between duration of ECMO support and CL or V (data not shown).
Dose-exposure relationship
Prophylactic arm (4 mg/kg IV every 24h)
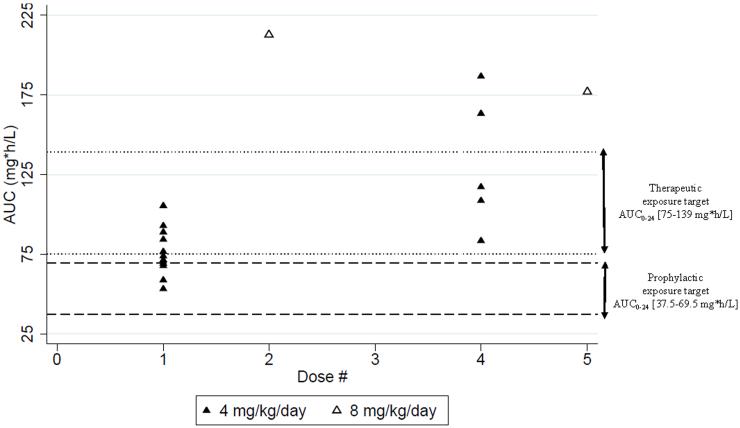
Median AUC0-24 were 74 mg*h/L (53, 106) and 117 mg*h/L (84, 187) after dose 1 and 4, respectively (Figure 2). After a single dose, all infants were at or higher than the prophylaxis range (Figure 2). After dose 4, all infants sampled (5/5) exceeded the prophylactic range. Although infants in the prophylactic arm were not treated for active fungal infection, 5/11 (45%) achieved the therapeutic exposure target (75-139 mg*h/L) after a single dose, while the remaining infants were below the lower limit of the therapeutic exposure target (Figure 2). Among the 5 infants who were sampled around dose 4, 3/5 (60%) were within the therapeutic range, and 2 were supratherapeutic. Four infants had a hemofilter in line (Table 2). When a hemofilter was in line, all infants (4/4) achieved AUC0-24 within or above the prophylactic target range after dose 1 and 4. Two/4 (50%) infants with a hemofilter achieved AUC0-24 within or above the therapeutic target range after dose 1 and 4.
Figure 2. Micafungin Exposure.
AUC: Area under the concentration-time curve 0-24h
Treatment arm (8 mg/kg IV every 24h)
The one infant (ID#2) who received micafungin for suspected Candida infection had AUC0-24 of 213 and 178 mg*h/L after doses 2 and 5, respectively. This infant achieved an AUC0-24 that exceeded both prophylactic and therapeutic target ranges after doses 2 and 5 (Figure 2).
Dose-exposure simulations
A daily dose of 2.5 mg/kg infused over 1 h achieved the prophylactic target (AUC0-24 of 37.5 - 69.5 mg*h/L) within 24 h in 100% of the cohort. At steady state all children achieved an AUC0-24 of at least 37.5 mg*h/L, with 3 (25%) above the target range (AUC0-24 of 84, 88, 94 mg*h/L).
A daily dose of 5 mg/kg infused over 1 h achieved the therapeutic target range (AUC0-24 of 75 - 139 mg*h/L) within 24 h in 100% of the cohort. At steady state all children achieved an AUC0-24 of at least 75 mg*h/L, with 3 (25%) above the target range (AUC0-24 of 169, 177, 189 mg*h/L).
Safety
Five infants died during the study period. None of the deaths were considered related to micafungin. Two infants died following severe intracranial hemorrhage in the setting of systemic anticoagulation with heparin while on ECMO, and 1 died of refractory pulmonary hypertension after ECMO decannulation. Three additional AEs, unrelated to micafungin, were observed in 2 infants; 1 infant had severe intracranial hemorrhage but survived, and another had necrotizing enterocolitis likely related to prematurity and anticoagulation resulting in mild gastrointestinal bleeding.
Discussion
This is the first PK trial of micafungin in infants supported with ECMO. Infants on ECMO had altered PK requiring higher doses to achieve the same exposure as historical controls not on ECMO.15,19,24-26 We suggest an alternate dosing regimen of 2.5 and 5 mg/kg/day for prophylaxis and treatment of invasive candidiasis, respectively. However, preterm neonates at high risk for hematogenous Candida meningoencephalitis should be excluded from this recommendation because we did not evaluate the higher dosages (e.g., 10mg/kg) usually recommended in this populations.27,28
The V of micafungin in our cohort was 20-90% higher than that reported in infants not on ECMO (0.34 – 0.54 L/kg).15,25 There are three probable explanations for the increased V: 1) the large volume of blood required to prime the ECMO circuit (~450mL) relative to an infant’s native blood volume (80 mL/kg); 2) direct adsorption of micafungin by components of the ECMO circuit,12-14 and 3) altered physiology (e.g., anasarca, inflammation) commonly seen in critically ill infants. The impact of the ECMO prime volume is most pronounced in smaller infants where an infant’s circulating blood volume may be doubled or even tripled. Because the impact of prime volume is directly related to the ratio between prime volume and native blood volume, our results should not be extrapolated to older children where the ratio of exogenous (450 mL) to native blood volume (~2000-5000 mL) is much lower. Our findings are consistent with previous literature reporting increased V due to the ECMO prime volume in infants on fluconazole, vancomycin and gentamicin.10,18,20,29-32
Drug adsorption to the ECMO circuit itself, which is well described in ex vivo studies, is also likely to contribute to the increase in V. Drug characteristics including lipophilicity and high protein binding are key factors driving adsorption.13,14 While micafungin is not lipophilic (logP −1.5), it is >99% protein bound.33 Other drugs with comparable protein binding and logP such as caspofungin were substantially adsorbed (56%) in an isolated ex vivo ECMO circuit.14 Preliminary results from a micafungin ECMO ex vivo study conducted by our group showed that micafungin concentrations dropped by 58% over 24h (Watt unpublished data). In summary, our study was not designed to isolate the source of altered V, but the increase in V was probably due to a combination of circuit prime volume, circuit adsorption of drug, and patient disease state.
In the current study, total CL after the first dose (0.041 L/kg/h [0.026, 0.062]) was in the upper range of previously reported values for infants not supported by ECMO (0.020-0.039 L/kg/h).19,24-26 The increase in CL may be explained by altered drug metabolism. Micafungin is excreted primarily unchanged into bile and feces (71%) via hepatic transporter proteins.34 Limited metabolism also occurs in the liver by arylsulfatase and catechol-O-methyltransferase into metabolites with little or no antifungal activity.15 While the impact of ECMO on renal dysfunction is well described,35 the effect of ECMO on hepatic transporters and metabolic capacity is unknown. Micafungin PK studies in adults with severe hepatic dysfunction, revealed lower micafungin exposure and higher CL compared with healthy controls.36,37 This phenomenon was attributed to lower albumin concentrations in adults with hepatic dysfunction, resulting in higher availability of the unbound fraction to be cleared. In our cohort, no subjects had hepatic dysfunction, but critically ill infants on ECMO typically have serum albumin concentrations in the lower range of normal. In our study, we did observe an inverse relationship between clearance and serum albumin (see figure, Supplemental Digital Content 2), with a higher micafungin CL in infants who had low serum albumin. Finally, we did not find a relationship between PK parameters and serum creatinine. This is consistent with previous literature and the product label that states no adjustment is necessary for renal dysfunction.15,36
Higher apparent micafungin elimination with ECMO may also be due to non-specific and irreversible drug adsorption by the circuit if adsorption is an ongoing process.12-14 As described above, substantial extraction was observed over 24h in ex vivo circuits (58%).12 The origin of this loss is still under investigation but could be due to circuit adsorption. It could also be explained by light degradation because micafungin is light-sensitive and the ECMO circuit is not protected from light, as opposed to drug vials and infusion bags.
The type of ECMO support could also impact micafungin disposition. Veno-venous ECMO, in which blood is drained and returned to the venous system via a single, double lumen cannula, is subject to a recirculation phenomenon. In recirculation, a portion of the oxygenated blood returned to the venous system is immediately taken back into the ECMO circuit via the drainage lumen of the cannula.38 The impact of recirculation on drug PK is unknown. However, this phenomenon increases the time drug spends in the circuit; and for drugs that are adsorbed by the circuit, this could decrease exposure (AUC). In our cohort, only 1 infant (ID #6) was on VV ECMO and had a high CL and a low AUC0-24h compared to the rest of the cohort. In veno-arterial (VA) ECMO deoxygenated blood is drained from the venous system and pumped directly to the arterial circulation, completely bypassing the heart and lungs. The return flow is non-pulsatile. Non-pulsatile blood flow may be associated with reduced renal function, but its impact on a drug cleared by transporters in the liver such as micafungin is unknown.39
PK changes described above translated into micafungin exposure within or above the prophylaxis target range for infants who received 4 mg/kg/day. Thus, a dose 4 mg/kg/day may be too high for prophylaxis. However, assuming linear micafungin PK, standard prophylaxis dosing (1 mg/kg/day) recommended for older children would likely result in underexposure in infants on ECMO.15,21,23,24 Based on the dose-exposure simulations in our cohort, a dose of 2.5 mg/kg/day appears to be the most appropriate dose for Candida prophylaxis. Although simulations showed that some children will likely become supratherapeutic on 2.5 mg/kg/day, we believe that it is better to err on the higher side of exposure for two reasons: 1) invasive candidiasis is exceedingly difficult to treat in this population and 2) the highest simulated exposure on 2.5 mg/kg/day (94 mg*h/L) was well below the mean (standard deviation) exposure of 438 mg*h/L (99) that was deemed safe in a neonatal trial of 15 mg/kg/day.26 This dosing regimen needs to be prospectively evaluated.
The one infant who received micafungin 8 mg/kg/day for suspected Candida infection achieved AUC0-24 above the therapeutic target range, and survived until hospital discharge. Based on these findings, and on dose-exposure simulations performed in all infants of the cohort, we recommend a dose of 5 mg/kg/day for invasive candidiasis therapy. Dose-exposure simulations were performed in all infants because PK is linear in this dosing range.21,40 On 5 mg/kg/day some children may achieve exposures exceeding the target range. Similar to the prophylactic regimen, we feel it is better to target higher exposure because these exposures are still in a “safe” range; and higher exposures may be needed to treat through potential fungal biofilms. Moreover, target exposure used in this study, was derived from a phase III efficacy study in which >85% of adults had their central catheter removed during candidemia treatment.22 In the setting of ECMO, removal of catheter is impossible and therefore, micafungin exposure required to clear the infection may be higher. This dosing regimen also needs prospective validation.
Although dosing in the current study was higher than recommended in term infants not on ECMO, micafungin had a favourable safety profile without any observed drug-related AEs. This is consistent with previous literature describing the safe use of micafungin up to 15 mg/kg/day in young preterm infants.26 Safety assessment is however limited by our small sample size.
Our study is the first PK trial of micafungin in infants supported by ECMO. Because our study population includes vulnerable infants, enrollment was challenging and resulted in a limited sample size. In spite of the small sample size and critical illness of our population, interindividual variability in PK parameters was as expected. Other factors may affect micafungin PK in this population, including transfusion of blood products which we did not collect. Our data were also limited by the lack of documentation of site of drug administration in 4 infants. However, the effect of site of drug administration on PK is unknown, and PK parameters of those 4 infants (ID #5; 8-10) were similar to those with complete documentation. Site of PK sampling was also lacking in 1 infant (ID #5), but our protocol did not allow drawing PK samples from site of drug administration. Therefore we do not expect any significant impact on PK results. Finally, our assessment of the ECMO impact on micafungin PK was limited by the lack of controls.
Conclusion
In this cohort of infants supported by ECMO, micafungin was well tolerated and the PK model indicated that V was higher and CL was in the upper range of what had been described previously in infants not on ECMO. In order to match exposure observed in adults, we recommend dosing of 2.5 and 5 mg/kg/day for prophylaxis and treatment of invasive candidiasis, respectively. These dosing regimens need prospective validation.
Supplementary Material
Acknowledgements
We thank the staff and research team in the pediatric critical care units at Duke University for their support of this study. We also thank Desiree Bonadonna for her help regarding the ECMO circuit configuration.
Disclosure of funding: This research was supported by the NIH (1K23HD075891 [PI Watt]; Pediatric Critical Care and Trauma Scientist Development Program, 5K12HD047349 [PI Dean]; Duke-UNC Clinical Pharmacology Training Grant, 1T32GM086330 [PIs Brouwer, Benjamin]) and the Thrasher Research Fund (PI Watt).
Footnotes
Address for reprints: 2400 Pratt Street, Durham, NC, 27705
References
- 1.Bartlett RH, Gattinoni L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva anestesiologica. 2010;76:534–540. [PubMed] [Google Scholar]
- 2.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2010;12:277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 3.Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infection and immunity. 2004;72:6023–6031. doi: 10.1128/IAI.72.10.6023-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eppes SC, Troutman JL, Gutman LT. Outcome of treatment of candidemia in children whose central catheters were removed or retained. Pediatr Infect Dis J. 1989;8:99–104. [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda F, Wakai Y, Matsumoto S, et al. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob Agents Chemother. 2000;44:614–618. doi: 10.1128/aac.44.3.614-618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko Y, Ohno H, Fukazawa H, et al. Anti-Candida-biofilm activity of micafungin is attenuated by voriconazole but restored by pharmacological inhibition of Hsp90-related stress responses. Med Mycol. 2010;48:606–612. doi: 10.3109/13693780903426721. [DOI] [PubMed] [Google Scholar]
- 8.Tawara S, Ikeda F, Maki K, et al. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother. 2000;44:57–62. doi: 10.1128/aac.44.1.57-62.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996;40:1139–1142. doi: 10.1128/aac.40.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watt KM, Benjamin DK, Jr., Cheifetz IM, et al. Pharmacokinetics and safety of fluconazole in young infants supported with extracorporeal membrane oxygenation. Pediatr Infect Dis J. 2012;31:1042–1047. doi: 10.1097/INF.0b013e31825d3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33:1018–1024. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 12.Watt KMC-WM, Williams D, Bonadonna D, Cheifetz I, Benjamin DK, Jr, Brouwer KL. Antifungal extraction by the extracorporeal membrane oxygenation (ECMO) circuit ex vivo; Paper presented at: American Society for Clinical Pharmacology and Therapeutics Annual meeting; New Orleans, LA. Mar 3-7, 2015. 2015. [Google Scholar]
- 13.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive care medicine. 2010;36:2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shekar K, Roberts JA, McDonald CI, et al. Critical care (London, England) 2015;19:164. doi: 10.1186/s13054-015-0891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astellas Mycamine (micafungin) [package insert] 2013 http://www.astellas.us/docs/mycamine.pdf. Accessed 03-04-2015, 2015.
- 16.Use EMAEoMfH ASSESSMENT REPORT FOR Mycamine. 2008 [Google Scholar]
- 17.Lat A, Thompson GR, 3rd, Rinaldi MG, Dorsey SA, Pennick G, Lewis JS., 2nd Micafungin concentrations from brain tissue and pancreatic pseudocyst fluid. Antimicrob Agents Chemother. 2010;54:943–944. doi: 10.1128/AAC.01294-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1992;12:28–32. [PubMed] [Google Scholar]
- 19.Hope WW, Kaibara A, Roy M, et al. Population pharmacokinetics of micafungin and its metabolites M1 and M5 in children and adolescents. Antimicrob Agents Chemother. 2015;59:905–913. doi: 10.1128/AAC.03736-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Southgate WM, DiPiro JT, Robertson AF. Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1989;33:817–819. doi: 10.1128/aac.33.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seibel NL, Schwartz C, Arrieta A, et al. Safety, tolerability, and pharmacokinetics of Micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother. 2005;49:3317–3324. doi: 10.1128/AAC.49.8.3317-3324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–1527. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 23.van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 24.Heresi GP, Gerstmann DR, Reed MD, et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. Pediatr Infect Dis J. 2006;25:1110–1115. doi: 10.1097/01.inf.0000245103.07614.e1. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin DK, Jr., Smith PB, Arrieta A, et al. Safety and pharmacokinetics of repeat-dose micafungin in young infants. Clin Pharmacol Ther. 2010;87:93–99. doi: 10.1038/clpt.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith PB, Walsh TJ, Hope W, et al. Pediatr Infect Dis J. 2009;28:412–415. doi: 10.1097/INF.0b013e3181910e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez M, Moylett EH, Noyola DE, Baker CJ. Candidal meningitis in neonates: a 10-year review. Clin Infect Dis. 2000;31:458–463. doi: 10.1086/313973. [DOI] [PubMed] [Google Scholar]
- 28.Hope WW, Seibel NL, Schwartz CL, et al. Population pharmacokinetics of micafungin in pediatric patients and implications for antifungal dosing. Antimicrob Agents Chemother. 2007;51:3714–3719. doi: 10.1128/AAC.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1998;18:1082–1086. [PubMed] [Google Scholar]
- 30.Cohen P, Collart L, Prober CG, Fischer AF, Blaschke TF. Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J. 1990;9:562–566. doi: 10.1097/00006454-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Hoie EB, Swigart SA, Leuschen MP, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. 1990;9:711–715. [PubMed] [Google Scholar]
- 32.Munzenberger PJ, Massoud N. Pharmacokinetics of gentamicin in neonatal patients supported with extracorporeal membrane oxygenation. ASAIO Trans. 1991;37:16–18. doi: 10.1097/00002480-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Wishart DS, Knox C, Guo AC, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic acids research. 2008;36:D901–906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanni SB, Augustijns PF, Benjamin DK, Jr., Brouwer KL, Thakker DR, Annaert PP. In vitro investigation of the hepatobiliary disposition mechanisms of the antifungal agent micafungin in humans and rats. Drug Metab Dispos. 2010;38:1848–1856. doi: 10.1124/dmd.110.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta P, Carlson J, Wells D, et al. Relationship between renal function and extracorporeal membrane oxygenation use: a single-center experience. Artificial organs. 2015;39:369–374. doi: 10.1111/aor.12379. [DOI] [PubMed] [Google Scholar]
- 36.Hebert MF, Smith HE, Marbury TC, et al. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J Clin Pharmacol. 2005;45:1145–1152. doi: 10.1177/0091270005279580. [DOI] [PubMed] [Google Scholar]
- 37.Undre N, Pretorius B, Stevenson P. Pharmacokinetics of micafungin in subjects with severe hepatic dysfunction. European journal of drug metabolism and pharmacokinetics. 2015;40:285–293. doi: 10.1007/s13318-014-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2015;61:115–121. doi: 10.1097/MAT.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 39.Nam MJ, Lim CH, Kim HJ, et al. A Meta-Analysis of Renal Function After Adult Cardiac Surgery With Pulsatile Perfusion. Artificial organs. 2015;39:788–794. doi: 10.1111/aor.12452. [DOI] [PubMed] [Google Scholar]
- 40.Tabata K, Katashima M, Kawamura A, Tanigawara Y, Sunagawa K. Linear pharmacokinetics of micafungin and its active metabolites in Japanese pediatric patients with fungal infections. Biol Pharm Bull. 2006;29:1706–1711. doi: 10.1248/bpb.29.1706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.