Abstract
Introduction
Based on clinical observations, we hypothesized that in infiltrative high-grade brainstem neoplasms, such as diffuse intrinsic pontine glioma (DIPG), longitudinal metabolic evaluation of the tumor by magnetic resonance spectroscopy (MRS) may be more accurate than volumetric data for monitoring the tumor’s biological evolution during standard treatment.
Methods
We evaluated longitudinal MRS data and corresponding tumor volumes of 31 children with DIPG. We statistically analyzed correlations between tumor volume and ratios of Cho:NAA, Cho:Cr, and NAA:Cr at key time points during the course of the disease through the end of the progression-free survival period.
Results
By the end of RT, tumor volume had significantly decreased from the baseline (P < .0001) and remained decreased through the last available follow-up magnetic resonance imaging study (P = .007632). However, the metabolic profile of the tumor tissue (Cho:Cr, NAA:Cr, and Cho:NAA ratios) did not change significantly over time.
Conclusions
Our data show that longitudinal tumor volume and metabolic profile changes are dissociated in patients with DIPG during progression-free survival. Volume changes, therefore, may not accurately reflect treatment-related changes in tumor burden. This study adds to the existing body of evidence that the value of conventional MRI metrics, including volumetric data, needs to be reevaluated critically and, in infiltrative tumors in particular, may not be useful as study end-points in clinical trials. We submit that advanced quantitative MRI data, including robust, MRS-based metabolic ratios and diffusion and perfusion metrics, may be better surrogate markers of key end-points in clinical trials.
Keywords: Magnetic Resonance Imaging, proton spectroscopy, diffuse intrinsic pontine glioma, brainstem tumor, response criteria
Graphical Abstract
Introduction
In children, tumors arising from the brainstem (i.e., midbrain, pons, and medulla oblongata) constitute nearly 11% of all central nervous system (CNS) tumors [1]. Approximately 58% to 85% of brainstem tumors are diffusely infiltrative high-grade gliomas (DIPG), associated with a distinctively poor prognosis [2–4]. Conventional MRI plays an important role in the imaging diagnosis and follow-up of DIPG. Most investigators agree that the conventional MRI appearance of DIPG is virtually pathognomonic. Currently, the diagnosis is usually made by MRI, and biopsy is rarely performed. Follow-up MRI in patients receiving standard treatment (i.e., conformal radiation therapy [RT] and neo-adjuvant therapies) shows a very characteristic volumetric decrease of the brainstem lesion in conjunction with a decrease of the abnormal T2-weighted imaging (T2WI) signal within the lesion area, often associated with clinical improvement. This appearance is generally considered to represent a favorable response to treatment; unfortunately, it is temporary and is almost invariably followed by relapse and a dismal outcome. The time of progression-free survival (PFS) is a common end-point in clinical trials and is usually determined based on a combination of clinical and MRI data. Traditionally, a volume increase exceeding 25% is considered to be progressive disease from the imaging point of view, whereas clinically the need of increasing doses of steroids to maintain a stable neurological status may be indicative of the same.
During the clinical imaging care of patients with DIPG, we have frequently observed a discrepant evolution of conventional MRI and magnetic resonance spectroscopy (MRS) findings. That is, the early volumetric improvement during treatment is not mirrored by similar changes in the metabolic profile of the tumor; notably, Cho:NAA ratios remained relatively stable during treatment before worsening towards the end of the progression-free period. This phenomenon has also been demonstrated by other investigators [5]. Previous reports show that conventional imaging parameters do not have predictive value for outcome and may, therefore, not be suitable surrogate imaging markers for longitudinal imaging evaluation of DIPG [6, 7]. Conversely, advanced MRI techniques, such as MRS or perfusion-weighted imaging, capture important biological aspects of the neoplastic process (e.g., cell proliferation, angiogenesis) [8]. Several studies of the diagnostic and prognostic yield of MRS in DIPG have shown that metabolic ratios are correlated with the overall survival of these patients [9–11]. Therefore, MRS has the potential to predict disease progression prior to clinical or even imaging progression, when the latter is based on the evaluation of conventional MRI features [5, 10].
We hypothesized that the temporary volumetric improvement in DIPG during the first months of treatment may not accurately reflect changes in tumor biology and the burden induced by anti-tumor therapy, but instead constitute an epiphenomenon. The purpose of our study was to determine the relationship between the evolution of tumor volume and MRS metrics in patients with DIPG who receive conformal RT and anti–vascular endothelial growth factor treatment.
Patients and Methods
Study description
A total of 35 children with newly diagnosed DIPG were enrolled in a prospective phase I clinical trial between June 2007 and August 2009. This trial was designed to estimate the maximum tolerated dose and dose-limiting toxicity of Vandetanib (ZD6474, Zactima, AstraZeneca, Wilmington, DE) when used in conjunction with conformal RT [12]. Fractionated RT (54 Gy total) was given over approximately 6 weeks while Vandetanib was administered at 5 different dose levels during the entire duration of the study period. As per protocol stipulations, partial response to treatment was defined as a greater-than or equal- to 50% reduction in the sum of the product of the maximum perpendicular diameters of the tumor size at baseline by MRI and a stable or decreasing dose of dexamethasone accompanied by a stable or improving neurologic examination maintained for at least 6 weeks. Conversely, progression free survival was defined as the time interval from the start of therapy to disease progression or death. Progressive disease, indicating the end of PFS, was defined in the protocol as a) neurologic abnormalities or worsening neurologic status not explained by causes unrelated to tumor progression (e.g., seizures, anticonvulsant toxicity, electrolyte disturbances, sepsis) or b) a greater-than 25% increase in the product of the maximum perpendicular diameters of the tumor lesion by imaging or c) increasing doses of dexamethasone required to maintain a stable neurologic status.
MR techniques
MRI was performed by using a prospectively designed imaging protocol from enrollment through the end of PFS at specific time points. The first MRI study was performed at enrollment in the trial (baseline); followed by additional studies at approximately 1, 3, and 6 weeks (i.e., shortly after the end of RT) after initiation of treatment; and every 2 months thereafter. Most of the MRI studies were performed by using a 3 Tesla MRI scanner (Trio Tim, Siemens Healthcare, Erlangen, Germany). For logistic reasons, 14 examinations included in the final data evaluation were performed on a 1.5 Tesla system (Avanto, Siemens Healthcare, Erlangen, Germany). If deemed necessary, patients were scanned while under general anesthesia.
Conventional MRI sequences used for this study included non-enhanced axial T1-weighted gradient-echo, axial T2WI fast spin-echo, and axial contrast-enhanced T1-weighted gradient-echo imaging. Single-voxel spectroscopy (SVS) was used to acquire most MRS data; however, a few patients were scanned by using two-dimensional chemical shift imaging (2D-CSI) (i.e., multi-voxel spectroscopy [MVS]) at baseline and during follow-ups. All SVS studies were performed on the 3T platform, and all but one of the 2D CSI studies were performed on the 1.5T scanner. For SVS, the following parameters were used: vector size 1024; voxel size, 20×20×20 mm3; repetition time, 2000 ms; echo time, 135 ms; signal averages, 144; and bandwidth, 1200 Hz. All SVS were performed on a 3T magnet. A 2D CSI was used if the examination was performed on a 1.5T platform. The following MRI settings used differed from those used for SVS: volume-of-interest 55–80×55–80×15 mm3; voxel size, 10×10×15 mm3; repetition time, 1700 ms; signal averages, 5; and bandwidth, 1000 Hz/pixel.
Appropriately positioned saturation bands were used to eliminate artifacts from the skull and subcutaneous fat. For SVS, automatic in-line processing of the MRS data was performed by using the scanners’ proprietary software. For 2D-CSI MRS data processing, a dedicated postprocessing workstation from the same vendor (Leonardo Multimodality Workstation, Siemens, Erlangen, Germany) was used. In three instances, the spectra required manual editing using linear and constant phase-correction to ensure optimized spectral resolution of the Cho and Cr peaks and an optimal baseline.
Patient cohort
MRS data of 35 patients enrolled in the treatment protocol were screened for availability and quality of MRI/MRS data. One patient was eliminated from the evaluation because, in retrospect, the imaging appearance of the patient’s tumor at baseline was not characteristic for DIPG. In another patient, a large necrotic-hemorrhagic area within the tumor field rendered MRS data uninterpretable; hence, this patient was eliminated from the analysis, too. Two other patients were excluded from the evaluation because some of their studies were not performed at the required time points or were incomplete due to scheduling or other restraints (e.g., anesthesia, long scan time). Overall, 31 patients (17 girls, 14 boys; age range, 34 months to 16 years; mean age 7.3 ± 3.8 years) qualified for inclusion in this research.
All patients gave informed consent prior to inclusion in this study and the study was approved by the local ethics committee and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Data collection
The volumetric tumor evaluation included the calculation of tumor volume by manual segmentation of the hyperintense area within the pons on transverse T2WI. Extrapontine lesion extensions (i.e., into midbrain, cerebellar peduncles, or medulla oblongata) – when present – were also included.
MRS data of all patients were checked for quality by an experienced, board-certified neuroradiologist. A total of 131 spectra met our criteria of adequacy (good spectral and peak resolution) and were included in the final data analysis. Up to 12 datasets per patient were available, with a mean ± SD of 4 ± 2 per patient. Of the 131 data sets, 111 scans were acquired by using SVS; 20 scans were acquired by MVS. A total of 19 patients had analyzable MRS data from baseline scans. For the remaining 12 patients, who did not have MRS scans performed prior to initiation of treatment, the first on-treatment scan was defined as “baseline” for statistical evaluation; those datasets were obtained on days 5, 7, 8, 9, 11, or 12 after initiation of therapy. For the time point after the end of RT, 19 patients had analyzable MRS datasets. The total number of MRI scans available at each time point were as follows: baseline, 19; 2 weeks, 22; 4 weeks, 18; 8 weeks, 19; 16 and 24 weeks, 16 each; 32 weeks, 9; 40 weeks,6; 48, 64, and 72 weeks, 2 each; and 56 weeks, 3.
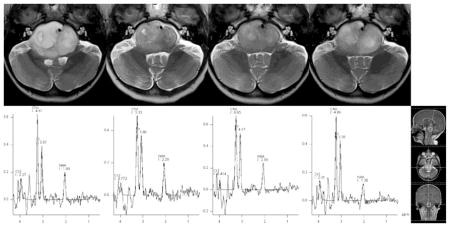
To ensure an optimal correspondence between SVS and MVS data, several contiguous voxels (typically 3 or 4) from the MVS dataset were selected from the lesion area, and mean values of Cho, Cr, and NAA were calculated for those. The calculated means were subsequently used to calculate the actual Cho:NAA, Cho:Cr, and NAA:Cr ratios in those cases. (Fig 1)
Fig 1.
Illustration of the longitudinal evolution of conventional MRI features and MRS in a patient with DIPG. Images and MR spectra were obtained at baseline and at 8 weeks, 6 months, and 9 months after treatment initiation (left to right). An example of voxel placement for SVS is shown for the 9-month-scan (far right). Note the simultaneous decrease of the tumor size and signal intensity during treatment and their subsequent increase during follow-up. Cho:NAA ratios are relatively stable or minimally increase during treatment (baseline, 2.27; 8 weeks, 2.34; 6 months, 2.71) until the last scan prior to the patient being taken off study due to progressive disease (9 months, 3.59).
Statistical analysis
Descriptive statistics and graphical tools were used to describe the longitudinal evolution of the Cho:NAA, Cho:Cr, and NAA:Cr ratios for each patient separately. The Wilcoxon signed-rank test was used to evaluate changes in tumor volume and MRS metrics over time. Pairwise comparisons were made to examine the changes between the follow-up points and baseline. The P values were adjusted using the Bonferroni method to account for multiple testing problems when pairwise comparisons were made. To test for a correlation of tumor volume and MRS metrics, we used a generalized estimating equation model that accounts for intra-patient variability because longitudinal MRI data were collected. MRS parameters were “response variables,” and tumor volume was the “independent variable.” The results were adjusted by age; baseline ratios of Cho:NAA, Cho:Cr, and NAA:Cr; and the elapsed time effect. All of the statistical analyses were conducted by using SAS 9.3. A P value of 0.05 or less was considered to indicate statistical significance.
Results
Longitudinal development of tumor volume
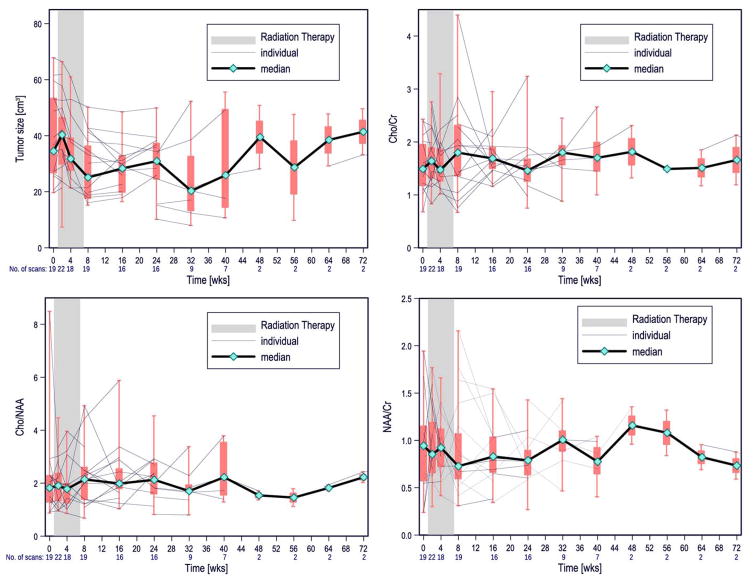
Data recorded for the tumor volume are summarized in Table 1 and shown in Fig 2A. The median tumor size increased slightly from baseline to the first scan after initiation of treatment but steadily decreased thereafter during RT. The size of the tumor was significantly smaller at the end of RT than at baseline (P < .0001) After a short period of stability, the median tumor size began to increase; however, tumor volume remained significantly smaller than at baseline through the last available scan (P = .007632). The increase in tumor size for the entire cohort was not statistically significant between the end of RT and the last available time point.
Table 1.
Longitudinal evolution of tumor volume and MRS parameters
| Tumor size [cm3] | Baseline | Post RT | Last |
|---|---|---|---|
| Min | 7.40 | 15.20 | 8.00 |
| Max | 67.80 | 50.20 | 55.60 |
| Median | 36.60 | 25.10 | 26.05 |
| SD | 14.36 | 11.25 | 14.28 |
|
| |||
| Cho:Cr | |||
|
| |||
| Min | 0.68 | 0.67 | 0.75 |
| Max | 3.29 | 4.40 | 3.24 |
| Median | 1.50 | 1.80 | 1.61 |
| SD | 0.62 | 0.88 | 0.63 |
|
| |||
| NAA:Cr | |||
|
| |||
| Min | 0.24 | 0.31 | 0.27 |
| Max | 1.94 | 2.16 | 1.46 |
| Median | 0.95 | 0.73 | 0.83 |
| SD | 0.46 | 0.50 | 0.35 |
|
| |||
| Cho:NAA | |||
|
| |||
| Min | 0.87 | 0.68 | 0.80 |
| Max | 8.49 | 4.93 | 5.88 |
| Median | 1.87 | 2.14 | 2.13 |
| SD | 1.53 | 1.14 | 1.25 |
Note: Min = minimum, Max = maximum, SD = standard deviation, RT = radiation therapy
Fig 2.
Box plots depicting the longitudinal evolution of tumor volume (A) and MRS metrics (B–D) in our patient cohort. Included are median (black line), interquartile range (box plots), and lowest and highest data points in the cohort (whiskers). Note: A single data point at week 80 and one at week 88 each are not depicted.
Applying the response criteria defined for this trial, we found that compared to tumor size at baseline 4 of the 31 patients (12.90%) had a greater than 50% reduction in tumor volume by the end of RT. Another 6 patients (19.35%) had a greater than 50% reduction in tumor volume by 30 weeks after the initiation of treatment. A greater than 50% reduction in tumor volume occurred in one patient 38 weeks into treatment, this patient completed treatment with an overall survival of 3.69 years.
MRS evaluation of the longitudinal evolution of the tumors’ metabolic profile
Table 1 also summarizes the MRS data at baseline, after RT and at the last time point. From baseline to post RT, both a slight increase of the median Cho:Cr ratio and a decrease of the median NAA:Cr ratios were observed. However, neither these nor longitudinal changes of the Cho:NAA ratio were found to be significant using Wilcoxon signed-rank test. The graphs of the longitudinal development of the MRS ratios show that these variables usually remain relatively stable over time (Fig 2B–D) although, occasionally, an increase in Cho:NAA was noted on an individual basis (in 4 patients).
Correlation of MRS metrics with tumor volume
No statistically significant correlation was found between the tumor volume and metabolic ratios (Cho:NAA, Cho:Cr, and NAA:Cr) during the study period.
Discussion
The discrepancy between the prominent longitudinal tumor volume changes and the relatively stable MRS parameters suggests that the temporary volumetric (and T2WI signal) improvement observed by conventional MRI during treatment may not be truly representative of the actual therapy-induced tumor control in pediatric patients with DIPG [5, 10]. In contrast to a previous study reporting an increase of choline and Cho:NAA, and decrease in NAA after RT [10], we did not observe statistically significant changes in Cho:Cr, NAA:Cr, or Cho:NAA ratios over time. However, a slight increase of median Cho:Cr levels from 1.50 at baseline to 1.80 after RT and a decrease of NAA:Cr from 0.95 to 0.73 occurred. Cho:NAA ratios slightly increased from a median value of 1.87 to respective values of 2.14 and 2.13 at the end of RT and at the last follow-up MRI. Because increased choline levels indicate increased cell membrane turnover, decreased levels of NAA are related to a loss of integrity of the neuroaxonal unit (both occur and are characteristic in parenchymal brain tumors), and Cr is considered to be a relatively constant “reference metabolite”. The minimal, statistically non-significant variations of the metabolic ratios over time through the end of RT suggest, at best, a “steady-state” or temporary “arrest” of the neoplastic process during RT, followed by worsening ratios consistent with a subsequent progression of disease indicative of the end of PFS [13]. These findings also show that MRS data more closely match key events in the clinical course of the disease. In addition, the finding that 11 of 31 (35%) patients had a greater than 50% reduction in tumor volume at least one point during follow-up supports our conclusions that volumetric evaluation alone may lead to overestimation of the response to therapy in patients with DIPG. Perhaps, this would be applicable to other infiltrative high-grade gliomas as well.
We submit that intratumoral (mainly interstitial) water content (i.e., vasogenic edema) fluctuations may be a dominant factor producing the volume (and T2WI signal intensity) variations within the tumor lesion area over time. As a function of their neurological status, patients commonly receive steroids as adjuvant therapy during the first few weeks or months of their treatment. It is likely that significant reduction in water content (edema removal) within the tumor may occur as a synergetic result of the various therapeutic measures, including RT [14], adjuvant steroid therapy [15], and anti-VEGF [16]. These therapeutic measures likely affect mechanisms regulating tissue water homeostasis in various ways, such as inducing corrective bulk water shifts from the interstitial compartment to the intravascular compartment, stabilizing vessel wall permeability, and/or favorably modifying the proportion of leaky (angioneogenetic) versus non-leaky (normal) vessels which ultimately leads to decreased water leakage from tumor vessels and increased resorption of interstitial fluid [16–19]. In keeping with this hypothesis, a previous study in patients with DIPG showed that DSC and ASL perfusion metrics (cerebral blood volume and flow) improved from baseline through the end of RT [20]. Our findings obviously do not imply that RT is ineffective in tumor control and does not produce a reduction in tumor cells within the lesion; but the actual changes to tumor burden per se may not be assessed directly and accurately by conventional MRI metrics (e.g., T2WI signal or lesion volume). Therefore, we propose that in infiltrative tumors (i.e., DIPG and likely also other infiltrative gliomas), the actual tumor response is not adequately captured by tumor volume measurements and T2WI signal changes, leading to a gross overestimation of tumor response in most cases and making conventional MRI metrics inadequate as response criteria for study endpoints in clinical trials [5, 8, 21, 22].
This conclusion is further underscored by well documented data showing that conventional MRI metrics in DIPG have no value for prognostication or the prediction of response to treatment [6, 7]. The Response Assessment in Neuro-Oncology Criteria (RANO) [23, 24], and their modified version for pediatric tumors, Response Assessment in Pediatric Neuro-Oncology (RAPNO [25], continue to use conventional MRI-based criteria. For example, disease progression is defined as a 25% increase in the product of the largest perpendicular diameters, and 25% or more decrease indicates response [8]. The challenges with making tumor volume measurements in infiltrative tumors of the CNS lie in the poor definition of the tumor’s boundaries and uncertainties about the density and distribution of tumor cells within the lesion area. Tumor cells in infiltrative gliomas may extend far beyond the area of enhancement [26]. Furthermore, because cerebral neoplasms are often associated with more or less perilesional vasogenic edema, the extent of T2WI signal abnormalities may not accurately delineate the tumor either, leading to potentially considerable intraobserver ambiguities and interobserver variations. Conversely, advanced MRI markers (e.g., apparent diffusion coefficient, cerebral blood volume, Cho:NAA ratio) may capture more relevant aspects of tumor histoarchitecture and pathophysiology, making them more suitable for tumor delineation and assessment of the actual tumor burden at initial diagnostic work-up and during treatment [27–29].
Some limitations of our study need to be addressed. Although the total numbers of patients and data points were quite considerable for a DIPG study, the numbers steadily decreased markedly towards the end of the evaluation period as patients reached the study’s endpoint and dropped out of the study. Some technical issues further compounded this complication. Although saturation bands were used to minimize fat contamination and susceptibility artifacts from the skull base and the calvarium, a certain number of spectra had to be excluded from the final evaluation because of quality concerns. Therefore, the number of data points for individual patients was inconsistent, which may have affected the statistical evaluation. Additionally, some of the spectroscopic datasets were acquired by using SVS, others by using 2D-CSI (i.e., 18% of data sets). However, we think that our workaround solution of merging and averaging spectra from several voxels from the 2D-CSI dataset adequately addressed this problem since it has been shown previously that there is a strong correlation between SVS and 2D-CSI [30]. Furthermore, in this study, we focused on only the most robust brain metabolites; therefore, other potentially relevant substances, such as lactate [13], citrate, myoinositol, glutamate, glutamine, and lipids, were not evaluated. Also, metabolic ratios instead of absolute values for MRS were used; this method was chosen for its robustness and clinical reproducibility.
Conclusion
Our results suggest that longitudinal changes in tumor volume have limited value for evaluating the response of pediatric DIPG (and perhaps of other infiltrative CNS tumors) during treatment. Consequently, volume estimates based on the RANO or RAPNO criteria may not be suitable biomarkers to assess response to treatment in clinical trials because they are not representative of actual tumor control. Response assessment using bi-dimensional, tri-dimensional, or volumetric measurements of infiltrative tumors, on the basis of T2WI signal abnormalities alone, may be inadequate to serve as endpoints in clinical trials. We therefore challenge the appropriateness of using conventional MRI metrics to monitor the response to therapy in DIPG, both in routine clinical settings and in clinical trials, and highlight the importance of advanced MRI techniques, including MRS, DWI, dynamic contrast-enhanced perfusion, dynamic susceptibility contrast perfusion, and arterial spin labeling, which have also been advocated by many other investigators [5, 31–33].
Acknowledgments
This work was supported in part by Grant P30 CA021765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). The authors thank Dr. Cherise Guess of the Department of Scientific Editing at St. Jude Children’s Research Hospital for editing the manuscript.
Footnotes
Compliance with ethical standards
We declare that all human studies have been approved by the local ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
AB received partial financial support from AstraZeneca to conduct this clinical trial.
References
- 1.CBTRUS. CBTRUS. Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006 2010 [Google Scholar]
- 2.Albright AL, Price RA, Guthkelch AN. Brain stem gliomas of children. A clinicopathological study. Cancer. 1983;52:2313–2319. doi: 10.1002/1097-0142(19831215)52:12<2313::aid-cncr2820521226>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Epstein F, McCleary EL. Intrinsic brain-stem tumors of childhood: surgical indications. J Neurosurg. 1986;64:11–15. doi: 10.3171/jns.1986.64.1.0011. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan AM, Albright AL, Zimmerman RA, et al. Brainstem gliomas in children. A Children’s Cancer Group review of 119 cases. Pediatr Neurosurg. 1996;24:185–192. doi: 10.1159/000121036. [DOI] [PubMed] [Google Scholar]
- 5.Thakur SB, Karimi S, Dunkel IJ, et al. Longitudinal MR spectroscopic imaging of pediatric diffuse pontine tumors to assess tumor aggression and progression. AJNR Am J Neuroradiol. 2006;27:806–809. [PMC free article] [PubMed] [Google Scholar]
- 6.Hargrave D, Chuang N, Bouffet E. Conventional MRI cannot predict survival in childhood diffuse intrinsic pontine glioma. J Neurooncol. 2008;86:313–319. doi: 10.1007/s11060-007-9473-5. [DOI] [PubMed] [Google Scholar]
- 7.Liu AK, Brandon J, Foreman NK, Fenton LZ. Conventional MRI at presentation does not predict clinical response to radiation therapy in children with diffuse pontine glioma. Pediatr Radiol. 2009;39:1317–1320. doi: 10.1007/s00247-009-1368-5. [DOI] [PubMed] [Google Scholar]
- 8.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 9.Hipp SJ, Steffen-Smith E, Hammoud D, et al. Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro-Oncol. 2011;13:904–909. doi: 10.1093/neuonc/nor076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panigrahy A, Nelson MD, Finlay JL, et al. Metabolism of diffuse intrinsic brainstem gliomas in children. Neuro-Oncol. 2008;10:32–44. doi: 10.1215/15228517-2007-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffen-Smith EA, Shih JH, Hipp SJ, et al. Proton magnetic resonance spectroscopy predicts survival in children with diffuse intrinsic pontine glioma. J Neurooncol. 2011;105:365–373. doi: 10.1007/s11060-011-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broniscer A, Baker JN, Tagen M, et al. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol. 2010;28:4762–4768. doi: 10.1200/JCO.2010.30.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamasaki F, Kurisu K, Kajiwara Y, et al. Magnetic resonance spectroscopic detection of lactate is predictive of a poor prognosis in patients with diffuse intrinsic pontine glioma. Neuro-Oncol. 2011;13:791–801. doi: 10.1093/neuonc/nor038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger PC, Mahley MS, Dudka L, Vogel FS. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979;44:1256–1272. doi: 10.1002/1097-0142(197910)44:4<1256::aid-cncr2820440415>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4:233–242. doi: 10.1586/ecp.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machein MR, Plate KH. VEGF in brain tumors. J Neurooncol. 2000;50:109–120. doi: 10.1023/a:1006416003964. [DOI] [PubMed] [Google Scholar]
- 17.Hedley-Whyte ET, Hsu DW. Effect of dexamethasone on blood-brain barrier in the normal mouse. Ann Neurol. 1986;19:373–377. doi: 10.1002/ana.410190411. [DOI] [PubMed] [Google Scholar]
- 18.Heiss JD, Papavassiliou E, Merrill MJ, et al. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest. 1996;98:1400–1408. doi: 10.1172/JCI118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulos MC, Saadoun S, Binder DK, et al. Molecular mechanisms of brain tumor edema. Neuroscience. 2004;129:1011–1020. doi: 10.1016/j.neuroscience.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Sedlacik J, Winchell A, Kocak M, et al. MR imaging assessment of tumor perfusion and 3D segmented volume at baseline, during treatment, and at tumor progression in children with newly diagnosed diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol. 2013;34:1450–1455. doi: 10.3174/ajnr.A3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poussaint TY, Kocak M, Vajapeyam S, et al. MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC) Neuro-Oncol. 2011;13:417–427. doi: 10.1093/neuonc/noq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadlbauer A, Moser E, Gruber S, et al. Improved delineation of brain tumors: an automated method for segmentation based on pathologic changes of 1H-MRSI metabolites in gliomas. NeuroImage. 2004;23:454–461. doi: 10.1016/j.neuroimage.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 24.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 25.Warren KE, Poussaint TY, Vezina G, et al. Challenges with defining response to antitumor agents in pediatric neuro-oncology: a report from the response assessment in pediatric neuro-oncology (RAPNO) working group. Pediatr Blood Cancer. 2013;60:1397–1401. doi: 10.1002/pbc.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly PJ, Daumas-Duport C, Scheithauer BW, et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62:450–459. doi: 10.1016/s0025-6196(12)65470-6. [DOI] [PubMed] [Google Scholar]
- 27.Lu S, Ahn D, Johnson G, et al. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology. 2004;232:221–228. doi: 10.1148/radiol.2321030653. [DOI] [PubMed] [Google Scholar]
- 28.Oh J, Cha S, Aiken AH, et al. Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging JMRI. 2005;21:701–708. doi: 10.1002/jmri.20335. [DOI] [PubMed] [Google Scholar]
- 29.Cohen BA, Knopp EA, Rusinek H, et al. Assessing global invasion of newly diagnosed glial tumors with whole-brain proton MR spectroscopy. AJNR Am J Neuroradiol. 2005;26:2170–2177. [PMC free article] [PubMed] [Google Scholar]
- 30.Steffen-Smith EA, Venzon DJ, Bent RS, et al. Single- and multivoxel proton spectroscopy in pediatric patients with diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2012;84:774–779. doi: 10.1016/j.ijrobp.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 32.Laprie A, Pirzkall A, Haas-Kogan DA, et al. Longitudinal multivoxel MR spectroscopy study of pediatric diffuse brainstem gliomas treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:20–31. doi: 10.1016/j.ijrobp.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Poussaint TY, Vajapeyam S, Ricci KI, et al. Apparent diffusion coefficient histogram metrics correlate with survival in diffuse intrinsic pontine glioma: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2015 Oct 20; doi: 10.1093/neuonc/nov256. pii: nov256 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]