Abstract
Di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), a non-phthalate plasticizer, was introduced commercially in 2002 as an alternative to ortho-phthalate esters because of its favorable toxicological profile. However, the potential health effects from DINCH exposure remain largely unknown. We explored the associations between urinary concentrations of metabolites of DINCH on markers of ovarian response among women undergoing in vitro fertilization (IVF) treatments.
Between 2011 and 2015, 113 women enrolled a prospective cohort study at the Massachusetts General Hospital Fertility Center and provided up to two urine samples prior to oocyte retrieval. The urinary concentrations of two DINCH metabolites, cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH) and cyclohexane-1,2-dicarboxylic acid monocarboxyisooctyl ester (MCOCH), were quantified by isotope dilution tandem mass spectrometry. We used generalized linear mixed models to evaluate the association between urinary metabolite concentrations and markers of ovarian response, accounting for multiple IVF cycles per woman via random intercepts.
On average, women with detectable urinary MHiNCH concentrations, as compared to those below LOD, had a lower estradiol levels (−325 pmol/l, p=0.09) and number of retrieved oocytes (−1.8, p=0.08), with a stronger association among older women. However, urinary MHiNCH concentrations were unrelated to mature oocyte yield and endometrial wall thickness.
In conclusion, we found suggestive negative associations between urinary MHiNCH concentrations and peak estradiol levels and number of total oocyte yields. This is the first study evaluating the effect of DINCH exposure on human reproductive health and raises the need for further experimental and epidemiological studies to better understand the potential effects of this chemical on health.
Keywords: DINCH metabolites, epidemiology, markers of ovarian response, phthalates, reproductive health
Introduction
Phthalates are chemicals widely used in consumer goods and personal care products (CDC 2009). Because of their widespread use, their urinary metabolites are generally detected in the U.S., European, and Canadian general populations (Dewalque et al. 2014, Saravanabhavan et al. 2013, Schutze et al. 2014, Tefre de Renzy-Martin et al. 2014, Zota et al. 2014). Because of their potential adverse effects on human health (Braun et al. 2013, Dodge et al. 2015, Hauser et al. 2015, Messerlian et al. 2016, Miodovnik et al. 2014), the use of certain phthalates in diverse products have been banned (or banned from use in high amounts) in the European Union (EU 2005), Canada (Canada 2012), and in the U.S. by the Consumer Product Safety Commission (CPSC 2008). Di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), a non-phthalate plasticizer, was introduced commercially in 2002 as a safer alternative to ortho-phthalate esters because of a more favorable toxicological profile (EFSA 2006, SCENIHR 2015). The use of DINCH was approved by the European Food Safety Authority in 2006 (EFSA 2006).
DINCH is used in many polyvinyl chloride (PVC) products particularly in food packaging and in the manufacturing of toys, building materials and medical devices (EFSA 2006). Before 2002, cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH) and cyclohexane-1,2-dicarboxylic acid monocarboxyisooctyl ester (MCOCH), two oxidative DINCH metabolites, were not detected in urine collected from convenience samples of German and US adults (Schutze et al. 2014, Silva et al. 2013). However, MHiNCH was detected in 19.3% of urine samples from National Health and Nutrition Examination Survey (NHANES) 2011–2012 participants (CDC 2015) and in the urine of 98% of German business students during 2012 (Schutze et al. 2014). Moreover, two recent publications showed that MHiNCH was also detected in pooled urine samples collected from a convenience group of Australians (Gomez Ramos et al. 2016) and a group of 27–80 month old children in Germany (Fromme et al. 2016). Therefore, taken together, these findings show that there is ongoing exposure to the non-phthalate plasticizer DINCH; due to its increased use in various products, higher exposure is expected in the future.
DINCH was not considered an endocrine disruptor or reproductive toxicant (EFSA 2006). No evidence was found of developmental or reproductive toxicity in rats subchronically gavaged for 13 weeks up to the highest dose of 1 g/kg bw/day; similarly, thyroid hyperplasia and renal toxicity occurred only at high doses (1 g/kg bw/day in female rats and 300 mg/kg bw/day in male rats) (EFSA 2006). Similarly, a recent in vitro experimental study by Boisvert and colleagues has shown no consistent effect of DINCH on Leydig and spermatogonial mouse testicular cell lines (Boisvert et al. 2016). In addition, based on another recent in vitro study using rat preadipocytes, Campioli and coworkers also found no effect of DINCH itself, but they suggested that cyclohexane-1,2-dicarboxylic acid monoisononyl ester (MINCH), a minor metabolite of DINCH (Koch et al. 2013), is a potent peroxisome proliferator-activated receptor (PPAR)-α agonist and a metabolic disruptor capable of inducing stromal vascular fraction preadipocyte differentiation, which may interfere with the endocrine system (Campioli et al. 2015). Whether DINCH behaves as an endocrine disruptor and has toxicological effects on reproduction is unclear due to the scarce data on experimental animals (Boisvert et al. 2016, Campioli et al. 2015, Campioli and Papadopoulos 2016, Otter 2016). Moreover, there are no data on the potential human effects of DINCH on reproductive health.
We recently published that urinary concentrations of the metabolites of di-(2-ethylhexyl) phthalate (DEHP), the most common PVC phthalate plasticizer (Hauser and Calafat 2005) were negatively associated with oocyte yield, clinical pregnancy and live birth among women following in vitro fertilization (IVF) treatments in an academic fertility clinic in Boston, MA (Hauser et al. 2015). In the current study, we explored the associations between urinary concentrations of DINCH metabolites on IVF outcomes in a subsample of women from the same study cohort with urinary concentrations of DINCH measured.
Methods
Study population
Study participants were women enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort established to evaluate environmental and dietary determinants of fertility (Hauser et al. 2006). Women between 18 and 45 years old were eligible to participate and approximately 60% of those contacted by the research nurses enrolled. Although DINCH was introduced commercially in 2002 and participants were recruited into this study beginning in 2004, analytical methods for measurement of urine DINCH metabolites were developed several years later (CDC 2015) and therefore, the measurement of DINCH metabolites in urine did not begin until June 2011, when these chemicals were added to the study protocol. The current analysis includes 113 women who completed at least one IVF cycle between June 2011 and June 2015 (n=151 cycles) at the Massachusetts General Hospital (MGH) Fertility Center, and had provided at least one urine sample for the measurement of DINCH metabolites per IVF cycle. We excluded from this analysis IVF cycles for which women used an egg donor (n=1) and cryo-thaw cycles (n=29). The study was approved by the Human Studies Institutional Review Boards of the MGH, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC). Participants signed an informed consent after the study procedures were explained by a trained research study staff and all questions were answered.
Urine sample collection and DINCH metabolite measurements
Women provided up to two spot urine samples per IVF cycle. The first specimen (not necessarily a fasting sample) was collected between Day 3 and Day 9 of the stimulation phase; the second, always a fasting sample, was collected on the day of oocyte retrieval, prior to the procedure or administration of IV fluids. Urine was collected in a sterile, clean polypropylene specimen cup at the MGH Fertility Center. Specific gravity (SG) was used to correct DINCH metabolites concentrations for urine dilution. SG was measured at room temperature and within several hours (typically within one hour) of the urine being produced using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) calibrated with deionized water before each measurement. The urine was then divided into aliquots, frozen at −20°C, and then stored long-term at −80 °C. Samples were shipped on dry ice overnight to the CDC where they were stored at or below −40 °C until analysis. The urinary concentrations of MHiNCH and MCOCH were measured using online solid-phase extraction (SPE) coupled with isotope dilution- high-performance liquid chromatography (HPLC)- tandem mass spectrometry (MS/MS) as described before (Silva et al. 2013). Briefly, urine (0.1 mL) was spiked with an internal standard solution of stable-isotope labeled standards of the target metabolites and a buffered β-glucuronidase solution (E. coli-K12; 25 μL, pH 6.5/1 M), and incubated for a minimum of 120 min. After injection (450 μL), the target analytes in the spiked urine were extracted using online SPE (Chromolith high resolution RP-18e guard column, 4.6 mm × 5 mm, Merck KGaA, Germany), chromatographically resolved by HPLC (Betasil phenyl HPLC column, 3 μm, 150 mm × 2.1 mm, ThermoHypersil-Keystone, Bellefonte, PA, USA) using a nonlinear solvent gradient from 100% mobile phase A (0.1% water) to 100% mobile phase B (0.1% acetic acid in acetonitrile) at a flow rate of 0.35 mL/minute, and detected using negative ion electrospray-ionization MS/MS (Thermo Scientific TSQ Vantage Triple Quadrupole Mass Spectrometer, ThermoFinnigan, Bellefonte, PA, USA). The limit of detection (LOD) were 0.4 μg/L (MHiNCH) and 0.5 μg/L (MCOCH). In addition to study samples, each analytical run included low-concentration and high-concentration quality control urine pools and reagent blanks to assure the accuracy and reliability of the data (Silva et al. 2013). DINCH metabolite concentrations were adjusted for dilution using the following formula: Pc = P[(1.015 − 1)/SG − 1], where Pc is the SG-corrected DINCH metabolite concentration (μg/L), P is the measured DINCH metabolite concentration (μg/L) of the urine sample, and 1.015 is the mean SG concentration in the study population (Smith et al. 2012). The geometric mean of the SG-adjusted DINCH metabolite concentrations from two spot urine samples collected during each IVF cycle was used as a measure of cycle-specific urinary DINCH metabolites concentration. For cycles with only one urine sample (~19%), the DINCH metabolite concentration for that single urine sample was used as the cycle-specific urinary concentration.
Clinical management and assessment of outcomes
Clinical information was abstracted from the patient’s electronic medical record by trained research staff. As part of the infertility work-up all patients had blood collected on the third day of the menstrual cycle. The serum concentrations of follicle stimulating hormone (FSH) and estradiol (E2) were measured using an automated electrochemiluminescence immunoassay at the MGH Core Laboratory, as previously described (Mok-Lin et al. 2010). E2 levels, defined as the highest level of E2 prior to oocyte retrieval, was obtained on the day of trigger with human Chorionic Gonadotropin (hCG). Subsequent to an infertility evaluation, each patient was assigned an infertility diagnosis by a physician at the MGH Fertility Center according to the Society for Assisted Reproductive Technology (SART) definitions as previously described (Mok-Lin et al. 2010). The participant’s date of birth was collected at entry, and weight and height were measured by trained study staff. Body mass index (BMI) was calculated as weight (in kilograms) per height (in meters) squared.
Women underwent one of three controlled ovarian stimulation IVF treatment protocols after completing a cycle of oral contraceptives: 1) luteal phase GnRH-agonist protocol, 2) follicular phase GnRH-agonist/Flare protocol, or 3) GnRH-antagonist protocol. FSH/hMG and GnRH-agonist or GnRH-antagonist was continued the night before of the day of trigger with hCG. Follicular response was monitored with serial ultrasounds and estradiol levels. The latter were determined at the MGH Core Laboratory using the Elecsys Estradiol II reagent kit (Roche Diagnostics). hCG was used to trigger oocyte maturation 36 hours before the scheduled oocyte retrieval and was administered when at least 3 follicles 16–18 mm in diameter were detected by transvaginal ultrasonography and when the peak E2 level reached at least 800 pg/mL. Patients were monitored during gonadotropin stimulation for serum E2, follicle size measurements and counts, and endometrial thickness through 2 days before egg retrieval. Details of egg retrieval have been previously described (Mok-Lin et al. 2010). Embryologists determined the total number of oocytes retrieved per cycle and classified them as germinal vesicle, metaphase I, metaphase II (MII) or degenerated.
Statistical analysis
Demographic and baseline reproductive characteristics of the women were presented using median ± interquartile ranges (IQRs) or percentages. Women’s exposure to DINCH was categorized into 2 groups of urinary MHiNCH concentrations (below and above LOD). MCOCH was not considered for analysis because of the low percentage of detectable concentrations (9%). Associations of urinary MHiNCH concentrations with demographics and baseline reproductive characteristics were evaluated using Kruskal–Wallis tests for continuous variables and chi-squared tests for categorical variables (or Fisher’s exact test where appropriate). An intraclass correlation coefficient (ICC) was calculated to explore variability of urinary MHiNCH concentrations within woman. Multivariable generalized linear mixed models were used to evaluate the association between urinary MHiNCH concentrations and markers of ovarian response, accounting for multiple IVF cycles per woman using random intercepts. A Poisson distribution and log link function were specified for oocyte counts, and a normal distribution and identity link function were specified for endometrial wall thickness and E2 trigger levels. To allow for better interpretation of the results, population marginal means (Searle et al. 1980) were presented adjusting for all the covariates in the model, at the mean levels of all covariates (including categorical measures).
Confounding was assessed using prior knowledge on biological relevance through descriptive statistics from our study population. The variables considered as potential confounders included factors previously related to ovarian outcomes in this and other studies, and factors associated with DINCH exposure and ovarian outcomes in this study, regardless of whether they had been previously described as predictors of ovarian outcomes (Table 1). Final models were adjusted for age (continuous), year of treatment (continuous), and infertility diagnosis (male factor, female factor, unexplained). Endometrial wall thickness, total and mature oocyte yields models were further adjusted by Day3 FSH levels (continuous). All tests were two-tailed and the level of statistical significance was set at 0.05. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
Table 1.
Baseline characteristicsa of 113 women in the EARTH by urinary MHiNCH concentrations (μg/L).
| Baseline characteristics | Total cohort n=113 |
Urinary MHiNCH concentrations (μg/L) | ||
|---|---|---|---|---|
| <LOD (n=80) | >LOD (n=33) | P,valueb | ||
| Age, years | 35.0 (31.0, 38.0) | 33.5 (31.0, 37.0) | 37.0 (33.0, 39.0) | 0.03 |
| Race/Ethnic group, n (%) | 0.44 | |||
| White/Caucasian | 90 (79.7) | 62 (77.5) | 28 (84.8) | |
| Black | 3 (2.7) | 3 (3.8) | 0 (0) | |
| Asian | 15 (13.3) | 10 (12.5) | 5 (15.2) | |
| Other | 5 (4.3) | 5 (6.2) | 0 (0) | |
| Body Mass Index, kg/m2 | 23.2 (21.1, 26.3) | 23.1 (21.0, 25.8) | 24.6 (21.4, 27.3) | 0.24 |
| Ever smoker, n (%) | 25 (22.1) | 17 (21.3) | 8 (24.2) | 0.80 |
| Education, nc (%) | 0.53 | |||
| < College graduate | 8 (7.1) | 6 (8.6) | 2 (21.4) | |
| College graduate | 30 (30.6) | 24 (34.3) | 6 (21.4) | |
| Graduate degree | 59 (60.2) | 39 (55.7) | 20 (71.4) | |
| Baseline Reproductive characteristics | ||||
| Initial infertility diagnosis, n (%) | 0.96 | |||
| Male factor | 33 (29.2) | 24 (30.0) | 9 (27.3) | |
| Female factor | 36 (31.9) | 24 (30.0) | 12 (36.4) | |
| Diminished Ovarian Reserve | 4 (3.5) | 3 (3.8) | 1 (3.0) | |
| Endometriosis | 8 (7.1) | 5 (6.2) | 3 (9.2) | |
| Ovulation Disorders | 10 (8.9) | 6 (7.5) | 4 (12.1) | |
| Tubal | 14 (12.4) | 10 (12.5) | 4 (12.1) | |
| Uterine | 0 (0) | 0 (0) | 0 (0) | |
| Unexplained | 44 (38.9) | 32 (40.0) | 12 (36.4) | |
| Initial treatment protocol, n (%) | 0.14 | |||
| Antagonist | 17 (15.0) | 10 (12.5) | 7 (21.2) | |
| Flared | 13 (11.5) | 7 (8.9) | 6 (18.2) | |
| Luteal phase agoniste | 83 (73.5) | 63 (78.8) | 20 (60.6) | |
Values are presented as median (IQR) unless otherwise noted.
From Kruskal-Wallis test for continuous variables and chi-squared tests (or Fisher’s exact test where appropriate) for categorical variables.
This variable has missing data.
Follicular phase GnRH-agonist/Flare protocol.
Luteal phase GnRH-agonist protocol.
Results
Most of the 113 women were Caucasian (80%), had a college degree (60%) and had never smoked (78%) (Table 1). Participants had a median (IQR) age and BMI of 35 years (31, 38) and 23.2 kg/m2 (21.1, 26.3), respectively. Unexplained infertility (39%) was the primary SART diagnosis at enrollment and luteal phase GnRH-agonist was the most used initial treatment protocol (74%). Women were older across increasing urinary MHiNCH concentrations (p value=0.03), but no other covariates differed across urinary DINCH metabolite concentrations (Table 1).
The SG-adjusted GM and median MHiNCH urinary concentration for the 274 samples provided by the 113 women (contributing 151 IVF cycles) was <LOD (0.40 μg/L), with a 29% detection rate (Table 2). The SG-adjusted GM and median MCOCH urinary concentrations was also <LOD (0.50 μg/L), and 9% of them had detectable concentrations. Two urine samples were collected in 81% (123/151 for MHiNCH) of the IVF cycles. The intraclass correlation coefficient (ICC) (95% CI) was 0.22 (0.12, 0.38), which demonstrates high within woman variability.
Table 2.
Distribution of cycle-specific urinary concentrations of DINCH metabolites among 113 women in the EARTH undergoing 151 IVF cycles.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Detection frequency | N Urines | 50th | Percentile | ||||
| 75th | 90th | 95th | Max | ||||
| MHiNCH | 29 | 274 | <LOD | 0.47 | 0.80 | 1.30 | 67.2 |
| SG-adj | <LOD | 0.44 | 0.68 | 1.12 | 42.8 | ||
| MHiNCH | |||||||
| MCOCHa | 9 | 167 | <LOD | <LOD | <LOD | 0.72 | 25.1 |
| SG-adj | <LOD | <LOD | <LOD | 0.98 | 16.0 | ||
| MCOCH | |||||||
MHiNCH LOD=0.40 μg/L and MCOCH LOD=0.50 μg/L. All concentrations below LOD were assigned a value equal to the LOD divided by √2.
69 women contributing to 99 IVF cycles with data for MCOCH since this metabolite was added to the protocol later than MHiNCH.
In unadjusted and adjusted models, there were negative associations between SG-adjusted urinary MHiNCH concentrations and total oocyte yields and peak E2 levels (Table 3). Although these relationships were of borderline statistical significance, in adjusted models, women with detectable urinary MHiNCH concentrations (≥0.40 μg/L) had a mean decrease of 1.8 total oocytes (p, trend= 0.08) and a mean decrease of 325 pmol/l E2 levels (p, trend=0.09), respectively, compared with women in non-detectable urinary MHiNCH concentrations (<0.40 μg/L). However, SG-adjusted urinary MHiNCH concentrations were unrelated to other markers of ovarian reserve such as mature oocyte yields and endometrial wall thickness (Table 3).
Table 3.
Specific gravity adjusted cycle-specific urinary MHiNCH concentrations in relation to markers of ovarian responsea among 113 women contributing to 151 fresh IVF cycles in the EARTH.
| Urinary MHiNCH concentrations (μg/L) | |||
|---|---|---|---|
| <LOD (n=110) | >LOD (n=50) | P,value | |
| Total Oocyte Yield, n | |||
| Unadjusted | 12.9 (11.7, 14.2) | 11.4 (9.9, 13.1) | 0.13 |
| Adjusteda | 12.9 (11.7, 14.2) | 11.1 (9.7, 12.8) | 0.08 |
| MII Oocyte Yield, n | |||
| Unadjusted | 10.3 (9.3, 11.3) | 9.4 (8.1, 10.8) | 0.28 |
| Adjusteda | 10.2 (9.2, 11.2) | 9.2 (8.0, 10.7) | 0.25 |
| Endometrial wall thickness, mm | |||
| Unadjusted | 10.4 (9.9, 11.0) | 10.6 (9.8, 11.3) | 0.75 |
| Adjusteda | 10.5 (10.0, 11.0) | 10.5 (9.7, 11.3) | 0.99 |
| Peak E2 levels, pmol/l | |||
| Unadjusted | 2344 (2109, 2580) | 2038 (1709, 2367) | 0.09 |
| Adjusteda | 2342 (2102, 2582) | 2017 (1681, 2354) | 0.09 |
Data are presented as predicted marginal means (95% CI) adjusted for age (continuous), year of treatment (continuous), and infertility diagnosis (male, female and unexplained). Endometrial wall thickness, total and mature oocyte yields models were further adjusted by Day3 FSH levels (continuous). LOD for MHiNCH is 0.40 μg/L.
We also explored whether age modified the associations between urinary concentrations and markers of ovarian reserve. The association between SG-adjusted urinary MHiNCH concentrations and total oocyte yields was stronger among women who were ≥37 years old as compared to younger women (Figure 1); however, the p-value for interaction was borderline (p-interactions=0.13). Women who were ≥37 years old and and had SG-adjusted urinary MHiNCH concentrations above LOD, on average, had 2.9 fewer total oocytes (p, value=0.05) compared women who were <37 years old and had SG-adjusted urinary MHiNCH concentrations below LOD. This comparable difference in younger women was however, 0.4 fewer total oocytes (p, value= 0.80) (Figure 1).
Fig 1. Age modification on the association between urinary MHiNCH concentrations and total oocyte yield among 113 women contributing to 151 fresh IVF cycles in the EARTH.
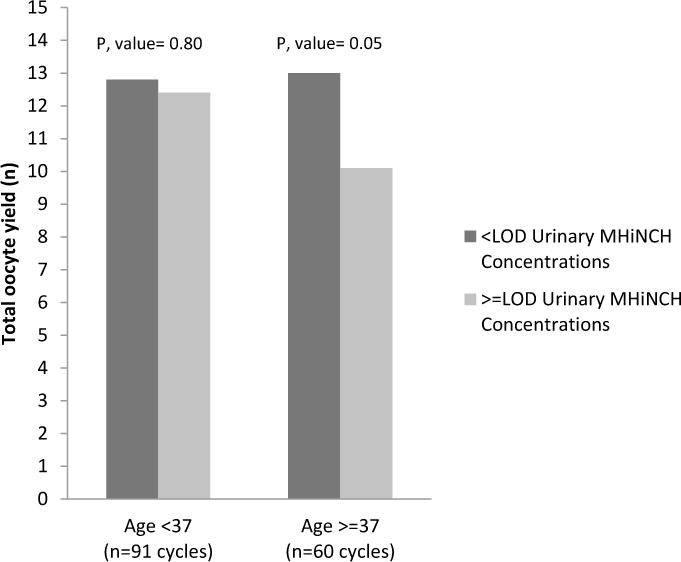
Models are adjusted for age (continuous), year of treatment (continuous), Day3 FSH levels (continuous), and infertility diagnosis (male, female and unexplained). P, interaction=0.13. LOD for MHiNCH is 0.40 μg/L.
Finally, because DINCH has been used as a substitute for DEHP in certain applications, we explored correlations between urinary DINCH and DEHP metabolites and also further adjusted our models for urinary concentrations of DEHP metabolites. We previously reported that urinary DEHP concentrations were negatively associated with oocyte yield (Hauser et al. 2015) and therefore, the negative association between urinary MHiNCH concentrations and total oocyte yield could be partially driven by DEHP exposure. As expected, urinary MHiNCH and DEHP metabolite concentrations were moderately correlated (r=0.65, p=0.03). However, in models additionally adjusted for sum of DEHP metabolites, results for the association of MHiNCH and oocyte yield did not change (data not shown).
Discussion
As far as we are aware, this is the first study exploring the association of DINCH metabolites with human reproductive health. We found suggestive associations of urinary MHiNCH concentrations with markers of ovarian response including the number of total oocyte and E2 levels collected and measured at retrieval; however, MHiNCH was unrelated to other markers of ovarian response. The negative association between urinary MHiNCH concentrations and total oocyte yield was stronger in older women (≥37 years old) compared with younger women (<37 years old). This interesting finding could be explained by the important role of age in predicting infertility. Age has been considered the main predictor of infertility in women trying to become pregnant; the percentage of women experiencing infertility increases markedly with age, from 7%–9% among those aged 15–34 years to 25% among those aged 35–39 years and 30% among those aged 40–44 years (CDC 2014). As women age, the risk of infertility rises because of diminished oocyte quality and ovulatory function. The stronger association between higher urinary MHiNCH concentrations with lower total oocyte yield among older women may represent age as a marker of increased sensitivity to DINCH exposure. Interestingly, we also found that older women had higher urinary MHiNCH concentrations compared with younger women. It is unclear why they had higher urinary concentrations and this is worthy of further investigation in other cohors.
It is difficult to interpret the potential pathways and mechanisms through which DINCH may impact oocyte yield given the dearth of in vitro studies and studies in experimental animals on this chemical and these outcomes. In a recent experimental study, Boisvert and coworkers showed differential in vitro effects of DINCH on Leydig and spermatogonial mouse testicular cell lines(Boisvert et al. 2016). While high concentrations of DINCH (10−4 M) were found to decrease Leydig cell steroid production, lower DINCH concentrations (10−8–10−5 M) had a stimulatory effect on the production of these cells. Also, no consistent effects were found on Leydig cell viability, proliferation, and mitochondrial integrity, and spermatogonial cell viability and proliferation. Campioli and colleagues reported that DINCH itself had no toxicological effect; however, MINCH, a minor DINCH metabolite, promoted differentiation in a primary culture model of rat preadipocytes, which is mediated by PPAR-α in its first stage. Therefore, if MINCH acts as a potential PPAR-α agonist and is able to alter hormone signaling, it may be considered a metabolic disruptor interfering with the endocrine system (Campioli et al. 2015). However, they did not study MHiNCH and it is unknown if this metabolite has similar activities as MINCH. The woman in our study with the highest urinary MHiNCH and MCOCH concentrations (SG-adjusted=42.8 and 16.0 μg/L, respectively) also had a reduced number of total (4) and mature (3) retrieved oocytes. However, it is difficult to make conclusions based on a single participant.
The MHiNCH median in our study population was below the LOD of 0.4 μg/L, in agreement with data from NHANES 2011–2012 adult participants (CDC 2015), as well as other North American (Silva et al. 2013) and German (Schutze et al. 2014) populations. It is important to note that only in samples collected in 2012 among the Germans, the MHiNCH median was above the LOD (Schutze et al. 2014). Interestingly, frequency of detection of MHiNCH among convenience groups of adults changed from 0% to 19% in the U.S. (Silva et al. 2013) and from 0% to 98 % in Germany (Schutze et al. 2014) over a 12–13 year period (1999–2000 to 2012). The increased frequency of detection of MHiNCH in these studies is also consistent with a 21% increase observed in our study population between 2011 and 2015. Therefore, higher MHiNCH detection frequencies and urinary concentrations are expected in the future as DINCH becomes more widely used.
The present study has several limitations including small sample size. Due to its design, it may not be possible to generalize findings to couples conceiving without medical intervention. However, these findings may be applicable to other women seeking infertility treatment (Stephen and Chandra 2000). Also, misclassification of DINCH exposure based on urinary concentrations of MHiNCH from spot samples is possible because DINCH metabolites have relatively short elimination half-lives (Koch et al. 2013) and exposures to DINCH are likely to be episodic in nature. However, we collected multiple urine samples per participant and this will reduce exposure misclassification that would potentially lead to attenuated associations. Lastly, only 29% of urine samples had detectable MHiNCH concentrations suggesting limited exposure to DINCH in our population. Strengths of our study include its prospective design which minimizes the possibility of reverse causation and the comprehensive adjustment for possible confounding variables.
In conclusion, we found suggestive negative associations between urinary MHiNCH concentrations and peak E2 levels and number of total oocyte yields, with stronger associations among older women. Because this is the first study evaluating the effect of DINCH exposure on human reproductive health, further experimental and epidemiological studies will be useful to better understand the effects of this chemical on health.
Highlights.
Women with detectable urinary MHiNCH concentrations, as compared to those below LOD, had a lower estradiol levels and number of retrieved oocytes
The negative association between urinary MHiNCH concentrations and total oocyte yield was stronger in older women (≥37 years old) compared with younger women (<37 years old).
Urinary MHiNCH concentrations were unrelated to mature oocyte yield and endometrial wall thickness.
Acknowledgments
The authors gratefully acknowledge Manori Silva, James Preau, Ella Samandar, and Tao Jia of CDC for their technical assistance with DINCH metabolite measurements. We also acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research nurses Jennifer Ford and Myra G. Keller, research staff Ramace Dadd and Patricia Morey, physicians and staff at Massachusetts General Hospital fertility center and a special thanks to all the study participants.
Study Funding
NIH grants R01ES022955, R01ES009718, and R01ES000002 from the National Institute of Environmental Health Sciences (NIEHS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s Contribution
R.H. was involved in study concept and design, and critical revision for important intellectual content of the manuscript. P.L.W was involved in study concept and design, and critical revision for important intellectual content of the manuscript and provided statistical expertise. L.M.A analyzed data, drafted the manuscript and had a primary responsibility for final content; L.M.A, I.S, P.L.W. and R.H. interpreted the data; Y-H.C. reviewed the statistical analysis; I.S, J.B.F, X.Y and A.M.C. were involved in acquisition of the data. All authors were involved in the critical revision of the manuscript and approved the final manuscript.
Conflict Of Interest
None of the authors has any conflicts of interest to declare. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References
- Boisvert A, Jones S, Issop L, Erythropel HC, Papadopoulos V, Culty M. In vitro functional screening as a means to identify new plasticizers devoid of reproductive toxicity. Environmental research. 2016;150:496–512. doi: 10.1016/j.envres.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children’s health. Current opinion in pediatrics. 2013;25:247–254. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioli E, Duong TB, Deschamps F, Papadopoulos V. Cyclohexane-1,2-dicarboxylic acid diisononyl ester and metabolite effects on rat epididymal stromal vascular fraction differentiation of adipose tissue. Environmental research. 2015;140:145–156. doi: 10.1016/j.envres.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Campioli E, Papadopoulos V. Cyclohexane-1,2-dicarboxylic acid diisononyl ester and metabolite effects on rat epididymal stromal vascular fraction differentiation of adipose tissue (2015) Environmental Research 140: 145–156 Reply to the letter by Otter R. Environmental research. 2016;144:167–169. doi: 10.1016/j.envres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Canada H. Industry guide to health Canada’s safety requirements for children’s toys and related products, 2012. Ottawa, Ontario: Health Canada; 2012. Available from: http://www.hc-sc.gc.ca/cps-spc/alt_formats/pdf/pubs/indust/toys-jouets/toys-jouets-eng.pdf [accessed 2016 2 Feb] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National Public Health Action Plan for the Detection, Prevention, and Management of Infertility. 2014 Available from: http://www.cdc.gov/reproductivehealth/infertility/pdf/drh_nap_final_508.pdf [accessed 1 August 2016]
- Centers for Disease Control and Prevention (CDC) Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; National Center for Environmental Health; Atlanta, GA: 2009. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables (February, 2015) Centers for Disease Control and Prevention; National Center for Environmental Health; Atlanta, GA: 2015. http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf [accessed 2016 2 Feb] [Google Scholar]
- Consumer Product Safety Improvement Act of 2008. Washington, DC: Consumer Product Safety Commission (CPSC); 2008. Available from: http://www.cpsc.gov/en/Regulations-Laws-Standards/Statutes/The-Consumer-Product-Safety-Improvement-Act [accessed 2016 2 Feb] [Google Scholar]
- Dewalque L, Pirard C, Charlier C. Measurement of urinary biomarkers of parabens, benzophenone-3, and phthalates in a Belgian population. BioMed research international. 2014;2014:649314. doi: 10.1155/2014/649314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge LE, Williams PL, Williams MA, Missmer SA, Souter I, Calafat AM, Hauser R. Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment. Reproductive toxicology. 2015;58:184–193. doi: 10.1016/j.reprotox.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 12th list of substances for food contact materials. EFSA J. 2006:395–401. 1–21. [Google Scholar]
- EU. Directive 2005/84/EC of the European Parliament and of the Council of 14 December 2005 amending for the 22nd time Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations (phthalates in toys and childcare articles) Council, European Parliament. 2005 Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005L0084&from=EN [accessed 2016 2 Feb]
- Fromme H, Schutze A, Lahrz T, Kraft M, Fembacher L, Siewering S, Burkardt R, Dietrich S, Koch HM, Volkel W. Non-phthalate plasticizers in German daycare centers and human biomonitoring of DINCH metabolites in children attending the centers (LUPE 3) International journal of hygiene and environmental health. 2016;219:33–39. doi: 10.1016/j.ijheh.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Gomez Ramos MJ, Heffernan AL, Toms LM, Calafat AM, Ye X, Hobson P, Broomhall S, Mueller JF. Concentrations of phthalates and DINCH metabolites in pooled urine from Queensland, Australia. Environment international. 2016;88:179–186. doi: 10.1016/j.envint.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occupational and environmental medicine. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, Meeker JD, Calafat AM, Williams PL. Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing Fertilization: Results from the EARTH Study. Environmental health perspectives. 2015 doi: 10.1289/ehp.1509760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Koch HM, Schutze A, Palmke C, Angerer J, Bruning T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH((R))) in humans after single oral doses. Archives of toxicology. 2013;87:799–806. doi: 10.1007/s00204-012-0990-4. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Souter I, Gaskins AJ, Williams PL, Ford JB, Chiu YH, Calafat AM, Hauser R. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Human reproduction (Oxford, England) 2016;31:75–83. doi: 10.1093/humrep/dev292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Edwards A, Bellinger DC, Hauser R. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology. 2014;41:112–122. doi: 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. International journal of andrology. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter R. The publication “Cyclohexane-1,2-dicarboxylic acid diisononyl ester and metabolite effects on rat epididymal stromal vascular fraction differentiation of adipose tissue” by Enrico Campioli, Tam B. Duong, Francois Deschamps, Vassilios Papadopoulos, Environmental Research 140 (2015), 145–156, merits some critical comments. Environmental research. 2016;144:165–166. doi: 10.1016/j.envres.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Saravanabhavan G, Guay M, Langlois E, Giroux S, Murray J, Haines D. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian Health Measures Survey (2007–2009) International journal of hygiene and environmental health. 2013;216:652–661. doi: 10.1016/j.ijheh.2012.12.009. [DOI] [PubMed] [Google Scholar]
- SCENIHR. Opinion on: The safety of medical devices containing DEHP-plasticized PVC or other plasticizers on neonates and other groups possibly at risk (2015 update) 2015 doi: 10.1016/j.yrtph.2016.01.013. Available from: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_047.pdf [accessed 2016 2 Feb] [DOI] [PubMed]
- Schutze A, Kolossa-Gehring M, Apel P, Bruning T, Koch HM. Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll(R) DINCH(R) in 24 h urine samples from the German Environmental Specimen Bank. International journal of hygiene and environmental health. 2014;217:421–426. doi: 10.1016/j.ijheh.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34:216–221. [Google Scholar]
- Silva MJ, Jia T, Samandar E, Preau JL, Jr, Calafat AM. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000–2012) Environmental research. 2013;126:159–163. doi: 10.1016/j.envres.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, Ye X, Ford J, Keller M, Meeker JD, et al. Predictors and Variability of Urinary Paraben Concentrations in Men and Women, Including before and during Pregnancy. Environmental health perspectives. 2012;120:1538–1543. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen EH, Chandra A. Use of infertility services in the United States: 1995. Fam Plann Perspect. 2000;32:132–137. [PubMed] [Google Scholar]
- Tefre de Renzy-Martin K, Frederiksen H, Christensen JS, Boye Kyhl H, Andersson AM, Husby S, Barington T, Main KM, Jensen TK. Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction. 2014;147:443–453. doi: 10.1530/REP-13-0461. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental health perspectives. 2014;122:235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]


