Abstract
Luteinizing Hormone (LH)/human chorionic gonadotropin (hCG) stimulates progesterone biosynthesis in the corpus luteum by activating cAMP /protein kinase A (PKA) cascade. Recent studies have shown that cAMP-mediated activation of PKA interacts with the mammalian target of rapamycin (mTOR) signaling pathways. Furthermore, the use of mTOR inhibitors for immunosuppression in transplant patients has shown adverse effects in reproductive functions. This study examined whether the mTOR pathway plays any role in LH-mediated regulation of progesterone production. Human granulosa lutein cells were isolated from follicular aspirates of women undergoing in vitro fertilization. Cells were cultured for 72 hours and treated with hCG (50 ng/ml) for different time periods with or without pretreatment with mTORC1 inhibitor, rapamycin, (20 nM) for 1 hour. Expression of steroidogenic enzymes including steroidogenic acute regulatory protein (STAR), cholesterol side chain cleavage enzyme (CYP11A1) and 3β-hydroxysteroid dehydrogenase type 1 (HSD3B1) mRNA were examined by real-time PCR after 6 hours of hCG treatment. Expressions of phospho-ribosomal protein S6 kinase (p-S6K1) and CYP11A1 were analyzed after 15 minutes and 24 hours of hCG treatment, respectively. Progesterone production was analyzed by an Enzyme Immunoassay kit after hCG (50 ng/ml) or forskolin (10 μM) treatment for 24 hours. Treatment with hCG increased the expression of downstream targets of mTORC1 as well as CYP11A1, HSD3B1, and STAR mRNAs. These increases were inhibited by rapamycin pretreatment. Increased progesterone production in response to treatment with hCG or forskolin was also blocked by rapamycin pretreatment. Our findings support a role for mTORC1 in regulating steroidogenesis in human granulosa lutein cells.
Keywords: Mammalian Target of Rapamycin Complex 1 (mTORC1), Human Granulosa Lutein Cells, Steroidogenesis
Introduction
It has been well established that progesterone production in mammalian ovaries, including human, is regulated by the receptor-mediated activation of cyclic AMP production by luteinizing hormone (LH) [1–4]. Cyclic AMP then activates protein kinase A, which culminates in increased progesterone production. The mechanism of increased progesterone production by cyclic AMP involves activation of multiple steps in steroidogenesis, including uptake of plasma-derived lipoprotein-associated cholesterol into the cytoplasm [5,6], STAR-mediated transport of cholesterol from cytosol to mitochondria [7–9], and the movement of cholesterol from the outer to the inner mitochondrial membranes [10–12] where the initial reaction in steroidogenesis—the conversion of cholesterol to pregnenolone—occurs [13,14]. It has also been well established that transcriptional activity of CYP11A1 is increased in response to LH treatment [15]. These events culminate in increased transformation of cholesterol to pregnenolone, and subsequent conversion to progesterone, in the cytosol. At higher concentrations of LH/hCG, a second pathway involving Gq-mediated activation of phospholipase C has also been reported [16–19]. More recent studies have revealed that the signaling pathways are more complex than previously recognized. For instance, in rodent ovaries, protein kinase A can activate other kinases including ERK1/2 and Akt [20–22]. Akt is a known activator of mTOR, increasing transcriptional and translational events that lead to increased cell size and number [23,17,24]. Furthermore, we have shown that in rat ovarian theca-interstitial cells, LH can activate steroidogenic enzyme expression and androstenedione production via activation of the mTOR signaling pathway. mTOR is an atypical member of the PI-3 kinase family of serine/threonine kinases [25–28] that plays a central role in mediating cell growth and proliferation [29]. Growth-promoting hormones have been recognized to activate mTOR via activation of the protein kinase Akt. The mechanism of mTOR activation uses a complex pathway. The mTOR kinase exists in two physically distinct protein complexes-mTORC1 and mTORC2. mTORC1 is composed of three essential components-mTOR, raptor, and mTST8-and is inhibited by rapamycin, whereas mTORC2 is insensitive to rapamycin [30]. Under unstimulated conditions, mTOR exists in an inactive state, which is maintained by the GDP-bound Rheb. Under hormonal stimulation, Rheb gets activated by conversion to the GTP-bound form, which then activates mTOR. In the activated state, mTOR phosphorylates downstream ribosomal proteins S6K1 and 4EBP1. These processes lead to activation of protein translation and increased transcription, culminating in cell growth, as well as in the regulation of metabolic pathways [27,28]. Although the mTOR signaling pathway is well-recognized as regulating proliferative pathways, it can also exert regulatory roles in lipid synthesis and the regulation of metabolic pathways, including glycolysis [31,32]. In this study, we examined the possible involvement of mTOR signaling in progesterone production by human granulosa lutein cells.
Materials and Methods
McCoy's 5A medium and 0.4% trypan blue were purchased from Invitrogen/GIBCO (Carlsbad, CA). Penicillin-streptomycin was purchased from Roche Diagnostics (Indianapolis, IN). BSA and anti-β-tubulin antibody were purchased from Sigma Chemical Co. (St. Louis, MO). Purified hCG was purchased from Dr. A. F. Parlow (National Hormone and Peptide Program, Torrance, CA). Forskolin was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). Rapamycin, anti-mouse or anti-rabbit IgG horseradish peroxidase conjugates, anti-p-S6K1, and anti-S6K1 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-CYP11A1 was obtained from Abcam (Cambridge, MA). Femto Super Signal Chemiluminescence reagent and Restore stripping buffer were purchased from Pierce (Rockford, IL). Progesterone Enzyme Immunoassay (EIA) kit was purchased from Cayman Chemical (Ann Arbor, MI). Real-time PCR reagents, as well as the primers and probes for STAR (assay id: Hs00264912_m1), CYP11A1 (assay id: Hs00167984_m1), HSD3B1 (assay id: Hs01084547_gH), and 18S rRNA (assay id: Hs99999901_s1) were purchased from Applied Biosystems (Foster City, CA). All other reagents used were conventional commercial products.
Granulosa lutein cell isolation and culture
Human granulosa lutein cells were isolated from follicular aspirates obtained at the time of oocyte retrieval in women undergoing in vitro fertilization. In these women, ovulation had been induced by hCG injection following ovarian stimulation with recombinant human FSH and LH. Follicles were aspirated 36 hours after hCG administration and remaining aspirates were pooled after the oocytes had been isolated. The cell suspension was made free of red blood cells by centrifugation with Ficoll-Paque PLUS, as previously described [33]. Briefly, the interface between the media and the Ficoll containing the granulosa cells was aspirated and resuspended in a fresh medium. The cells were washed twice to remove any residual blood cell contamination and resuspended in the final culture medium. Cell viability was tested by trypan blue dye exclusion [34]. The cells were then counted, plated, and cultured for 72 hours to allow the granulosa cells to recover from LH receptor downregulation before further treatment with test substances.
Rapamycin effect on cell viability
To test the possible adverse effect of rapamycin on cell viability, cells were seeded into 12-well plates and cultured with McCoy's medium containing 10% fetal bovine serum (FBS). The attached cells were serum-starved for 1 day and pretreated with 5, 10, 20 and 40 nM rapamycin dissolved in 3μl of dimethyl sulfoxide (DMSO) for 1 hour, followed by hCG (50 ng/ml) for 24 hours. The control group received 3μl of DMSO. After the treatment periods, cell viability was determined by Trypan Blue exclusion. Both the unstained (viable) and stained (nonviable) cells were counted and the percentages of viable cells were calculated.
Real-time PCR
After the different incubation periods, cells were harvested and total RNA was extracted using TRIzol reagent following manufacturer's instructions. Reverse-transcription was carried out in a final volume of 20 μl containing 1 or 2 ug RNA, 2.5 μM random hexamer, 2 mM deoxynucleotide triphosphates, 5.5 mM MgCl2, 8 U ribonuclease inhibitor, and 25 U Multiscribe reverse transcriptase in a PTC-100 thermal controller. The resulting cDNA was diluted and subjected to real-time PCR reactions using predesigned primers and probes. The changes in gene expressions were calculated using the ΔΔCt method, with 18S rRNA as the internal control.
Western blot analysis
After various treatments, cell monolayers were washed with PBS and homogenized using a radio immunoprecipitation assay (RIPA) buffer (PBS containing 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate). The protein content of the cell lysates were then determined. Samples (10–30 μg) were incubated with an SDS sample loading buffer and subjected to 10% SDS-PAGE under reducing conditions, followed by Western blot analysis as previously described [35]. Briefly, membranes were blocked in 5% fat-free milk for 1 hour and then incubated overnight at 4°C with a primary antibody. After several washes with Tris-buffered saline containing Tween 20 (TBST), membranes were incubated in a peroxidase-conjugated secondary antibody for 1 hour at room temperature. Following further washes with TBST, membrane-bound antibodies were detected using a chemiluminescence reagent. The total protein content of loading was monitored by reprobing the same blots with tubulin (loading control).
Assay of progesterone
To determine the involvement of the mTORC1 signaling pathway in hCG or forskolin-stimulated progesterone production, human granulosa cells were seeded in 35-mm plates with McCoy's medium containing 10% FBS. After attachment, the cells were cultured for 72 hours and pretreated with rapamycin (20 nM) for 1 hour followed by treatment with hCG (50 ng/ml) or forskolin (10 μM), a pharmacological activator of adenylyl cyclase, for 24 hours. At the end of the treatments, media were collected and progesterone was assayed using an EIA kit (Cayman, US) according to the manufacturer's instructions. Briefly, trace amounts of [3H]- progesterone (10,000 cpm) were added to the media samples (500 μl) to monitor recovery of extraction. The media were then extracted with methylene chloride (3×) and suspended in the ELISA buffer, after evaporating methylene chloride. 50 μl of the sample or standard(s) were added to the wells of the ELISA plate and treated with Ellman's reagent followed by tracer (progesterone-acetylcholinesterase conjugate). After 60–90 minute incubation in the dark, readings were taken at a wavelength of 412 nm using a plate reader. The progesterone values are expressed as pg/ ml of the reconstituted extract. The extraction recovery of the assay was greater than 82%. The assay has a range of 7.8–1000 pg/ml and sensitivity (80% B/B0) of approximately 10 pg/ml. The intra-assay and inter-assay coefficients of variation for progesterone were 4.9–54.5% and 1.5–16.4%, respectively.
Statistical analysis
Statistical analysis was carried out using one-way ANOVA, followed by the Tukey multiple comparison test. Values were considered statistically significant at p < 0.05. Each experiment was repeated at least 3 times, with similar results. Blots are representative of 3 experiments, and graphs represent the mean ± SE of 3 independent experiments.
Results
LH/hCG stimulates progesterone synthesis related gene expression
Prior to determining the role of mTOR signaling in hCG-stimulated steroidogenic enzyme protein and mRNA, the time course of hCG responsiveness on steroidogenic enzyme mRNA expression was determined. To test this, human granulosa lutein cells isolated from IVF retrieval fluids were cultured in the absence or presence of hCG for different time intervals, and the expression of CYP11A1, HSD3B1 and STAR mRNA were examined by real-time PCR. Experiments were performed in triplicate on each set of cells. In all samples, 50 ng/ml hCG treatment resulted in a time-dependent increase in the expression of steroidogenic enzymes HSD3B1, STAR, and CYP11A1. Representative results are shown in Figure 1. Levels of HSD3B1 increased 1.8-fold after 3 hours of treatment with hCG and 2.5-fold after 6 hours of treatment. Levels of CYP11A1 increased 2.1-fold after 3 hours of treatment with hCG and 2.7-fold after 6 hours of treatment. Levels of STAR increased 2.4-fold after 3 hours of treatment with hCG and 4.2-fold after 6 hours of treatment. Based on these results, 6-hour incubation with 50 ng/ml of hCG was chosen for subsequent experiments.
Fig. 1. HCG increases progesterone synthesis related gene expression in a time-dependent manner.
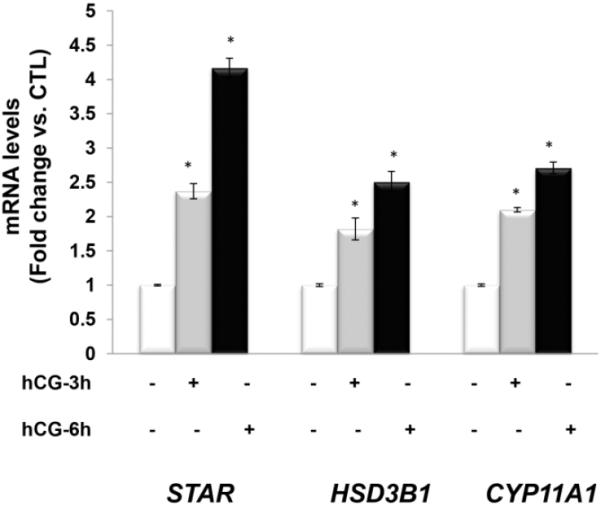
Human granulosa lutein cells were incubated with hCG (50 ng/ml) for 0, 3, or 6 hours. Cells were then harvested at different time periods for total RNA extraction followed by reverse transcription. The real-time PCR reactions were carried out using the diluted cDNA in triplicate with predesigned primers and probes for human STAR, CYP11A1 and HSD3B1, using 18S RNA as a control. *P<.05 vs. control.
HCG-stimulated progesterone synthesis related gene expression is blocked by the mTORC1 inhibitor
Since rapamycin is potentially toxic to the cells, initial experiments to determine the optimum dose of rapamycin that would produce no effect on cell viability revealed that a dose of 20 nM concentration of rapamycin produced no cell death. To test the role of mTORC1 signaling in LH/hCG action, granulosa lutein cells were pretreated with the mTORC1 inhibitor rapamycin (20 nM) for 1 hour, followed by incubation with hCG for an additional 6 hours. At the end of the incubation, the cells were processed for total RNA extraction and mRNA levels of steroidogenic enzymes (STAR, CYP11A1, and HSD3B1) were measured by real-time PCR. As shown in Figures 2A–C, hCG treatment resulted in a significant increase in the levels of progesterone synthesis related genes (CYP11A1, HSD3B1, and STAR), while pretreatment with rapamycin blocked these increases. After establishing the inhibitory effect of rapamycin on hCG-induced expression of steroidogenic enzyme mRNA expression, the inhibitory effect of rapamycin was then tested on CYP11A1 protein expression in cell lysates after 24 hours of incubation with hCG, by Western Blot analysis. The results presented in Figure 2D show that hCG treatment produced a 4.6-fold increases in CYP11A1 protein, but pretreatment with rapamycin significantly reduced this increase. These results suggest that mTORC1 signaling is involved in hCG-induced steroidogenic enzyme expression. The results also show that rapamycin treatment at 20 nM concentration did not reduce cell viability compared to the control group without rapamycin (Fig 3).
Fig. 2. HCG-stimulated progesterone synthesis related gene expression is significantly reduced by pretreatment with rapamycin.
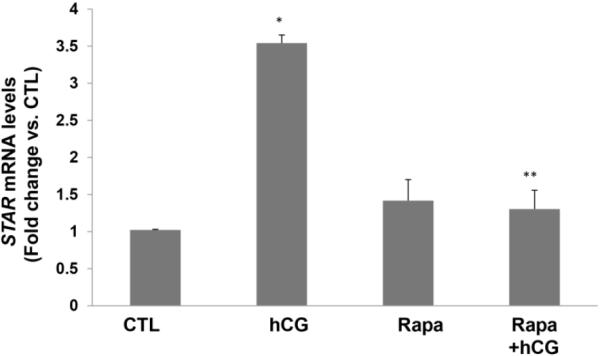
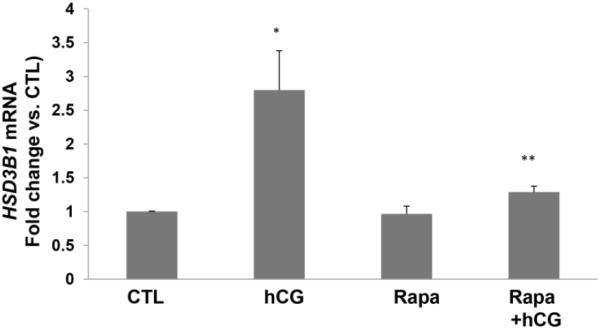
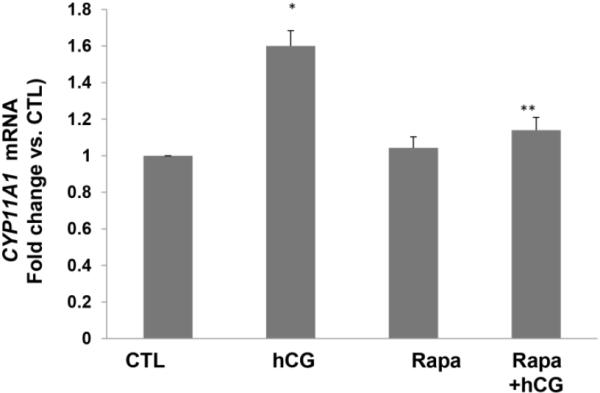
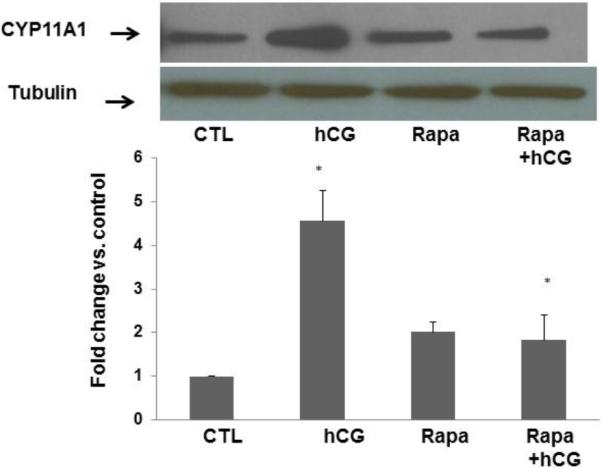
Human granulosa lutein cells were incubated with hCG (50 ng/ml) with or without pretreatment with rapamycin (rapa; 20 nM) for 1 hour. After 6h of incubation with hCG, cells were harvested and total RNA was extracted and reverse transcribed into cDNA, which was subjected to real-time PCR reactions with predesigned primers and probes for human STAR (A), HSD3B1 (B), and CYP11A1 (C) using 18S RNA as a control. After 24h of incubation, another set of cells were harvested, and CYP11A1 protein expression (D) was analyzed using Western Blot analysis. Protein loading was monitored by reprobing the same blots with tubulin antibody. *P<.05 vs. control, **P<.01 vs. hCG.
Fig. 3. Rapamycin treatment does not affect cell viability.
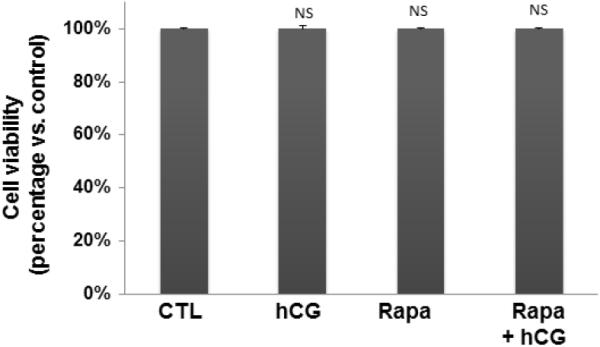
Human granulosa lutein cells were incubated with hCG (50 ng/ml) with or without pretreatment with rapamycin (rapa; 20 nM) for 1 hour. To test viability, the cells were harvested after 24 h of hCG and subjected to Trypan blue exclusion assay. NS - P>0.05 vs. control
Effect of hCG on mTOR signaling
The ability of hCG to activate mTOR signaling was then tested using the same dose that produced an increase in the expression of steroidogenic enzymes and mRNAs. Since the ribosomal protein S6 kinase is one of the important downstream targets of mTOR signaling [27,28], the effect of hCG on the formation of Phospho S6 kinase in human granulosa lutein cells was then determined. The cells were incubated with or without hCG for 6 hours and the cell lysates were examined for p-S6K1 by Western Blot analysis. The results presented in Figure 4 show that hCG treatment produced 8.7-fold increases in p- S6K1 formation. Pretreatment with rapamycin significantly reduced the stimulation produced by hCG. These results show that mTOR signaling pathway is operative in human granulosa lutein cells and that this pathway is activated by hCG. Thus, the blockade of hCG- stimulated activation of steroidogenic enzymes and mRNA in human granulosa lutein cells is consistent with a role of mTOR signaling pathway in hCG-mediated activation of steroidogenesis.
Fig. 4. HCG-induced phosphorylation of p-S6K1, the downstream target of mTOR signaling, is also significantly reduced by rapamycin pretreatment.
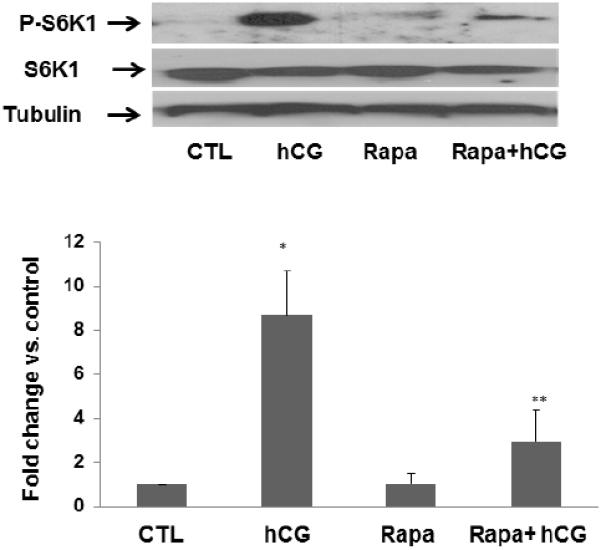
Human granulosa lutein cells were incubated with or without rapamycin (rapa; 20 nM) for 1 hour, followed by hCG (50 ng/ml) for 15 minutes. At the end of the incubation, the cells were harvested, and the p-S6K1 (lane 1) expression was analyzed using Western Blot analysis. Protein loading was monitored by reprobing the same blots with total S6k and tubulin antibodies (lanes 2 and 3). *P<.05 vs. control, **P<.01 vs. hCG.
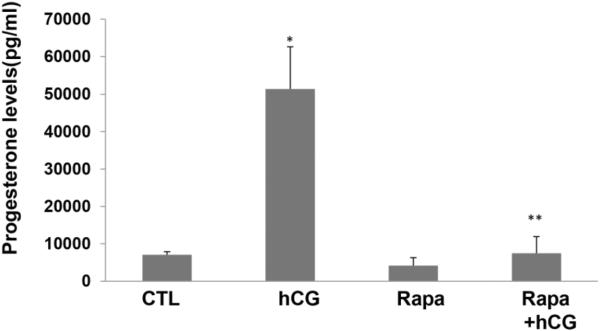
Rapamycin blocks LH/hCG or forskolin-stimulated progesterone synthesis
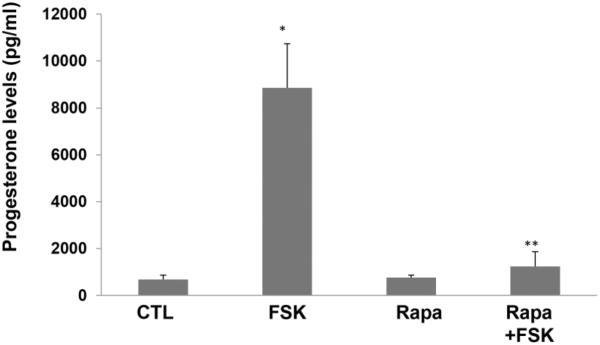
Primary cultures of human granulosa lutein cells were pretreated with or without rapamycin (20 nM) for 1 hour, followed by stimulation with hCG for 24 hours. The incubation media were collected and progesterone levels were assayed as described in detail in the methods. The results showed that, as seen in Figure 5, progesterone production, as expected, was increased more than 5-fold by hCG treatment compared to the control group. This pronounced increase was significantly reduced by pretreatment with rapamycin. Similarly, as shown in Figure 6, progesterone levels increased greater than 10-fold in forskolin-treated human granulosa lutein cells, but this increase was also significantly diminished by pretreatment with rapamycin, suggesting that the site of action of mTOR lies at a step after cyclic AMP production.
Fig. 5. HCG-induced production of progesterone is significantly reduced by rapamycin pretreatment.
Human granulosa lutein cells were treated with hCG (50 ng/ml) with or without pretreatment with rapamycin (rapa; 20 nM; for 1 hour). After 24h of hCG, progesterone production was determined by EIA as described in detail in the Methods. *P<.05 vs. control, **P<.01 vs. hCG.
Fig. 6. Forskolin-induced production of progesterone is significantly reduced by rapamycin pretreatment.
Human granulosa lutein cells were pretreated with or without rapamycin (rapa; 20 nM) for 1 hour, followed by treatment with forskolin (FSK; 10 μM), a pharmacological activator of adenylate cyclase, for 24 hours. At the end of the treatments, progesterone production was determined using an EIA kit. *P<.05 vs. control, **P<.01 vs. hCG.
Discussion
Our study examined the hCG-stimulated signaling network involved in the regulation of steroidogenesis in human granulosa lutein cells. The results show that cultured human granulosa lutein cells isolated from the retrieval fluids from follicles respond to hCG with increased expression of CYP11A1, HSD3B1 and STAR mRNA and protein. The extent of stimulation of CYP11A1 and HSD3B1 mRNA was comparable at 3 hrs and 6 hours of hCG treatment. The magnitude of responsiveness of STAR mRNA to hCG stimulation was higher (4.2-fold) than that seen for CYP11A1 (2.7 fold) and HSD3B1 (2.5 fold). The reason for differences in the extent of stimulation of mRNA and the corresponding protein for CYP11A1 and HSD3B1 is not understood although it could be attributed to the relatively higher instability of mRNA. The magnitude of progesterone response to hCG (5- fold) and forskolin (10-fold) was higher than that seen for the CYP11A1 and HSD3B1 mRNA transcripts. This difference could be partly due to longer incubation period (24 hours) used for measuring progesterone response. It would also be possible that increased expression of STAR mRNA in response to hCG treatment might contribute to increased progesterone production by facilitating cholesterol transport from outer to the inner mitochondrial membrane where cholesterol undergoes side chain cleavage to produce pregnenolone which is then converted to progesterone. Rapamycin reduced hCG-mediated increases in CYP11A1 protein 2-fold while reducing progesterone production 6–7 fold. This would suggest that rapamycin-mediated inhibition of STAR probably contributes more effectively to reduce progesterone production.
Since ovarian function is regulated by the anterior pituitary hormone LH and its placental counterpart hCG, previous studies have focused on the signaling pathways involved in the action of these two hormones through Gs protein-coupled receptors. While this is still true that both LH and hCG act primarily through interaction with Gs-coupled signaling pathways [36], the cAMP- mediated responses might interact with other signaling pathways in granulosa cells. Here we present evidence showing that LH/hCG-mediated cAMP regulates progesterone production by interacting with the mTOR signaling pathway, since inhibition of mTOR signaling by rapamycin significantly reduced the expression of CYP11A1, as well as progesterone production. The inhibitory effect of rapamycin on progesterone synthesis was also seen when granulosa cells were treated with forskolin, an agent that increases cyclic AMP production, bypassing the LH/hCG receptor, which suggests that the site of the inhibitory action lies at a step after cyclic AMP production.
The mechanism by which cyclic AMP activates the mTOR signaling pathway is thought to occur via activation of Akt [23]. It has now been well established that Akt is the upstream activator of mTOR (reviewed in [37]). Akt itself exists in an inactive form in most cells and signaling cascade emanating from hormones like insulin has been shown to activate inactive Akt to the active form by the PIP3-dependent protein kinase PDK1 [38]. It is possible that activation of PKA in response to LH/hCG stimulation in the ovary might prompt PDK1 to activate Akt. In fact, activation of Akt by FSH in the ovarian follicles has been previously reported [39,40]. In our previous studies, we have shown that hCG can activate Akt in rat theca-interstitial cells and that this activation can be blocked by both PKA and Akt inhibitors [41]. Thus, it is conceivable that LH/hCG-mediated activation of protein kinase A might activate Akt via PDK1 or through a yet unknown pathway. Activated Akt then activates mTORC1 and its downstream targets, 4EBP1 and S6K1, leading to transcriptional and/or translational activation of progesterone synthesis related genes. In this context, we have previously shown, using ChIP assays, that hCG increases the recruitment of cAMP response element-binding protein (CREB) to the proximal promoter of CYP17A1 gene, and that this increase was significantly reduced by rapamycin [42]. It is likely that the expression of other steroidogenic enzymes might also be activated through a similar mechanism.
In summary, we show here that mTOR signaling plays a crucial role in progesterone production in human granulosa lutein cells in response to stimulation by LH/hCG. This finding may explain the clinical observation that renal transplant patients treated with rapamycin (also called sirolimus) for immunosuppression showed diminished testosterone production, while their LH and FSH levels remained high [43,44]. Similarly, it has been reported that female renal transplant patients treated with rapamycin exhibit gonadal dysfunction and infertility, with increased FSH and LH and decreased progesterone levels [45]. Interestingly, menses returned and hormone levels returned to normal after cessation of rapamycin [45]. The present study, in addition to providing new information on the signaling pathways regulating progesterone production in granulosa lutein cells, also provides a mechanistic explanation for these clinical findings and may assist in patient selection and counseling.
Acknowledgments
The authors wish to express appreciation to the staff at IVF facility in the University of Michigan for providing ovarian follicular aspirates. We are grateful to Sarah Block for critical reading of the manuscript and many helpful suggestions.
Funding: This work was funded by National Institutes of Health (HD 06656-40).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: Exception granted since the material used are otherwise discarded.
References
- 1.Azhar S, Clark MR, Menon KM. Regulation of cyclic adenosine 3', 5' -mono-phosphate dependent protein kinase of rat ovarian cells by luteinizing hormone and human chorionic gonadotropin. Endocr Res Commun. 1976;3(2):93–104. doi: 10.3109/07435807609052925. [DOI] [PubMed] [Google Scholar]
- 2.Clark MR, Menon KM. Regulation of ovarian steroidogenesis. The disparity between 125I-labelled choriogonadotropin binding cyclic adenosine 3',5'-monophosphate formation and progesterone synthesis in the rat ovary. Biochim Biophys Acta. 1976;444(1):23–32. doi: 10.1016/0304-4165(76)90220-8. [DOI] [PubMed] [Google Scholar]
- 3.Williams MT, Marsh JM. Estradiol inhibition of luteinizing hormone-stimulated progesterone synthesis in isolated bovine luteal cells. Endocrinology. 1978;103(5):1611–1618. doi: 10.1210/endo-103-5-1611. doi:10.1210/endo-103-5-1611. [DOI] [PubMed] [Google Scholar]
- 4.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28(1):117–149. doi: 10.1210/er.2006-0022. doi:10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 5.Gwynne JT, Strauss JF., 3rd The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3(3):299–329. doi: 10.1210/edrv-3-3-299. doi:10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 6.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 7.Clark BJ, Stocco DM. Expression of the steroidogenic acute regulatory (StAR) protein: a novel LH-induced mitochondrial protein required for the acute regulation of steroidogenesis in mouse Leydig tumor cells. Endocr Res. 1995;21(1–2):243–257. doi: 10.3109/07435809509030440. [DOI] [PubMed] [Google Scholar]
- 8.Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267(5205):1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 9.Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod. 2009;15(6):321–333. doi: 10.1093/molehr/gap025. doi:10.1093/molehr/gap025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh DK, Dunham WR, Sands RH, Menon KM. Regulation of cholesterol side-chain cleavage enzyme activity by gonadotropin in rat corpus luteum. Endocrinology. 1987;121(1):21–27. doi: 10.1210/endo-121-1-21. doi:10.1210/endo-121-1-21. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh DK, Peegel H, Dunham WR, Sands RH, Menon KM. Modulation of progesterone synthesis and cytochrome P450 levels in rat luteal cells during human chorionic gonadotropin-induced desensitized state. Endocrinology. 1988;123(1):514–522. doi: 10.1210/endo-123-1-514. doi:10.1210/endo-123-1-514. [DOI] [PubMed] [Google Scholar]
- 12.Yago N, Ichii S. Submitochondrial distribution of components of the steroid 11 beta-hydroxylase and cholesterol sidechain-cleaving enzyme systems in hog adrenal cortex. J Biochem. 1969;65(2):215–224. [PubMed] [Google Scholar]
- 13.Cheng B, Hsu DK, Kimura T. Utilization of intramitochondrial membrane cholesterol by cytochrome P-450-dependent cholesterol side-chain cleavage reaction in bovine adrenocortical mitochondria: steroidogenic and non-steroidogenic pools of cholesterol in the mitochondrial inner membranes. Mol Cell Endocrinol. 1985;40(2–3):233–243. doi: 10.1016/0303-7207(85)90179-0. [DOI] [PubMed] [Google Scholar]
- 14.Kimura T. ACTH stimulation on cholesterol side chain cleavage activity of adrenocortical mitochondria. Transfer of the stimulus from plasma membrane to mitochondria. Mol Cell Biochem. 1981;36(2):105–122. doi: 10.1007/BF02354909. [DOI] [PubMed] [Google Scholar]
- 15.Hickey GJ, Oonk RB, Hall PF, Richards JS. Aromatase cytochrome P450 and cholesterol side-chain cleavage cytochrome P450 in corpora lutea of pregnant rats: diverse regulation by peptide and steroid hormones. Endocrinology. 1989;125(3):1673–1682. doi: 10.1210/endo-125-3-1673. doi:10.1210/endo-125-3-1673. [DOI] [PubMed] [Google Scholar]
- 16.Davis JS. Mechanisms of hormone action: luteinizing hormone receptors and second-messenger pathways. Curr Opin Obstet Gynecol. 1994;6(3):254–261. [PubMed] [Google Scholar]
- 17.Hou X, Arvisais EW, Davis JS. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology. 2010;151(6):2846–2857. doi: 10.1210/en.2009-1032. doi:10.1210/en.2009-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mejia R, Waite C, Ascoli M. Activation of Gq/11 in the mouse corpus luteum is required for parturition. Molecular endocrinology. 2015;29(2):238–246. doi: 10.1210/me.2014-1324. doi:10.1210/me.2014-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munshi UM, Peegel H, Menon KM. Palmitoylation of the luteinizing hormone/human chorionic gonadotropin receptor regulates receptor interaction with the arrestin-mediated internalization pathway. Eur J Biochem. 2001;268(6):1631–1639. doi: 10.1046/j.1432-1327.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- 20.Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18(9):1351–1359. doi: 10.1016/j.cellsig.2006.02.011. doi:10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunzicker-Dunn ME, Lopez-Biladeau B, Law NC, Fiedler SE, Carr DW, Maizels ET. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc Natl Acad Sci U S A. 2012;109(44):E2979–2988. doi: 10.1073/pnas.1205661109. doi:10.1073/pnas.1205661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Maizels ET, DeManno D, St Clair E, Adam SA, Hunzicker-Dunn M. A stimulatory role of cyclic adenosine 3',5'-monophosphate in follicle-stimulating hormone-activated mitogen-activated protein kinase signaling pathway in rat ovarian granulosa cells. Endocrinology. 1996;137(3):967–974. doi: 10.1210/endo.137.3.8603610. doi:10.1210/endo.137.3.8603610. [DOI] [PubMed] [Google Scholar]
- 23.Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem. 2004;279(19):19431–19440. doi: 10.1074/jbc.M401235200. doi:10.1074/jbc.M401235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayampilly PP, Menon KM. Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology. 2007;148(8):3950–3957. doi: 10.1210/en.2007-0202. doi:10.1210/en.2007-0202. [DOI] [PubMed] [Google Scholar]
- 25.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126(Pt 8):1713–1719. doi: 10.1242/jcs.125773. doi:10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. doi:10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laplante M, Sabatini DM. mTOR Signaling. Cold Spring Harb Perspect Biol. 2012;4(2) doi: 10.1101/cshperspect.a011593. doi:10.1101/cshperspect.a011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. doi:10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. doi:10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6(11):1122–1128. doi: 10.1038/ncb1183. doi:10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 31.Gu L, Xie L, Zuo C, Ma Z, Zhang Y, Zhu Y, Gao J. Targeting mTOR/p70S6K/glycolysis signaling pathway restores glucocorticoid sensitivity to 4E-BP1 null Burkitt Lymphoma. BMC Cancer. 2015;15:529. doi: 10.1186/s12885-015-1535-z. doi:10.1186/s12885-015-1535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YJ, Hsiao PW, Lee MT, Mason JI, Ke FC, Hwang JJ. Interplay of PI3K and cAMP/PKA signaling, and rapamycin-hypersensitivity in TGFbeta1 enhancement of FSH-stimulated steroidogenesis in rat ovarian granulosa cells. J Endocrinol. 2007;192(2):405–419. doi: 10.1677/JOE-06-0076. doi:10.1677/JOE-06-0076. [DOI] [PubMed] [Google Scholar]
- 33.Nair AK, Peegel H, Menon KM. The role of luteinizing hormone/human chorionic gonadotropin receptor-specific mRNA binding protein in regulating receptor expression in human ovarian granulosa cells. J Clin Endocrinol Metab. 2006;91(6):2239–2243. doi: 10.1210/jc.2005-2739. doi:10.1210/jc.2005-2739. [DOI] [PubMed] [Google Scholar]
- 34.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001;Appendix 3:Appendix 3B. doi: 10.1002/0471142735.ima03bs21. doi:10.1002/0471142735.ima03bs21. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Nair AK, Menon KM. Ribonucleic acid binding protein-mediated regulation of luteinizing hormone receptor expression in granulosa cells: relationship to sterol metabolism. Molecular endocrinology. 2007;21(9):2233–2241. doi: 10.1210/me.2007-0102. doi:10.1210/me.2007-0102. [DOI] [PubMed] [Google Scholar]
- 36.Menon KM, Gunaga KP. Role of cyclic AMP in reproductive processes. Fertil Steril. 1974;25(8):732–750. doi: 10.1016/s0015-0282(16)40577-7. [DOI] [PubMed] [Google Scholar]
- 37.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. doi:10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 38.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8(1):55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 39.Cottom J, Salvador LM, Maizels ET, Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent P, Kawakatsu H, Ogata M, Hunzicker-Dunn M. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem. 2003;278(9):7167–7179. doi: 10.1074/jbc.M203901200. doi:10.1074/jbc.M203901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Molecular endocrinology. 2000;14(8):1283–1300. doi: 10.1210/mend.14.8.0500. doi:10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- 41.Palaniappan M, Menon KM. Human chorionic gonadotropin stimulates theca-interstitial cell proliferation and cell cycle regulatory proteins by a cAMP-dependent activation of AKT/mTORC1 signaling pathway. Molecular endocrinology. 2010;24(9):1782–1793. doi: 10.1210/me.2010-0044. doi:10.1210/me.2010-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palaniappan M, Menon KM. Luteinizing hormone/human chorionic gonadotropin-mediated activation of mTORC1 signaling is required for androgen synthesis by theca-interstitial cells. Molecular endocrinology. 2012;26(10):1732–1742. doi: 10.1210/me.2012-1106. doi:10.1210/me.2012-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S, Coco M, Greenstein SM, Schechner RS, Tellis VA, Glicklich DG. The effect of sirolimus on sex hormone levels of male renal transplant recipients. Clin Transplant. 2005;19(2):162–167. doi: 10.1111/j.1399-0012.2005.00257.x. doi:10.1111/j.1399-0012.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 44.Fritsche L, Budde K, Dragun D, Einecke G, Diekmann F, Neumayer HH. Testosterone concentrations and sirolimus in male renal transplant patients. Am J Transplant. 2004;4(1):130–131. doi: 10.1046/j.1600-6135.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 45.Boobes Y, Bernieh B, Saadi H, Raafat Al Hakim M, Abouchacra S. Gonadal dysfunction and infertility in kidney transplant patients receiving sirolimus. Int Urol Nephrol. 2010;42(2):493–498. doi: 10.1007/s11255-009-9644-8. doi:10.1007/s11255-009-9644-8. [DOI] [PubMed] [Google Scholar]