Abstract
Background
Experimental and epidemiological studies suggest that gestational exposure to Bisphenol A (BPA), an ubiquitous endocrine disrupting chemical, may lead to neurobehavioral problems in childhood; however, not all results have been consistent. We previously reported a positive association between prenatal BPA exposure and symptoms of anxiety/depression reported by the mother at child age 7–9 years in boys, but not girls.
Objectives
Here, in the same birth cohort, we investigated the association of prenatal BPA exposure with symptoms of depression and anxiety self-reported by the 10–12 year olds, hypothesizing that we would observe sex-specific differences in anxiety and depressive symptoms.
Methods
African-American and Dominican women living in Northern Manhattan and their children were followed from mother’s pregnancy through children’s age 10–12 years. BPA was quantified in maternal urine collected during the third trimester of pregnancy and in child urine collected at ages 3 and 5 years. Children were evaluated using the Revised Children’s Manifest Anxiety Scale (RCMAS) and Children’s Depression Rating Scale (CDRS). We compared the children in the highest tertile of BPA concentration to those in the lower two tertiles. Associations between behavior and prenatal (maternal) BPA concentration or postnatal (child) BPA concentration were assessed in regression models stratified by sex.
Results
Significant positive associations between prenatal BPA and symptoms of depression and anxiety were observed among boys. Postnatal BPA exposure was not significantly associated with outcomes. There was substantial co-occurrence of anxiety and depressive symptoms in this sample.
Conclusion
These results provide evidence that prenatal BPA exposure is associated with more symptoms of anxiety and depression in boys but not in girls at age 10–12 years.
Keywords: Prenatal, Bisphenol A, child behavior, sex-specific
Introduction
Because of their increasing prevalence, early onset, and impact on the child, family, and community, mental and behavioral disorders in children are a growing public health concern (Perou et al. 2013). An estimated 17 percent of US adolescents aged 13–18 experience an emotional, mental, or behavioral disorder, including anxiety and depression (Merikangas et al. 2010). These mental and behavioral disorders in persons <24 years of age impose an annual cost in the United States of $247 billion or $2,380 per person (Perou et al. 2013; Eisenberg and Neighbors 2007). Their etiology is complex, including familial, genetic and environmental factors (Reinherz et al. 2000; Fendrich et al. 1990; López et al. 1999; Merikangas et al. 1999).
Bisphenol A (BPA) is a ubiquitous endocrine disrupting chemical commonly used to manufacture polymers found in food and drink containers, certain dental sealants (Maserejian et al. 2012), thermal receipt paper (Vandenberg et al. 2007), medical devices and sales receipts (Biedermann et al. 2010; Geens et al. 2011). According to a national survey, 93% of persons 6 years or older who were sampled had detectable levels of BPA in their urine; higher BPA urinary concentrations were seen among women and low-income individuals (Nelson et al. 2012; Calafat et al. 2008).
Experimental studies in laboratory animals have reported associations between BPA exposure and sex-specific changes in brain structure, function, alterations in DNA methylation of the genome, and behavioral problems (including anxiety-like behavior, inhibition of spatial skills, problems in spatial learning, and aggressiveness) (Wolstenholme et al. 2011; Patisaul et al. 2006; Rubin et al. 2006; Patisaul et al. 2007; Cox et al. 2010; Nakagami et al. 2009; Palanza et al. 2008; Kundakovic et al. 2013; Peluso et al. 2014). In mice, prenatal BPA induced lasting changes in DNA methylation in the transcriptionally relevant region of the gene encoding BDNF (brain derived neurotrophic factor), which plays an important role in fetal brain development; these changes were consistent with BDNF changes in the cord blood of children exposed to BPA in utero (Kundakovic et al. 2015). Similarly, the various adverse effects observed in these studies may be occurring in a sex-specific, dose-dependent manner via changes in DNA methylation and gene expression in estrogen signaling pathways and estrogen receptors (Kundakovic et al. 2013; Naciff et al. 2002; Vandenberg et al. 2009; Wetherill et al. 2007).
Epidemiologic studies have reported sex-specific differences in child behavior associated with increased prenatal BPA/maternal urinary concentrations (Perera et al. 2012; Harley et al. 2013; Braun et al. 2011a; Braun et al. 2009; Evans et al. 2014; Roen et al. 2015). However, the associations and sex-specific relationships observed in epidemiological studies have not always been consistent (Perera et al. 2012; Harley et al. 2013; Braun et al. 2011a; Braun et al. 2009)(see details in Discussion).
Using assessment instruments with reporting by the child, rather than by the parent, to elicit information on symptoms of anxiety and depression, we hypothesized that we would continue to observe sex-specific associations between prenatal BPA urinary concentrations and symptoms of both anxiety and depression at 10–12 years of age. Further, we hypothesized that we would observe extensive comorbidity of anxiety and depression in this inner city cohort, as has been reported in many other studies (Cummings et al. 2014).
Methods
Sample selection
A complete description of the NYC cohort and study design appears elsewhere (Perera et al. 2003; Perera et al. 2006). Briefly, participants included mothers and children participating in the Columbia Center for Children’s Environmental Health (CCCEH) prospective cohort study. Between 1998 and 2006, 727 pregnant women residing in Washington Heights, Harlem and the South Bronx were recruited in prenatal clinics to participate in the study. Only women ages 18–35 years, non-smokers, non-users of other tobacco products and/or illicit drugs, those generally in good health (free of known diabetes, hypertension and HIV), and those who initiated prenatal care by 20 weeks of pregnancy were included in the study. In-person postnatal questionnaires were given when the child was 6 months and annually thereafter, with developmental questionnaires administered every 1–2 years. Informed consent was provided for children by the mothers until age 7 after which age the children gave their assent to participate. The Institutional Review Boards of the Columbia University Medical Center and the Centers for Disease Control and Prevention (CDC) approved this study.
Chemical measures
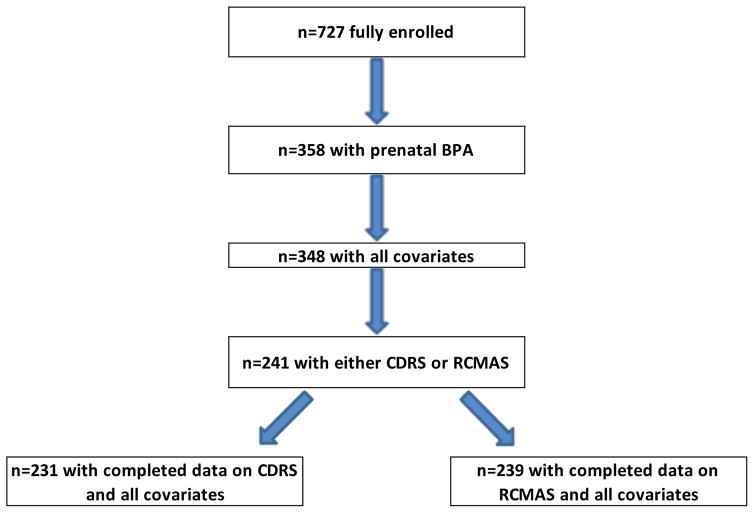
Collection of spot urine samples during the third trimester of pregnancy began in 1999 (Hoepner et al. 2013), the year after initial recruitment and data collection began. 348 mothers provided urine samples during pregnancy for measurement of BPA and mono-n-butyl phthalate (MnBP), as a major phthalate metabolite. Not all participants had BPA measures because of limitations in amount of urine available and limited funding for these analyses. A subset of the 348 children was followed through ages 10–12, when data from the Revised Children’s Manifest Anxiety Scale (RCMAS) and Children’s Depression Rating Scale-Revised (CDRS) were obtained on 239 and 231 children, respectively (Figure 1). Urine samples were collected at ages 3 and/or 5 years during the same visit when questionnaires were administered. 218 of the 241 children included in this analysis had data on postnatal BPA concentrations: age 5 urinary BPA measures were used for 181 children and age 3 year measures were used for 37 children who were missing the 5 year sample.
Figure 1.
Selection criteria for children included in the analysis1
1Due to limitations in available maternal urine and limited funding for analysis of BPA concentrations, BPA data were available only for a subset of participants enrolled in the parent study
After collection, the samples were sent to the CCCEH laboratory, inventoried, stored at −80°C, and subsequently shipped to the CDC for analysis. Total (free plus conjugated) urinary concentrations of BPA and MnBP were measured separately using online solid-phase extraction coupled with high-performance liquid chromatography–isotope dilution–tandem mass spectrometry as described previously (Ye et al. 2005; Kato et al. 2005), with appropriate quality control samples in each run. The limits of detection (LOD) were 0.4 μg/L (BPA) and 0.4–1.1 μg/L (MnBP). Concentrations below the LOD were given a value of LOD/2 for statistical analysis. To adjust for urinary dilution, BPA and phthalate metabolite values were adjusted for specific gravity (SG) obtained using a handheld refractometer (Urine-Specific-Gravity-Refractometer-PAL-10-S-P14643C0; TAGO USA, Inc., Bellevue, WA). We used the formula: Chemicalc = BPA or MnBP × [(mean SG − 1)/(individual SG − 1)] where Chemicalc is the SG-corrected chemical concentration (μg/L), BPA or MnBP is the measured chemical concentration (μg/L), SG is the specific gravity of the urine sample, and mean SG is the mean SG in the study population calculated separately for maternal, child age 3 and child age 5 samples (Hauser et al. 2004).
Behavioral outcomes
When children reached ages 10–12 years, they completed a semi-structured interview using the CDRS administered by a trained researcher, according to the standard method of administration.
The CDRS is the most widely used scale for assessing severity of depression in children. The CDRS assesses the severity of depressive symptoms on a 17-item scale. The scale encompasses cognitive, somatic, affective, and psychomotor symptoms. Items are rated for severity on a 7-point scale (1 to 7) for 14 items and on a 5-point scale (1 to 5) for three items. A total score for each measure is generated based on the child’s answers to questions related to the different categories (such as school work, capacity to have fun, social withdrawal) that assess symptom severity (Mayes et al. 2010; Poznanski et al. 1979). The psychometric properties of the scale are strong for the child age group (ages 6–12 years) (Poznanski and Mokros 1996). The internal consistency (Cronbach’s α) in children is good (α = 0.85) (Mayes et al., 2010). Test-retest reliability is good (Wisniewski et al. 1987). Convergent validity with a global assessment of depression by two child psychiatrists has also been shown (0.92) (Poznanski and Mokros 1996). For the CDRS, a raw score of ≥40 is indicative of depression, whereas a score ≤28 is often used to define minimal or no symptoms (Mayes et al. 2010).
The Revised Children’s Manifest Anxiety Scale was self-administered at age 10–12 years. This self-report instrument was designed to measure anxiety for children and adolescents older than 6 years (Reynolds and Richmond 1985). It consists of 37 questions answered as yes/no. The Total Anxiety score and the Anxiety subscale scores are determined by the number of “yes” responses to the anxiety items (Reynolds and Richmond 1985). The lie score is used to determine if the child was making a valid attempt to respond (Lee et al. 1988). Psychometric properties (reliability and validity) of the RCMAS have been well characterized. Reliability estimates for internal consistency (Cronbach’s α) are good (0.79 for males, 0.85 for females, and 0.82 for the total sample of males and females) (Reynolds et al. 1980). There is substantive research confirming the validity of the RCMAS as a measure of chronic manifest anxiety in children, dating back to the original article reporting the development of the RCMAS (Reynolds and Richmond 1978). A meta-analytic review showed that it discriminates well between youth with anxiety disorders and youth without disorders (Seligman et al. 2004); and a previous study shows good discriminant validity between boys with anxiety and normal controls (Perrin and Last 1992). For RCMAS Total Anxiety, a raw score of > 18 is indicative of clinically significant anxiety (Stallard et al. 2001). However, severity ratings for anxiety and depression are not able to effectively distinguish youth with anxiety disorders and major depression because of the high rate of comorbidity between the two disorders in youth. Moreover, dimensional ratings of symptom severity are not intended to be diagnostic of anxiety vs. depression, but simply to measure the severity of the respective symptoms (Seligman et al. 2004).
Statistical analysis
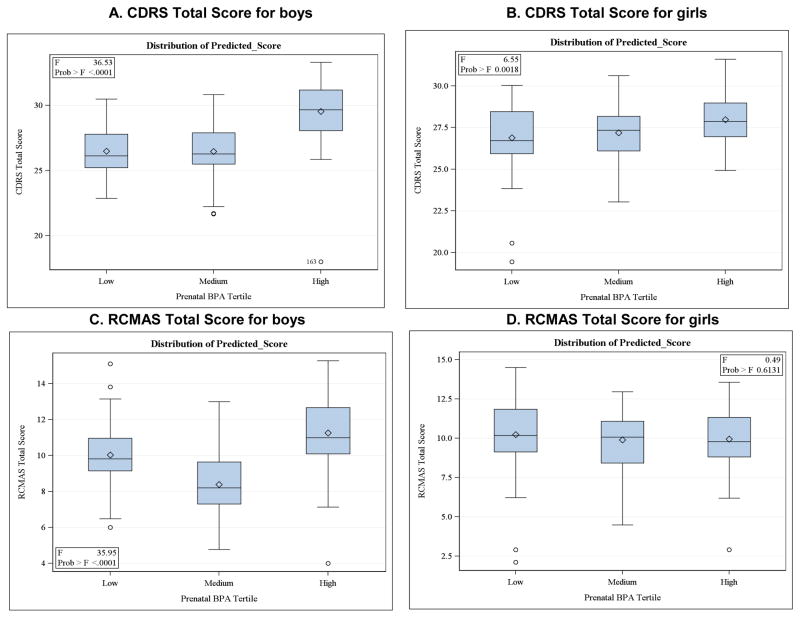
Because of the uncertainty in the measurement of the BPA level in a single spot urine as an indicator of chronic exposure, prenatal and postnatal BPA concentrations were divided into tertiles. Although several samples are preferable to properly characterize BPA exposure (Quiros-Alcala et al. 2013; Meeker et al. 2013; Braun et al. 2012), a single spot urine sample can adequately classify individuals in terms of exposure groups by tertiles (Mahalingaiah et al. 2008), despite low intra-class correlations for concentrations from multiple samples (Braun et al. 2011b) and high daily variance (Ye et al. 2011). For these reasons, as in our prior analyses (Perera et al. 2012; Roen et al. 2015), we compared the upper to the two lower tertiles based on the distribution of predicted scores, which did not strongly support an alternative U-shaped curve (Figure 2). To be consistent, postnatal BPA concentration, which was included as an independent variable in a supplemental analysis, was treated in the same manner as prenatal BPA in the analyses of CDRS and RCMAS scores.
Figure 2.
Comparison of prenatal BPA tertile groups by CDRS Total and RCMAS Total score for Boys and Girls
On the RCMAS, the Total Anxiety Score is expressed as a raw score and a T-score (M=50, sd=10); the subscales are expressed as raw scores and scaled scores (M=10, sd=3). Both raw scores and T-scores are generated on the CDRS. We analyzed the raw scores as continuous variables in order to assess associations across the full range of outcomes in this non-clinical population. The CDRS and RCMAS raw scores were normally distributed; we therefore used linear regression to analyze the relationship between scores and BPA urinary concentrations. Analyses were performed stratifying on sex and then combining sexes to assess BPA exposure x sex interaction.
As in our prior reports (Perera et al. 2012; Roen et al. 2015), we adjusted for potential confounding variables that are known or suspected risk factors based on the literature and our previous results. These included ethnicity, gestational age of the child (based on medical record data), mother’s intelligence (measured by the Test of Nonverbal Intelligence 3rd Edition, TONI-3 (Brown et al. 1997)), maternal education (completion of high school prior to birth of this child vs. no completion), maternal demoralization (measured by the Psychiatric Epidemiology Research Instrument–Demoralization (PERI-D) scale) (Dohrenwend et al. 1978), child age (in months) at testing, quality of the child’s home environment at 3 years of age (measured by the HOME (Home Observation for Measurement of the Environment) Inventory (Bradley 1994), prenatal exposure to environmental tobacco smoke (ETS, yes/no), and SG-adjusted MnBP in maternal urine collected during the third trimester of pregnancy (Adibi et al. 2008). In contrast to MnBP, exposure to polycyclic aromatic hydrocarbons (PAH) monitored in air during pregnancy was not correlated with BPA (Pearson Correlation Coefficient r=0.012, p=0.86) and was not included in the models. We used an established multiple imputation method (Rubin 1987) to provide missing values for the small percentage of the children who lacked particular information, as shown in the table below. In no case were data lacking for more than 6.2% of participants.
To determine if postnatal BPA was a potential confounder, we compared the adjusted and unadjusted associations in the subset having both BPA measures (n=210) and found that results were materially unchanged. Parallel analyses with postnatal BPA urinary concentrations as the predictor variable were also conducted. To evaluate the potential for selection bias due to loss to follow-up, we conducted sensitivity analyses using inverse probability weighting (IPW).
We examined the BPA x sex interaction on the CDRS and RCMAS scores using linear regression, including the main effects of BPA and sex, and an interaction term defined as significant by having a regression coefficient that differed from 0 with a p-value < 0.05. A negative interaction term indicates that the observed effect of BPA on depression or anxiety is smaller in girls compared to that in boys. All regression coefficient estimates and p-values were generated using SAS (version 9.3 SAS Institute Inc., Cary, NC). Although our analyses involved multiple tests, we did not perform Bonferroni adjustment, in order to reduce the possibility of making a type II error (Rothman 1990). Rather, our focus was on the sex-specific patterns of the observed relationships and their consistency with those seen in our cohort children at an earlier age (Perera et al. 2012; Roen et al. 2015). We note that there were no material differences in results before and after conducting imputation.
We examined the extent to which CDRS and RCMAS total scores were correlated with each other (Pearson Correlation). To determine the degree to which prenatal BPA was associated with symptoms of anxiety independent of depression, in separate analyses, we: 1) ran the model for RCMAS adjusting for CDRS total score and 2) treating the residuals obtained from regression of RCMAS on CDRS as the outcome variable.
Results
The 241 children included in this analysis did not differ in demographic or exposure characteristics from all others in the parent study (N=471) who were not included, except for having an earlier age at assessment on CDRS and RCMAS and a higher average HOME inventory score (p<0.05). (Table 1) The same was true when we compared the subset analyzed (N=241) with the group having prenatal BPA measures but missing outcome or postnatal BPA or MnBP data (N=107). Age at assessment was included in the model.
Table 1.
Comparison of the characteristics of children included (N=241) and excluded (N=471) in the present analysis, among 712 fully enrolled non-smokers
| Variable | Subjects in the analysis (n=241) | Subjects NOT in the analysis (n=471) | P-value |
|---|---|---|---|
| Prenatal BPA urinary concentration (μg/L)1, 2, 3 | 3.11 ± 4.04 | 3.04 ± 5.67 | 0.90 |
| Prenatal mono-n-butyl phthalate concentration (μg/L)1, 4 | 61.54 ± 68.78 | 54.88 ± 48.35 | 0.24 |
| Percent female | 52.28 | 50.74 | 0.70 |
| Percent African American | 36.93 | 33.76 | 0.40 |
| Percent with prenatal ETS exposure5 | 30.29 | 34.82 | 0.16 |
| Percent >= high school education | 60.17 | 60.30 | 0.25 |
| Gestational age at birth (weeks)1 | 39.32 ± 1.23 | 39.28 ± 1.44 | 0.67 |
| Maternal TONI score1 | 19.93 ± 8.25 | 20.83 ± 7.41 | 0.14 |
| HOME inventory1 | 39.97 ± 5.74 | 39.05 ± 5.27 | 0.03* |
| Maternal demoralization score1 | 1.12 ± 0.64 | 1.16 ± 0.6 | 0.37 |
| Age at CDRS assessment1 | 124.88 ± 11.44 | 133.52 ± 9.24 | <.0001* |
| Age at RCMAS assessment1 | 125.3 ± 11.33 | 133.9 ± 9.38 | <.0001* |
mean±SD
lowest tertile of exposure= 0.93 ± 0.30, middle tertile of exposure = 1.97 ± 0.38, highest tertile of exposure 6.07 ± 5.67
LOD= 0.4 μg/L
LOD= 0.4–1.1 μg/L
Environmental tobacco smoke
p< 0.05
The present study sample in the analysis of RCMAS included 239 children (114 boys and 125 girls). The analysis of CDRS included 231 children (109 boys and 122 girls). A total of 235/241 (97.51%) prenatal samples had detectable BPA concentrations and 215/218 (98.6 %) postnatal samples (at age 3 or 5 years) had detectable BPA concentrations. The ranges and percentile distribution of prenatal BPA urinary concentrations are shown in Table 2. A wide range of prenatal and postnatal BPA concentrations was observed within our cohort with higher geometric mean concentrations observed during childhood.
Table 2.
Descriptive statistics of prenatal and postnatal BPA concentrations among 115 boys and 126 girls included in the analysis (LOD=0.4μg/L)
| Percentile | Geometric mean | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time of BPA collection | Minimum | 10th | 25th | 50th | 75th | 90th | Maximum | Mean | |
| Prenatal BPA | |||||||||
| No specific gravity adjustment (μg/L) | |||||||||
| All children | <LOD | 0.60 | 1.10 | 1.90 | 3.60 | 6.50 | 30.00 | 3.05 | 1.93 |
| Girls | <LOD | 0.60 | 1.00 | 1.80 | 3.50 | 8.30 | 30.00 | 3.19 | 1.92 |
| Boys | <LOD | 0.60 | 1.20 | 2.00 | 3.70 | 6.00 | 19.10 | 2.90 | 1.95 |
| Specific gravity adjusted (μg/L) | |||||||||
| All children | 0.25 | 0.86 | 1.25 | 2.09 | 3.55 | 5.79 | 38.97 | 3.11 | 2.14 |
| Girls | 0.25 | 0.86 | 1.25 | 2.20 | 3.67 | 5.79 | 32.24 | 3.20 | 2.18 |
| Boys | 0.29 | 0.86 | 1.25 | 2.01 | 3.35 | 5.82 | 38.97 | 3.01 | 2.10 |
| Postnatal BPA | |||||||||
| No specific gravity adjustment (μg/L) | |||||||||
| All children | <LOD | 1.00 | 1.80 | 3.05 | 6.60 | 11.90 | 41.20 | 5.28 | 3.29 |
| Girls | 0.40 | 1.00 | 1.50 | 2.90 | 6.40 | 10.70 | 41.20 | 5.35 | 3.10 |
| Boys | <LOD | 1.00 | 2.20 | 3.30 | 7.10 | 11.90 | 27.70 | 5.20 | 3.53 |
| Specific gravity adjusted (μg/L) | |||||||||
| All children | 0.25 | 1.25 | 2.03 | 3.17 | 6.47 | 13.73 | 404.00 | 8.40 | 3.74 |
| Girls | 0.25 | 1.25 | 1.80 | 3.19 | 7.14 | 15.69 | 404.00 | 11.01 | 3.82 |
| Boys | 0.29 | 1.31 | 2.06 | 3.14 | 6.08 | 11.71 | 33.34 | 5.49 | 3.63 |
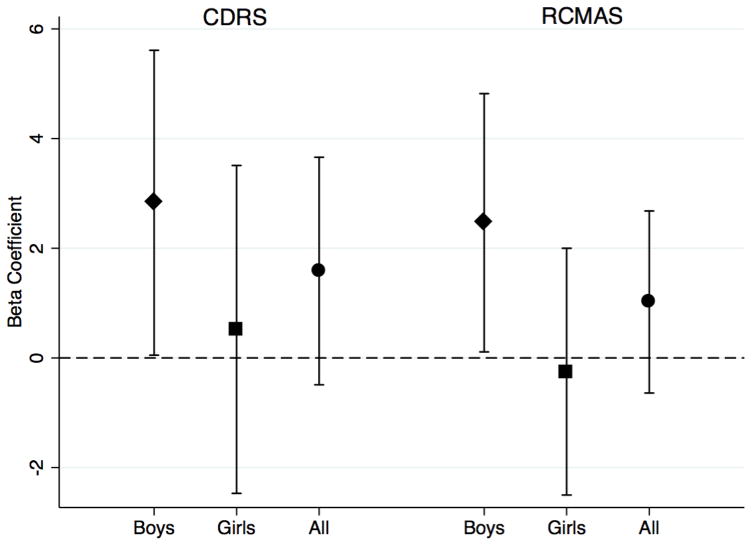
Supplemental Tables 3 and 4 provide the mean scores for CDRS and RCMAS by tertile of BPA exposure, for boys and girls, respectively. As shown in Figure 3 and summarized in Supplemental Table 5, boys in the highest prenatal BPA concentration tertile experienced higher CDRS Total Scores (more symptoms of depression) compared to those in the lower two tertiles (β=2.83, p=0.05). No significant association was detected in girls. As noted above, a raw score of ≥40 is indicative of depression, whereas a score ≤28 is often used to define minimal or no symptoms (Mayes et al., 2010). The mean raw CDRS score for the boys in the highest BPA tertile in our study was 29.5. 5/38 (13.2%) of the boys in the highest BPA tertile scored ≥40, compared to 1/33 (3.0%) in the lowest tertile and 1/38 (2.6%) in the middle tertile.
Figure 3.
Association between prenatal BPA exposure (highest tertile vs. lower two) and anxiety and depression symptoms measured by the CDRS and RCMAS in boys (diamond markers), girls (square markers), and all children (circle markers) comparing the highest BPA tertile to the lower two
We observed significant positive associations between prenatal BPA concentration and the RCMAS Total Score (β=2.47, p=0.04) and the social concern subscale (β=0.83, p=0.025) in boys (Figure 3 and Supplemental Table 5). No significant associations were seen in girls (Supplemental Table 5). 5/39 (12.8%) of boys in the highest BPA tertile scored >18, compared to 2/34 (5.9%) in the lowest tertile and 3/41 (7.3%) in the middle tertile.
Results for both sexes combined are shown in Supplemental Table 5 along with the interaction terms. The positive significant associations seen overall (all children) were driven mainly by the effects in boys. The interaction terms (BPA x sex) were not significant: for the CDRS Total Score (β=−2.61, p=0.194); for the RCMAS Total Score or the subscale scores (βs between −0.79 and −2.66).
After accounting for potential selection bias based on the inverse probability weighting method, we found that the associations between prenatal BPA concentration and CDRS and RCMAS scores in boys were not materially changed (Supplemental Table 6). Additionally, because it may not be appropriate to use age 3 measures of BPA for 37 children who lacked the 5 year measure, we conducted a sensitivity analysis that did not show material differences in results when those 37 children were included vs. excluded.
The CDRS and RCMAS Total scores were significantly correlated (Pearson Correlation, r=0.554, p<0.0001). Adjustment of RCMAS for CDRS in the model with prenatal BPA resulted in loss of significance, although the directions of association remained the same (Supplemental Table 7). Treating the residuals obtained from regression of RCMAS on CDRS as the outcome variable, the associations with prenatal BPA were nonsignificant for both sexes (data not shown).
The associations between postnatal BPA and RCMAS or CDRS outcomes were generally not significant in either boys or girls, except for the interaction term (BPA x sex) for RCMAS subscale oversensitivity (β=1.67, p=0.04) (see Supplemental Table 8). However, as in our earlier reports (Perera et al. 2012; Roen et al. 2015), the directions of the associations were opposite to those seen with prenatal BPA.
Discussion
The aim of this study in a non-clinical population was to assess the associations between BPA exposure and continuous measures of depression and anxiety. We observed significant positive associations between prenatal BPA urinary concentrations and self-reported symptoms of anxiety and depression among 10–12 year old boys in our NYC cohort of Dominican and African-American children; in contrast no significant associations were seen among girls. The associations with postnatal BPA were generally inverse but none were significant. This suggests that the critical window of susceptibility is the period of fetal development. These findings, with respect to prenatal exposure, are consistent with results in the same cohort using the maternal report on the CBCL at child ages 7–9 years that showed positive associations between maternal urinary BPA concentrations and internalizing and externalizing composite scores and specific syndrome scales including anxiety/depression in boys (Perera et al. 2012; Roen et al. 2015). They are also consistent with our previous finding that maternal urinary BPA concentrations were generally inversely associated with scores among girls 7–9 years of age (Perera et al. 2012; Roen et al. 2015). In a primarily Hispanic cohort in Salinas Valley, California, Harley et al. also reported positive associations between prenatal BPA urinary concentrations and internalizing problems, including symptoms of anxiety and depression as well as increased aggressive behavior in boys at 7 years of age using the Behavioral Assessment Scale for Children (BASC-2). In girls, these authors observed inverse associations between prenatal BPA and many symptoms, although none were significant (Harley et al. 2013). In a multi-center cohort in four US cities, Evans et al. observed increased behavior problems in school age boys, but not girls, related to prenatal BPA when restricting to children of mothers with detectable urinary prenatal BPA (Evans et al., 2014). In contrast, in a largely white cohort in Cincinnati, Ohio, Braun et al. reported that prenatal BPA urinary concentrations were linked to a decrease in hyperactivity in boys and increases in anxiety, depression, hyperactivity and externalizing symptoms in girls at ages 2–3 years using the BASC (Braun et al. 2011a; Braun et al. 2009).
The discrepancy among studies may be due to differences in timing of exposure measurement, instruments used in outcome assessment, statistical treatment of exposure variables, and populations studied. Ethnic differences in co-exposures, genetic factors, and social stressors experienced by the mothers and children in the respective cohorts and differences in age at which the children were tested may also contribute.
The observed extensive co-occurrence of anxiety and depressive symptoms in this sample is consistent with other studies (Cummings et al. 2014).
In this study, higher levels of prenatal exposure to BPA are associated with higher more reported symptoms of depression and anxiety in boys. A higher percentage of the boys in the higher prenatal exposure group scored in the clinically significant range than among boys with lower prenatal exposure; however, a definitive clinical diagnosis requires additional assessment, which is being conducted in the cohort at a later follow-up. We note that the level of symptoms are largely in the typical range and not indicative of major mental health problems.
Regarding possible mechanisms by which BPA could exert endocrine disrupting effects on symptom expression, a recent experimental study suggests that prenatal BPA exposure alters expression of genes encoding the estrogen receptors, ER-α, ER-β and estrogen-related receptor-γ, with corresponding changes in mRNA levels of DNA methyltransferases DNMT1 and DNMT3A and methylation of the ER-α gene in the cortex and hypothalamus of male and female mice, respectively. As noted earlier, another study showed that prenatal BPA exposure induced lasting DNA methylation changes in the BDNF gene (Kundakovic et al. 2015, 2014). Additional studies have shown that rodents exposed to BPA prenatally had increased levels of anxiety (Cox et al. 2010; Patisaul and Bateman 2008; Ryan and Vandenbergh 2006; Tian et al. 2010; Yu et al. 2011; Xu et al. 2014; Luo et al. 2014), although the sex-specificity of these associations was not always consistent between studies (Ishido et al. 2004; Ishido et al. 2007; Masuo et al. 2004; Xu et al. 2007). Sex-specific effects on spatial learning and memory outcomes have also been linked to developmental BPA exposure (Ryan and Vandenbergh 2006; Xu et al. 2007). Taken together, these studies suggest that epigenetic mechanisms may underlie some of the effects of BPA on behavior.
Our findings on the effect of prenatal BPA exposure in boys are consistent with prior epidemiologic data suggesting a greater vulnerability of the male brain during prenatal development (Perera et al. 2012; Harley et al. 2013). A number of studies assessing outcomes, such as behavior problems and reduced IQ, have reported evidence that boys may be more affected than girls by prenatal exposure to BPA (Harley et al. 2013), lead (Jedrychowski et al. 2009), and chlorpyrifos (Horton et al. 2012). However, the sex-dependent effect of these chemicals likely depends on the nature and timing of exposure. The finding that results differ between boys and girls is plausible given the endocrine-disrupting characteristic of BPA.
The present study has a number of strengths including the prospective cohort design and the large number of variables collected from medical records, questionnaires and biological samples collected across the span of our study, allowing us to control for multiple potential confounders at different time points, and the fact that symptoms are now self-reported by the child rather than reported by the parent.
The limited sample size and the assessment of prenatal BPA exposure based on a single measurement collected prenatally are limitations. Because BPA has a short half-life and exposures are likely episodic in nature, the single spot urine sample collected may not adequately describe a participant’s chronic exposure. Recent work shows that repeated urinary specimens are ideal to better characterize BPA exposure (Cox et al. 2016). However, as noted, the urinary concentration from a single urine sample has moderate sensitivity in estimating an individual’s BPA tertile categorization (Mahalingaiah et al. 2008), mitigating exposure misclassification. Furthermore, given the potential for noise in these measurements, we would expect the result to be biased towards the null. We adjusted for a number of known factors that may confound the association between BPA exposure and behavioral outcomes; however, it is possible that residual confounding remains. Although generalizability is reduced by the ethnicity of our cohort (African-American and Dominican), these ethnic groups comprise a large and growing fraction of the US population of women of reproductive age. There is the possibility of selection bias in any such study; however, analysis using IPW did not show evidence of a biased sample.
Conclusion
The etiology of behavioral problems in children is complex and multi-factorial; however, these results suggest that prenatal BPA exposure may contribute to sex-specific effects on anxiety and depressive symptoms in our inner city population. They add to other reports raising concern about the pervasiveness of this chemical and its potential effects on children from early-life exposures. Other studies have linked anxiety and depression with adverse learning outcomes and poor school performance (Bub et al. 2007; Grimm et al. 2010), both of which adversely impact future economic and emotional well-being. Other research also indicates that the rate of symptom expression is only beginning to increase at ages 9–12 years and that elevated symptoms at these ages may be harbingers of subsequent more serious clinical disorders related to anxiety and depression that will manifest in adolescence and young adulthood (Merikangas et al. 2010; Simonoff et al. 1997; Costello et al. 2006; Paus et al. 2008; Patton and Viner 2007). We continue to follow the children to assess the relation between prenatal BPA exposure and clinical disorders in adolescence in our cohort.
Supplementary Material
Highlights.
We assessed sex-specific associations between Bisphenol A (BPA) and symptoms of anxiety and depression.
We measured BPA in maternal urine during pregnancy and in child urine at ages 3–5.
BPA exposure was classified by tertile of pre and postnatal urinary concentrations.
We administered the RCMAS and CDRS to assess symptoms.
Significant positive associations between prenatal BPA and symptoms of depression and anxiety were observed among boys.
Acknowledgments
Funding Sources:
Funding was provided by the National Institute for Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency (US EPA): NIEHS/EPA P01ES09600/R82702701, NIEHS/EPA P01ES09600/RD83214101, NIEHS/EPA P01ES09600/RD83450901, NIEHS R01ES08977, NIEHS R01ES015579, and NIDA R01DA027100. This publication was also made possible in part by the John and Wendy Neu Family Foundation, the New York Community Trust, and the Trustees of the Blanchette Hooker Rockefeller Fund.
The authors gratefully acknowledge the assistance of Xiaoyun Ye, Xiaoliu Zhou, Josh Kramer, and Lily Jia in measuring the urinary concentrations of BPA, Valerie Thomas for collecting the psychological assessment data, Joanne Chin and Kylie Wheelock in preparing the manuscript.
Footnotes
Human subjects research:
All activities were approved by the Institutional Review Boards at the Columbia University Medical Center under human subjects protocol number AAAA-6110, and by the Centers for Disease Control and Prevention.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding from all the institutions listed was used towards the design and conduct of the study, collection, management, analysis and interpretation of the data, and the preparation, review, and approval of the manuscript. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA or the National Institutes of Health. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, et al. Mental health surveillance among children--United States, 2005–2011. Morbidity and mortality weekly report. Surveillance summaries. 2013;62(Suppl 2):1–35. [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Neighbors K. Economic and policy issues in preventing mental disorders and substance abuse among young people. presentation for the IOM Committee on the Prevention of Mental Disorders and Substance Abuse; October 31st, 2007; Department of Health Management and Policy School of Public Health, University of Michigan; 2007. [Google Scholar]
- Reinherz HZ, Giaconia RM, Hauf AM, Wasserman MS, Paradis AD. General and specific childhood risk factors for depression and drug disorders by early adulthood. J Am Acad Child Adolesc Psychiatry. 2000;39(2):223–231. doi: 10.1097/00004583-200002000-00023. [DOI] [PubMed] [Google Scholar]
- Fendrich M, Warner V, Weissman MM. Family risk factors, parental depression, and psychopathology in offspring. Developmental Psychology. 1990;26(1):40–50. [Google Scholar]
- López JF, Akil H, Watson SJ. Role of biological and psychological factors in early development and their impact on adult life: Neural circuits mediating stress. Biological Psychiatry. 1999;46(11):1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Avenevoli S, Dierker L, Grillon C. Vulnerability factors among children at risk for anxiety disorders. Biol Psychiatry. 1999;46(11):1523–1535. doi: 10.1016/s0006-3223(99)00172-9. [DOI] [PubMed] [Google Scholar]
- Maserejian NN, Trachtenberg FL, Hauser R, McKinlay S, Shrader P, Tavares M, et al. Dental composite restorations and psychosocial function in children. Pediatrics. 2012;130(2):e328–338. doi: 10.1542/peds.2011-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reproductive Toxicology. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Analytical and bioanalytical chemistry. 2010;398(1):571–576. doi: 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- Geens T, Goeyens L, Covaci A. Are potential sources for human exposure to bisphenol-A overlooked? International journal of hygiene and environmental health. 2011;214(5):339–347. doi: 10.1016/j.ijheh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Nelson JW, Scammell MK, Hatch EE, Webster TF. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003–2006. Environ Health-Glob. 2012:11. doi: 10.1186/1476-069X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Hormones and behavior. 2011;59(3):296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicology and teratology. 2006;28(1):111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28(1):1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Hormones and behavior. 2010;58(5):754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami A, Negishi T, Kawasaki K, Imai N, Nishida Y, Ihara T, et al. Alterations in male infant behaviors towards its mother by prenatal exposure to bisphenol A in cynomolgus monkeys (Macaca fascicularis) during early suckling period. Psychoneuroendocrinology. 2009;34(8):1189–1197. doi: 10.1016/j.psyneuen.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environmental research. 2008;108(2):150–157. doi: 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(24):9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MEM, Munnia A, Ceppi M. Bisphenol-A exposures and behavioural aberrations: Median and linear spline and meta-regression analyses of 12 toxicity studies in rodents. Toxicology. 2014;325(0):200–208. doi: 10.1016/j.tox.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(22):6807–6813. doi: 10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ, et al. Gene expression profile induced by 17alpha-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicological sciences : an official journal of the Society of Toxicology. 2002;68(1):184–199. doi: 10.1093/toxsci/68.1.184. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In vitro molecular mechanisms of bisphenol A action. Reproductive Toxicology. 2007;24(2):178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environmental health perspectives. 2012;120(8):1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environmental research. 2013 doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics. 2011a;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal Bisphenol A Exposure and Early Childhood Behavior. Environmental health perspectives. 2009;117(12):1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SF, Kobrosly RW, Barrett ES, Thurston SW, Calafat AM, Weiss B, et al. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology. 2014;45:91–99. doi: 10.1016/j.neuro.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roen EL, Wang Y, Calafat AM, Wang S, Margolis A, Herbstman J, et al. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environmental research. 2015 doi: 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological bulletin. 2014;140(3):816–845. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environmental health perspectives. 2003;111(2):201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental health perspectives. 2006;114(8):1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepner LA, Whyatt RM, Just AC, Calafat AM, Perera FP, Rundle AG. Urinary concentrations of bisphenol A in an urban minority birth cohort in New York City, prenatal through age 7 years. Environmental research. 2013;122:38–44. doi: 10.1016/j.envres.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77(9):2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environmental health perspectives. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. J Child Adolesc Psychopharmacol. 2010;20(6):513–516. doi: 10.1089/cap.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64(4):442–450. [PubMed] [Google Scholar]
- Wisniewski JJ, Mulick JA, Genshaft JL, Coury DL. Test-retest reliability of the Revised Children’s Manifest Anxiety Scale. Percept Mot Skills. 1987;65(1):67–70. doi: 10.2466/pms.1987.65.1.67. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. Revised children’s manifest anxiety scale. Western Psychological Services; Los Angeles: 1985. [Google Scholar]
- Lee SW, Piersel WC, Friedlander R, Collamer W. Concurrent validity of the Revised Children’s Manifest Anxiety Scale (RCMAS) for adolescents. Educational and Psychological Measurement. 1988;48(2):429–433. [Google Scholar]
- Reynolds CR, Bradley M, Steele C. Preliminary norms and technical data for use of the Revised-Children’s Manifest Anxiety Scale with kindergarten children. Psychology in the Schools. 1980;17(2):163–167. [Google Scholar]
- Reynolds CR, Richmond BO. What I think and feel: A revised measure of children’s manifest anxiety. Journal of abnormal child psychology. 1978;6(2):271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- Seligman LD, Ollendick TH, Langley AK, Baldacci HB. The utility of measures of child and adolescent anxiety: a meta-analytic review of the Revised Children’s Manifest Anxiety Scale, the State-Trait Anxiety Inventory for Children, and the Child Behavior Checklist. J Clin Child Adolesc Psychol. 2004;33(3):557–565. doi: 10.1207/s15374424jccp3303_13. [DOI] [PubMed] [Google Scholar]
- Perrin S, Last CG. Do childhood anxiety measures measure anxiety? Journal of abnormal child psychology. 1992;20(6):567–578. doi: 10.1007/BF00911241. [DOI] [PubMed] [Google Scholar]
- Stallard P, Velleman R, Langsford J, Baldwin S. Coping and psychological distress in children involved in road traffic accidents. Br J Clin Psychol. 2001;40(Pt 2):197–208. doi: 10.1348/014466501163643. [DOI] [PubMed] [Google Scholar]
- Quiros-Alcala L, Eskenazi B, Bradman A, Ye X, Calafat AM, Harley K. Determinants of urinary bisphenol A concentrations in Mexican/Mexican--American pregnant women. Environment international. 2013;59:152–160. doi: 10.1016/j.envint.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, Variability, and Predictors of Urinary Concentrations of Phenols and Parabens among Pregnant Women in Puerto Rico. Environ Sci Technol. 2013;47(7):3439–3447. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of Urinary Phthalate Metabolite and Bisphenol A Concentrations before and during Pregnancy. Environmental health perspectives. 2012;120(5):739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environmental health perspectives. 2008;116(2):173–178. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Xiaoyun Y, Silva MJ, et al. Variability and Predictors of Urinary Bisphenol A Concentrations during Pregnancy. Environmental health perspectives. 2011b;119(1):131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. Variability of Urinary Concentrations of Bisphenol A in Spot Samples, First-morning Voids, and 24-Hour Collections. Environmental health perspectives. 2011;119(7):983–988. doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK. Test of nonverbal intelligence. 3. Bensenville, IL: Scholastic Testing Service, Inc; 1997. [Google Scholar]
- Dohrenwend BS, Krasnoff L, Askenacy A, Dohrenwend B. Exemplification of a method for scaling life events: the PERI life events scale. J Health Soc Behavior. 1978;19:205–229. [PubMed] [Google Scholar]
- Bradley RH. The Home Inventory: review and reflections. Adv Child Dev Behav. 1994;25:241–288. doi: 10.1016/s0065-2407(08)60054-3. [DOI] [PubMed] [Google Scholar]
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of Phthalate Exposure among Pregnant Women Assessed by Repeat Air and Urine Samples. Environmental health perspectives. 2008;116(4):467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proceedings of the National Academy of Sciences of the United States of America; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Hormones and behavior. 2008;53(4):580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Hormones and behavior. 2006;50(1):85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Tian Y-H, Baek J-H, Lee S-Y, Jang C-G. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64(6):432–439. doi: 10.1002/syn.20746. [DOI] [PubMed] [Google Scholar]
- Yu C, Tai F, Song Z, Wu R, Zhang X, He F. Pubertal exposure to bisphenol A disrupts behavior in adult C57BL/6J mice. Environmental toxicology and pharmacology. 2011;31(1):88–99. doi: 10.1016/j.etap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Xu X, Dong F, Yang Y, Wang Y, Wang R, Shen X. Sex-specific effects of long-term exposure to bisphenol-A on anxiety- and depression-like behaviors in adult mice. Chemosphere. 2014;120C:258–266. doi: 10.1016/j.chemosphere.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Luo G, Wang S, Li Z, Wei R, Zhang L, Liu H, et al. Maternal bisphenol a diet induces anxiety-like behavior in female juvenile with neuroimmune activation. Toxicological sciences : an official journal of the Society of Toxicology. 2014;140(2):364–373. doi: 10.1093/toxsci/kfu085. [DOI] [PubMed] [Google Scholar]
- Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurosci Res. 2004;76(3):423–433. doi: 10.1002/jnr.20050. [DOI] [PubMed] [Google Scholar]
- Ishido M, Yonemoto J, Morita M. Mesencephalic neurodegeneration in the orally administered bisphenol A-caused hyperactive rats. Toxicol Lett. 2007;173(1):66–72. doi: 10.1016/j.toxlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Morita M, Oka S, Ishido M. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regulatory peptides. 2004;123(1–3):225–234. doi: 10.1016/j.regpep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu Y, Sadamatsu M, Tsutsumi S, Akaike M, Ushijima H, et al. Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene. Neurosci Res. 2007;58(2):149–155. doi: 10.1016/j.neures.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera F, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, et al. Gender specific differences in neurodevelopmental effects of prenatal exposure to very low-lead levels: The prospective cohort study in three-year olds. Early Hum Dev. 2009;85(8):503–510. doi: 10.1016/j.earlhumdev.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicology and teratology. 2012;34(5):534–541. doi: 10.1016/j.ntt.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KJ, Porucznik CA, Anderson DJ, Brozek EM, Szczotka KM, Bailey NM, et al. Exposure Classification and Temporal Variability in Urinary Bisphenol A Concentrations among Couples in Utah-The HOPE Study. Environmental health perspectives. 2016;124(4):498–506. doi: 10.1289/ehp.1509752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub KL, McCartney K, Willett JB. Behavior problem trajectories and first-grade cognitive ability and achievement skills: A latent growth curve analysis. J Educ Psychol. 2007;99(3):653–670. [Google Scholar]
- Grimm KJ, Steele JS, Mashburn AJ, Burchinal M, Pianta RC. Early Behavioral Associations of Achievement Trajectories. Dev Psychol. 2010;46(5):976–983. doi: 10.1037/a0018878. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Meyer JM, Silberg JL, Maes HH, Loeber R, et al. The Virginia Twin Study of Adolescent Behavioral Development. Influences of age, sex, and impairment on rates of disorder. Arch Gen Psychiatry. 1997;54(9):801–808. doi: 10.1001/archpsyc.1997.01830210039004. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Foley DL, Angold A. 10-Year Research Update Review: The Epidemiology of Child and Adolescent Psychiatric Disorders: II. Developmental Epidemiology. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(1):8–25. doi: 10.1097/01.chi.0000184929.41423.c0. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.