Abstract
Objectives
Cartilage is a highly mechano-responsive tissue. Chondrocytes undergo a series of complex changes, including proliferation and metabolic alteration as the target of external biomechanical and biochemical stimuli. IL-1β is known to regulate chondrocyte metabolism and play an important role in the pathogenesis of osteoarthritis. The objective of this study was to employ low-intensity pulsed ultrasound (LIPUS) as a localized mechanical stimulus and assess its effects on chondrocyte migration, proliferation, metabolism, and differentiation, as well as its ability to suppress IL-1β mediated catabolism in cartilage.
Methods
Human cartilage explants and chondrocytes were stimulated by LIPUS in presence and absence of of IL-1β to asses cartilage degradation, chondrocytes metabolism, migration and proliferation. Western blot analyses were conducted to study IL-1β the associated NFκB pathway in chondrocytes.
Results
LIPUS stimulation increased the proteoglycan content in human cartilage explants and inhibited IL-1β induced loss of proteoglycans. LIPUS stimulation increased rates of chondrocyte migration and proliferation, and promoted chondrogenesis in mesenchymal stem cells. Further, LIPUS suppressed IL-1β induced activation of phosphorylation of NFκB-p65 and IκBα leading to reduced expression of MMP13 and ADAMT5 in chondrocytes.
Conclusions
Collectively, these data demonstrate the potential therapeutic effects of LIPUS in preventing cartilage degradation and treating osteoarthritis via a mechanical stimulation that inhibits the catabolic action of IL-1β and stimulates chondrocyte migration, proliferation, and differentiation.
Keywords: Osteoarthritis, Low Intensity Pulsed Ultrasound, Interleukin 1β, Cartilage Metabolism, Chondrocytes
Introduction
Osteoarthritis (OA) is an articular pathology, characterized by a progressive loss of cartilage, and the development of arthralgia, stiffness, and restricted motion. OA is the most common cause of disability in the American population. According to recent estimates, arthritis treatment costs approximately $128 billion per year to the US economy alone1. While many etiological factors (e.g., obesity, aging, and genetics) are correlated with OA2,3, an understanding of the precise factors contributing towards OA onset remains elusive. However, several pro-inflammatory cytokines are strongly implicated in initiating and aggravating OA lesions. The most notable cytokine with relation to joint destruction is interleukin-1 beta (IL-1β). IL-1β is highly expressed in OA patients and plays a critical role in the pathogenesis of OA4,5. The activation of IL-1β is regulated by the IL-1 receptor type 1 (IL-1R) which has been shown to be highly expressed in human OA chondrocytes and synovial fibroblasts, relative to normal cells6. Activation of IL-1β is mediated through several downstream pathways including c-Jun/P38 MAPKs and, more importantly, the nuclear factor κB (NFκB) pathway7–9. IL-1β stimulates the production of nitric oxide synthase (NOS), soluble phospholipase A2, cyclooxygenase 2(Cox-1) and microsomal prostaglandin E synthase 2, contributing to enhanced production of catabolic enzymes such as matrix metalloproteinase 13 (MMP13) and A disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) while inhibiting anabolic matrix proteins such as collagens and proteoglycans9,10. Overall increased expression of IL-1β is one of the most detrimental factors leading to cartilage degradation in OA pathology11.
Articular cartilage is a highly mechanosensitive tissue and physiological mechanical stimulation of cartilage has been shown to increase the anabolic activity of chondrocytes12,13. In vitro and ex vivo studies have shown increased matrix deposition and chondrocyte proliferation after dynamic stimulation14,15. Similarly, increased aggrecan, collagen II (Col II), and proteoglycan expression have been observed following the application of cyclic pressure and hydrostatic pressure in vitro and using explanted chondrocyte cultures16–18. Moderate physical exercise has also been associated with delayed onset and decreased progression of OA in humans19,20.
Low-intensity pulsed ultrasound (LIPUS) is an acoustic pressure wave capable of providing localized mechanical stimulus to cells. It has been approved by the FDA as a clinical therapy to promote fracture healing. LIPUS has been reported to enhance chondrocyte proliferation and matrix production through the upregulation of the integrin/P13K/AKT pathway21 and the reduction of mRNA expression of MMP13 in the presence of IL-1β through the integrin-mediated P38 MAPK pathway22. LIPUS stimulation has also been shown to increase Col II expression in a rat OA model23 and downregulate MMP-13, ERK1/2, and p38 in a rabbit OA model24. LIPUS also enhances chondrogenesis by increasing Col II, Sox9, and aggrecan expression in human mesenchymal stem cells25. Overall LIPUS shows both anabolic and anti-catabolic effects in settings of cartilage degradation.
OA is a multifactorial articular disease that results in increased expression of IL-1β, which in turn activates signaling pathways that cause progressive cartilage degradation. In this study, we examined the chondro-protective effects of LIPUS via its inhibition of IL-1β induced activation of the NFκB pathway.
METHODS
Ultrasound Setup
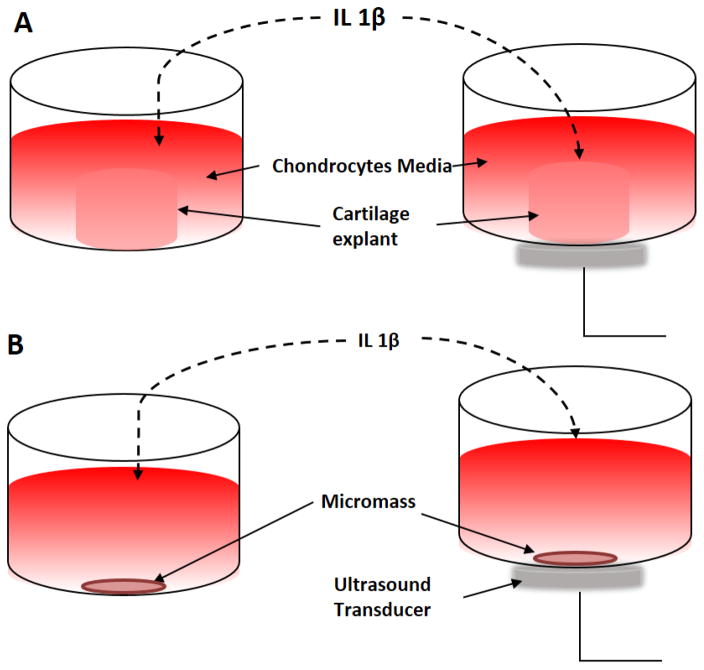
LIPUS stimulations were applied using Sonicator® 740× (Mettler Electronics, Anaheim, CA) with 10cm2 transducer. Cells and explants were cultured in 35mm plates and were stimulated 20min per day for the duration of the experiment at 30mW/cm2, at a frequency of 1 MHz with a pulse duration of 200μs repeated at 100Hz, as shown in Figure 1. The intensity and pulse duration were selected in line with FDA approved parameters for bone healing. The stimulations were conducted in a sterile environment at room temperature. The acoustic gel was used as a coupler between the transducer and cell plate to ensure optimal ultrasound exposure26.
Figure 1.
LIPUS stimulation setup. A) Human explant culture with and without IL-1β and LIPUS stimulation at 30mW/cm2/per day for the duration of experiment. B) Micromass culture with and without IL-1β and LIPUS stimulation at 30mW/cm2, 20min per day for the duration of the experiment.
Preparation of cartilage explant cultures
The cartilage explants were harvested from patients receiving total knee joint replacement surgery for OA at New York University Hospital for Joint Diseases (New York, USA). Informed consent was collected from each patient before surgery. The protocol was approved by the Institutional Review Board (IRB#12758). Our studies followed the guidelines of the Institutional Review Board of New York University (NYU) School of Medicine for the use of surgically discarded human tissues. Careful examination of cartilage samples was conducted and only healthy cartilage samples were used in the current study. Cartilage explants were examined for cartilage thickness uniformity (~2mm, with superficial, middle, and deep zones intact), surface morphology, and the presence of osteophytes. Articular cartilage was harvested from the tibial plateau of the discarded knees and cut into 3-mm discs using a biopsy punch by Acu-Punch (Acuderm Inc., Ft. Lauderdale, FL). Explanted discs were placed in 6-well plates with Chondrocyte Growth Medium (Cell Applications Inc., San Diego, CA) supplemented with 10% FBS and 1% Penn-Strep.
Micromass cultures
To study the effects of LIPUS on chondrocyte metabolism C-28/I2 immortalized human chondrocytes (provided by Dr. Goldring) were seeded at 2×106 cell/cm2 and grown in chondrogenic media supplemented with 10% FBS. Cells were divided into four groups (n=4): 1) Control, 2) LIPUS 3) IL-1β (10ng/ml) and 4) LIPUS + IL-1β.
To study the effects of LIPUS on chondrogenesis C3H10T1/2 cells (ATCC, VA, USA) were used at 2×106 cell/cm2 seeding density. Cell micromasses were cultured in chondrogenic media supplemented with 10% FBS and 100ng/ml BMP2. Cells were divided into 4 groups (n=4) as described above. All in vitro experiments were conducted in biological triplicates.
The explants and cells were maintained at 37C and 5% CO2, the medium was changed every other day.
Safranin O Staining
Ex-vivo Cartilage Explants
Human articular cartilage was collected from 5 patients ranging from ages 55 – 75. 3mm cartilages biopsies were randomly distributed into four groups (n=3): 1) Control, 2) LIPUS 3) IL-1β (10ng/ml) and 4) LIPUS + IL-1β. After 7 days of treatment, explants were fixed in 10% formalin overnight at 4C and embedded in paraffin. Longitudinal sections of 10 microns were made using a microtome. Slides were stained with Safranin O for 30 min with Weigert’s Iron Hematoxylin as a counterstain.
Micromass Cell Cultures
C-28/I2 were fixed in 0.1% glutaraldehyde in PBS for 20 min at room temperature and stained with 1% Safranin O Stain for 15 min.
GAG Release Quantification
Cartilage was collected from 8 patients’ age ranging 55 – 75. Five (3mm) cartilage discs were cultured in each well with 2ml media per well with 4 biological replicates (n=4). Media were collected at Days 1, 3, 5, and 7 and pooled to assay glycosaminoglycan (GAG) degradation over time. GAG release was quantified using Dimethylmethylene Blue Assay (DMMB) as described by Stone et al.27. Chondroitin 6-suplhate (Sigma-Alrich, USA) concentrations were used for standard curves.
Picosirius Red Staining and Quantification
C-28/I2 cell were cultured as micromass and treated with IL-1β and/or LIPUS for 7 days. Cells were fixed with 70% ethanol and stained with 0.1% picosirius red (Electron Microscopes Sciences, PA) for 1hr at room temperature. Excess stain was washed with acidified water. Images were captured at 40× using a Nikon inverted microscope (Nikon Inc, USA). Staining was eluted using extraction buffer (Chondrex Inc., WA) and quantified at 540nm.
Quantitative Real-Time PCR
Chondrocytes were cultured in 6 well plates (3 wells per group) and exposed to IL-1β and LIPUS. After 10 mins of stimulation, mRNA was collected from the cells using RNeasy Mini Kit (Qiagen), RNA was pooled and cDNA was synthesized using SuperScript® Reverse Transcriptase (Invitrogen). SYBR® Green PCR Master Mix (Applied Biosystems) and used to perform real-time PCR in StepOnePlus™ Real-Time PCR Systems (Applied Biosystems). PCR reactions were repeated 3 times. qRT-PCR analyses were performed for MMP13 (5′CTTCACGATGGCATTGCTGA3′, 3′AACTCATGCGCAGCAACAAG 5′), ADAMTS4 (5′ AGAAGAAGTTTGACAAGTG 3′, 3′ GCGTGTATTCACCATTGAG 5′), ADAMTS5(5′ ATCACCCAATGCCAAGG 3′, 3′ AGCAGAGTAGGAGACAAC 5′), Col II (5′ TGGTGGAGCAGCAAGAGCAA 3′, 3′ CCGTGGACAGCAGGCGTAGGAA 5′), COMP (5′ TGCGACGACGACGACGACAA 3′, 3′ CTTGTCTACCACCTTGTCTG5′) to assess chondrocyte metabolism: Sox9 (5′ CGAAATCAACGAGAAACTGGAC 3′,3′ ATTTAGCACACTGATCACACG 5′) and Col II for chondrogenesis. All data were normalized to 18s (5′ GTAACCCGTTGAACCCCATT 3′, 3′ CCATCCAATCGGTAGTAGCG 5′) as a housekeeping gene for relative gene expression.
Migration Scratch Assay
C-28/I2 cells were grown to 90% confluency as a monolayer in 35mm tissue culture plates (n=3). Scratches were made through the center of each plate using 200ul pipette tips and were observed for cell migration at 24, 48, and 72 hr. in the presence of IL-1β (10ng/ml) with and without LIPUS (30mW/cm2 every 24 hours). Migration was captured through images using phase contrast microscopy. The images were analyzed with ImageJ. This enabled us to analyze the scratched area in comparison to the covered area as well as the number of migrated cells.
Proliferation Assay
C-28/I2 cells were seeded at 0.2 × 106 cells per 35mm plate. Cells were treated with IL-1β with and without LIPUS stimulation and untreated cultures were used as controls (n=4). Samples were collected at Day 1, 3, and 5 and treated with MTT reagent (Life technologies, USA). Cells were lysed and OD was measured at 570nm as per manufacturer’s instructions.
Western blots
To further study the activation and inhibition of the IL-1β pathway in presences of IL-1β and LIPUS respectively, NFκB pathway activation was assayed using Western blots. Human cartilage explants from 3 patients (age 55–75) were randomly distributed into four groups (n=3 with 3/well). Explants were incubated with IL-1β with and without LIPUS. After 45 mins of stimulation, explants were snap frozen in liquid nitrogen and total protein was extracted using RIPA buffer. The protein samples were pooled and concentrations were quantified using a Bradford assay. Lysates were separated by SDS-PAGE and proteins were transferred to nitrocellulose membranes (Bio-Rad. CA). The membranes were incubated overnight at 4C with primary antibodies specific to NFκB-p65 (Santa Cruz, 1:1000 dilution), pNFκB-p65 (Cell Signaling Technology, 1:1000 dilution), IκBα (Santa Cruz, 1:1000 dilution), pIκBα (Santa Cruz, 1;1000 dilution), IL-1R1 (Abcam, 1:1000 dilution), and GAPDH (Santa Cruz, 1:1000 dilution) was used a housing keeping protein to assess relative protein expression. Appropriate secondary antibodies were used to visualize the protein signals, which were detected by enhanced chemiluminescence (Bio-Rad, CA). Western blots were run seven times to assess the regulation of NFκB pathway by IL-1β and LIPUS.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Statistically, significant differences between experimental and control groups were determined using Median tests. Western blot data was analyzed using One-Way ANOVA and p values were calculated using Fisher’s Least Significant Difference (LSD) post hoc test, p-values less than 0.05 were deemed as significant changes.
Results
LIPUS antagonizes IL-1β induced chondrocyte matrix depletion
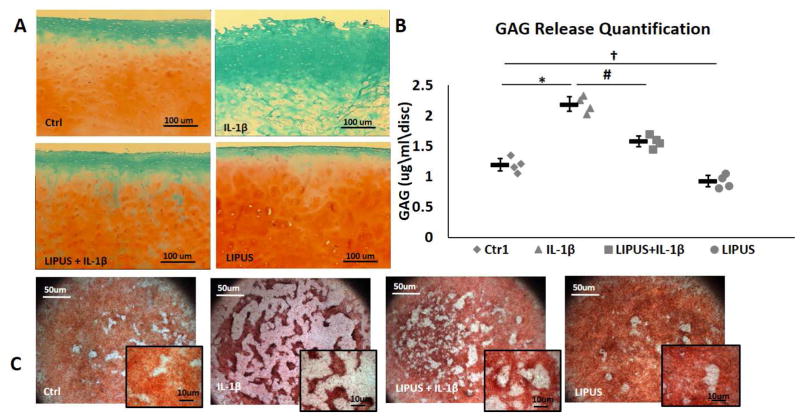
Human chondrocytes explants were treated with LIPUS and LIPUS + IL-1β for 7 days. IL-1β treatment caused a marked reduction in the amount of proteoglycans present in the matrix of these explants with little to no visible Safranin O staining relative to control samples (Fig. 2A). In contrast, LIPUS stimulated explants had relatively intense staining, indicating an increase in proteoglycans in the matrix. LIPUS also abrogated the IL-1β induced loss of proteoglycans, with LIPUS treated explants staining similar to control samples (Fig. 2A). GAG release was highest in IL-1β treated samples and LIPUS stimulation in conjunction with IL-1β significantly reduced the GAG release from cartilage explants. LIPUS treated explants exhibited significantly less GAG release relative to non-treated controls (Fig 2B).
Figure 2.
LIPUS protects against IL-1 ation of out IL-1 culture with and without IL-1 tal depletion of GAG content in human cartilage explants (n=3). LIPUS increased GAG content and rescued the IL-1d induced depletion of GAG from the cartilage matrix. B) Chondrocyte micromass cultures show disoriented and patchy GAG content in the presence of IL-1β, LIPUS protects GAG content in the presence of IL-1β and shows an apparent increase in GAG secretion in LIPUS treated samples. C) IL-1 inontent and rescuednts shows increased release of GAG contents into culture media. LIPUS reduced GAG breakdown in cartilage explants and significantly reduced IL-1βts shows GAG depletion. * represents significant difference between Ctrl and IL-1AG contents insignificant difference between IL-1 and IL-1AG contents into, and † significant difference between Ctrl and LIPUS groups, p=0.028. Bars indicate group means and error bars show 95% confidence intervals.
C-28/I2 micromass cultures showed similar trends, with evenly distributed Safranin O staining observed in control cultures. The addition of IL-1β resulted in scattered staining with visible area of minimal or no proteoglycans (Fig. 2C). LIPUS stimulation increased the staining intensity in the cultures without IL-1 β and rescued proteoglycan staining in cultures exposed to IL-1β.
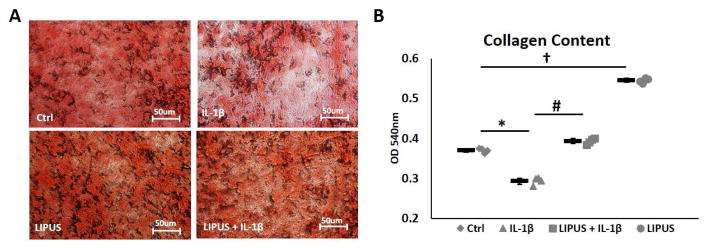
Collagen plays an important role in matrix integrity. IL-1β exposure reduced collagen content in C-28/I2 micromass cultures. Picosirius red staining shows a lack of collagen fiber alignment and density relative to control cultures (Fig 3A). LIPUS stimulation increased collagen fiber density and protected collagen matrix integrity against the detrimental effects of IL-1β (Fig 3A). Collagen content quantification at 540nm showed a significant decrease in collagen levels in IL-1β treated cultures. LIPUS retained collagen levels in the presence of IL-1β and enhanced matrix collagen content in LIPUS only treated cultures relative to non-treated controls (Fig 3B).
Figure 3.
LIPUS treated micromass cultures retain collagen. A) IL-1β treated cultures show reduced collagen staining with lack of collagen fiber orientation. LIPUS stimulation intensified collagen staining and retain collagen fibers intensity and integrity in IL-en. A) IL-1β treated cultures show reduced collagen staining with lack of collagen fiber orientation. LIPUS stimulation intensified collagen staining and t and inhibited IL-1ensity and integrity in IL-en. A * represents significant difference between Ctrl and IL-1ntegrity in ILsignificant difference between IL-1 and IL-1ntegrity in IL-en. and †nsignificant difference between Ctrl and LIPUS groups, p=0.028. Bars indicate group means and error bars show 95% confidence intervals.
LIPUS antagonizes IL-1β induced chondrocyte catabolism
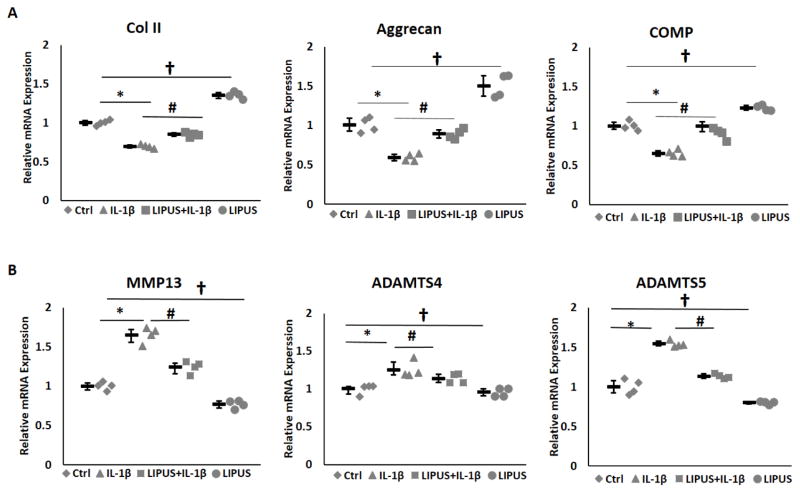
On the level of gene expression, quantitative real-time PCR demonstrated significant increases in mRNA expression MMP13 and ADAMTS5 in IL-1β treated chondrocytes (Fig. 4A). Stimulation with LIPUS inhibited IL-1β induced expression of MMP13 and ADAMTS5 to levels comparable to non-treated control samples. LIPUS treated cells also showed reduced mRNA expression of MMP13 and ADAMTS5 relative to non-treated samples. Furthermore, stimulation with LIPUS significantly increased the expression of anabolic marker genes Col II, aggrecan, and cartilage oligomeric matrix protein (COMP) (Fig. 4B). IL-1β exposure significantly reduced the expression of these anabolic markers while LIPUS stimulation inhibited IL-1β induced suppression of Col II, aggrecan, and COMP. LIPUS alone significantly increased Col II, aggrecan and COMP mRNA expression in chondrocytes. Collectively these data illustrate that LIPUS has an anabolic as well as a protective role against IL-1β induced cartilage degradation.
Figure 4.
LIPUS regulates chondrocyte metabolism (n=3). A) IL-1β significantly increases MMP3 and ADAMTS5 mRNA expression in C-28/I2 cells. LIPUS stimulation significantly reduces IL-1β induced MMP13 and ADAMTS5 mRNA expression. B) LIPUS significantly increase Col II, aggrecan and COMP expression in chondrocytes and protects against IL-1 stimulation intensified collagen staining and represents significant difference between Ctrl and IL-1β, p=0.028, # significant difference between IL-1 and IL-1β, p=0.028, # LIPU and †nsignificant difference between Ctrl and LIPUS groups, p=0.028. Bars indicate group means and error bars show 95% confidence intervals.
LIPUS enhances cell migration and proliferation
Increased expression of IL-1β is associated with reduced chondrocyte migration4. We next sought to examine the effects of LIPUS on the migration and proliferation of C-28/I2 chondrocytes. The scratch migration assay revealed little or no migration of cells in the presence of IL-1β over 72hr (Fig. 5A). LIPUS stimulation increased the rate of migration into the scratched region with or without the presence of IL-1β. Image J analysis of images indicated a significant increase in the number of cells and area covered by migrated cells in LIPUS treated cultures. LIPUS stimulation also inhibited IL-1β induced abrogation of chondrocyte migration (Fig. 5BC).
Figure 5.
LIPUS enhances C-28/I8 cells migration and proliferation (n=3) A) IL-1βIinhibits chondrocyte migration, LIPUS stimulation enhances chondrocyte migration into the empty region and suppresses IL-1β anti-migratory effects. B) Scratch area remains mostly cell-free in IL-1βacultures, LIPUS reverses the IL-1β effects as more than 90% of the area is covered in LIPUS treated cultures. C) ImageJ analysis shows significantly higher number of cells (> 500 cells) migrated into scratch area in LIPUS treated cultures in comparison to <50 cells migrated in IL-1β treated culture. D) Proliferation study shows no significant differences at D1 and D3 but LIPUS stimulated cultures show significant increase at D5. IL-1β cultures show no or little effect on chondrocyte proliferation. * represents significant difference between Ctrl and IL-1 representndroc significant difference between IL-1 and IL-1 representndrocyte pand †nsignificant difference between Ctrl and LIPUS groups, p=0.028. Bars indicate group means and error bars show 95% confidence intervals.
Chondrocyte proliferation was assessed using an MTT assay over 5 days of in vitro culture with and without IL-1β and LIPUS stimulation. IL-1β showed no effects on cell proliferation from D1 to D5. However, LIPUS stimulation significantly increased the rate of chondrocyte proliferation by D5 (Fig. 5D). Collectively, the migration and proliferation data show an increase in the rates of migration and proliferation in LIPUS treated cells and an inhibition of IL-1β induced abrogation of chondrocyte migration.
LIPUS attenuates IL-1β suppression of chondrogenesis
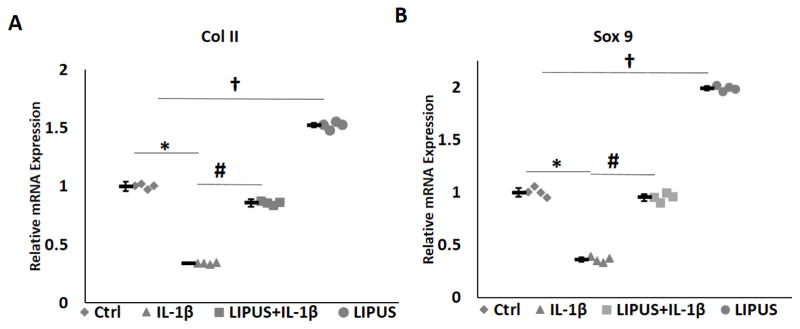
Current OA treatments include intra-articular injection of mesenchymal stem cells (MSC) and microfracture, both of which greatly depend on the differentiation of stem cells into chondrocytes. We next determined whether LIPUS affects chondrocyte differentiation. For this purpose, micromass cultures of C3H10T1/2 mesenchymal stem cells were cultured in the presence of IL-1β with or without LIPUS stimulation. Quantitative real-time PCR analysis showed LIPUS significantly increased BMP2-induced expression of Col II and Sox 9. In contrast, IL-1β inhibited the expression of Col II and Sox9. LIPUS stimulation antagonized IL-1β inhibition of Col II and Sox 9. Furthermore, LIPUS alone stimulation enhanced Col II and Sox 9 expression in chondrocytes relative to the expression level of control, non-treated chondrocytes (Fig. 6).
Figure 6.
LIPUS enhances chondrogenic differentiation of C3H10T1/2. LIPUS significantly increases the expressions of Sox9 and Col II in C3H10T1/2 cells and suppresses IL-1βImediated inhibition of Sox9, and Col II. * represents significant difference between Ctrl and IL-1 Col II. * repsignificant difference between IL-1 and IL-1 Col II. * represe and †asignificant difference between Ctrl and LIPUS groups, p=0.028. Bars indicate group means and error bars show 95% confidence intervals.
LIPUS inhibited IL-1β mediated activation of NFκB
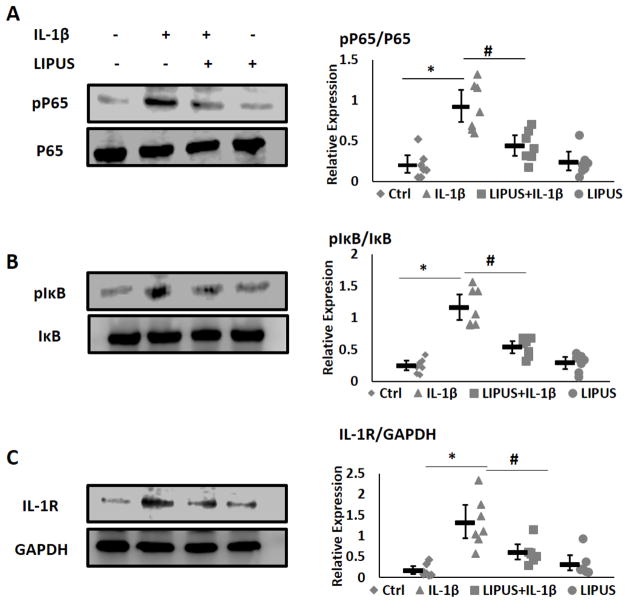
IL-1β is known to regulate the expression of the catabolic enzymes MMP13, ADAMTS4, and ADAMTS5 through the activation of the NFκB pathway6. We next examined whether LIPUS was able to inhibit IL-1β mediated activation of NFκB pathway in chondrocytes. Briefly, cartilage explants were treated with IL-1β with or without LIPUS stimulation. Protein expression was normalized to GAPDH and relative phosphorylation was quantified using ImageJ gel analyses. The Western blot analysis showed an increase in phosphorylation of NFκB-p65 and IκBα in IL-1β treated cells. LIPUS treatment suppressed the IL-1β induced phosphorylation of NFκB and Iκβα (Fig. 7AB). LIPUS stimulation alone had little effect on phosphorylation of NFκB-p65 and IκBα. Expression of IL-1R was elevated in the presence of IL-1β (Fig 7AC). LIPUS stimulation significantly reduced IL-1β induced IL-1R expression. Collectively LIPUS antagonized activation of IL-1β induced activation of NFκB pathway and IL-1R expression.
Figure 7.
LIPUS downregulates IL-1s and error bars show 95NFPU pathway (n=3). A) Phosphorylation of NF and error bars show 95% confidence intervals.cantly increases the expressions of Sox9 and Col II in C3H10T1/2 cells and suppresses IL-1 into scraκB-p65. B) LIPUS suppress IL-1β induced phosphorylation of I-pα-p65. B) LIPUS suppres. C) IL-1) LIPUS suppress IL-1β induced phosphorylati. Stimulation with LIPUS significantly reduces the expression of IL-1R. Expression levels were normalized to GAPDH expression levels. * represents significant difference between Ctrl and IL-1ized to GAPDH and # significant difference between IL-1 and IL-1ized to GABars indicate group means and error bars show 95% confidence intervals.
Discussion
OA is a multifactorial degenerative disease that affects hyaline cartilage. It is associated with age, diabetes, obesity, and lifestyle28. The onset of OA remains elusive but numerous studies have shown the critical role of IL-1β in the degradation of cartilage. IL-1β inhibits extracellular matrix protein synthesis and promotes its degradation through the increased expression of MMP-13 and ADAMTS 4/510,29. Increased levels of IL-1β have also been associated with decreased expression of both aggrecan and collagen type II. In addition to activation of catabolic enzymes through the NFκB pathways, IL-1β also induces other inflammatory cytokines, such as IL-6 and IL-830. The culminating synergism of these cytokines produces degenerative effects. Recent studies have shown encouraging results using an IL-1R antagonist (IL-1ra) in OA animal models and clinical trials31,32. IL-1ra may potentially slow OA progression but its effects are limited to suppressing IL-1β induced matrix degradation, with no anabolic effects demonstrated.
Ultrasound is a mechanical stimulus known to activate mechano-transductive pathways associated with annexin V, integrin, stretch activation, and ion channels33–35. Ultrasound-stimulated C-28/I2 chondrocytes have shown the activation of integrin and stretched activated channels leading to elevated mRNA expression pathways and increased expressions of collagen type II and aggrecan33. The integrin-associated pathways have been associated with cell survival, proliferation, and differentiation36,37. Recent studies have also shown that LIPUS may play a potential chondroprotective role in chondrocyte metabolism29,38. Ito et al., have shown significant inhibition of MMP13 and MMP1 mRNA expression in LIPUS treated articular cartilage in the presence of IL-1β29. LIPUS stimulation also significantly reduced IL-1β induced expression of Cox-2 in mandibular condylar chondrocytes through inhibition of IL-1β activated ERK1/2 phosphorylation39. LIPUS at an intensity of 30mW/cm2 for 20 min/day has been shown to significantly increase osteogenic differentiation, osteoblast proliferation, and bone healing in both in-vitro and in vivo models26,40.
In this study, we explored the potential of employing low-intensity pulsed ultrasound as a potential IL-1β antagonist, pro-anabolic, and chondroprotective therapy. LIPUS stimulation showed a significant increase in the expression of Col II, and aggrecan mRNA in human cartilage explants while IL-1β reduced the expression of Col II and aggrecan concomitant with increased mRNA expression of MMP13 and ADAMTS4. In human cartilage explants treated with IL-β and LIPUS, LIPUS restored Col II and aggrecan expression while suppressing IL-1β induced expression of MMP13 and ADAMTS5. Furthermore, LIPUS stimulation reduced the expression of MMP13 and ADAMTS5 mRNA in C-28/I2 cells, demonstrating potential anti-catabolic effects of LIPUS. Similar trends were observed in Safranin O staining of human cartilage explants after 7 days of exposure to LIPUS with or without IL-1β. GAG release analysis showed a significant increase in the degradation of cartilage matrix in IL-1β treated explants. Application of LIPUS significantly slowed down matrix degradation induced by IL-1β. Further, LIPUS treated samples showed higher retention of GAGs relative to control samples. LIPUS protected against IL-1β induced GAGs depletion of the extracellular cartilage matrix.
Cartilage regeneration is highly dependent on chondrocyte proliferation and migration. The current study has shown that IL-1β significantly reduced chondrocyte migration but had little or no effect on chondrocyte proliferation. The application of LIPUS enhanced chondrocyte migration and rescued the IL-1β treated chondrocytes. LIPUS stimulation also increased chondrocyte proliferation after 5 days of stimulation with little differences in first 3 days of stimulation. Delayed proliferative effects of C-28/I2 cells may be attributed to the slow proliferative tendency of chondrocytes. C-28/I2 cell migration was studied for 72 hours. Control and LIPUS stimulated cells fully migrated into the scratch region while little or no migration was observed in IL-1β treated samples. Application of LIPUS restored cell migration, fully inhibiting IL-1β ablation of cellular migration. Cell migration and proliferation are important for cartilage regeneration and repair. Cumulatively, these results indicate that LIPUS may present an avenue for the enhancement of cell migration and proliferation; two processes integral to cartilage repair that are, at least partially, hampered by IL-1β.
In the clinic, arthroscopic microfracture is used as a therapeutic approach in the treatment of osteoarthritis. However, the success rate of this approach is limited due to the adverse effect of high IL-1β levels on MSC differentiation. Simsa-Maziel et al., have shown that an increased amount of IL-1β significantly reduces the rate chondrocyte differentiation in ATDC5 cells by inhibiting expressions of alkaline phosphatase, Runx2, Sox 9, Col II and X, mediated by activation of P38 MAPK and NFκB pathways41. Our current study confirms the published results of Simsa-Maziel et al., as IL-1β treated C3H10T1/2 cells showed a significant reduction in the expression of Col II, and Sox 941. IL-1β was shown to increase cell proliferation in ATDC5 cells, which was not observed in current study with C-28/I2 chondrocytes. This difference is probably attributable to the difference in cell types tested41. LIPUS stimulation increased the level of expression of Col II, and Sox 9 in C3H10T1/2 cell and antagonized the detrimental effects of IL-1β. IL-1β suppresses the level of Col II, and aggrecan expression through activation of NFκB pathway. Treating cartilage explants with LIPUS suppressed the IL-1β induced phosphorylation of NFκB-p65 and Iκβα. Furthermore, LIPUS inhibited the expression of IL-1R in presence of IL-1β, thereby making chondrocytes less susceptible to the catabolic and inflammatory effects of IL-1β
The underlying mechanism of ultrasound stimulation on gene regulation remains unclear. Mechanotransductive studies have shown that membrane-bound mechanosensors such as stretch-activated channels, integrins, ion channels, and gap junctions can play important roles in regulating gene expression through several pathways. The most notable pathways are P13K/AKT, MAPK/ERK1/2, P38 and JNK21,34,35,42. A recent study by Whitney et al., shows that the phosphorylation of FAK/Src/CrkII complex and activation MAPK/ERK pathways potentially leading to altered gene expression, cell proliferation, migration, and survival35. Jang et al., have also shown activation of focal adhesion kinase pathways in chondrogenic progenitor cells migration43. The data from our current study show that LIPUS not only can suppress IL-1β induced cartilage degradation through MMP13 and ADAMTS 5 but also can facilitate cartilage regeneration through chondrocyte migration, proliferation, and differentiation. Our assays also demonstrate that LIPUS stimulation largely suppresses the activation of IL-1β induced NFκB activation. LIPUS reduced the expression of NFκB associated IL-1β receptor, IL-1R, and also reduced phosphorylation of downstream IκBα and NFκB-p65.
Recent studies show that LIPUS stimulation can decrease the expression of MMP13 mRNA expression and reduce matrix degradation. The chondro-regenerative effects of LIPUS may result from other mechano-transductive pathways associated with the janusi-activated kinase (JAK), Signal transducer and activator of transcription (STAT) pathway, Rac activation, calcium and nitric oxide signaling through SIC, G-coupled receptors, or growth factor receptors44. LIPUS stimulation has been shown to activate MAPK-ERK1/2, calcium ion signaling, and P13/AKt pathways in osteoblast and mesenchymal lineage cells45–47. Further studies are required to understand the underlying mechanism of LIPUS effects on cartilage metabolism and regeneration. It is also noted that the current study was limited to in vitro and ex vivo assessment of the effects of LIPUS in cartilage and chondrocytes.
In summary, this study indicates that LIPUS suppress IL-1β mediated cartilage degeneration and increase levels of extracellular matrix proteins (Col II and aggrecan), facilitates chondrocyte proliferation, migration, and chondrogenic differentiation. Thus, LIPUS may have the potential to be an anabolic and chondo-protective therapeutic treatment for OA and other cartilage degenerative disorders. Further studies to validate the chondroprotective role of LIPUS and to understand the underlying mechanism with in vivo animal models are warranted.
Acknowledgments
We cordially thank Dr. Young-Su Yi and Dr. Jian-lu Wei for their contribution in protein expression data. This study is supported by NIH 1R56AI100901, 5R01AR061484, 1R01AR062207, and ACR Research and Education Foundation.
Footnotes
Authors Contribution
SU and CJ designed the experiments. SU conducted all experiments and wrote the manuscript. RB conducted safranin O staining. YD and AH ran western blots. SU, DK, YQ, and CJ did the data analysis and reviewed the manuscript.
Competing Financial Interest Statement: The authors have no conflict of interest and competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15:S230–235. 11991 [pii] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 3.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22:351–384. doi: 10.1016/j.berh.2008.02.001. S1521-6942(08)00013-2 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Joos H, Wildner A, Hogrefe C, Reichel H, Brenner RE. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis research & therapy. 2013;15:R119. doi: 10.1186/ar4299. ar4299 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB. Osteoarthritis, and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 6.Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. The Journal of rheumatology. 2008;35:2306–2312. doi: 10.3899/jrheum.080346. 08/13/1022 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix biology : journal of the International Society for Matrix Biology. 2002;21:251–262. doi: 10.1016/s0945-053x(02)00007-0. S0945053X02000070 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis and rheumatism. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Sylvester J, El Mabrouk M, Ahmad R, Chaudry A, Zafarullah M. Interleukin-1 induction of aggrecanase gene expression in human articular chondrocytes is mediated by mitogen-activated protein kinases. Cell Physiol Biochem. 2012;30:563–574. doi: 10.1159/000341438. 000341438 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Pratta MA, Scherle PA, Yang G, Liu RQ, Newton RC. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis and rheumatism. 2003;48:119–133. doi: 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- 11.Blumenfeld I, Livne E. The role of transforming growth factor (TGF)-beta, insulin-like growth factor (IGF)-1, and interleukin (IL)-1 in osteoarthritis and aging of joints. Exp Gerontol. 1999;34:821–829. doi: 10.1016/s0531-5565(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 12.Lammi MJ. Current perspectives on cartilage and chondrocyte mechanobiology. Biorheology. 2004;41:593–596. [PubMed] [Google Scholar]
- 13.Sanchez-Adams J, Leddy HA, McNulty AL, O’Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep. 2014;16:451. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2012;18:715–724. doi: 10.1089/ten.TEA.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo V, Cadova M, Gallo LM. Mechanical behavior of bovine nasal cartilage under static and dynamic loading. Journal of biomechanics. 2013;46:2137–2144. doi: 10.1016/j.jbiomech.2013.07.001. S0021-9290(13)00308-4 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Angele P, et al. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2003;21:451–457. doi: 10.1016/S0736-0266(02)00230-9. S0736026602002309 [pii] [DOI] [PubMed] [Google Scholar]
- 17.De Croos JN, Dhaliwal SS, Grynpas MD, Pilliar RM, Kandel RA. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix biology : journal of the International Society for Matrix Biology. 2006;25:323–331. doi: 10.1016/j.matbio.2006.03.005. S0945-053X(06)00041-2 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Toyoda T, Seedhom BB, Kirkham J, Bonass WA. Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology. 2003;40:79–85. [PubMed] [Google Scholar]
- 19.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. 00003677-200510000-00008 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Sutton AJ, Muir KR, Mockett S, Fentem P. A case-control study to investigate the relation between low and moderate levels of physical activity and osteoarthritis of the knee using data collected as part of the Allied Dunbar National Fitness Survey. Annals of the rheumatic diseases. 2001;60:756–764. doi: 10.1136/ard.60.8.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng K, et al. Effects of low-intensity pulsed ultrasound on integrin-FAK-PI3K/Akt mechanochemical transduction in rabbit osteoarthritis chondrocytes. Ultrasound in medicine & biology. 2014;40:1609–1618. doi: 10.1016/j.ultrasmedbio.2014.03.002. S0301-5629(14)00149-5 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Xia P, et al. Low-Intensity Pulsed Ultrasound Affects Chondrocyte Extracellular Matrix Production via an Integrin-Mediated p38 MAPK Signaling Pathway. Ultrasound in medicine & biology. 2015;41:1690–1700. doi: 10.1016/j.ultrasmedbio.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Naito K, et al. Low-intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28:361–369. doi: 10.1002/jor.20995. [DOI] [PubMed] [Google Scholar]
- 24.Li X, et al. Effect of low-intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell biochemistry and biophysics. 2011;61:427–434. doi: 10.1007/s12013-011-9206-4. [DOI] [PubMed] [Google Scholar]
- 25.Guha Thakurta S, Budhiraja G, Subramanian A. Growth factor and ultrasound-assisted bioreactor synergism for human mesenchymal stem cell chondrogenesis. Journal of tissue engineering. 2015;6 doi: 10.1177/2041731414566529. 2041731414566529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin SM, Qin YX. Enhancement of Osteogenic Differentiation and Proliferation in Human Mesenchymal Stem Cells by a Modified Low Intensity Ultrasound Stimulation under Simulated Microgravity. PloS one. 2013;8:e73914. doi: 10.1371/journal.pone.0073914. PONE-D-13-17793 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone JE, Akhtar N, Botchway S, Pennock CA. Interaction of 1,9-dimethylmethylene blue with glycosaminoglycans. Ann Clin Biochem. 1994;31(Pt 2):147–152. doi: 10.1177/000456329403100206. [DOI] [PubMed] [Google Scholar]
- 28.Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3:257–264. doi: 10.1023/a:1020185404126. 5090807 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Ito A, et al. Low-intensity pulsed ultrasound inhibits messenger RNA expression of matrix metalloproteinase-13 induced by interleukin-1beta in chondrocytes in an intensity-dependent manner. Ultrasound in medicine & biology. 2012;38:1726–1733. doi: 10.1016/j.ultrasmedbio.2012.06.005. S0301-5629(12)00364-X [pii] [DOI] [PubMed] [Google Scholar]
- 30.Attur MG, Patel IR, Patel RN, Abramson SB, Amin AR. Autocrine production of IL-1 beta by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proc Assoc Am Physicians. 1998;110:65–72. [PubMed] [Google Scholar]
- 31.Chevalier X, et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. The Journal of rheumatology. 2005;32:1317–1323. 0315162X-32-1317 [pii] [PubMed] [Google Scholar]
- 32.Yang KG, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16:498–505. doi: 10.1016/j.joca.2007.07.008. S1063-4584(07)00260-9 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Choi BH, et al. Mechanotransduction pathways of low-intensity ultrasound in C-28/I2 human chondrocyte cell line. Proc Inst Mech Eng H. 2007;221:527–535. doi: 10.1243/09544119JEIM201. [DOI] [PubMed] [Google Scholar]
- 34.Hsu HC, et al. Ultrasound induces cyclooxygenase-2 expression through integrin, integrin-linked kinase, Akt, NF-kappa B and p300 pathway in human chondrocytes. Cellular Signalling. 2007;19:2317–2328. doi: 10.1016/j.cellsig.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Whitney NP, Lamb AC, Louw TM, Subramanian A. Integrin-mediated mechanotransduction pathway of low-intensity continuous ultrasound in human chondrocytes. Ultrasound in medicine & biology. 2012;38:1734–1743. doi: 10.1016/j.ultrasmedbio.2012.06.002. S0301-5629(12)00339-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi BH, Woo JI, Min BH, Park SR. Low-intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J Biomed Mater Res A. 2006;79:858–864. doi: 10.1002/jbm.a.30816. [DOI] [PubMed] [Google Scholar]
- 37.Nishikori T, et al. Effects of low-intensity pulsed ultrasound on proliferation and chondroitin sulfate synthesis of cultured chondrocytes embedded in Atelocollagen gel. Journal of biomedical materials research. 2002;59:201–206. doi: 10.1002/jbm.1226. [pii] [DOI] [PubMed] [Google Scholar]
- 38.Park K, Hoffmeister B, Han DK, Hasty K. Therapeutic ultrasound effects on interleukin-1beta stimulated cartilage construct in vitro. Ultrasound in medicine & biology. 2007;33:286–295. doi: 10.1016/j.ultrasmedbio.2006.08.009. S0301-5629(06)01804-7 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Iwabuchi Y, et al. Effects of low-intensity pulsed ultrasound on the expression of cyclooxygenase-2 in mandibular condylar chondrocytes. J Oral Facial Pain Headache. 2014;28:261–268. doi: 10.11607/ofph.1156. [DOI] [PubMed] [Google Scholar]
- 40.Uddin SMZ, Cheng JQ, Lin W, Qin YX. Low-Intensity Amplitude Modulated Ultrasound Increases Osteoblastic Mineralization. Cellular and Molecular Bioengineering. 2011;4:81–90. doi: 10.1007/s12195-010-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simsa-Maziel S, Monsonego-Ornan E. Interleukin-1beta promotes proliferation and inhibits differentiation of chondrocytes through a mechanism involving down-regulation of FGFR-3 and p21. Endocrinology. 2012;153:2296–2310. doi: 10.1210/en.2011-1756. en.2011-1756 [pii] [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi R, et al. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: a basic science study. Arthritis research & therapy. 2008;10:R77. doi: 10.1186/ar2451. ar2451 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang KW, et al. Low-intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway. Ultrasound in medicine & biology. 2014;40:1177–1186. doi: 10.1016/j.ultrasmedbio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. nrm2597 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Gusmao CVB, Pauli JR, Saad MJA, Alves JM, Belangero WD. Low-intensity Ultrasound Increases FAK, ERK-1/2, and IRS-1 Expression of Intact Rat Bones in a Noncumulative Manner. Clinical orthopaedics and related research. 2010;468:1149–1156. doi: 10.1007/s11999-009-1146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou CH, Hou SM, Tang CH. Ultrasound Increased BMP-2 Expression Via PI3K, Akt, c-Fos/c-Jun, and AP-1 Pathways in Cultured Osteoblasts. Journal of cellular biochemistry. 2009;106:7–15. doi: 10.1002/Jcb.21934. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S, Cheng J, Qin YX. Mechanobiological modulation of cytoskeleton and calcium influx in osteoblastic cells by short-term focused acoustic radiation force. PloS one. 2012;7:e38343. doi: 10.1371/journal.pone.0038343. PONE-D-11-26111 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]