Abstract
Human papillomavirus (HPV) infection is the causative agent in cervical cancer, and is associated with numerous other genital cancers, including vulvar, vaginal, and anal cancer. Primary prevention with HPV vaccination is safe and efficacious, and a recently approved HPV vaccine will provide even more extensive protection against several oncogenic HPV strains. Screening strategies for HPV are rapidly evolving, reflecting the essential role that HPV infection plays in cervical cancer. This article highlights new evidence regarding the efficacy of the recently approved 9-valent HPV (9vHPV) vaccine and the use of primary high-risk HPV testing in cervical cancer screening. We consider the utility of urinary HPV testing in routine clinical practice and review current guidelines regarding anal HPV screening.
KEY WORDS: HPV, vaccination, screening, cervical cancer, prevention
INTRODUCTION
In the United States, approximately 33,000 cases of human papilloma virus (HPV)-associated cancers are diagnosed annually.1 Oncogenic HPV strains are detected in almost all cases of cervical cancer and in the majority of vulvar, vaginal, and anal cancers.2 Vaccination against high-risk HPV (hrHPV) subtypes is safe and effective, and is recommended routinely for females and males starting at age 11 or 12 years.3 Secondary prevention through cervical cancer screening and follow-up of detected abnormalities has reduced the incidence of cervical cancer in the United States to fewer than 13,000 cases annually.4 However, there have been many new developments in recent years in both primary and secondary prevention of cervical cancer. Three vaccines are now approved for prevention of infection with hrHPV, and new guidelines endorse screening for HPV infection as well as cytologic abnormalities. Clinicians may struggle to incorporate all of this new evidence into practice, wondering which vaccine to choose, and how and with what frequency they should screen for HPV. In this narrative review article, we discuss the updates in HPV vaccination and screening that are most relevant to the general internist in their everyday clinical care.
CASE (cont.)
Ms. Jones is a 26-year-old nursing student presenting for routine primary care. She has not seen a health care provider in several years. She has no medical problems and does not take any medications. She is sexually active with one male partner and has an IUD in place for contraception. She exercises regularly, eats a well-balanced diet, and has received her tetanus and pertussis vaccine. She has been reading about a new HPV vaccine and wonders whether it is an appropriate option for her.
Vaccine Types
The bivalent HPV vaccine targets subtypes 16 and 18, which cause 70 % of cervical cancers; the quadrivalent vaccine (4vHPV) extends this coverage by additionally targeting subtypes 6 and 11, which cause 90 % of genital warts. Both vaccines are highly effective in preventing cervical dysplasia.5,6 Countries that have achieved high coverage with the 4vHPV vaccine have seen dramatic reductions in genital warts and infection with HPV 16 and 18.7,8
The Advisory Committee on Immunization Practices (ACIP) recommends routine HPV vaccination starting at age 11 or 12 years, though the series can be started as early as 9 years of age. Vaccination is also recommended for females ages 13 through 26 years and for males ages 13 through 21 years who have not completed the three-dose series. Men up to age 26 should also be vaccinated if they have sex with men or are immunocompromised.3
Despite the proven effectiveness of the HPV vaccines in preventing cervical cancer, most girls in the United States, including our patient, have not been fully immunized. According to data from the 2014 National Immunization Survey, only 39.7 % of girls ages 13–17 in the United States had completed the recommended three-dose HPV vaccination series; 60 % had received at least one dose. Although HPV vaccination coverage among females appears to be slowly increasing, it still lags behind the tetanus, diphtheria, pertussis (Tdap), and meningococcal vaccines, indicating missed opportunities for administering HPV vaccine at visits when these other vaccines are given.9
A new 9-valent HPV vaccine (9vHPV) was approved by the FDA in December 2014 for females ages 9–26 and males ages 9–15. In addition to the four HPV subtypes (6, 11, 16, and 18) found in the quadrivalent vaccine (i.e. the subtypes that cause 70 % of cervical cancer and 90 % of genital warts), it includes five additional oncogenic HPV subtypes (31, 33, 45, 52, and 58), which cause an additional 15 % of cervical cancer.
Joura et al. describe the results of a randomized trial that compared the efficacy of the 9vHPV and quadrivalent vaccines among 14,000 women ages 16–26 years who were followed for 4.5 years.10 Women received either the quadrivalent or 9vHPV vaccine, each given in the same three-dose series (day 1, repeated in 2 and 6 months); there was no placebo control arm. The investigators performed a “per-protocol” analysis, which included only those participants who were negative at study entry for all five of the additional HPV subtypes included in the 9vHPV and who received all three doses of their assigned vaccine. Among the women in this group, the new vaccine was 96.7 % effective for preventing significant dysplasia (cervical intraepithelial neoplasia 2 or higher) caused by those five subtypes. In contrast, in the intention-to-treat analysis, which evaluated the effectiveness of the vaccine in all study women regardless of their baseline HPV status, the efficacy of the 9vHPV vaccine was equivalent to that of the quadrivalent vaccine (14 cases of dysplasia per 1000 women). Thus, the 9vHPV vaccine was shown to extend the coverage to additional oncogenic subtypes, but only among women who were naïve to the additional subtypes at the time of vaccine administration. The safety profile of the 9vHPV resembles that of the quadrivalent vaccine, with the exception of greater redness and swelling at the injection site with the newer vaccine.11
Should we offer the 9vHPV vaccine to our patient instead of the quadrivalent vaccine? Does the fact that she is already sexually active affect our recommendation? For our patient, if both the 4vHPV and 9vHPV vaccines were equally priced and available, we would recommend using the 9vHPV vaccine and starting the immunizations immediately. If there were any barriers to getting the 9vHPV today, we would administer the quadrivalent vaccine today. A cost-effectiveness analysis revealed that using the 9vHPV in both males and females was cost-saving when compared with the quadrivalent vaccine for both sexes, assuming that the 9vHPV cost only $13 more per dose than the quadrivalent vaccine.12
However, the cost of the 9vHPV vaccine as of February 2016 was $17–26 more per dose than the quadrivalent vaccine.13
In March 2015, the ACIP updated their guidelines to allow substitution of the 9vHPV vaccine for the quadrivalent vaccine.11 These recommendations indicate that providers may use the 9-strain vaccine to complete any series for male or female patients who have received one or two doses of the earlier vaccines. However, administration of the 9vHPV vaccine is not recommended if the patient has received all three doses of another HPV vaccine (although available data show no serious safety concerns in persons who were vaccinated with the 9vHPV vaccine after having received a three-dose series of the quadrivalent HPV vaccine at least 12 months earlier).14 Cervical cancer screening is recommended beginning at age 21 years and continuing through age 65 years for both vaccinated and unvaccinated women, and an abnormal cervical cancer screening test (cytology or hrHPV test) should be treated the same way regardless of whether a woman has received an HPV vaccination. Completing an HPV vaccination series is recommend for all females ages 9–26, even if they have already had an abnormal pap smear and/or positive hrHPV screen, since they may still benefit from immunity to the other subtypes in the vaccine.
CASE (cont.)
Ms. Jones returns for follow-up 4 years after her initial visit. She received just two of the three vaccine doses that were recommended. She has a new sexual partner and is concerned about whether she might have acquired HPV from him, especially since she forgot about her third vaccination. She would like you to perform the “most up-to-date test” to screen for HPV and cervical cancer.
Number of Doses and Vaccine Efficacy
The three-dose HPV vaccination schedule poses challenges in ensuring that individuals receive all the recommended doses. One question frequently arises: if an individual receives fewer than the recommended number of doses, how much protection does he/she receive?
A recent population-based study assessed the relationship between the number of doses of the quadrivalent vaccine received and the incidence of condyloma (genital warts).15 Although the main goal of HPV vaccination is to prevent cervical cancer, the quadrivalent vaccine also prevents genital condyloma, a clinical outcome which typically develops earlier than cervical cancer. A cohort of over one million females aged 10–24 years living in Sweden (where subsidized and free vaccination programs for girls were in place) were followed between 2006 and 2010 for the incidence of condyloma. Ninety-nine percent of those vaccinated received the quadrivalent vaccine. Condyloma cases were identified through the use of the Swedish patient register, and linked with the Prescribed Drug Register, which contained information on the number of HPV vaccine doses received. The main outcomes were the incidence rate ratios and the incidence rate differences between vaccinated and unvaccinated females at the various doses.
Receipt of three doses of the vaccine was associated with the greatest reduction in number of condyloma cases (459 fewer per 100,000 person-years). However, a significant reduction was also seen with receipt of only one or two doses (384 and 400 fewer per 100,000 person-years, respectively), suggesting that a significant amount of vaccine efficacy is achieved with even the initial dose of the vaccine. Thus, our patient can be reassured that the amount of vaccine she received likely afforded her significant protection against HPV infection. However, this study did not answer the important question regarding the relationship between the number of vaccine doses and the incidence of cervical cancer, which is the main goal of HPV vaccination.
HPV and Cervical Cancer Screening
The current guidelines for cervical cancer screening among average-risk women are summarized in Table 1. For women over age 30, co-testing with cytology and hrHPV can be used to extend the screening interval from 3 to 5 years.16
Table 1.
| Society recommendations | Screening method and time interval | ||
|---|---|---|---|
| Age 21–24 years | Age 25–29 years | Age 30–65 years | |
| United States Preventive Services Task Force (USPSTF) 2012 | Cytology every 3 years (HPV testing not recommended) |
Cytology every 3 years (HPV testing not recommended, Grade D recommendation by USPSTF) |
Cytology every 3 years or co-testing every 5 years |
| American Congress of Obstetricians and Gynecologists (ACOG) 2012 | |||
| American Cancer Society/American Society for Colposcopy and Cervical Pathology/American Society for Clinical Pathology 2012 | |||
| American College of Physicians 2015 | |||
| ACOG 2015 | Cytology or hrHPV alone every 3 years | Options above or hrHPV alone every 3 years | |
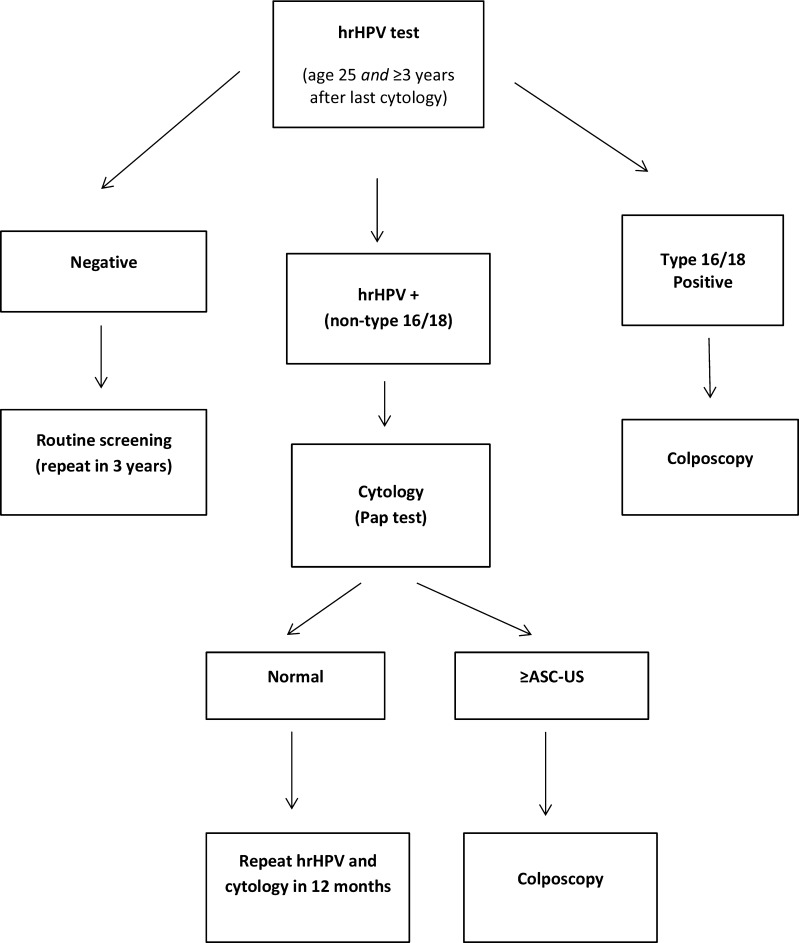
Since virtually all cases of cervical cancer are associated with hrHPV infection, studies have focused on the efficacy of HPV-only screening for cervical cancer. In 2008, the ATHENA study was initiated to compare the sensitivity and specificity of one round of primary hrHPV screening (for women 25 years and older) to either cytology alone or a “hybrid” strategy (cytology alone for ages 25–29 and co-testing beginning at age 30).17 The study was funded by the makers of the specific hrHPV test studied (Roche cobas HPV Test, US FDA-approved in April 2014). Approximately 41,000 women received hrHPV testing and cytology at baseline, and were followed intensively each year for the development of dysplasia, including a colposcopy for most women at the end of the study. Over a 3-year follow-up period, 319 cases of cervical intraepithelial neoplasia 3 (CIN 3), 20 cases of carcinoma in situ, and 8 invasive cancers were found. The sensitivity for detecting CIN 3 or more severe disease (CIN 3+) was 47.8 % for cytology alone, 61.7 % for the hybrid strategy, and 76.1 % for primary hrHPV testing, respectively (Table 3). Notably, the increased sensitivity of the primary hrHPV testing strategy was due to earlier initiation of HPV screening (age 25 for the primary hrHPV group, age 30 for the hybrid group). Instead of referring all hrHPV-positive women for colposcopy, cytology (which had been collected at the time of the hrHPV test) was used to triage patients according to the protocol shown in Figure 1. Despite the use of cytology for triage, the increased sensitivity of hrHPV testing led to a greater number of colposcopies than the other screening strategies (Table 2). However, the number of colposcopies required to detect a single case of severe dysplasia (CIN 3+) was the same for the primary hrHPV and hybrid strategies. A limitation of the study is that it encompassed only one round of screening. Optimal frequency of hrHPV-only screening is unknown, and it is unclear whether the detection of cases before age 30 improves morbidity or mortality risk. Cost-effectiveness studies are also needed, especially comparing primary hrHPV testing to the hybrid strategy.
Table 3.
Test Characteristics of Urinary HPV: Pooled Estimates24
| Test | Pooled sensitivity (95 % CI) | Pooled specificity (95 % CI) |
|---|---|---|
| Urinary detection of any HPV | 87 % (78–92 %) | 94 % (82–98 %) |
| Urinary detection of any high-risk HPV* | 77 % (68–84 %) | 88 % (58–97 %) |
| Urinary detection of HPV 16 and 18 | 73 % (56–86 %) | 98 % (91–100 %) |
*High-risk HPV includes the following HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82
Figure 1.
Primary HPV screening algorithm proposed by expert panel*†.19 *Expert panel comprising 13 experts from the following groups: Society of Gynecologic Oncology, American Society for Colposcopy and Cervical Pathology, American College of Obstetricians and Gynecologists, American Cancer Society, American Society of Cytopathology, College of American Pathologists, American Society for Clinical Pathology. †Only the Roche cobas HPV Test has been approved by the FDA for this testing. Abbreviations: hrHPV, high-risk human papillomavirus; ASC-US, atypical squamous cells of undetermined significance.
Table 2.
Comparison of Screening Strategies for the Detection of Cervical Abnormalities (CIN 3+) in One Round of Screening of 40,901 Women17
| Screening strategy: | No. of cases of CIN 3+ detected | Sensitivity (%) | Specificity (%) | Colposcopies required |
|---|---|---|---|---|
| Cytology alone age 25+ | 179 | 47.8 | 97.1 | 1934 |
| Cytology alone age 25–29 and co-testing age 30+ | 240 | 61.7 | 94.6 | 3097 |
| Primary hrHPV testing age 25+ | 294 | 76.1 | 93.5 | 3769 |
CIN 3+ = CIN 3, carcinoma in situ, or invasive cancer
The results of the ATHENA trial, as well as additional studies similarly demonstrating improved sensitivity of primary hrHPV testing compared to cytology,18 prompted an expert panel to publish interim clinical guidelines in 2015 regarding the use of primary hrHPV screening in clinical practice.19 These guidelines suggest that (1) hrHPV screening may be considered as an alternative to a cytology-based screening strategy, (2) hrHPV screening should begin no earlier than age 25 and no sooner than 3 years after the last normal cytology test, and (3) re-testing after a negative primary hrHPV screen should occur no sooner than 3 years. For performing hrHPV screening, only an HPV assay approved for primary screening should be used (only the Roche cobas HPV Test is FDA-approved); performance characteristics vary among the four available tests, and thus they cannot be used interchangeably at this time. The panel stated that, based on limited evidence, the triage protocol shown in Figure 1 was reasonable.
The panel had concerns about harms, stating that “progression to cancer is uncommon, and detection of most of the disease found in the 25–29-year age group can be safely deferred until age 30 and older.” They pointed out that cytology alone or co-testing are currently the only screening options recommended by all major societies, which include the American Society for Colposcopy and Cervical Pathology, the American Cancer Society, the United States Preventive Services Task Force, and the American Congress of Obstetricians and Gynecologists.16,20,21 In addition, the more recent Best Practice Advice released in June 2015 by the American College of Physicians included the recommendation that hrHPV testing should not begin before age 30.22
Urinary HPV Screening
Detection of cervical HPV in the urine represents a potentially more accessible and acceptable method, by avoiding the need for a pelvic examination.23 However, how accurate is this method for the detection of cervical HPV? This was evaluated in a recent meta-analysis,24 in which studies eligible for inclusion compared the detection of HPV in urine with detection in the cervix in any sexually active woman concerned about HPV infection or the development of cervical cancer. Urine positivity was compared with clinician-collected cervical swab positivity for any HPV, any hrHPV, and HPV 16 and 18.
A total of 14 studies were included in the meta-analysis. The sensitivity and specificity of urinary HPV for cervical HPV detection are shown in Table 3. The accuracy of urine HPV for detecting cervical HPV was significantly higher with “first void” urine specimens, primarily because of higher sensitivity.
This study of diagnostic accuracy did not assess the important outcome of invasive cancer or even of CIN. In addition, although the overall test sensitivity and specificity were reasonably high, the individual sensitivity and specificity varied significantly among studies. Based on these data, urine HPV testing will likely be an acceptable alternative to cervical HPV testing in the future for hard-to-reach patient populations.
For our patient, we would continue to recommend cervical sampling for HPV detection, and consider urinary HPV testing only if cervical testing were not feasible. Since Ms. Jones is now 30, it would be reasonable to perform co-testing with cytology and hrHPV screening, according to current guidelines.
CASE (cont.)
You review the current guidelines with Ms. Jones. Since she is 30, she can choose to be screened with cytology alone or cytology and hrHPV testing. She chooses the latter option. Ms. Jones’ screening test demonstrates normal cervical cytology, but her HPV test is positive, and you call her to discuss follow-up recommendations. She is very anxious about this result and what it means for her future health. In particular, her aunt had been diagnosed with anal cancer, and Ms. Jones has read that HPV can be associated with this disease. She wonders if she should receive additional screening and evaluation.
The incidence of anal cancer has been increasing in recent years. While the highest rates have been observed in men who have sex with men (MSM), especially those who are HIV-positive,25 more than 3000 cases are diagnosed annually among US women.26 HPV infection of the anal canal is detected in more than 80 % of anal cancers, and is closely linked to the development of anal precursor lesions, including anal intraepithelial neoplasia (AIN) 1, 2, and 3.27 Women with HPV-associated lower genital tract pathology are at increased risk for anal cancer,28 but the relationship between cervical HPV infection, cervical dysplasia, and anal HPV has not been clearly defined.
In an attempt to more clearly delineate this association, Sehnal et al. investigated the prevalence of anal HPV infection among women referred for colposcopy.29 Subjects were categorized as “low-risk” if the biopsy specimen was consistent with CIN 1 or a non-neoplastic diagnosis (endometrial polyp, irregular bleeding), and were categorized as “high-risk” if the biopsy showed CIN 2, CIN 3, or more severe disease. Concurrent cervical and anal HPV infections were more common in the high-risk than the low-risk group, increasing with the severity of disease (CIN 1: 15.8 %, CIN 2: 27.8 %, CIN 3: 48.5 %). Fifty-one percent of cervical and 48.6 % of anal HPV infections were related to HPV 16. Notably, in this study, concurrent infection was not associated with smoking, history of genital warts, unprotected vaginal intercourse, or anal intercourse. No evidence was provided regarding the management or outcomes among women diagnosed with anal HPV infections.
A recently published systematic review provided additional information regarding the natural history of anal HPV in women.26 One of the included studies reported on the incidence and clearance of anal HPV infection among 431 sexually active women (average age 39 years). Approximately 50 % of women had anal HPV infections, but the mean duration of infection was only 5 months, suggesting rapid clearance of the infection in most women. In contrast to the Sehnal study, publications included in this systematic review found that anal intercourse, lifetime number of partners, and genital warts were associated with anal HPV infection. The prevalence of high-grade anal lesions and anal cancer among HIV-positive women was significantly higher than among HIV-negative women (high-grade anal lesions: 3–26 % vs. 0–3 %; anal cancer: standardized incidence ratio 18.5 vs. 0). Women with cervical cancer or severe cervical dysplasia also had a higher incidence of anal cancer, with incidence ratios ranging between 0.8 and 63.8 per 100,000 person-years.
Researchers pooled similar data on the incidence and progression of anal HPV infection among HIV-positive and HIV-negative MSM.25 Results revealed that hrHPV anal infection was more common among HIV-positive than HIV-negative MSM (pooled prevalence 73.5 % [CI 63.9–83.0 %] vs. 37.2 % [CI 27.4–47.0 %]). Similarly, anal cytologic abnormalities were more prevalent among HIV-positive individuals (pooled prevalence 63.1 % [CI 52.6–73.6 %] vs. 29.2 % [CI 12.3–46.2 %]). Nine of the included studies provided data regarding the incidence of anal cancer, which was significantly higher among HIV-positive MSM (pooled incidence 45.9 per 100,000 men vs. 5.1 per 100,000 men).
Although hrHPV anal infection is associated with anal cytological abnormalities, it is unclear whether screening for these reduces the risk of anal cancer. Little is known about the natural history of anal dysplasia, including the frequency of progression from low-grade to high-grade abnormalities and cancer, and whether follow-up high-resolution anoscopy improves outcomes.30 As a result, the Centers for Disease Control and Prevention (CDC) does not currently recommend routine screening for hrHPV with anal cytology, although the guidelines acknowledge that some clinicians may choose to perform anal pap testing among certain high-risk patients (such as persons with HIV infection and MSM), and to follow up abnormal results with high-resolution anoscopy.31 Transgender women in particular have high rates of HIV infection and may engage in risky sexual behavior (unprotected receptive anal intercourse, multiple casual partners),32 and the CDC recommends taking a careful anatomical and sexual history to fully assess the risk for sexually transmitted infections in this group of individuals. In contrast to the CDC guidelines, the New York City Department of Health and Mental Hygiene does specifically recommend annual anal pap testing among HIV-positive MSM as well as among HIV-positive women with a history of cervical or vulvar dysplasia or genital warts.33
Based on the evidence above, we can counsel our patient that many women with anal HPV will clear their infection rapidly, and based on current national guidelines, there is no role for anal pap testing. However, we would continue to recommend safe sexual practices, including the use of condoms during intercourse.
DISCUSSION
HPV infection is common and is associated with numerous genital cancers. Vaccination is effective for the primary prevention of HPV infection, and the recently approved 9vHPV vaccine will provide protection against multiple hrHPV subtypes when given early, prior to the onset of sexual activity. Increasing evidence suggests that primary hrHPV screening beginning at age 25 is more sensitive for detecting high-grade cervical dysplasia compared to cytology alone, and can detect high-grade dysplasia earlier than co-testing with cytology and hrHPV beginning at age 30. The increased sensitivity associated with primary hrHPV testing also leads to an increased number of colposcopies, and thus increased costs, without clearly improving health outcomes. Major US guidelines continue to recommend cytology or co-testing for screening. Urinary hrHPV testing is a new screening modality, but is not yet available in all labs and should be used only in women who cannot tolerate or access cervical screening. Anal HPV infection has been associated with anal dysplasia, but the natural history of the progression to anal cancer is unclear, and anal pap testing is not currently recommended.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
References
- 1.Wu X, Watson M, Wilson R, Saraiya M, Cleveland JL, Markowitz L. Human papillomavirus–associated cancers—United States, 2004–2008. MMWR. 2012;61:41–5. [Google Scholar]
- 2.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2014;63(5):1–30. [PubMed] [Google Scholar]
- 4.Thaxton L, Waxman AG. Cervical cancer prevention immunization and screening 2015. Med Clin N Am. 2015;99:469–477. doi: 10.1016/j.mcna.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 5.FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 6.Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 7.Hariri S, Markowitz LE, Dunne EF, Unger ER. Population impact of HPV vaccines: summary of early evidence. J Adolesc Health. 2013;53:679–82. doi: 10.1016/j.jadohealth.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032. [DOI] [PubMed]
- 9.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR. 2015;64(29):784–92. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joura EA, Giuliano AR, Iversen OE. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 11.Petrosky D, Bocchini JA, Hariri S. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 12.Brisson M, Laprise JF, Chesson HW, Drolet M, Malagon T, Boily MC, et al. Health and economic impact of swithing from a 4-valent to a 9-valent HPV vaccination program in the United States. JNCI J Natl Cancer Inst. 2016;108(1): djv282. doi:10.1093/jnci/djv282. [DOI] [PMC free article] [PubMed]
- 13.CDC Vaccine Price List. Available at http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html Accessed April 11, 2016
- 14.MMWR 2015. Additional guidance for providers regarding 9-valent HPV vaccine use among persons who previously received HPV vaccination. Available at http://www.cdc.gov/hpv/downloads/9vHPV-guidance.pdf Accessed April 20, 2016
- 15.Heirweijer E, Leval A, Ploner A, et al. Association of varying number of doses of quadrivalent human papillomavirus vaccine with incidence of condyloma. JAMA. 2014;311(6):597–603. doi: 10.1001/jama.2014.95. [DOI] [PubMed] [Google Scholar]
- 16.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;62:147–172. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 17.Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197. doi: 10.1016/j.ygyno.2014.11.076. [DOI] [PubMed] [Google Scholar]
- 18.Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 19.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–7. doi: 10.1097/AOG.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 20.Moyer VA, U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880–91,W312. [DOI] [PubMed]
- 21.Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol. 2012;120:1222–38. [DOI] [PubMed]
- 22.Sawaya GF, Kulasingam S, Denberg TD, Qaseem A, Clinical Guidelines Committee of the American College of Physicians Cervical cancer screening in average-risk women: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;162(12):851–9. doi: 10.7326/M14-2426. [DOI] [PubMed] [Google Scholar]
- 23.Sellors JW, Mahony JB, Kaczorowski J, Lytwyn A, Bangura H, Chong S, et al. Prevalence and predictors of human papillomavirus infection in women in Ontario, Canada. CMAJ. 2000;163(5):503–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Pathak N, Dodds J, Zamora J, Khan K. Accuracy of urinary human papillomavirus testing for cervical HPV: systematic review and meta-analysis. BMJ. 2014;349:g5264. [DOI] [PMC free article] [PubMed]
- 25.Machalek DA, Poynten M, Jin F, Fairley CK, Fransworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 26.Steier EA, Sebring MC, Mendez AE, Ba FS, Trimble DD, Chiao EY. Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: a systematic review. Am J Obstet Gynecol. 2015;213(3):278–309. doi: 10.1016/j.ajog.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shvestov YB, Hernandez BY, McDuffie K, et al. Duration and clearance of anal human papillomavirus (HPV) infection among women: the Hawaii HPV cohort study. Clin Infect Dis. 2009;48:536–46. doi: 10.1086/596758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valari O, Koliopoulos G, Karakitsos P, et al. Human papillomavirus and mRNA positivity of the anal canal in women with lower genital tract HPV lesions: predictors and clinical implications. Gynecol Oncol. 2011;122:505–8. doi: 10.1016/j.ygyno.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 29.Sehnal B, Dusek L, Cibula D, et al. The relationship between the cervical and anal HPV infection in women with cervical intraepithelial neoplasia. J Clin Virol. 2014;59(1):18–23. doi: 10.1016/j.jcv.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Smyczek P, Singh AE, Romanowski B. Anal intraepithelial neoplasia: review and recommendations for screening and management. Int J STD AIDS. 2013;24(11):843–851. doi: 10.1177/0956462413481527. [DOI] [PubMed] [Google Scholar]
- 31.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64(3):1–138. [PMC free article] [PubMed] [Google Scholar]
- 32.Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1–17. doi: 10.1007/s10461-007-9299-3. [DOI] [PubMed] [Google Scholar]
- 33.New York City Department of Health and Mental Hygiene. Prevent Sexual Transmit Infect. 2013;32(4):19–27. Available at http://www.nyc.gov/html/doh/html/data/chi32-4_screening.html Accessed April 11, 2016.