Abstract
Introduction
Predicting response to proton-pump inhibitor (PPI) therapy in patients with laryngeal symptoms is challenging. The Restech Dx-pH probe is a transnasal catheter that measures oropharyngeal pH. In this study, we aimed to investigate the prognostic potential of oropharyngeal pH monitoring to predict responsiveness to PPI therapy in patients with laryngeal symptoms.
Methods
We conducted a physician blinded prospective cohort study at a single academic institution between 1/2013–10/2014. Adult patients with Reflux Symptom Index scores (RSI) ≥13 off PPI therapy were recruited. Patients underwent video laryngoscopy and 24-hour oropharyngeal pH monitoring, followed by an 8–12 week trial of omeprazole 40 mg daily. Prior to, and following PPI therapy, patients completed various symptom questionnaires. The primary outcome was the association between PPI response and oropharyngeal pH metrics. PPI response was separated into three subgroups based on post-treatment RSI score and % RSI response: Non-response = RSI ≥ 13; Partial response = post-treatment RSI < 13 and change in RSI < 50%; and Complete response = post-treatment RSI < 13 and change in RSI ≥ 50%. The primary analysis utilized a multinomial logistic regression controlling for pre-treatment RSI score. A secondary analysis assessed the relationship between change in RSI (post-pre) and oropharyngeal pH metrics via ordinary least square regression.
Results
Thirty-four patients completed the study and were included in final analysis. Symptom response to PPI therapy was as follows: 50% no response, 15% partial-response and 35% complete-response. Non-responders had a higher pre-treatment RSI (P < 0.01). There were no significant differences in oropharyngeal acid exposure (below pH of 4.0, 5.0, 5.5, 6.0 and RYAN scores) between responder types. The secondary analysis noted a trend between lower PPI response and greater total percent time below pH of 5.0 (P = 0.03), upright percent time below pH of 5.0 (p = .07) and RYAN supine (corrected) (P = 0.03), as well as an association between PPI response and greater decreases in the Anxiety Sensitivity Inventory (P < 0.01), Brief Symptom Inventory-18 (P < 0.01), and Negative Affect Scale (P < 0.01).
Discussion
Oropharyngeal pH testing did not predict laryngeal symptom response to PPI therapy. Contrary to hypothesis, our study signaled that the degree of oropharyngeal acid exposure is inversely related to PPI response. In addition, reduction in negative affect and psychological distress parallels PPI response.
Keywords: Extraesophageal reflux, Laryngopharyngeal reflux, Gastroesophageal reflux, Oropharyngeal pH testing
Introduction
Discerning whether patients with laryngeal symptoms will respond to proton-pump inhibitor (PPI) therapy is clinically challenging. Although chronic laryngitis due to gastroesophageal reflux, referred to as laryngopharyngeal reflux (LPR), is an established extraesophageal reflux syndrome, there exists significant controversy about the causative role of reflux and the clinical algorithm for LPR.1, 2 Current methods to detect LPR and predict response to treatment are of limited utility.3, 4 While commonly utilized, laryngoscopy is nonspecific and the reflux finding score (RFS), a validated laryngoscopic diagnostic tool to semi-quantify severity of findings, suffers from poor inter-rater relability.5, 6 Similarly, 24-hour dual probe ambulatory pH monitoring lacks sensitivity and specificity, with concerns over placement variability.7 Though an area of interest, outcome studies are lacking for combined pH impedance monitoring and its clinical significance is currently undefined.1, 8 In the setting of unreliable diagnostic tests, empiric PPI trials are commonly used to assess for symptom response. However, less than half of patients will respond to PPI therapy, with no significant improvement when compared to placebo.9–11 The deficiency of a well-defined clinical algorithm for LPR often results in overutilization of PPI therapy and diagnostic tests. The reported cost of diagnosing and managing extraesophageal reflux is estimated at $5,000 per patient, and actual cost burden is likely even greater. This is a major healthcare issue as LPR is an increasingly diagnosed entity, postulated to comprise up to 50% of laryngeal complaints in an otolaryngology practice alone.4, 12
The Restech Dx-pH system (Respiratory Technology Corporation, San Diego, CA, USA) is a minimally invasive oropharyngeal pH monitoring device which detects pH in both liquid and aerosolized droplets.13, 14 Studies demonstrate that oropharyngeal pH monitoring is highly sensitive in detecting acid reflux in the posterior oropharynx and more quickly reaches pH equilibrium compared to a standard pH catheter.13–16 However, data evaluating the agreement between esophageal pH impedance and oropharyngeal pH are mixed17–20, and unified normative values for oropharyngeal pH monitoring are lacking, limiting its current role as a diagnostic tool.21–24 In addition, whether oropharyngeal pH testing is a reliable prognostic tool for medical therapy and surgical outcomes is of great interest.15
This study aimed to determine if oropharyngeal pH monitoring predicts PPI response in patients with laryngeal symptoms. We hypothesized that patients with higher oropharyngeal acid exposure would be more likely to respond to PPI therapy.
Methods
Setting & Subjects
We conducted a prospective observational cohort study at an academic medical center between January 2013 and October 2014. The Institutional Review Board approved the study and a ClinicalTrials.gov record was maintained (ClinicalTrials.gov Identifier: NCT01755221). Patients between the ages of 18 and 89 presenting to the otolaryngology clinic for a standard of care evaluation for laryngeal symptoms were recruited to participate. Eligible patients had symptoms classically associated with LPR for greater than one month and a total Reflux Symptom Index (RSI) score greater than or equal to 13.6 Patients were excluded if PPI use was contraindicated or if they were currently taking a PPI and unable or unwilling to stop the medication for a minimum of two weeks before oropharyngeal pH probe placement. Patients were also excluded if they were pregnant, unable to stop anticoagulant use, or unwilling or unable to undergo an oropharyngeal pH assessment.
Study Design
Patients received a flexible fiber optic video laryngoscopy as part of their standard of care evaluation and the RFS was collected. Patients completed several self-administered questionnaires to obtain a baseline assessment of LPR symptom severity and duration, GERD symptom frequency, and symptom-specific anxiety, discomfort, affect, and stress. The questionnaire set included the RSI, a validated patient-reported laryngeal symptom questionnaire,6, 25 GerdQ, a validated instrument for evaluating GERD symptoms,26 Visceral Sensitivity Index (VSI), a validated instrument for gastrointestinal symptom-specific anxiety,27 and the Brief Symptom Inventory (BSI-18), a validated psychosocial self-report symptom scale.17 In addition, the Heartburn Vigilance Scale (HVS), adapted from the validated Pain Vigilance and Awareness Questionnaire, and the Heartburn Catastrophizing Scale (HCS), adapted from the Pain Catastrophizing Scale were used to examine reflux-specific thought and discomfort.28, 29 The Anxiety Sensitivity Inventory (ASI), a validated instrument that assesses beliefs about the social and somatic consequences of anxiety symptoms, and the Discomfort Intolerance Scale (DIS), a self-report measure of the ability to tolerate uncomfortable sensations, were used to evaluate gastrointestinal symptom-specific anxiety.30–32 Patients also completed the Positive and Negative Affect Schedule, a valid measure of positive and negative affect and the Perceived Stress Scale-4 (PSS-4), a shortened version of the Perceived Stress Scale, a validated measure of global perceived stress in relation to health outcomes.33–36
Patients underwent oropharyngeal pH assessment with the Restech Dx-pH system. Members of the research team prepared the device by calibrating it at pH levels of four and seven according to the manufacturer’s instructions. The pH probe was placed transnasally with the probe resting one cm below the uvula as recommended by the manufacturer. Patients were instructed to carry out their usual routine while the probe was in place, with the exception of vigorous physical activity. Patients recorded symptom events such as coughing, heartburn, and throat clearing in a written diary and on a wireless transponder they carried with them during the assessment period. The next day (24 hours later), patients returned to the clinic for probe removal. Studies were excluded if less than 16 hours of analyzable data was available. In a blinded fashion, physicians analyzed the oropharyngeal pH tracings according to manufacturer recommendations.
After probe removal, patients initiated an eight to twelve week course of omeprazole 40mg daily, thirty minutes prior to the final meal of the day, per the otolaryngology standard of care practice at our institution. At the conclusion of the PPI therapy course, patients returned for a standard of care follow-up visit and completed the aforementioned questionnaire set to obtain a post-PPI therapy assessment of symptom burden.
Definitions
Currently there is no standard definition of PPI response for LPR; thus, PPI response was defined by separating patients into sub-groups based on percent response and post-treatment RSI scores. Non-response was defined as post-treatment RSI ≥ 13; Partial response was defined as a post-treatment RSI < 13 but a percent change in RSI < 50%; and Complete response was defined as a post-treatment RSI < 13 and percent change in RSI ≥ 50%.
Outcomes
The primary outcome was the association between PPI response and oropharyngeal pH testing parameters. Oropharyngeal pH tracings were analyzed at pH levels of 4.0, 5.0, 5.5, and 6.0 in the upright position and over the total time period. Based on a previous study, which detected a post-supine lag artifact with the software, adjustments were made to calculate corrected time below pH 5.5 in total and upright positions.3 Additionally, composite scores for oropharyngeal pH testing (RYAN scores) were calculated in the upright and supine positions.22
The secondary outcomes were the associations between PPI response and psychosocial questionnaire results, including baseline and change (post-pre) results.
Statistical Analysis
The primary analysis evaluated PPI response as a dependent variable, considering patients in three sub-groups: Non-responder, Partial responder, and Complete responder. Differences in baseline clinical variables, oropharyngeal pH testing and questionnaire data were analyzed via Chi Square analysis for categorical variables and multinomial logistic regression.
In addition, we performed a secondary analysis to assess the relationships between change in RSI score (post-pre) and baseline clinical variables, oropharyngeal pH testing and questionnaire data via an ordinary least square (OLS) regression. Hypothetically, the OLS regression may identify associations that were obscured in the categorical primary analysis.
Baseline RSI was used as a covariate in each generalized logit and multiple linear regression model. Each outcome variable was tested in a separate model. Since we did not adjust for multiple hypothetical tests, P-values less than 0.01 were considered significant and P-values between 0.01–0.10 were considered marginally significant. All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). Multinomial logistic was run using PROC LOGISTIC with the GLOGIT link function for generalized logit.
Results
Of 236 patients with suspected LPR screened for the study, 29 did not meet inclusion criteria and 110 met exclusion criteria. Of the 97 eligible patients, 42 (43.3%) consented and enrolled. The thirty-four (81%) of the 42 patients who had 16 to 24 hours of analyzable oropharyngeal pH data and completed the appropriate questionnaires after PPI therapy were included in the final analysis. [Figure 1]
Figure 1. Patient flow diagram.
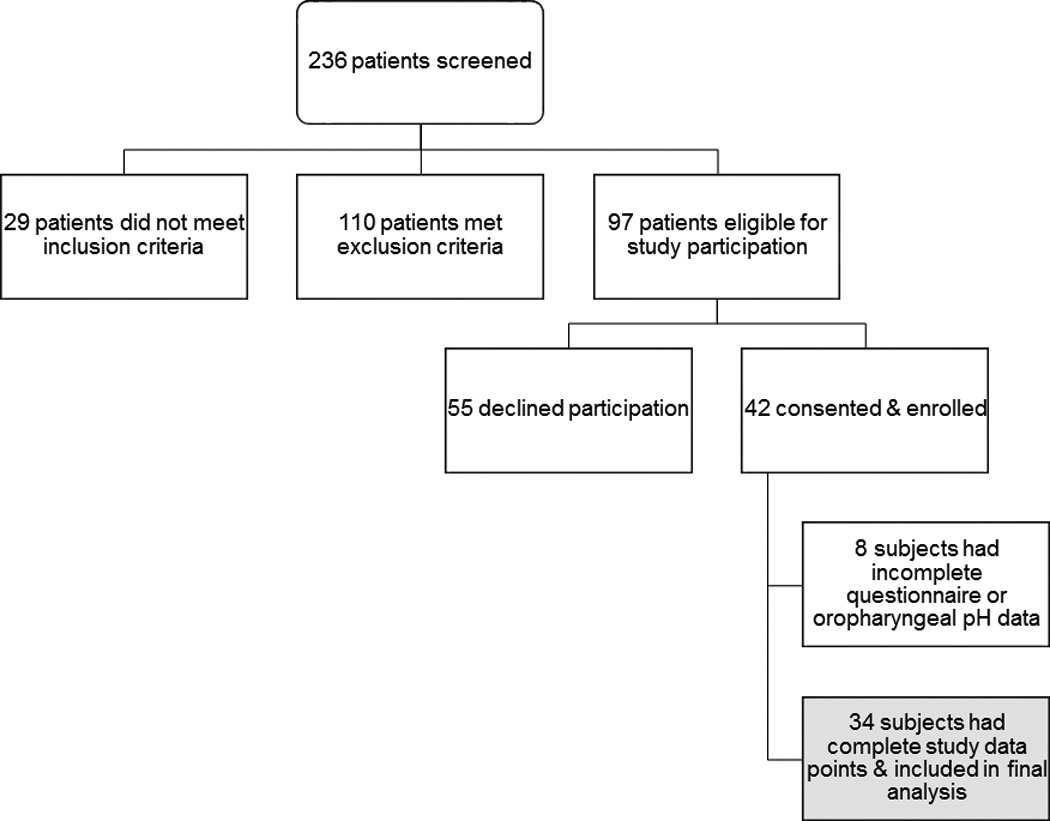
Of 236 patients screened, 139 were not eligible for study participation: 29 did not meet inclusion criteria and 110 met exclusion criteria. Of the 97 eligible patients, 42 (43%) consented & enrolled in the study. Of those enrolled, 34 (81%) had complete study data points and were included in the final analysis.
Baseline Findings
Twenty-four (71%) patients were female gender with an overall mean age of 45.5 ± 13.3 years and mean BMI of 27.2 ± 6.9 kg/m2. Commonly presenting symptoms included throat clearing (79.4%), post-nasal drip (79.4%), hoarseness (70.6%), chronic cough (55.9%), globus (47.1%), dysphagia (29.4%), chronic sore throat (23.5%), and difficulty breathing (20.6%). Less than one-third of patients complained of heartburn (32.4%). The overall mean RSI was 23.2 ± 7.3, with a mean GerdQ of 3.8 ± 4.2. Laryngoscopy findings were recorded for 19 patients, with a mean RFS of 7.9 ± 2.7.
Rates of PPI Response
Overall, the mean change (post-pre) in RSI score was a decrease of 8.9 ± 9.2 points, and mean percent change in RSI was reduction by 37.0 ± 39.7%. Seventeen (50%) were non-responders, while 5 (15%) met the definition for partial response and twelve (35%) for complete response [Figure 2]. Baseline clinical variables between these three subgroups did not significantly differ; however, 88% of non-responders were female while 40% of partial responders and 58% of complete responders were female (P = 0.06). Additionally, only two patients were smokers and both did not respond to PPI therapy [Table 1].
Figure 2. Distribution of patient response type based on pre- and post-RSI scores.
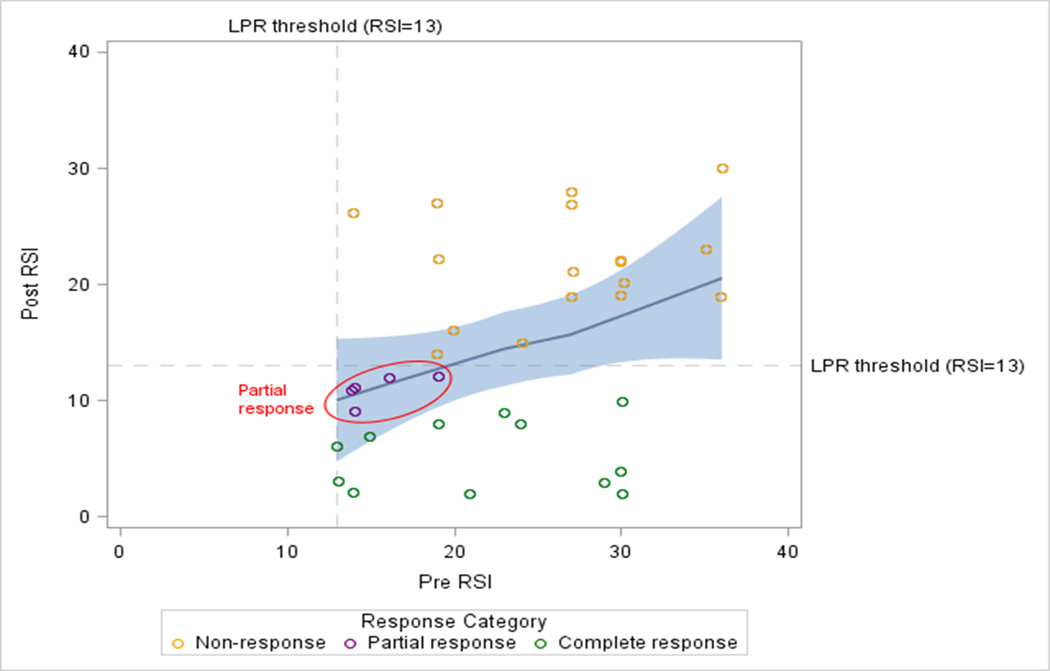
Non-response was defined as post-treatment RSI ≥ 13; Partial response was defined as a post-treatment RSI < 13 with percent change in RSI < 50%; and Complete response was defined as a post-treatment RSI < 13 with percent change in RSI ≥ 50%.
Table 1.
Baseline clinical data reported for PPI responder group.
| No response (n 17) |
Partial response (n 5) |
Complete Response (n 12) |
P- value |
|
|---|---|---|---|---|
| Age (years), Mean (SD) | 42.9 (12.1) | 47.4 (11.1) | 48 (15.9) | 0.58 |
| Female Gender, n (%) | 15 (88%) | 2 (40%) | 7 (58%) | 0.06 |
| BMI (kg/m2), Mean (SD) | 28.5 (7.30 | 25.0 (1.1) | 26.4 (7.7) | 0.53 |
| Smoker, n (%) | 2 (11.8%) | 0 (0%) | 0 (0%) | 0.34 |
| Pre-RSI, Mean (SD) | 26.5 (6.5) | 15.4 (2.2) | 21.8 (6.9) | 0.004 |
| Pre-GerdQ, Mean (SD) | 3.9 (4.0) | 1.4 (1.3) | 4.9 (5.0) | 0.3 |
| RFS, Mean (SD) | 8.3 (3.7) | 9.7 (2.1) | 7.0 (1.1) | 0.3 |
P-values calculated via generalized logit model with pre-RSI as a covariate.
Unable to test due to 0 subjects in two groups.
RFS available only for 19 subjects.
Body Mass Index (BMI); Reflux Symptom Index (RSI); Reflux Finding Score (RFS).
Pre-treatment Variables
The pre-treatment RSI score was significantly higher in the non-responder group (P < 0.01); as such pre-RSI was considered a covariate in study analyses. Differences in pre-treatment psychosocial questionnaire responses were not significant in primary or secondary analyses.
Oropharyngeal pH testing
There were no significant differences in baseline oropharyngeal acid exposure between the three sub-groups in primary analysis. Table 2 depicts the data for oropharyangeal pH measurements below pH values of 5.0, 5.5 (corrected) and RYAN scores (corrected); additional pH descriptive data are presented in the Supplemental Table. In secondary (OLS) analysis, we noted a trend between lower PPI response and greater total percent time below pH of 5.0 (P = 0.03), upright percent time below pH of 5.0 (P = 0.07) and RYAN supine (corrected) (P = 0.03); otherwise no significant associations were detected.
Table 2.
Baseline oropharyngeal pH metrics based on PPI response.
| Pre-Treatment Oropharyngeal pH Parameters |
No response (n 17) |
Partial response (n 5) |
Complete Response (n 12) |
Prim ary Anal ysis* P- value |
Seco ndary Analy sis+ P- value |
|---|---|---|---|---|---|
| Total % time pH < 5.0 | 4.3 (9.2) | 1.4 (2.2) | 0.4 (0.5) | 0.5 | 0.03 |
| Upright % time pH < 5.0 |
2.1 (5.4) | 3.0 (4.6) | 0.4 (0.7) | 0.5 | 0.07 |
| Total % time pH <5.5, Corrected |
8.6 (14.8) | 11.4 (20.2) | 4.0 (4.7) | 0.5 | 0.1 |
| Upright % time pH <5.5, Corrected |
3.3 (6.9) | 8.5 (17.6) | 0.8 (1.0) | 0.6 | 0.2 |
| RYAN Score, Upright, Corrected |
130.7 (232.0) |
271.0 (535.1) |
42.7 (48.4) | 0.5 | 0.2 |
| RYAN Score, Supine, Corrected |
10.8 (15.0) | 7.4 (11.7) | 3.0 (1.6) | 0.5 | 0.03 |
Both analyses conducted with pre-RSI as a covariate.
Generalized logit model;
Ordinary least square regression.
Post-treatment Variables
Changes in GerdQ were not associated with PPI response. In secondary analysis (OLS regression), PPI response was associated with greater decreases in ASI (P < 0.01), BSI-18 (P < 0.01), Negative Affect Scale (P < 0.01), and PSS4 (P < 0.04); these trends were not significant in the primary analysis (generalized logit model). PPI response was not associated with changes in DIS, VSI, HVS, or HCS in both analyses [Figure 3].
Figure 3. Change in Psychosocial Scores Based on Response Type.
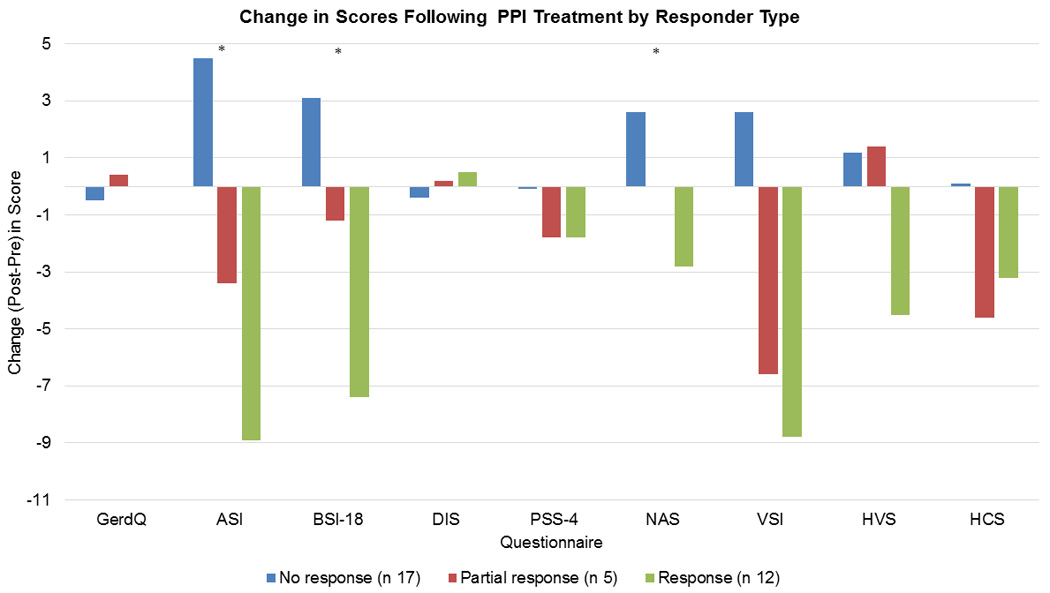
In primary analysis, no significant differences was seen in change (post – pretreatment) in psychosocial scores by responder type. In secondary analysis, responders had a significantly greater decrease in ASI, BSI-18 and NAS scores. * indicates P < 0.01. Anxiety Sensitivity Inventory (ASI); Brief Symptom Inventory (BSI-18); Discomfort Intolerance Scale (DIS); Perceived Stress Scale-4 (PSS-4); Negative Affect Schedule (NAS); Visceral Sensitivity Index (VSI); Heartburn Vigilance Scale (HVS); Heartburn Catastrophizing Scale (HCS).
Discussion
In this physician-blinded prospective observational cohort study, neither oropharyngeal pH testing nor pre-treatment psychosocial factors predicted laryngeal symptom response to PPI therapy, and our results do not support the use of these as clinical surrogates for predicting response to PPI therapy.
Contrary to our hypothesis that greater acid exposure would predict PPI response, our secondary analysis (OLS regression) suggested that a higher oropharyngeal acid burden (defined as less than pH level of 5.0) exists for PPI non-responders. Although not found in primary analysis when comparing responder types, this phenomenon is thought provoking, suggesting that those with higher acid exposure between pH of 4.0 to 5.0 are increasingly refractory to the therapeutic benefits of PPI therapy. Perhaps this reflects that the laryngopharynx is most sensitive to exposure below pH levels of 5.0, and that PPIs are less effective in reducing pH below this level. As seen in our prior study, an oropharyngeal pH level of 5.0 in the context of laryngeal symptoms appears to be most discriminatory, and further work to determine thresholds at this pH level are needed.3 Despite this relationship, there was an absence of measurable events below a pH level of 5.0 in the majority of PPI non-responders. As expected, higher pre-treatment RSI scores were associated with PPI non-response, suggesting that severity of initial symptoms is associated with refractoriness to PPI treatment.
Our findings differ from other studies examining the relationship between oropharyngeal pH testing and PPI response. In a retrospective case control study of 170 patients with LPR symptoms, Friedman et al. reported that PPI therapy based on a positive RYAN score compared to empiric PPI therapy resulted in significantly increased symptom response and compliance to therapy; however, in both the control and case groups the RSI significantly decreased following treatment, with mean post-treatment RSIs less than 13.37 A prospective study of 22 patients with LPR symptoms reported a 59% response rate to PPI therapy, and described that oropharyngeal pH testing has a 69% sensitivity and 100% specificity for predicting PPI response. However, response was defined as at least a 5-point decrease in RSI; derivation of this 5-point decrease threshold is unclear. Furthermore the statistical model did not control for pre-RSI or examine percentage change in RSI or RSI thresholds.38 These studies, including ours, highlight the need for clarifying thresholds and normative values for oropharyngeal pH testing and RSI prior to studying the predictive role of oropharyngeal pH testing. In particular, we previously found that one-third of healthy volunteers manifested abnormal upright RYAN scores, questioning the validity of the RYAN score and urging reexamination of normal and abnormal cutoff values.24 In an attempt to circumvent these issues our study defined response based on RSI thresholds and response rates reported in the literature, and additionally conducted a secondary analysis to examine associations. Moreover, our analysis assessed percent time of oropharyngeal acid exposure in place of normative thresholds.
The question of whether GERD is a co-factor in LPR remains unanswered. PPI responders in our study did not have higher initial GerdQ nor heartburn vigilance or heartburn catastrophizing scores than non-responders, suggesting that all patients with laryngeal symptoms who derive benefit from PPI therapy do not have classic co-morbid GERD. Factors beyond acidic gastroesophageal reflux are known to contribute to laryngeal pathology. For instance, allergen exposure is related to supraglottic eosinophilia.39 Work in esophageal eosinophilia suggests that the therapeutic role of PPIs may extend beyond an antacid mechanism and involve anti-inflammatory properties; future studies should examine the association between laryngeal eosinophilia and PPI response.40. Additionally, tobacco is associated with elevated subglottic mucin and smokers are at risk of developing chronic laryngeal disease and cancer outside of their acid exposure.39 Another theory is that acidic reflux is not the primary driver of laryngeal symptoms. Higher salivary pepsin concentrations have been detected in patients with laryngeal complaints without evidence of higher oropharyngeal acid exposure.3 Pepsin is postulated to be a potential biomarker of GERD,41 and it is possible that either a non-acidic or higher volume of refluxate drives symptoms or that 24-hour monitoring may not capture the original insult.
The role of psychosocial factors in the presentation of laryngeal symptoms is an area of interest. In our study, PPI response paralleled the reduction in psychosocial scores of anxiety, stress and negative affect, however the psychosocial scores did not predict PPI response. Laryngeal hypersensitivity may play a role in symptom recognition in laryngeal irritation, similar to the concept of esophageal hypersensitivity in patients with persistent troublesome GERD symptoms.42, 43 At the same time, it is possible that patients enrolled in a clinical trial may be more likely to feel better with regards to health related outcomes, translating to improvements in psychosocial questionnaire results. While this chicken and egg phenomenon is unclear, our results do support the interplay between psychosocial factors and symptom generation. These considerations reiterate the importance of tailoring management to symptom genesis, as PPI therapy as a primary treatment may not be indicated, and therapeutic strategies for certain sub-groups should include cognitive behavioral therapy and neuromodulation.
Although limited by a small sample size, these results suggest that oropharyngeal pH testing is unlikely to have sufficient specificity to replace empiric PPI therapy in the initial treatment algorithm for patients with suspected LPR. Furthermore, this study was not designed to measure the actual effect of PPI therapy as on-treatment pH measurements were not performed and this question would be best evaluated in a larger randomized controlled fashion with a placebo arm. Our patients also had low baseline GerdQ scores, and GERD symptom response to PPI therapy was thus difficult to interpret. Finally, we examined several oropharyngeal pH parameters and psychosocial factors which introduces the possibility of familywise error. While none of the pH parameters were strongly statistically significant or would stand up to familywise adjustment, P-values should be interpreted cautiously.
In conclusion, our results do not support the use of oropharyngeal pH testing or psychosocial questionnaires to predict symptom response to PPI therapy. This study importantly cautions against clinical reliance on oropharyngeal pH monitoring as a prognostic tool in suspected LPR. Unexpectedly, our study signals that the degree of oropharyngeal acid exposure may be inversely related to PPI response; as such, thresholds and the relationship between acid exposure and PPI response need to be clarified before utilizing this technology to guide management decisions of long-term PPI therapy or antireflux procedures. In addition, further work is needed to understand the mechanisms involved in laryngeal symptom genesis. This study suggests a relationship between laryngeal symptoms and psychosocial factors, providing groundwork for future studies to incorporate these therapeutic targets into clinical algorithms.
Supplementary Material
Study Highlights.
1. WHAT IS CURRENT KNOWLEDGE
It is difficult to predict which patients with laryngeal complaints will respond to proton-pump inhibitor (PPI) therapy
Current diagnostic methods are not reliable in detecting laryngopharyngeal reflux
The Restech Dx-pH system is an oropharyngeal pH monitoring device
2. WHAT IS NEW HERE
Oropharyngeal pH monitoring with the Restech Dx-pH system did not predict laryngeal symptoms response to PPI therapy
This study suggested that the degree of oropharyngeal acid exposure is inversely related to PPI response
Acknowledgments
The exception of: RY: Supported by T32 DK101363-02 grant; JEP: Consults for Covidien, Sandhill Scientific, and Given.
Abbreviations
- PPI
Proton-pump inhibitor
- LPR
Laryngopharyngeal Reflux
- RFS
Reflux Finding Score
- RSI
Reflux Symptom Index
- VSI
Visceral Sensitivity Index
- BSI-18
Brief Symptom Inventory
- HVS
Heartburn Vigilance Scale
- HCS
Heartburn Catastrophizing Scale
- ASI
Anxiety Sensitivity Inventory
- DIS
Discomfort Intolerance Scale
- PSS-4
Perceived Stress Scale-4
- OLS
Ordinary least square
Footnotes
Disclosures/Conflicts of Interest: The authors do not have any disclosures or potential conflicts of interest
Author Contributions:
Rena Yadlapati: Study oversight, study concept & design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
John E. Pandolfino: Study concept & design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Alcina K. Lidder: Study concept & design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Nadine Shabeeb: Study concept & design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Diana-Marie Jaiyeola: Study concept & design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Christopher Adkins: Study concept & design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Neelima Agrawal: Study concept & design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Andrew Cooper: Analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Caroline PE Price: Study concept & design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Jody D. Ciolino: Analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Andrew J. Gawron: Study concept & design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
Stephanie S. Smith: Acquisition of data; critical revision of the manuscript for important intellectual content
Michiel Bove: Acquisition of data; critical revision of the manuscript for important intellectual content
Bruce K. Tan: Study concept & design; acquisition of data; drafting of manuscript; critical revision of the manuscript for important intellectual content
References
- 1.Hom C, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease. Gastroenterol Clin North Am. 2013;42:71–91. doi: 10.1016/j.gtc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 1943. [DOI] [PubMed] [Google Scholar]
- 3.Yadlapati R, Adkins C, Jaiyeola DM, et al. Abilities of Oropharyngeal pH Tests and Salivary Pepsin Analysis to Discriminate Between Asymptomatic Volunteers and Subjects with Symptoms of Laryngeal Irritation. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108:905–911. doi: 10.1038/ajg.2013.69. [DOI] [PubMed] [Google Scholar]
- 5.Chang BA, MacNeil SD, Morrison MD, et al. The Reliability of the Reflux Finding Score Among General Otolaryngologists. J Voice. 2015;29:572–577. doi: 10.1016/j.jvoice.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS) Laryngoscope. 2001;111:1313–1317. doi: 10.1097/00005537-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Vaezi MF, Schroeder PL, Richter JE. Reproducibility of proximal probe pH parameters in 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol. 1997;92:825–829. [PubMed] [Google Scholar]
- 8.Kavitt RT, Yuksel ES, Slaughter JC, et al. The role of impedance monitoring in patients with extraesophageal symptoms. Laryngoscope. 2013;123:2463–2468. doi: 10.1002/lary.23734. [DOI] [PubMed] [Google Scholar]
- 9.Noordzij JPAK, Evans BA, Desper E, Mittal RK, Reibel JF, Levine PA. Evaluation of omeprazole in the treatment of reflux laryngitis: A prospective, placebo-controlled, randomized, double-blind study. Laryngoscope. 2001;111:2147–2151. doi: 10.1097/00005537-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HBLP, Buchner A, Inadomi JM, Gavin M, McCarthy DM. Lansoprazole treatment of patients with chronic idiopathic laryngitis:a placebo-controlled trial. Am J Gastroenterol. 2001;96:979–983. doi: 10.1111/j.1572-0241.2001.03681.x. [DOI] [PubMed] [Google Scholar]
- 11.Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131:342–350. doi: 10.1016/j.otohns.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Asaoka D, Nagahara A, Matsumoto K, et al. Current perspectives on reflux laryngitis. Clin J Gastroenterol. 2014;7:471–475. doi: 10.1007/s12328-014-0535-x. [DOI] [PubMed] [Google Scholar]
- 13.Friedman M, Hamilton C, Samuelson CG, et al. The Value of Routine pH Monitoring in the Diagnosis and Treatment of Laryngopharyngeal Reflux. Otolaryngol Head Neck Surg. 2012 doi: 10.1177/0194599812436952. [DOI] [PubMed] [Google Scholar]
- 14.Ayazi S, Lipham JCH, J A, Tang AL, Zehetner J, Leers JM, Oezcelik A, Abate E, Banki F, DeMeester SR, DeMeester TR. A New Technique for Measurement of Pharyngeal pH: Normal Values and Discriminating pH Threshold. J Gastrointest Surg. 2009;13:1422–1429. doi: 10.1007/s11605-009-0915-6. [DOI] [PubMed] [Google Scholar]
- 15.Patel DA, Harb AH, Vaezi MF. Oropharyngeal Reflux Monitoring and Atypical Gastroesophageal Reflux Disease. Curr Gastroenterol Rep. 2016;18:12. doi: 10.1007/s11894-016-0486-0. [DOI] [PubMed] [Google Scholar]
- 16.Yuksel ES, Slaughter JC, Mukhtar N, et al. An oropharyngeal pH monitoring device to evaluate patients with chronic laryngitis. Neurogastroenterol Motil. 2013;25:e315–e323. doi: 10.1111/nmo.12109. [DOI] [PubMed] [Google Scholar]
- 17.Andrews TM, Orobello N. Histologic versus pH probe results in pediatric laryngopharyngeal reflux. Int J Pediatr Otorhinolaryngol. 2013;77:813–816. doi: 10.1016/j.ijporl.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Chiou E, Rosen R, Jiang H, et al. Diagnosis of supra-esophageal gastric reflux: correlation of oropharyngeal pH with esophageal impedance monitoring for gastro-esophageal reflux. Neurogastroenterol Motil. 2011;23:717-e326. doi: 10.1111/j.1365-2982.2011.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ummarino D, Vandermeulen L, Roosens B, et al. Gastroesophageal reflux evaluation in patients affected by chronic cough: Restech versus multichannel intraluminal impedance/pH metry. Laryngoscope. 2013;123:980–984. doi: 10.1002/lary.23738. [DOI] [PubMed] [Google Scholar]
- 20.Becker V, Graf S, Schlag C, et al. First agreement analysis and day-to-day comparison of pharyngeal pH monitoring with pH/impedance monitoring in patients with suspected laryngopharyngeal reflux. J Gastrointest Surg. 2012;16:1096–1101. doi: 10.1007/s11605-012-1866-x. [DOI] [PubMed] [Google Scholar]
- 21.Sun G, Muddana S, Slaughter JC, et al. A new pH catheter for laryngopharyngeal reflux: Normal values. Laryngoscope. 2009;119:1639–1643. doi: 10.1002/lary.20282. [DOI] [PubMed] [Google Scholar]
- 22.Ayazi S, Lipham JC, Hagen JA, et al. A new technique for measurement of pharyngeal pH: normal values and discriminating pH threshold. J Gastrointest Surg. 2009;13:1422–1429. doi: 10.1007/s11605-009-0915-6. [DOI] [PubMed] [Google Scholar]
- 23.Chheda NN, Seybt MW, Schade RR, et al. Normal values for pharyngeal pH monitoring. Ann Otol Rhinol Laryngol. 2009;118:166–171. doi: 10.1177/000348940911800302. [DOI] [PubMed] [Google Scholar]
- 24.Yadlapati R, Adkins C, Jaiyeola DM, et al. Abilities of Oropharyngeal pH Tests and Salivary Pepsin Analysis to Discriminate Between Asymptomatic Volunteers and Subjects With Symptoms of Laryngeal Irritation. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1–78. doi: 10.1002/lary.1991.101.s53.1. [DOI] [PubMed] [Google Scholar]
- 26.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030–1038. doi: 10.1111/j.1365-2036.2009.04142.x. [DOI] [PubMed] [Google Scholar]
- 27.Labus JS, Mayer EA, Chang L, et al. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- 28.Roelofs J, Peters ML, McCracken L, et al. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. 2003;101:299–306. doi: 10.1016/S0304-3959(02)00338-X. [DOI] [PubMed] [Google Scholar]
- 29.Osman A, Barrios FX, Gutierrez PM, et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 30.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 31.RL PRAH. The anxiety sensitivity index: Construct validity and factor analytic structure. Journal of Anxiety Disorders. 1987;1:117–121. [Google Scholar]
- 32.Schmidt NB, Richey JA, Fitzpatrick KK. Discomfort intolerance: Development of a construct and measure relevant to panic disorder. J Anxiety Disord. 2006;20:263–280. doi: 10.1016/j.janxdis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich M, Verdolini Abbott K, Gartner-Schmidt J, et al. The frequency of perceived stress, anxiety, and depression in patients with common pathologies affecting voice. J Voice. 2008;22:472–488. doi: 10.1016/j.jvoice.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Levenstein S, Prantera C, Varvo V, et al. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res. 1993;37:19–32. doi: 10.1016/0022-3999(93)90120-5. [DOI] [PubMed] [Google Scholar]
- 35.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 36.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 37.Friedman M, Maley A, Kelley K, et al. Impact of pH monitoring on laryngopharyngeal reflux treatment: improved compliance and symptom resolution. Otolaryngol Head Neck Surg. 2011;144:558–562. doi: 10.1177/0194599811399240. [DOI] [PubMed] [Google Scholar]
- 38.Vailati C, Mazzoleni G, Bondi S, et al. Oropharyngeal pH monitoring for laryngopharyngeal reflux: is it a reliable test before therapy? J Voice. 2013;27:84–89. doi: 10.1016/j.jvoice.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Mouadeb DA, Belafsky PC, Birchall M, et al. The effects of allergens and tobacco smoke on the laryngeal mucosa of guinea pigs. Otolaryngol Head Neck Surg. 2009;140:493–497. doi: 10.1016/j.otohns.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 40.Richter JE. Current Management of Eosinophilic Esophagitis 2015. J Clin Gastroenterol. 2015 doi: 10.1097/MCG.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 41.Hayat JO, Gabieta-Somnez S, Yazaki E, et al. Pepsin in saliva for the diagnosis of gastro-oesophageal reflux disease. Gut. 2015;64:373–380. doi: 10.1136/gutjnl-2014-307049. [DOI] [PubMed] [Google Scholar]
- 42.Roman S, Keefer L, Imam H, et al. Majority of symptoms in esophageal reflux PPI non-responders are not related to reflux. Neurogastroenterol Motil. 2015;27:1667–1674. doi: 10.1111/nmo.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson JA. Work-associated irritable larynx syndrome. Curr Opin Allergy Clin Immunol. 2015;15:150–155. doi: 10.1097/ACI.0000000000000144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


