Abstract
In this study, the role of CX3CR1 in the progression of diabetic retinopathy (DR) was investigated. The retinas of wild type (WT), CX3CR1 null (CX3CR1gfp/gfp, KO) and heterozygous (CX3CR1+/gfp, Het) mice were compared in the presence and absence of streptozotocin (STZ) induced diabetes. CX3CR1 deficiency in STZ-KO increased vascular pathology at 4 months of diabetes, as a significant increase in acellular capillaries was observed only in the STZ-KO group. CX3CR1 deficiency and diabetes had similar effects on retinal neurodegeneration measured by an increase in DNA fragmentation. Retinal vascular pathology in STZ-KO mice was associated with increased numbers of monocyte-derived macrophages in the retina. Furthermore, compared to STZ-WT, STZ-KO mice exhibited increased numbers of inflammatory monocytes in the bone marrow and impaired homing of monocytes to the spleen. Induction of retinal IL-10 expression by diabetes was significantly less in KO mice, and when bone marrow-derived macrophages from KO mice were maintained in high glucose they expressed significantly less IL-10 and more TNF-α in response to LPS stimulation. These findings support that CX3CR1 deficiency accelerates the development of vascular pathology in DR through increased recruitment of proinflammatory myeloid cells that demonstrate reduced expression of anti-inflammatory IL-10.
Keywords: CX3CR1, diabetes, retinopathy, macrophages, apoptosis, IL-10
INTRODUCTION
Diabetic retinopathy (DR) is the leading cause of blindness in working-age adults. During the first two decades of the disease, nearly all individuals with type 1 diabetes and approximately 60% of individuals with type 2 diabetes will develop some degree of DR [1]. It is therefore imperative to understand the progression of the disease from the early to late stages. Currently, there are no treatment options for the early phase DR, with interventions only becoming available after vision has been compromised [2]. This limits management of DR risk to aggressive regulation of hyperglycemia, serum hyperlipidemia and hypertension [2].
Microvascular changes remain the clinical hallmark of DR and the current therapeutic target. Our understanding of its pathobiology includes inflammation and neurodegeneration in the early phase of the disease [3]. In the mouse streptozotocin (STZ) induced type 1 model, inflammation is detected within 2–8 weeks of initiation of diabetes, evidenced by activation of the endothelium, low-level elevation of pro-inflammatory cytokine expression and leukostasis [4]. At the same time, microglia, the endogenous immune cells of CNS and the retina, show evidence of activation, with decreased ramification and slightly increased expression of pro-inflammatory cytokines [5]. Apoptosis of retinal neurons is also initiated during this early stage of DR, leading to accumulative loss of retinal ganglion cells (RGC) [3,6–8]. Finally, after approximately 6 months of experimental diabetes, vascular pathology is evident in the murine diabetic model [4]. Thus, in the time window between 2 and 6 months the functions of resident microglia and invading monocytes, including their role in clearance of apoptotic cells, may be key determinants of maintaining retinal homeostasis and are critical to avoiding a severe inflammatory response. In analogy to other chronic neurodegenerative diseases [10,11], it is feasible that a first wave of the inflammatory cascade, including the expression of “on” cytokines such as TNFα and IL-1β, is counterbalanced by expression anti-inflammatory “off” cytokines such as IL-10 produced during clearance of apoptotic cells.
In peripheral tissues CX3CR1 is expressed by innate immune cells [12,13], including, a subset of Ly6Clo/CX3CR1hi monocytes that have the unconventional role of maintaining vascular endothelial cell homeostasis [14]. They patrol the luminal side of the endothelium, scavenge cellular debris, and are believed to occasionally extravagate and differentiate into inflammatory macrophages or dendritic cells [14]. These patrolling monocytes are associated with resolution of inflammation and are considered anti-inflammatory. In contrast, classical pro-inflammatory Ly6Chi/CCR2+ monocytes lack CX3CR1 expression. We have consistently shown that diabetes skews hematopoiesis towards a myeloid phenotype with an increased infiltration of inflammatory CCR2+ monocytes in the retina [15–19].
In the CNS and retina CX3CR1 is expressed by microglial cells [20]. Microglia are the endogenous retinal immune cells, distributed throughout the retinal parenchyma, continuously surveying their microenvironment with their long processes [5,21]. In response to infection or retinal damage they become activated, retract their processes and produce damaging neuroinflammatory mediators, including, inflammatory cytokines, nitric oxide and reactive oxygen species that can contribute to killing of neurons [5]. Microglia also maintain homeostasis of neural tissue by phagocytosis of apoptotic neurons and cell debris.
Recent studies suggest that the CX3CR1 and its ligand, CX3CL1, play an important role in suppression of neuroinflammation and controlling the fate of the neurons. CX3CL1 is expressed on the surface neurons in the CNS and endothelial cells in non-neuronal tissues [22]. In the mouse retina, CX3CL1 is expressed on ganglion cells and within the inner plexiform layer [23]. Activation and downstream effects of the CX3CL1-CX3CR1 signaling are complex, tissue-dependent and are likely influenced by many factors. For example, it has been implicated in suppression of inflammation in the eye [24–26] and exacerbation of macrovascular disease [27,28]. Furthermore, in the CNS, CX3CR1 can exert both neuroprotective and neurotoxic effects, depending on the pathological scenario [29]. Therefore, this chemokine pathway requires tissue- and disease-specific characterization.
Few publications report on the CX3CL1-CX3CR1 axis in DR. In these, diabetic mice have been shown to exhibit increased infiltration of CD11b+ cells with elevated expression of CX3CR1 in the retina [30,31]. These studies were done in murine models of diabetes at early times points (less than 2 months of diabetes). Additionally, a recent study by Cardona and coworkers found that CX3CL1 levels were increased in the retina of diabetic mice [32], while CX3CR1 deletion resulted in enhanced activation of microglia and increased loss of RGC and their axons. However, the vascular phenotype was not examined. Furthermore, a study by McMenamin’s group demonstrated that CX3CR1 deficiency exacerbated diabetes-associated activation of retinal microglia and accumulation macrophages in the vitreous and subretinal space [33].
In the present study, we investigated the effects of CX3CR1 absence on the development of DR. We chose 4 months as an endpoint for our studies in order to examine the effects of CX3CR1 deficiency in the window of time between inflammation and vascular defects and to determine if progression of vascular disease was affected by CX3CR1 deletion. We found that CX3CR1 deletion accelerated vascular pathology in the retina, increased retina cell apoptotic death with coincident activation of local microglia or infiltration of macrophages. We further observed that CX3CR1 deletion in bone marrow derived macrophages reduced their IL-10 expression, signifying a role for this cytokine at the critical homeostatic window of 4 months diabetes in the experimental STZ murine model.
RESEARCH DESIGN AND METHODS
Animal studies
All mice used in this study were bred in the University of Florida facility and were on the C57BL/6J genetic background. CX3CR1gfp/gfp and C57Bl/6J mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) CX3CR1gfp/gfp have been backcrossed to the C57Bl/6J background for more than 12 generations. CX3CR1+/gfp were generated by breeding CX3CR1gfp/gfp with C57BL/6J mice. The experimental groups include: CX3CR1gfp/gfp mice, referred to as “KO”; CX3CR1+/+ mice, referred to as “WT”, and CX3CR1gfp/+ mice, referred as “Het”. The studies presented included both male and female mice. The procedures were carried out in accordance with the guidelines of the University of Florida Institutional Animal Care and Use Committee (IACUC). Type 1 diabetes was induced by intraperitoneal injection of STZ (50 mg/kg in 100mM sodium citrate, adjusted to pH 4.5) for five consecutive days. Control mice were injected with vehicle (Veh) alone. Diabetes was verified two weeks later by measuring blood glucose (defined as >250 mg/dL) using a glucose meter (Glucometer Elite XL; Bayer Corp, Elkhart, IN) according to the manufacturer’s instructions. Six experimental groups were established: 1) Veh-WT, 2) Veh-Het, 3) Veh-KO, 4) STZ-WT 5) STZ-Het and 6) STZ-KO. Animals were maintained in a hyperglycemic state for 4 months. Glycated hemoglobin was measured at the end of the study and animals were considered diabetic if > 7.5%.
Trypsin digest preparation of retinal vasculature
Retinal vasculature was prepared as previously described [34] with minor modifications. Briefly, fixed retinas were digested in 3% trypsin (BD Biosciences, San Jose, CA) in 0.1M Tris buffer in 2–4 cycles of 30 min digestion at 37°C on a slow shaker and washed in water until all only vascular tissue remained. The vasculature was mounted on a clean slide, allowed to dry, stained with PAS-H&E (Sigma, St. Louis, MO), dehydrated and mounted in Permount mounting media (Sigma). Slides were scanned by Aperio CS slide-scanning system with Spectrum Plus information management system (Aperio Technologies, Inc. Vista, CA). Ten to 15 random, non-overlapping fields from each retina were imaged. Acellular capillaries of >50 μm in length were counted from images for each retina and expressed as number of acellular vessels per mm2.
Cell preparation
Retinas, and hypothalamus were shipped overnight to Duke University where single cell suspensions were prepared the next day. Spleens, hind legs and blood were processed the same day at University of Florida, according previous published methods [15]. Retinas from 8 mice per group were pooled together in 15 mL tubes and were digested with 2 mg/mL Collagenase D (Roche, Indianapolis, IN) at 37°C for 45 min according to O’Koren et al. 2016 [35]. Cells were washed twice and then stained with antibodies for flow cytometry as described in the following sections.
DNA fragmentation assay
Apoptotic DNA cleavage was assayed using an ELISA Kit (Cell Death Detection, Roche Applied Science, Indianapolis, IN) and normalized to retinal wet weight following exactly the method previously described by Abcouwer et al 2010 [36]. Relative DNA fragmentation was expressed as optical density (light absorbance at 405 nm with a 490 nm reference wavelength) normalized by retinal mass in each aliquot of retinal supernatant.
Flow cytometry and FACS analysis
Antibodies were purchased from BD Biosciences, San Jose, CA unless otherwise stated. Combinations of the following antibodies were used for staining: PerCPCy5.5- CD3e (145-2C11); PerCPCy5.5- CD19 (1D3); PerCPCy5.5- NK1.1 (PK136), PerCPCy5.5- Ly6G (1A8), APC- CD11c (HL3); APC- or Pacific Blue- F4/80 (BM8); APC- IA-b (AF6-120.1), PE- CCR2 (475301); PECy7-Ly6C (Al-21); APC-Cy7- or PE- CD11b (M1/7); PE-CF594- CD45 (30-F11) DAPI; fixable-viability dye efluor780 (ebiosciences). Retinal and brain samples were stained with antibodies against F4/80, PECy7, IA/IE, Ly6G, CD11b and CD45. Stained samples were acquired immediately after staining on a LSR II flow cytometer at the flow cytometry shared facility in University of Florida. Data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Bone marrow-derived macrophages (BMDMs)
Macrophages were derived from cultures of bone marrow cells (2×106 cells/mL) in non- treated plastic 100 mm plates with 10ng/mL recombinant M-CSF (Sigma-Aldrich, St. Louis, MO) in 10% FBS, DMEM (Gibco™) media with antibiotics (Gibco™). Media changes were performed at 3.5 days and at day 7. Macrophages were harvested with trypsin-EDTA treatment for 15 min at 37°C and seeded in 12 well plates for further polarization. Macrophages were stimulated for the next 24 h with or without LPS (10 ng/mL, Sigma) and murine IFN-γ (100 ng/mL, Sigma). Glucose was adjusted to 5 mM and 25 mM with the addition of 45% glucose solution (Gibco™). At the end of the experiment, cells were lysed and RNA was extracted as described in the methods.
RNA isolation and RT-PCR
RNA from BMDM and retina was isolated using the RNeasy Mini Kit (Qiagen, Germantown, MA) according to the manufacturer’s instructions. RNA was quantified with a Nanodrop 1000 (Thermo Scientific, Whaltman, Massachusetts). Reverse transcription was carried out using the Superscript VILO cDNA Synthesis Kit (Life Technologies, Carlsbad, California). Real-time PCR was performed on an ABI PRISM 7900HT Sequence Detection System, using TaqMan Fast Universal PCR Master Mix (2X) (Life Technologies) and TaqMan primer/probe gene expression assays. Relative quantification of gene expression was carried out using 18S endogenous control and the ΔΔCt method. All the results were expressed relative to the Veh-WT control.
Data analysis
All data were analyzed for outliers prior to treatment comparisons. Comparisons between the groups were assessed using a one-way ANOVA followed by the Tukey post-hoc test. A p-value <0.05 was considered significant.
RESULTS
Metabolic characteristics of experimental animals
Glycated hemoglobin and post-study body weights are displayed in Table 1. STZ-diabetes was associated with elevated glycated hemoglobin and decreased body weight in all groups and there was no significant effect of genotype.
Table 1.
Effect of CX3CR1 deficiency and STZ-diabetes on body weight and glycated hemoglobin.
| Veh WT (7) | Veh KO (7) | STZ WT (12) | STZ KO (11) | |
|---|---|---|---|---|
| Body weight (g) | 32.2 ± 2.1 | 30.5 ± 1.7 | 22.9 ± 5.2* | 21.05 ± 4.8* |
| Glycated hemoglobin (%) | 4.6 ± 0.2 | 4.9 ± 0.6 | 8.6 ± 1.6* | 9.4 ± 2.2* |
| Glycated hemoglobin (mmol/mol) | 27 ± < 9 | 30 ± < 9 | 70 ± < 9* | 79 ± < 9* |
Veh, non diabetic mice; STZ, streptozotocin- induced diabetic mice; WT, CX3CR1 homozygous wild type; KO, CX3CR1 homozygous knockout. Data represent mean ± SD. N=7–12 mice. Asterisks indicate statistical significance, p<0.015 compared to respective veh group.
Absence of CX3CR1 leads to acceleration of diabetic retinopathy
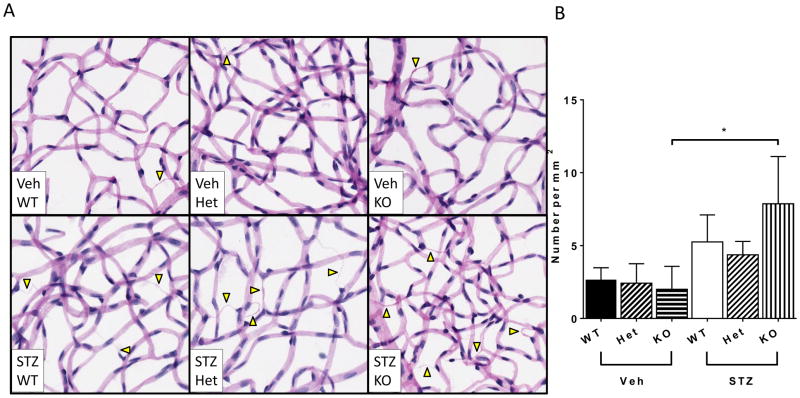
As expected, there was no significant difference between the number of retinal acellular capillaries in the WT, Het or KO animals that were treated with vehicle as all represented the corresponding nondiabetic controls. Furthermore, the number of acellular capillaries was not significantly increased in the diabetic WT (STZ-WT) compared to Veh-WT, nor in the STZ-Het compared to its Veh-Het control, as 4 months of diabetes is considered an early time for vascular pathology in WT mice. In contrast, diabetic KO (STZ-KO) mice had significantly higher numbers of acellular capillaries compared to vehicle treated KO (Veh-KO) mice (Figure 1), indicating acceleration of vascular pathology in the absence of CX3CR1. Because the Veh-Het and the STZ-Het responded similarly to the WT controls with regard to the primary endpoint of DR (acellular capillaries), we did not study these cohorts further and used the WT as the sole control for all subsequent studies.
Figure 1. Vascular Pathology in the retina.
(A) Representative images of trypsin-digested retinas for acellular capillaries (arrowheads) in nondiabetic Veh-WT, Veh-Het and Veh-KO mice and in STZ-WT, STZ-Het and STZ-KO mice. (B) Quantification of acellular capillaries in each group. Data are expressed per mm2 and represent mean ± SEM, n = 6. Asterisks indicate statistically significant difference p <0.05, one-way ANOVA. Scale bar = 50μm.
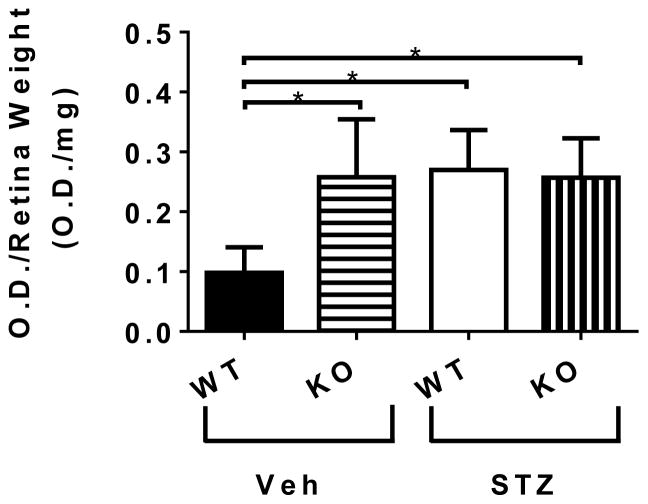
Absence of CX3CR1 and diabetes increased apoptosis in the retina
The absence of CX3CR1 in the retina led to increased apoptosis as measured by an increase in DNA fragmentation in the Veh-KO treated animals. A marked increase was also observed due to diabetes in the STZ- WT and STZ- KO mice (Figure 2).
Figure 2. Apoptosis in the retina.
Apoptosis was measured by ELISA, as DNA fragmentation in nondiabetic Veh-WT, Veh-KO mice and in diabetic STZ-WT, and STZ-KO mice. Data is expressed relative to retinal mass. Data represents mean ± SEM. n = 5; one per animal. Asterisks indicate statistically significant difference p <0.05, t-test).
Absence of CX3CR1 leads to increased myeloid cells in the neuronal tissues
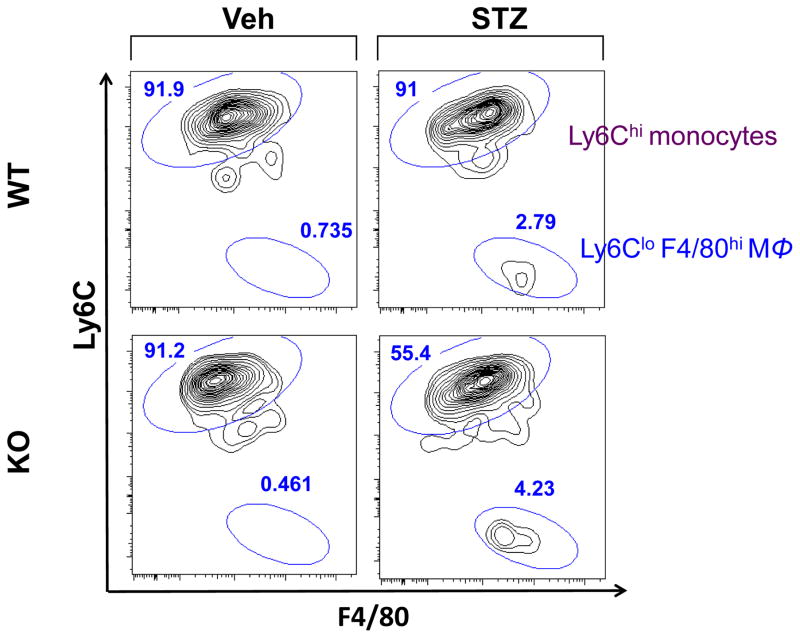
We observed slightly higher numbers of Iba-1+ cells in the STZ-KO compared to STZ-WT (data not shown). However, Iba-1 does not distinguish between microglia and bone derived macrophages. To assess whether the absence of CX3CR1 in diabetes leads to increased infiltration of bone marrow-derived myeloid cells, retinas and brains were analyzed by flow cytometry. Our gating strategy, which is summarized in the supplemental Fig. 1, separates resident microglia (CD45dim cells) from bone marrow derived macrophages (CD45high cells) and distinguish bone marrow derived macrophages as Ly6Clo F480hi cells [35]. In the retina of WT mice, bone marrow macrophage accumulation was 0.735% in Veh-WT and increased to 2.79% in STZ-WT, representing a 4-fold increase of macrophages with diabetes (Figure 3). In the absence of CX3CR1, diabetes had a greater effect resulting in an increase of macrophages from 0.461% in Veh-KO to 4.23% in STZ-KO. This represented a 10-fold accumulation of macrophages within the retina. Thus, the absence of CX3CR1 activated resident microglia and recruited bone marrow derived immune populations into the retina.
Figure 3. Macrophage infiltration in the retina.
Flow cytometry diagrams from n = 8 pooled retinas from Veh-WT, Veh-KO, STZ-WT and STZ KO mice. Gating strategy is described in Supplemental Fig. 1. Flow diagrams show: [live singlets/Ly6G-/CD45hi/F4/80+/− mononuclear cells.
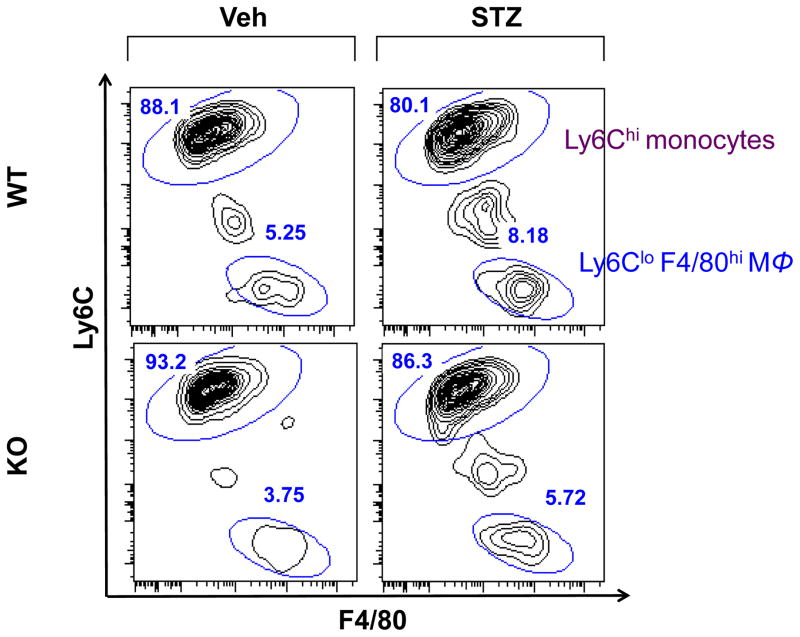
We next examined the brains in these cohorts of mice using the gating strategy described above. As shown in Figure 4, there were slightly fewer macrophages in Veh-KO compared to Veh-WT (3.75% vs. 5.25% respectively). STZ-KO brains contained 5.75% macrophages compared to 8.18% in STZ-WT, indicating that the increased recruitment of bone marrow cells was specific to the retina.
Figure 4. Macrophage infiltration in the hypothalamic regions of the brain.
Flow cytometry diagrams from n = 8 pooled brains from Veh-WT, Veh-KO, STZ-WT and STZ KO mice. Gating strategy is described in Supplemental Fig. 1. Flow diagrams show: [live singlets/Ly6G-/CD45hi/F4/80+/-] mononuclear cells.
Effect of CX3CR1 deletion on monocytes in the peripheral tissues
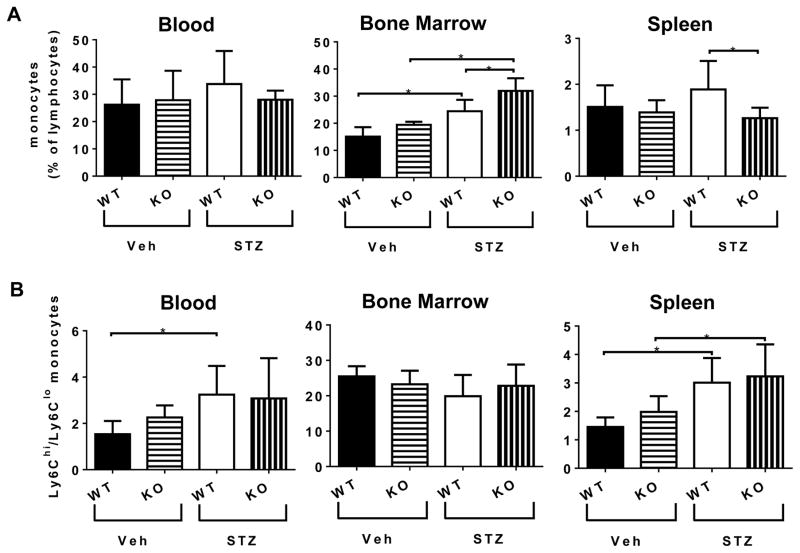
Monocyte populations from the blood, bone marrow and spleen (representing the major sources of circulating monocytes) were identified by flow cytometry using a gating strategy described in supplemental Fig. 2. CX3CR1 deficiency had no effect on the relative percentages of monocytes in the blood (Figure 5A, left panel); diabetes resulted in increased monocytes numbers in the bone marrow and this effect was even higher in the absence of CX3CR1 (Figure 5A, center panel). However the STZ-KO mice had significantly reduced monocytes in the spleen compared to STZ-KO (Figure 5A, right panel).
Figure 5. Peripheral monocyte homeostasis.
(A) Percentages of monocytes in the blood, bone marrow and spleen. (B) Ratio of percentages of Ly6Chi/Ly6Clo monocytes in the blood, bone marrow and spleen. Gating strategy is described in Supplemental Fig. 2. Flow diagrams show: [CD3e, CD19, NK1.1, Ly6G)-/(CD11c, F4/80, IA-b)-/CD11b+] mononuclear cells. Data represent mean ± SEM, n = 8–11. Asterisks indicate statistically significant difference p <0.05, one-way ANOVA.
Further characterization of the monocyte subsets revealed that diabetes had higher impact than CX3CR1 deletion. Diabetes resulted in increased ratio of inflammatory to patrolling monocytes (Ly6Chi to Ly6Clo ratio) in the blood (Figure 6B left panel), and spleen (Figure 6B right panel) but not in the bone marrow (Figure 6B center panel).
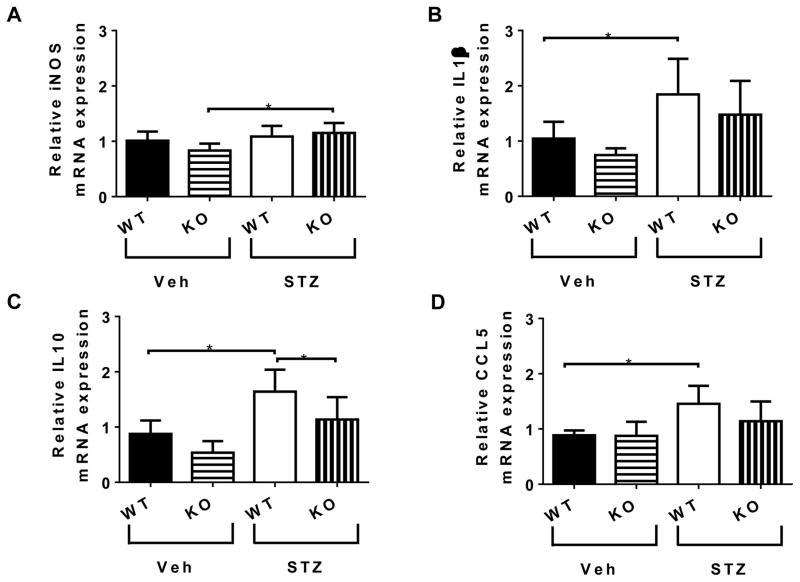
Figure 6. mRNA expression in the retina.
Relative (A) iNOS, (B) IL-1β, (C) IL-10, (D) CCL-5 mRNA expression. Data represent mean ± SEM, n = 5–6. Asterisks indicate statistically significant difference p <0.05, one-way ANOVA.
Changes in inflammatory and anti-inflammatory mediators in diabetic retina of KO and WT mice
To examine the role of CX3CR1 in the progression of DR, we performed RT-PCR analysis of mRNAs for a series of retinal genes: i) expressed by activated endothelium (ICAM-1, VCAM-1, VEGF), ii) expressed by inflammatory M1 macrophages (iNOS, CD11c), iii) expressed by alternative activated M2 macrophages (IL-10, Arg1); and iv) mediators of clearance of apoptotic cells by macrophages (IL-10, TGF-β1, TGF-β2, PTGES, MERTK, P2RX7, LXRa, ABCA1, TGM2, RETNL). We also examined retinal mRNA levels of chemoattractants (CCL2, CCL5, SDF-1) and inflammatory cytokines (TNF-α, IL-1β, IL-6). At 4 months of diabetes we did not observe changes in ICAM-1, VCAM-1, VEGF, TNF-α, IL-6 and SDF-1. However, CX3CR1 absence led to increased iNOS mRNA expression with diabetes (Figure 6A). The only inflammatory cytokine that remained increased with diabetes at 4 months was IL-1β. Levels were higher in STZ -WT compared to Veh-WT, however, IL-1β expression was not higher in STZ-KO compared to Veh-KO (Figure 6B). IL-10 was the only gene we found to be upregulated during resolution of inflammation and clearance of apoptotic cells in the retina of STZ-WT mice. Importantly, IL-10 was significantly reduced in the retinas of STZ-KO (Figure 6C). CCL5, a chemokine that attracts monocytes, was also increased only in the STZ-WT mice (Figure 6D) but unchanged in the STZ-KO.
Bone marrow-derived macrophages from KO mice produce less IL-10
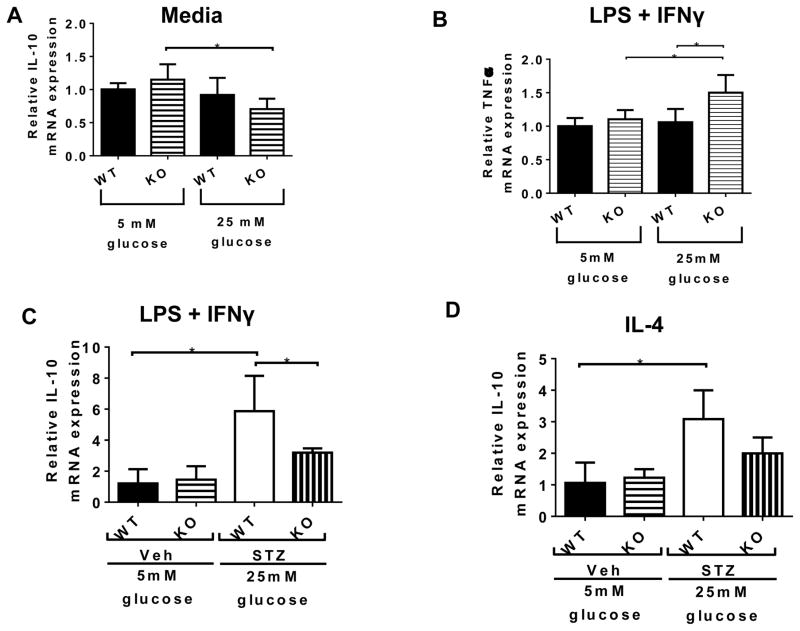
To examine the effect of CX3CR1 deletion on the function of infiltrated macrophages, we obtained bone marrow derived macrophages (BMDMs) and cultured these under high glucose conditions in vitro. BMDMs from Veh-WT and Veh-KO mice were derived under normal glucose media (5mM) for 7 days and then cultured for another 24 hours in normal (5mM) or high (25mM) glucose in the presence or absence of an inflammatory stimulus (LPS, 10ng/mL + IFNγ, 100ng/mL). As shown in Figure 7A, BMDMs from KO mice stimulated under high glucose conditions produced less anti-inflammatory IL-10 compared those in low glucose conditions. Furthermore, when they were stimulated with LPS + IFNγ, BMDMs from KO mice in high glucose conditions displayed significantly elevated TNFα production (Figure 7B). These data indicate that deletion of CX3CR1 predisposed macrophages towards a more inflammatory (higher TNFα) and less anti-inflammatory (less IL-10) phenotype.
Figure 7. Inflammatory and anti-inflammatory mediator production by bone marrow derived macrophages (BMDMs).
(A and B): BMDMs from Veh-WT and Veh-KO were derived in 5mM glucose for 8 days and then further stimulated with nothing (A) or stimulated with LPS (10 ng/ml) and IFNγ (100 ng/ml) (B) for 24 hours in low glucose (5mM) or high glucose (25 mM) media. Total RNA was isolated and the relative expression of IL-10 (A) and TNF-α (B) mRNAs was quantified by RT-PCR using 18S endogenous control and the ΔΔCt method. (C and D): BMDMs from Veh-WT and Veh-KO were derived in 5mM glucose and BMDMs from STZ-WT and STZ-KO were derived in 25mM glucose for 8 days and then further stimulated with LPS (10 ng/ml) and IFNγ (100 ng/ml) (C) or IL-4 (10 ng/ml) (D) for 24 hours in low glucose (5mM) or high glucose (25 mM) media. Total RNA was isolated and the relative expression of IL-10 (C and D) mRNAs was quantified by RT-PCR using 18S endogenous control and the ΔΔCt method. Data represent mean ± SEM, n = 5–6. Asterisks indicate statistically significant difference p <0.05, one-way ANOVA.
To verify the inability of macrophages from CX3CR1-KO mice to produce IL-10, we generated BMDMs from Veh-WT and Veh-KO in 5mM glucose media and compared them to BMDMs from STZ-WT and STZ-KO generated in 25mM glucose media. As shown in Figure 7C, the STZ-KO BMDMs expressed significantly reduced IL-10 compared to STZ-WT BMDMs in response to both LPS + IFNγ (Figure 7C) and IL-4 (Figure 7D). All together this data would suggest that CX3CR1 affects macrophage function and IL-10 expression.
DISCUSSION
Lack of CX3CR1 accelerated the progression of diabetic retinopathy in mice. At four months of diabetes, STZ-WT mice are not expected to display retinal vascular pathology, as measured by the formation of acellular capillaries, an assay indicative of retinal microvascular damage. Indeed, there was no significant difference between Veh-WT and STZ-WT at this time point; however, in the absence of CX3CR1, STZ-KO mice had significantly elevated numbers of acellular capillaries at 4 months of diabetes, compared to Veh-KO controls. We utilized WT rather than mice that were heterozygous for CX3CR1 in our studies. Previously published studies show that no difference existed between Het and WT mice regarding surface expression of CX3CR1 [32,37]. Furthermore, other studies have made direct comparisons between WT and KO [38–40].
We investigated if at 4 months, apoptosis was increased with diabetes. Loss of CX3CR1 had detrimental effects in the retina as, even in the absence of diabetes, deletion of CX3CR1 increased retinal apoptotic cell death. Since the CX3CR1 KO mice exhibited a high level of retinal cell apoptosis, we examined the retina for activated microglia and bone marrow derived macrophages, as these cells participate in resolution of inflammation and clearance of apoptotic cells. We found that STZ-KO mice had increased numbers of activated microglia and increased bone marrow derived macrophages in their retinas compared to the other groups. This increase in the homing of macrophages in the STZ-KO mice was observed in the retina but not in the brain. Furthermore, CX3CR1 deletion in the presence of diabetes caused perturbation of the myeloid population in the bone marrow. To reconcile the role of infiltrated macrophages in the retina of STZ-KO mice, we examined a series of genes known to be upregulated during inflammation and clearance of apoptotic cells by macrophages. At 4 months of diabetes when a high degree of apoptosis was detected, proinflammatory “on” cytokines were inhibited, however, in the STZ-KO retina iNOS expression was significantly higher.
Among the anti-inflammatory “off” cytokines that are upregulated during clearance of apoptotic cells, only IL-10 was significantly increased in the retinas of STZ-WT. However, IL-10 expression was not increased in retinas of STZ-KO mice. Furthermore, expression of IL-10 was reduced in bone marrow-derived macrophages from the STZ-KO mice generated and activated under high glucose conditions. The role of IL-10 in the progression of DR is not yet clear. Our research highlights that this anti-inflammatory molecule has important repercussions for the health of the retina. Overall, our studies suggest a role for CX3CR1 in retinal homeostasis during early stages of diabetes; its absence may compromise the ability of microglia and macrophages to promote clearance of apoptotic cells or to suppress inflammation following phagocytosis, thus leading to enhanced vascular pathology.
CX3CR1 can exert both neuroprotective and neurotoxic effects in the CNS, depending on the pathological scenario. Kezic et al. determined that CX3CR1 deficiency exacerbated the diabetes-associated activation of retinal microglia [33]. Loss of CX3CR1 signaling leads to failure of microglia homeostatic functions, including phagocytosis, immune surveillance, homing and injury response [41,24]. However, in this study the authors did not differentiate between microglia and tissue-derived macrophages and they did not relate microglia and recruited macrophage function with neurodegeneration. Finally, other studies have shown that even under normal conditions CX3CR1-deficient mice exhibit accumulation of subretinal microglia and macrophages [42,43,25]. Importantly, the prolonged presence of macrophages and microglia in the subretinal space is associated with retinal degeneration [44,42].
A limitation of our study is that we have only used a single time point for the analysis, 4 months. We do not have data for longer time points wherein the subtle differences we found at 4 months may have be more profound at 6 or 9 months. However, at the 4 months CX3CR1 deletion led to increased DNA fragmentation in the retinas of Veh-KO mice, even in the absence of diabetes. These data suggest that CX3CR1 has an important homeostatic role in the retina and disruption of this system potentially predisposes the retina to neurodegeneration. A likely explanation for the initiation of apoptosis observed even in Veh-treated KO mice is the absence of CX3CL1’s neurotrophic effects and the subsequent increase of apoptosis of the neuroretina. Our studies expand on this explanation: in the presence of diabetes, CX3CR1 deletion compromises the clearance of apoptotic cells from the retina. The observed accumulation of myeloid cells in STZ-KO mice likely occurs to facilitate the processing of the increased numbers of apoptotic cells. Our data (Figure 6) suggests that under high glucose conditions the CX3CR1-deficient macrophages are predisposed to adopting a more proinflammatory phenotype and to produce less IL-10. Together, these observations emphasize the notion that CX3CR1 is important for maintenance of retinal homeostasis both in basal as well as in pathological conditions.
To identify whether these changes are reflected in other peripheral tissues, monocyte populations in blood, bone marrow and spleen (major sources of circulating monocytes) were identified by flow cytometry. The contribution of bone marrow-derived cells to the acceleration of retinopathy is well established [45]. We also have shown that diabetes skews hematopoiesis towards the generation of more pro-inflammatory monocytes, fewer anti-inflammatory monocytes and lower numbers of reparative progenitor cells [15–19]. Bone marrow progenitors from diabetic mice generate higher numbers of myeloid colonies, which is attributed to increased levels of M-CSF and inflammatory cytokines such as IL-1β, IL-27 and IFN-γ in the bone marrow microenvironment [17]. These pro inflammatory monocytes become trapped within retinal capillaries [30,46] and activate the vasculature [45]. Accordingly, we showed that inflammatory CCR2+ monocytes accumulate in the retina of STZ diabetic mice, implicating the CCL2-CCR2 axis in the development of DR [17]. Our group has also examined the diurnal variation in the levels of inflammatory, Ly6Chi/CCR2+ monocytes and found that diabetes not only results in higher circulating levels but also a “phase advance” of the diurnal release of these cells into the circulation that coincides with an increase in expression of adhesion molecules on the vascular endothelium [17,18]. CX3CR1 deletion predisposed the bone marrow of STZ-KO mice to contain more monocytes, in particular more inflammatory Ly6Chi monocytes compared to bone marrow of STZ-WT mice. This would facilitate the accumulation of more inflammatory and less cells resolving inflammation in the diabetic retina.
In summary, our findings are consistent with the hypothesis that CX3CR1 activation mediates important protective effects at the eye. We show that these beneficial effects are evident, even in the absence of diabetes, and involve mitigating retinal cell apoptosis and modulating macrophage function in response to elevated apoptosis. Furthermore, in the presence of an inflammatory microenvironment, such as diabetes, the presence of CX3CR1 likely delays the onset of diabetic retinal pathology. The absence of CX3CR1 permits the deleterious effects of diabetes in the retina to occur in an accelerated manner. It is possible that CX3CR1 activation, at least in part, is an important mechanism responsible for delaying the onset of DR or slowing the progression of DR.
Supplementary Material
Supplemental Figure S1: Gating strategy for single cell suspension of brain and retina. Viable/Live singlets were gated on, CD45+, CD45hi recruits, and then F4/80+/− Ly6G− mononuclear cells. Using this gating strategy allowed subsequent analysis of Ly6C+ monocytes and Ly6C− monocyte/macrophage. Data shown are from brain and retina of STZ-diabetic WT mice.
Supplemental Figure S2: Gating strategy for single cell suspension of bone marrow and spleen. Splenic and bone marrow monocytes were gated in the lymphocyte gate based on forward and side scatter and identified as Lineage 1 (CD3e, CD19, NK1.1, Ly6G) negative, Lineage 2 (CD11c, F4/80, IA-b) negative and CD11b+ cells.
Key messages.
CX3CR1 deletion in STZ-diabetic mice accelerated the onset of diabetic retinopathy (DR).
The early onset of DR was associated with increased retinal cell apoptosis.
The early onset of DR was associated with increased recruitment of bone marrow-derived macrophages to the retina.
Bone marrow-derived macrophages from CX3CR1 KO diabetic mice expressed more TNF-α and less IL-10.
The role of IL-10 in protection from progression of DR is highlighted.
Acknowledgments
The authors would like to thank Neal Benson from the cytometry core at the ICBR in University of Florida. Research was supported by NIH grants: EY012601-15, EY007739-25, EY018358, DK 090730 and HL110170 (MBG).
References
- 1.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R American Diabetes A. Diabetic retinopathy. Diabetes Care. 2003;26(Suppl 1):S99–S102. doi: 10.2337/diacare.26.2007.s99. [DOI] [PubMed] [Google Scholar]
- 2.Simo R, Hernandez C. Prog Retin Eye Res. 2015;48:160–180. doi: 10.1016/j.preteyeres.2015.04.003. ç. [DOI] [PubMed] [Google Scholar]
- 3.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investigative ophthalmology & visual science. 2011;52(2):1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigsby JG, Cardona SM, Pouw CE, Muniz A, Mendiola AS, Tsin AT, Allen DM, Cardona AE. The role of microglia in diabetic retinopathy. Journal of ophthalmology. 2014;2014:705783. doi: 10.1155/2014/705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Investigative ophthalmology & visual science. 2004;45(9):3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53(5):971–979. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Mao D, Chen X, Zhao L, Tian Q, Liu C, Zhou BL. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Molecular vision. 2012;18:1411–1420. [PMC free article] [PubMed] [Google Scholar]
- 9.Kern TS, Tang J, Berkowitz BA. Validation of structural and functional lesions of diabetic retinopathy in mice. Molecular vision. 2010;16:2121–2131. [PMC free article] [PubMed] [Google Scholar]
- 10.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. Journal of neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. The EMBO journal. 2014;33(1):7–22. doi: 10.1002/embj.201386609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91(4):521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 13.Foussat A, Coulomb-L’Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. European journal of immunology. 2000;30(1):87–97. doi: 10.1002/1521-4141(200001)30:1<87::AID-IMMU87>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol. 2015;35(6):1306–1316. doi: 10.1161/ATVBAHA.114.304650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakravarthy H, Beli E, Navitskaya S, O’Reilly S, Wang Q, Kady N, Huang C, Grant MB, Busik JV. Imbalances in Mobilization and Activation of Pro-Inflammatory and Vascular Reparative Bone Marrow-Derived Cells in Diabetic Retinopathy. PloS one. 2016;11(1):e0146829. doi: 10.1371/journal.pone.0146829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravarthy H, Navitskaya S, O’Reilly S, Gallimore J, Mize H, Beli E, Wang Q, Kady N, Huang C, Blanchard GJ, Grant MB, Busik JV. Role of acid sphingomyelinase in shifting the balance between pro-inflammatory and reparative bone marrow cells in diabetic retinopathy. Stem cells. 2015 doi: 10.1002/stem.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazra S, Jarajapu YP, Stepps V, Caballero S, Thinschmidt JS, Sautina L, Bengtsson N, Licalzi S, Dominguez J, Kern TS, Segal MS, Ash JD, Saban DR, Bartelmez SH, Grant MB. Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia. 2013;56(3):644–653. doi: 10.1007/s00125-012-2781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu P, Thinschmidt JS, Yan Y, Hazra S, Bhatwadekar A, Caballero S, Salazar T, Miyan JA, Li W, Derbenev A, Zsombok A, Tikhonenko M, Dominguez JM, 2nd, McGorray SP, Saban DR, Boulton ME, Busik JV, Raizada MK, Chan-Ling T, Grant MB. CNS inflammation and bone marrow neuropathy in type 1 diabetes. The American journal of pathology. 2013;183(5):1608–1620. doi: 10.1016/j.ajpath.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yellowlees Douglas J, Bhatwadekar AD, Li Calzi S, Shaw LC, Carnegie D, Caballero S, Li Q, Stitt AW, Raizada MK, Grant MB. Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2012;31(5):481–494. doi: 10.1016/j.preteyeres.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 21.Wolf Y, Yona S, Kim KW, Jung S. Microglia, seen from the CX3CR1 angle. Front Cell Neurosci. 2013;7:26. doi: 10.3389/fncel.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zieger M, Ahnelt PK, Uhrin P. CX3CL1 (fractalkine) protein expression in normal and degenerating mouse retina: in vivo studies. PloS one. 2014;9(9):e106562. doi: 10.1371/journal.pone.0106562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Investigative ophthalmology & visual science. 2009;50(9):4444–4451. doi: 10.1167/iovs.08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raoul W, Feumi C, Keller N, Lavalette S, Houssier M, Behar-Cohen F, Combadiere C, Sennlaub F. Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice. Ophthalmic research. 2008;40(3–4):115–119. doi: 10.1159/000119860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu P, Li L, Kuno K, Wu Y, Baba T, Li YY, Zhang X, Mukaida N. Protective roles of the fractalkine/CX3CL1-CX3CR1 interactions in alkali-induced corneal neovascularization through enhanced antiangiogenic factor expression. Journal of immunology. 2008;180(6):4283–4291. doi: 10.4049/jimmunol.180.6.4283. [DOI] [PubMed] [Google Scholar]
- 27.Moatti D, Faure S, Fumeron F, Amara Mel W, Seknadji P, McDermott DH, Debre P, Aumont MC, Murphy PM, de Prost D, Combadiere C. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97(7):1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- 28.Kasama T, Wakabayashi K, Sato M, Takahashi R, Isozaki T. Relevance of the CX3CL1/fractalkine-CX3CR1 pathway in vasculitis and vasculopathy. Translational research : the journal of laboratory and clinical medicine. 2010;155(1):20–26. doi: 10.1016/j.trsl.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Lauro C, Catalano M, Trettel F, Limatola C. Fractalkine in the nervous system: neuroprotective or neurotoxic molecule? Annals of the New York Academy of Sciences. 2015 doi: 10.1111/nyas.12805. [DOI] [PubMed] [Google Scholar]
- 30.Serra AM, Waddell J, Manivannan A, Xu H, Cotter M, Forrester JV. CD11b+ bone marrow-derived monocytes are the major leukocyte subset responsible for retinal capillary leukostasis in experimental diabetes in mouse and express high levels of CCR5 in the circulation. The American journal of pathology. 2012;181(2):719–727. doi: 10.1016/j.ajpath.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PloS one. 2014;9(10):e108508. doi: 10.1371/journal.pone.0108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardona SM, Mendiola AS, Yang YC, Adkins SL, Torres V, Cardona AE. Disruption of Fractalkine Signaling Leads to Microglial Activation and Neuronal Damage in the Diabetic Retina. ASN neuro. 2015;7(5) doi: 10.1177/1759091415608204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kezic JM, Chen X, Rakoczy EP, McMenamin PG. The effects of age and Cx3cr1 deficiency on retinal microglia in the Ins2(Akita) diabetic mouse. Investigative ophthalmology & visual science. 2013;54(1):854–863. doi: 10.1167/iovs.12-10876. [DOI] [PubMed] [Google Scholar]
- 34.Bhatwadekar A, Glenn JV, Figarola JL, Scott S, Gardiner TA, Rahbar S, Stitt AW. A new advanced glycation inhibitor, LR-90, prevents experimental diabetic retinopathy in rats. The British journal of ophthalmology. 2008;92(4):545–547. doi: 10.1136/bjo.2007.127910. [DOI] [PubMed] [Google Scholar]
- 35.O’Koren EG, Mathew R, Saban DR. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Scientific reports. 2016;6:20636. doi: 10.1038/srep20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abcouwer SF, Lin CM, Wolpert EB, Shanmugam S, Schaefer EW, Freeman WM, Barber AJ, Antonetti DA. Effects of ischemic preconditioning and bevacizumab on apoptosis and vascular permeability following retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2010;51(11):5920–5933. doi: 10.1167/iovs.10-5264. [DOI] [PubMed] [Google Scholar]
- 37.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Molecular and cellular biology. 2000;20(11):4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu SJ, Calippe B, Lavalette S, Roubeix C, Montassar F, Housset M, Levy O, Delarasse C, Paques M, Sahel JA, Sennlaub F, Guillonneau X. Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1beta Secretion and Photoreceptor Neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(18):6987–6996. doi: 10.1523/JNEUROSCI.3955-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, Trautwein C, Tacke F. The fractalkine receptor CX(3)CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology. 2010;52(5):1769–1782. doi: 10.1002/hep.23894. [DOI] [PubMed] [Google Scholar]
- 40.Sennlaub F, Auvynet C, Calippe B, Lavalette S, Poupel L, Hu SJ, Dominguez E, Camelo S, Levy O, Guyon E, Saederup N, Charo IF, Rooijen NV, Nandrot E, Bourges JL, Behar-Cohen F, Sahel JA, Guillonneau X, Raoul W, Combadiere C. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO molecular medicine. 2013;5(11):1775–1793. doi: 10.1002/emmm.201302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raoul W, Keller N, Rodero M, Behar-Cohen F, Sennlaub F, Combadiere C. Role of the chemokine receptor CX3CR1 in the mobilization of phagocytic retinal microglial cells. Journal of neuroimmunology. 2008;198(1–2):56–61. doi: 10.1016/j.jneuroim.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. The Journal of clinical investigation. 2007;117(10):2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinnery HR, McLenachan S, Humphries T, Kezic JM, Chen X, Ruitenberg MJ, McMenamin PG. Accumulation of murine subretinal macrophages: effects of age, pigmentation and CX3CR1. Neurobiology of aging. 2012;33(8):1769–1776. doi: 10.1016/j.neurobiolaging.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Nakazawa T, Hisatomi T, Nakazawa C, Noda K, Maruyama K, She H, Matsubara A, Miyahara S, Nakao S, Yin Y, Benowitz L, Hafezi-Moghadam A, Miller JW. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2425–2430. doi: 10.1073/pnas.0608167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caballero S, Hazra S, Bhatwadekar A, Li Calzi S, Paradiso LJ, Miller LP, Chang LJ, Kern TS, Grant MB. Circulating mononuclear progenitor cells: differential roles for subpopulations in repair of retinal vascular injury. Investigative ophthalmology & visual science. 2013;54(4):3000–3009. doi: 10.1167/iovs.12-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fadini GP, de Kreutzenberg SV, Boscaro E, Albiero M, Cappellari R, Krankel N, Landmesser U, Toniolo A, Bolego C, Cignarella A, Seeger F, Dimmeler S, Zeiher A, Agostini C, Avogaro A. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia. 2013;56(8):1856–1866. doi: 10.1007/s00125-013-2918-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Gating strategy for single cell suspension of brain and retina. Viable/Live singlets were gated on, CD45+, CD45hi recruits, and then F4/80+/− Ly6G− mononuclear cells. Using this gating strategy allowed subsequent analysis of Ly6C+ monocytes and Ly6C− monocyte/macrophage. Data shown are from brain and retina of STZ-diabetic WT mice.
Supplemental Figure S2: Gating strategy for single cell suspension of bone marrow and spleen. Splenic and bone marrow monocytes were gated in the lymphocyte gate based on forward and side scatter and identified as Lineage 1 (CD3e, CD19, NK1.1, Ly6G) negative, Lineage 2 (CD11c, F4/80, IA-b) negative and CD11b+ cells.