Background
The CYP24A1 gene encodes a mitochondrial 24-hydroxylase that metabolizes both 25(OH)D and 1,25(OH)2D. The induction of CYP24A1 gene expression by both hypercalcemia and 1,25(OH)2D provides a safeguard against vitamin D-induced hypercalcemia.(1) Conversely, inactivating mutations in CYP24A1 can cause hypercalcemia. Following the introduction of vitamin D fortification of infant formula in Great Britain in the 1950s, Lightwood described an epidemic of idiopathic infantile hypercalcemia and postulated supranormal sensitivity to vitamin D as the culprit.(2,3) Subsequently, Schlingman identified loss-of-function mutations in CYP24A1 in a kindred of idiopathic infantile hypercalcemia.(4) CYP24A1 deficiency has since been reported to cause hypercalcemia in all age groups.(5)
During pregnancy, 25(OH)D 1-alpha hydroxylase is expressed by the placenta and upregulated in the maternal kidney, leading to a physiological doubling of maternal 1,25(OH)2D levels.(6) Since most prenatal vitamin supplements provide 400 IU/day of vitamin D, one might expect pregnancy to increase the phenotypic penetrance of CYP24A1 deficiency. However, only two cases of gestational hypercalcemia have been attributed to CYP24A1 deficiency.(7,8) Herein we report the case of a 20-year-old woman with a homozygous inactivating CYP24A1 mutation who developed recurrent gestational hypercalcemia and pancreatitis.
Case
A 20-year-old primipara was referred to our medical center at 23 weeks of gestation due to severe maternal hypertension and intrauterine growth restriction of twin fetuses. Following the demise of both fetuses, labor was induced at 26 weeks. Immediately postpartum, her mental status became altered and hypercalcemia was noted at 14 mg/dL (albumin 3.0 gm/dL). She was treated with saline infusion and one dose of subcutaneous calcitonin (4 units/kg). Biochemical workup revealed a suppressed PTH (6 pg/mL), an undetectable PTHrP (<2.8 pmol/L), a 25(OH)D of 45 ng/mL, and elevated 1,25(OH)2D levels (165 and 195 pg/mL on postpartum day 1 and 5, respectively). A subsequent review of her outside medical records revealed the presence of hypercalcemia (12.3 mg/dL) at 12 weeks of gestation that had not been further evaluated.
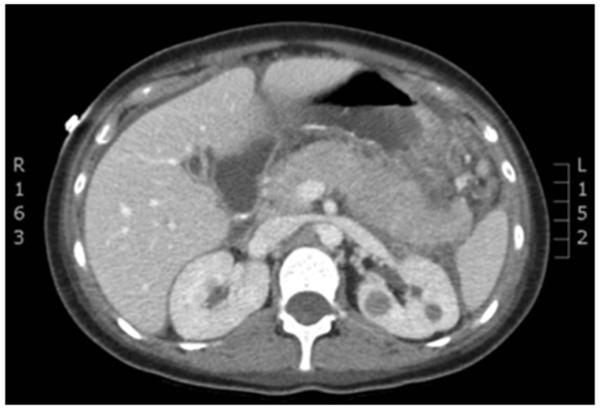
In addition to altered mental status, the patient developed fevers, tachycardia and epigastric pain. Acute pancreatitis was diagnosed on postpartum day 3 with a serum lipase level of 1,416 U/L. Her triglyceride levels were 140 mg/dL. An abdominal CT scan revealed an edematous pancreas and moderate ascites but no evidence of cholelithiasis (Fig. 1). Also noted was extensive mesenteric and omental thickening that, along with an abrupt drop in serum calcium (Fig. 3), suggested saponification associated with pancreatitis (Fig. 1).
Figure 1.
Abdominal CT scan showing an edematous pancreas, simple ascites, and renal cysts without evidence of nephrocalcinosis.
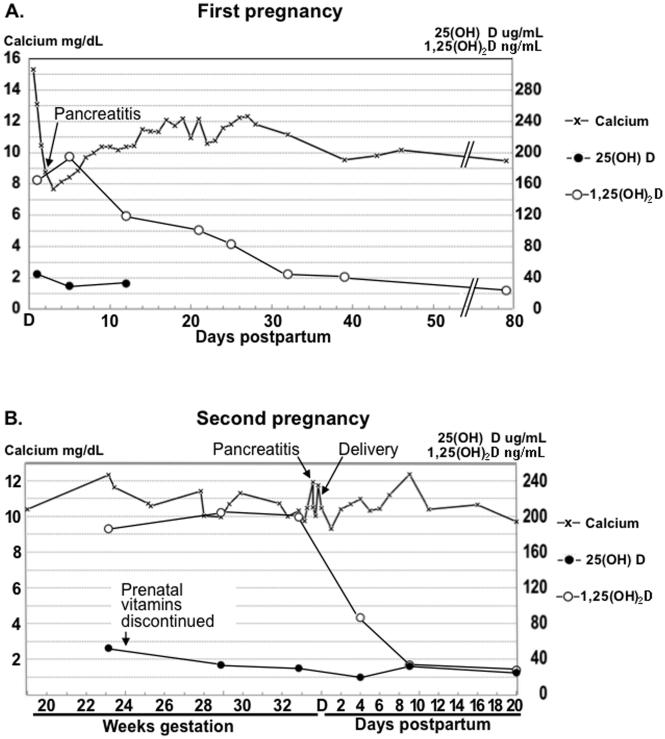
Figure 3.
Serum levels of calcium, 25(OH)D, and 1,25(OH)2D during first pregnancy (a) and second pregnancy (b).
Prior to the pregnancy, she weighed 41.3 kg (BMI 17.8 kg/m2) and did not receive regular medical care. She has had hypertension since childhood. Her medications during pregnancy consisted of a prenatal vitamin (discontinued postpartum) and labetalol (200 mg twice daily). She had no prior history of hypercalcemia, kidney stones, fractures, pancreatitis, or alcohol use. Renal ultrasound revealed simple cysts bilaterally but no nephrolithiasis or nephrocalcinosis. She was born in Mexico. Her parents were from the same town but not known to be blood-related. There was no family history of calcium disorders, kidney stones, or pancreatitis.
A search for potential causes of her vitamin D-mediated hypercalcemia revealed an angiotensin converting enzyme level of 8 U/L (reference range 9-13 U/L) and a positive QuantiFERON test. Her ascites fluid showed a serum-ascites albumin gradient of 0.3 gm/dL and tested negative in bacterial and mycobacterial cultures as well as acid-fast bacilli (AFB) stains. Her chest radiograph revealed a calcified granuloma (5 mm) in the right upper lobe. This was redemonstrated by a chest CT, which unexpectedly also revealed multiple pulmonary emboli but no mediastinal lymphadenopathy, active pulmonary tuberculosis, sarcoidosis or malignancy. A bone marrow biopsy revealed normal cellularity and hematopoiesis, without evidence of lymphoma, granuloma, mycobacteria, or growth in AFB culture. Under the suspicion that sarcoidosis might be responsible for her hypercalcemia (12.0 mg/dL), she was given one 40-mg dose of prednisone on postpartum day 26. The next day, her serum calcium levels remained unchanged. She was discharged on postpartum day 28 with instructions to continue an anticoagulant and amlodipine. Between one and three months postpartum, her serum calcium and 1,25(OH)2D levels decreased from 11.4 to 10.2 mg/dL and from 83 to 24 pg/mL respectively. Over the ensuing three months, her serum calcium remained between 10.0-10.2 mg/dL and her 24-hour urine calcium was 277 mg.
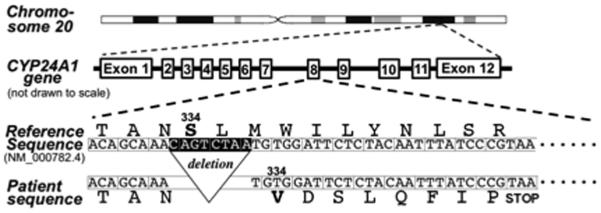
She became pregnant again six months postpartum and resumed prenatal vitamin supplementation. At 14 weeks of gestation, her calcium was 10.0 mg/dL (albumin 3.5 gm/dL) and her 1,25(OH)2D level exceeded 200 pg/mL. To establish the diagnosis of CYP24A1 deficiency, we found her 24,25(OH)2D level (both D2 and D3) to be below the detection limit of 2 ng/mL while a concurrent 25(OH)D level was 53 ng/mL. Exon sequencing of the CYP24A1 gene in peripheral leucocytes revealed a homozygous, 8-nucleotide deletion (c.999_1006del, p.Ser334Valfs*9) in exon 8, causing an S334V substitution and premature termination due to a frame shift (Fig. 2).
Figure 2.
The intron-exon structure of the CYP24A1 locus on chromosome 20. Deletion of the 8 nucleotides highlighted in exon 8 (c.999_1006del) causes a valine substitution of Ser334 followed by a premature stop codon (p.Ser334Valfs*9). This frameshift deletion corresponds to a minor CYP24A1 allele (SNP ID: rs770055617, http://www.ncbi.nlm.nih.gov/snp) with an apparent allele frequency of <0.0005. CYP24A1 homozygosity in the patient was confirmed by additional deletion/duplication analyses (data not shown).
Upon confirmation of CYP24A1 deficiency at 22 weeks of gestation, the patient was advised to discontinue prenatal vitamins and to minimize sun exposure and calcium-rich foods. To reduce intestinal calcium absorption, she was prescribed omeprazole (20 mg daily) and phosphate (250 mg of K-Phos Neutral with each meal). Despite these measures, her calcium levels rose to 11.5 mg/dL (albumin 3.5 gm/dL) at 33 weeks of gestation, when severe epigastralgia led to the diagnosis of acute pancreatitis. Her pancreas was edematous and enlarged on ultrasound. Her amylase and lipase levels peaked at 1,688 U/L and 4,440 U/L respectively. Since neither cholelithiasis nor dilatation of the biliary tree was found on ultrasound or magnetic resonance cholangiopancreatography, we attributed the pancreatitis to hypercalcemia.
She received aggressive saline infusion and one dose of subcutaneous calcitonin for her pancreatitis and hypercalcemia. Because of worsening hypertension, labor was induced at 34 weeks. The male newborn weighed 1,786 gm (14.5 percentile), had a cord blood calcium level of 9.2 mg/dL, and showed no signs of hypercalcemia or hypocalcemia during the 12 days of hospitalization. Comparisons of maternal serum (8 days antepartum) to cord blood showed a transplacental gradient of 30 to 14 ng/mL for 25(OH)D, and 199 to 39 pg/mL for 1,25(OH)2D. The patient was discharged on postpartum day 7 with a serum calcium level of 10.7 mg/dL and 1,25(OH)2D level of 87 pg/mL. She self-injected calcitonin (100 ug) on postpartum days 10, 13 and 18 while her calcium decreased from 12 to 9.8 mg/dL. Her calcium level was 10.0 mg/dL (albumin 4.5 gm/dL) six months postpartum.
Discussion
CYP24A1 mutations can cause hypercalcemia, nephrolithiasis, and nephrocalcinosis that manifest during infancy(4,9) or adulthood.(5,7,8,10) This report describes a CYP24A1-deficient young woman with gestational hypercalcemia during two consecutive pregnancies, each confounded by acute pancreatitis, a complication not previously attributed to CYP24A1 mutations. The pathogenic 8-nt deletion (c.999_1006del) has not been reported in CYP24A1-related hypercalcemia. This deletion is expected to abolish the 24-hydroxylase activity of CYP24A1 since the resulting premature stop codon is predicted to trigger degradation of the mRNA(11) and the deletion removes the heme-binding domain (aa 455-464) critical to enzymatic activity.(12)
A plausible explanation for the limited penetrance of her biallelic CYP24A1 mutation is that she has consistently avoided sun exposure since age 4, when the exposure was noted to cause a rash. Alternatively, she might have been protected from vitamin D-mediated hypercalcemia by compensatory mechanisms that downregulate 1,25(OH)2D production or upregulate its degradation through CYP24A1-independent pathways as have been proposed by mouse models of CYP24A1 deficiency.(13)
During pregnancy and peripartum, several factors could synergize to unmask the hypercalcemic effect of CYP24A1 deficiency. These include increased calciferol intake from prenatal vitamins, increased activation of 25(OH)D by the placenta and maternal kidney, the cessation of transplacental calcium flux at delivery,(14,15) and the production of PTHrP by the placenta (16) and lactating mammary gland.(17) We expect these gestation-specific hypercalcemic factors to be poorly tolerated in the CYP24A1-deficient state, where the already-suppressed PTH levels offer no room for further suppression.
Our patient shared several gestational complications with pregnant women who have PTH-mediated hypercalcemia. These include worsening hypertension, peripartum hypercalcemic crisis, pancreatitis (both pregnancies), and fetal demise (first pregnancy).(14,18) Although not previously linked to CYP24A1 deficiency, the pancreatitis in our patient was likely attributable to hypercalcemia since she did not have the usual risk factors like cholelithiasis, hypertriglyceridemia, or alcohol use.
Maternal PTH-mediated hypercalcemia is known to cause transient neonatal hypocalcemia.(19) Our patient’s baby was free from hypocalcemia presumably because of our multipronged approach to minimize gestational hypercalcemia. The baby was also free from hypercalcemia, in line with the typically recessive nature of CYP24A1-related hypercalcemia.(4) The normal 1,25(OH)2D level in the cord blood (39 ng/mL) despite a maternal level of 199 ng/ml was also in keeping with the limited placental transfer of this vitamin.(20)
The calcemic effect of breastfeeding has not been investigated in CYP24A1-deficient mothers. By stimulating mammary production of PTHrP, lactation often induces mild hypercalcemia(21) and occasionally causes symptomatic hypercalcemia.(22) We advised our patient to stop breastfeeding after colostrum production due to the concern that lactation-induced hypercalcemia might exacerbate ongoing hypercalcemia and acute pancreatitis. Indeed, her PTHrP level on postpartum day 4 was found to be 3.4 pmol/L, the upper limit of the normal range.
It is noteworthy that during the resolution of hypercalcemia after each pregancy, our patient transitioned through a phase when hypercalcemia was accompanied by 1,25(OH)2D levels that fell within the reference range (20–79 pg/mL) (Fig. 3). An analogous transitional phase has been observed in sarcoid patients with hypercalcemia, where glucocorticoid treatment normalizes 1,25(OH)2D levels ~1 week prior to the resolution of hypercalcemia (3-7 vs. 12-15 days).(23) This illustrates the caveat that normal levels of 1,25(OH)2D do not exonerate this hormone as the cause of hypercalcemia.
Our patient received one 40-mg dose of prednisone just before leaving the hospital following the first pregnancy, when sarcoidosis was presumed to cause her hypercalcemia. Although this trial of glucocorticoids was of inadequate duration to demonstrate a treatment effect, or lack thereof, available literature suggests that CYP24A1-associated hypercalcemia does not respond to glucocorticoids.(10,24)
Historically, the cause of 1,25(OH)2D-mediated hypercalcemia remains unidentified in up to 18% of cases.(25) Given the recent realization that many of these cases are due to CYP24A1 mutations(26), measurement of plasma 24,25(OH)2D level and its ratio over 25(OH)D should be included in the evaluation of vitamin D-mediated hypercalcemia. A low ratio (<0.04 in our case) suggests impaired vitamin D catabolism and may warrant sequencing of the CYP24A1 gene. As reported previously (7,8) and herein, hypercalcemia from CYP24A1 deficiency may be unmasked by the physiologic changes of pregnancy. It is therefore important to establish a pregnancy- or trimester-specific reference range for 24,25(OH)2D and its ratio over 25(OH)D.
In summary, hypercalcemia caused by CYP24A1 deficiency may manifest only during pregnancy in women without baseline hypercalcemia or nephrocalcinosis. Potential complications in pregnancy may include maternal hypertension, acute pancreatitis, and fetal demise. CYP24A1 deficiency should be considered in patients with otherwise unexplained vitamin D-mediated hypercalcemia, including those with “inappropriately normal” 1,25(OH)2D levels or subnormal levels of 24,25(OH)2D. Behavioral management of CYP24A1-related hypercalcemia includes the avoidance of sun exposure, vitamin D and dietary calcium. Future studies will investigate the indications and modalities of pharmacological management during pregnancy and the safety of breastfeeding.
Acknowledgments
Supported in part by an NIH T32 training grant (DK007044).
Footnotes
Authors’ roles: Data analysis: AS, HG, NC, NWC and RF. Data interpretation: GW and NWC. Manuscript preparation and revision: GW and NWC. Approving final version of manuscript: all authors.
REFERENCES
- 1.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523(1):9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Lightwood R, Stapleton T. Idiopathic hypercalcaemia in infants. Lancet. 1953;265(6779):255–6. doi: 10.1016/s0140-6736(53)90187-1. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton T, Macdonald WB, Lightwood R. The pathogenesis of idiopathic hypercalcemia in infancy. Am J Clin Nutr. 1957;5(5):533–42. doi: 10.1093/ajcn/5.5.533. [DOI] [PubMed] [Google Scholar]
- 4.Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365(5):410–21. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- 5.Molin A, Baudoin R, Kaufmann M, et al. CYP24A1 Mutations in a Cohort of Hypercalcemic Patients: Evidence for a Recessive Trait. J Clin Endocrinol Metab. 2015;100(10):E1343–52. doi: 10.1210/jc.2014-4387. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18(6):832–72. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 7.Dinour D, Davidovits M, Aviner S, et al. Maternal and infantile hypercalcemia caused by vitamin-D-hydroxylase mutations and vitamin D intake. Pediatr Nephrol. 2015;30(1):145–52. doi: 10.1007/s00467-014-2889-1. [DOI] [PubMed] [Google Scholar]
- 8.Shah AD, Hsiao EC, O'Donnell B, et al. Maternal Hypercalcemia Due to Failure of 1,25-Dihydroxyvitamin-D3 Catabolism in a Patient With CYP24A1 Mutations. J Clin Endocrinol Metab. 2015;100(8):2832–6. doi: 10.1210/jc.2015-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fencl F, Blahova K, Schlingmann KP, Konrad M, Seeman T. Severe hypercalcemic crisis in an infant with idiopathic infantile hypercalcemia caused by mutation in CYP24A1 gene. Eur J Pediatr. 2013;172(1):45–9. doi: 10.1007/s00431-012-1818-1. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs TP, Kaufman M, Jones G, et al. A lifetime of hypercalcemia and hypercalciuria, finally explained. J Clin Endocrinol Metab. 2014;99(3):708–12. doi: 10.1210/jc.2013-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JN, Pearce DA. Nonsense-mediated decay in genetic disease: friend or foe? Mutat Res Rev Mutat Res. 2014;762:52–64. doi: 10.1016/j.mrrev.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degtyarenko KN, Archakov AI. Molecular evolution of P450 superfamily and P450-containing monooxygenase systems. FEBS Lett. 1993;332(1-2):1–8. doi: 10.1016/0014-5793(93)80470-f. [DOI] [PubMed] [Google Scholar]
- 13.Byford V, Arabian A, et al. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146(2):825–34. doi: 10.1210/en.2004-1116. [DOI] [PubMed] [Google Scholar]
- 14.Dochez V, Ducarme G. Primary hyperparathyroidism during pregnancy. Arch Gynecol Obstet. 2015;291(2):259–63. doi: 10.1007/s00404-014-3526-8. [DOI] [PubMed] [Google Scholar]
- 15.Matthias GS, Helliwell TR, Williams A. Postpartum hyperparathyroid crisis. Case report. Br J Obstet Gynaecol. 1987;94(8):807–10. doi: 10.1111/j.1471-0528.1987.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 16.Curtis NE, Gillespie MT, King RG, Rice GE, Wlodek ME. Parathyroid hormone-related protein (PTHrP) mRNA splicing and parathyroid hormone/PTHrP receptor mRNA expression in human placenta and fetal membranes. J Mol Endocrinol. 1998;21(2):225–34. doi: 10.1677/jme.0.0210225. TR. [DOI] [PubMed] [Google Scholar]
- 17.Rude RK, Haussler MR, Singer FR. Postpartum resolution of hypocalcemia in a lactating hypoparathyroid patient. Endocrinol Jpn. 1984;31(3):227–33. doi: 10.1507/endocrj1954.31.227. [DOI] [PubMed] [Google Scholar]
- 18.Norman J, Politz D, Politz L. Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: a call for earlier intervention. Clin Endocrinol (Oxf) 2009;71(1):104–9. doi: 10.1111/j.1365-2265.2008.03495.x. [DOI] [PubMed] [Google Scholar]
- 19.Mestman JH. Parathyroid disorders of pregnancy. Semin Perinatol. 1998;22(6):485–96. doi: 10.1016/s0146-0005(98)80028-1. [DOI] [PubMed] [Google Scholar]
- 20.Greer FR. 25-Hydroxyvitamin D: functional outcomes in infants and young children. Am J Clin Nutr. 2008;88(2):529S–33S. doi: 10.1093/ajcn/88.2.529S. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40(4):795–826. doi: 10.1016/j.ecl.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Sato K. Hypercalcemia during pregnancy, puerperium, and lactation: review and a case report of hypercalcemic crisis after delivery due to excessive production of PTH-related protein (PTHrP) without malignancy (humoral hypercalcemia of pregnancy) Endocr J. 2008;55(6):959–66. doi: 10.1507/endocrj.k08e-092. [DOI] [PubMed] [Google Scholar]
- 23.Sandler LM, Winearls CG, Fraher LJ, Clemens TL, Smith R, O'Riordan JL. Studies of the hypercalcaemia of sarcoidosis: effect of steroids and exogenous vitamin D3 on the circulating concentrations of 1,25-dihydroxy vitamin D3. Q J Med. 1984;53(210):165–80. [PubMed] [Google Scholar]
- 24.Colussi G, Ganon L, Penco S, et al. Chronic hypercalcaemia from inactivating mutations of vitamin D 24-hydroxylase (CYP24A1): implications for mineral metabolism changes in chronic renal failure. Nephrol Dial Transplant. 2014;29(3):636–43. doi: 10.1093/ndt/gft460. [DOI] [PubMed] [Google Scholar]
- 25.Donovan PJ, Sundac L, Pretorius CJ, d'Emden MC, McLeod DS. Calcitriol-mediated hypercalcemia: causes and course in 101 patients. J Clin Endocrinol Metab. 2013;98(10):4023–9. doi: 10.1210/jc.2013-2016. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann M, Gallagher JC, Peacock M, et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab. 2014;99(7):2567–74. doi: 10.1210/jc.2013-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]