Abstract
Hyaluronan (HA) is a glycosamminoglican involved in cell biology as well as a relevant polymer for tissue engineering and regenerative medicine. Megakaryocytes (Mks) are immersed in a mesh of extracellular matrix (ECM) components that regulate their maturation in the bone marrow (BM) and the release of platelets into the bloodstream. While fibrous ECMs such as collagens and fibronectin have been demonstrated to differently regulate Mk function and platelet release, the role of HA, that fills the majority of the BM extracellular interstitial space, has not been investigated so far. Here we demonstrated that, although human Mks express HA receptors, they are not affected by HA in terms of in vitro differentiation, maturation and platelet formation. Importantly, chemical properties of HA were exploited to generate hydrogels with entrapped ECMs that represent a useful model to more closely mimic the tridimensional characteristics of the BM environment for studying Mk function. In conclusion, in this work we demonstrated that HA is an ideal candidate for a 3D ex vivo model of human BM ECM component environment.
Keywords: Megakaryocytes, Hyaluronic Acid, Hydrogels, Proplatelet Formation, Extracellular Matrix, Bone Marrow, Thrombopoiesis, Proteoglycans
Graphical abstract
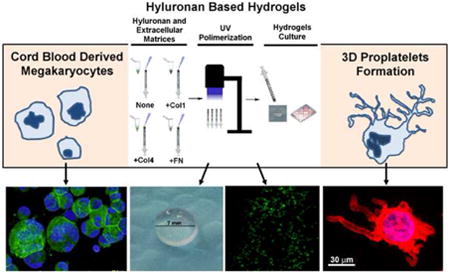
Introduction
Extracellular matrix (ECM) provides essential physical scaffolding for cells within bone marrow (BM) and generates biochemical and biomechanical cues that are required for Hematopoietic Stem Cell (HSC) differentiation and homeostasis (1-2). Megakaryocytes (Mks) differentiate from HSCs and are responsible for platelet biogenesis (3). During their differentiation, these cells migrate from the endosteal niche to vascular districts where platelets are shed into the bloodstream through the generation of branched cytoplasmatic protusions called proplatelets (4-5).
The BM environment contains soluble factors, chemokines and ECM components that are instrumental in driving all the steps required for platelet generation, such as Mk maturation, migration and positioning along the BM sinusoids (6). The ECM is composed of two main classes of macromolecules: fibrous proteins and proteoglycans (PGs), which are formed by glycosaminoglycans (GAGs) covalently attached to core proteins (7-8).
The major BM fibrous proteins such as fibronectin, laminin and collagens are differently located in the BM niches (9-10) and are involved in the regulation of Mk differentiation and function. Specifically, the endosteal type I collagen is considered a negative regulator of platelet release through activation of the Rho-ROCK-myosin IIA pathway (11-12). On the contrary, fibronectin and vascular basement membrane components laminin and type IV collagen support Mk maturation as well as platelet biogenesis (10-13). Further, biochemical and mechanical properties of these fibrous proteins differently regulate Mk migration and spreading in vitro (14-15). Importantly, PGs fill the majority of the BM extracellular interstitial space in the form of a hydrated gel and display a wide variety of functions that reflect their unique hydration and force-resistance properties (16). However, few studies attempted to analyze the role of PGs in the regulation of Mk and platelet development. Almost all GAGs, including hyaluronan, chondroitin sulfate, dermatan sulfate, heparin and heparan sulfate, were reported to stimulate at different extent both human and murine megakaryopoiesis in vitro and in vivo (17-21). As an example, administration of low-molecular weight heparin to mice increases both blood platelet count and Mk number in the BM, while sulfated GAGs were demonstrated to promote proplatelet formation by murine Mks in vitro (22, 23). However, mechanisms of action of GAGs on Mks are far from being elucidated.
Hyaluronan or hyaluronic acid (HA) is one of the major non-proteinaceous ECM components. HA differs from others GAGs in that it is a unique high molecular weight (HMW) and non-sulphated glycosaminoglycan that consists in an un-branched polysaccharide of repeating disaccharides of D-glucuronic acid and D-N-acetylglucosamine (25). Due to its hydrophilic properties, HA is very hydrated and ubiquitously expressed in the ECM of tissues. In mammals, HA synthesis occurs on the cellular plasma membrane by means of three hyaluronan synthase isoenzymes (HAS1, 2, and 3) that utilize the uridine diphosphate (UDP) glucuronic acid (UDP-GlcUA) and UDP N-acetylglucosamine (UDP-GlcNAc) as substrates (26-28). Hyaluronidases, oxygen radicals and HAS3 are instead responsible for the generation of low molecular weight fragments that display different biological activities in physiological or pathological conditions (29-30).
Binding of the HMW HA or fragments to specific proteins on cell surface called hyaladherins, such as CD44, RHAMM, HARE and toll like receptors (TLR) 2/4, has been implicated in the regulation of several biological processes, like cell proliferation, migration, tumorigenesis and inflammation (31-35). In the hematopoietic compartment, CD44 and HA cooperate with stromal derived factor 1 (SDF-1) in the trafficking of human CD34+ stem/progenitor cells to BM (36) and HA has recently emerged as a critical regulator of the spatial distribution of HSCs after transplantation in mice devoid of Has3-synthesized HA (37).
Beside HA role in biological processes, its properties as an inherently biocompatible, biodegradable and non-immunogenic compound, render HA a relevant biomaterial for tissue engineering and regenerative medicine (38-42). In particular, a variety of chemical modifications and cross-linking provide mechanically and chemically robust HA scaffolds that retain the biocompatibility and biodegradability of native HA (43-44).
In this work, we demonstrated that Mk behavior is uninfluenced by exogenous HMW HA treatment. Moreover, we showed that HA could be exploited as an ideal scaffold for mimicking the tridimensional BM environment to study Mk-fibrous ECM components 3D interactions.
Materials and Methods
Cell culture
Human cord blood was collected following normal pregnancies and deliveries upon informed consent of the parents, in accordance with the ethical committee of the IRCCS San Matteo Foundation and the principles of the Declaration of Helsinki. CD34+ cells were separated and cultured for 13 days in Stem Span media in the presence of 10ng/mL of human recombinant TPO, interleukin-11 and interleukin-6 (Peprotech, United Kingdom) as previously described (13). 10, 50 or 100 μg/mL of high molecular weight HA (average=5000 kDa) were used to stimulate cells (Healon ovd, Abbott Medical Optics) during 13 days of culture. Mks were identified on the basis of CD61 expression and Mk output was calculated as normalized percentages of Mks relative to control cultures. Proplatelet formation by HA untreated or treated Mks was evaluated onto fibrinogen-coated coverslips as previously described (13, 15). Briefly, 105 Mks at day 13 of differentiation were seeded on glass coverslips previously coated with 100μg/mL of human fibrinogen (Sigma Aldrich, Milan, Italy) and cultured for additional 16 hours in the presence of TPO. Cells were fixed with paraformaldehyde 4%, permeabilized with triton X-100 and stained with α-tubulin antibody (1:700, Sigma Aldrich, Milan, Italy) and CD61 (Immunotech, Marseille, France). Mks displaying proplatelets were counted and expressed as percentage on the total number of CD61+ Mks. Skin human fibroblasts were cultured in DMEM supplemented with 10% FBS, while peripheral blood platelets were purified as previously described (45).
Western blotting analysis
Western blotting were performed as previously described (10). Primary antibodies and dilutions used in this work are listed in Supplemental Table 1.
Hydrogel synthesis and megakaryocyte encapsulation
Metacrylated hyaluronan (MeHa) was synthetized with the method described by Nettles et al. (46). Briefly a 20 fold excess of methacrylic anhydride (Sigma-Aldrich, Milan, Italy) was added to a solution of 1% Hyaluronan (Lifecore, MW=51kDa) in deionized water and adjusted to pH 8. The solution was stirred at room temperature (RT), incubated overnight (o.n.) at 4 °C and then precipitated with cold ethanol to remove the excess of methacrylic anhydride. The final precipitate was lyophilized, re-suspended in sterile water and dialyzed in slide-A-Lyzer dialysis cassette (6-8 KDa molecular weight cut-off, Thermo Scientific). The final solution was lyophilized and stored at -20 °C. Hydrogels were fabricated using light initiated free radical cross-linking (47) by dissolving MeHa in Phosphate Buffer Saline (PBS) containing 0.05% 2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959, BASF) and then polymerized with the addition of 4 mW/cm2 ultraviolet light using a long-wave ultraviolet lamp (Black Ray, Model 100AP). Mks (0,5-1×106/hydrogel) were re-suspended in 50 μl of 2% MeHa 0,05% Irgacure2959 solution alone or containing 5 μg of purified bovine type 1 collagen (15), human type IV collagen (Sigma-Aldrich, Milan, Italy) or human plasma fibronectin (BD Biosciences, Milan, Italy), placed into sterile syringe tip mold and exposed to UV light for radical cross-linking. Hydrogels were finally plunged into wells of culture plate containing 1 mL of media. Phase contrast images of encapsulated Mks were acquired with an Olympus IX53 microscope (Olympus, Deutschland GmbH, Hamburg, Germany) using a 20×/0.40 PhC objective. The viability of Mks encapsulated within hydrogels was analyzed using a fluorescent live/dead staining kit (BioVision) following manufacture instructions. Alternatively, encapsulated cells were stained with CD61 and Hoechst 33258 (100 ng/mL in PBS) and visualized with a TCS SP2 confocal laser scanning microscope (Leica, Heidelberg, Germany).
FACS analysis
Cell ploidy analysis were performed as previously described (10). Data were analyzed using FCS express software. In order to evaluate the expression of CD44 and RHAMM, 2×105 cells at day 13 of culture were stained with anti-CD44 (eBioscience, Milan, Italy), anti-RHAMM (Thermo Scientific) and PE anti-CD61 (eBioscience) antibodies for 30 minutes at RT and samples analyzed with a Beckman Coulter Navios flow cytometer. For RHAMM and CD44 staining, cells were fixed with 4% PFA for 10 minutes. In the detection protocol for RHAMM, cells were also permeabilized with 0,5% Triton X-100 for 10 minutes at RT. Alexa Fluor 488 anti rabbit secondary antibody was then used to reveal RHAMM and CD44 staining.
Immunofluorescence analysis
Immunofluorescence analysis was performed as previously described (13). For intracellular hyaluronan staining, 105 living cells (No Triton X-100 condition) or fixed cells (Triton X-100 condition) at day 13 of culture were treated with hyaluronidase 200 units/mL at 37 °C for 15 minutes (48). Cells were cytospun on glass coverslips coated with 0.1% of Poly-L-lysine solution (Sigma Aldrich, Milan, Italy). Non-specific binding sites were saturated by incubating cells with 0,05% avidin and 0,005% biotin (Sigma-Aldrich) solutions both for 20 minutes at RT. Specimens were then incubated with the Hyaluronan Binding Protein (HABP) (Sigma-Aldrich) and CD61 o.n. at 4 °C, followed by staining for 1 hour at RT with 594 Alexa Fluor conjugated streptavidin and 488 Alexa Fluor conjugated antibody, respectively (Life Technologies, Milan, Italy). Nuclear counterstaining was performed using Hoechst 33258 (100 ng/mL in PBS). Images were acquired with an Olympus BX51 microscope (Olympus, Deutschland GmbH, Hamburg, Germany) using a 63×/1.25 UPlanF1 oil-immersion objective. Negative controls were routinely performed by omitting the primary antibody or HABP.
Real Time PCR
Retrotranscription (RT) was performed in a final volume of 20 μL reaction using the iScriptTM cDNA Synthesis Kit according to manufacturer instructions (BioRad Laboratories Inc, Milan, Italy). For quantitative Real Time PCR, RT samples were diluted up to 60 μL with ddH2O and 3 μL of the resulting cDNA was amplified in triplicate in 15 μL reaction mixture with 200 nM of each specific primer and SsoFast™ Evagreen® Supermix (Bio-rad Laboratories, Milan, Italy) at 1× as final concentration. The amplification reaction was performed in a CFX Real-time system (BioRad Laboratories Inc., Milan, Italy) as follows: 95°C for 5 minutes, followed by 35 cycles at 95°C for 10 seconds, 60°C for 15 seconds, 72°C for 20 seconds. Pre-designated KiCqStart™ primers for HAS1, HAS2 and HAS3 were purchased from Sigma-Aldrich (Milan, Italy). The BioRad CFX Manager® software 3.0 was used for the normalization of the samples (BioRad Laboratories Inc. Milan, Italy). β2 microglobulin gene expression was used for the comparative concentration analysis.
Statistics
T test was used to analyze data. Data are presented as mean ± S.D. A p value<0.05 was considered statistically different.
Results
Human megakaryocytes express hyaluronan and HAS isoenzymes
To unravel the role of HA in thrombopoiesis, we first evaluated the expression of HA and the enzymes involved in its synthesis in primary human Mks differentiated from cord blood CD34+ HSCs.
Firstly, real time PCR was used to measure the expression levels of HAS isoenzymes in cord blood derived CD34+ HSCs and differentiated Mks at day 13 of culture. As shown in Figure 1A, CD34+ HSCs expressed all the HAS isoenzymes, while differentiated Mks showed comparable levels of HAS2 and HAS3, but seemed not to express HAS1. The expression of the HAS3 isoenzyme was also evaluated by western blotting. Protein lysates from 5×105 mature Mks at day 13 of differentiation were tested with a commercially available anti HAS3 antibody. The 38 kDa band corresponding to the enzyme was detected in human fibroblasts used as controls and in Mks, however in Mk lysate the antibody recognized two additional bands around 35 and over 50 kDa (Figure 1B). Further, comparison of HAS3 protein levels in Mks and peripheral blood platelets revealed similar levels of the band over 50 kDa, but an increase in the 38kDa band, corresponding to HAS3, was observed in platelet lysates (Figure 1C). Quantification of HAS3 expression in platelets and Mks by densitometry analysis using β-actin as control, demonstrated a significant increase in the levels of HAS3 expression in platelets (p value<0.001) (Figure 1D). Expression of HAS3 was further confirmed by immunofluorescence staining of mature (day 13) Mks, identified using an antibody against the specific megakaryocytic marker CD61 (Figure 1E). Production of HA in Mks was further highlighted using a biotinylated HA Binding Protein (HABP) and fluorochrome conjugated-streptavidin (Figure 1F). HA localization was intra-cellular as staining did not occur in the absence of Triton X-100 treatment and HA was completely digested from Mk cytoplasm after hyaluronidase treatment in fixed cells. Overall these results demonstrated that expression of HA and related enzymes may occur in mature Mks.
Figure 1. Human Megakaryocytes express hyaluronan (HA) and HAS isoenzymes necessary for its biosynthesis.

A) Real Time qPCR analysis of HAS1, HAS2 and HAS3 expression in cord blood derived CD34+ HSCs and differentiated Mks. Results are expressed as fold increase of HAS isoenzymes expression in Mks with respect to CD34+ HSCs. β2 microglobulin gene expression was used for the comparative concentration analysis. At least three independent experiments were performed. n.d. = not detectable. B) Western blotting analysis of HAS3 expression in human fibroblasts and differentiated Mks. C) Western blotting analysis of HAS3 expression in differentiated Mks and human peripheral blood platelets. D) Densitometry analysis of HAS3 levels in Mks and peripheral blood platelets. β-actin was used as internal control of protein loading. At least three independent experiments were performed. E) Immunofluorescence staining of HAS-3 (red) (middle panel) in TPO differentiated CD61+ (green) (left panel) Mks. 1×105 Mks at day 13 of culture were cytospun onto poly-L-lysine coated glass coverslips and processed for immunofluorescence. Composite of images is shown on the right panel. Scale Bar=20μm. F) Immunofluorescence staining of hyaluronan (red) (middle panel) in TPO differentiated CD61+ (green) (left panel) Mks. Composite of images are shown on the right panel. 1×105 Mks at day 13 of culture were cytospun, fixed and permeabilized or not with Triton X-100 to demonstrate the intracellular localization of HA. Cells were stained with Hyaluronan Binding Protein (HABP) and CD61 antibody, followed by Alexa Fluor 594 coniugated streptavidin and Alexa Fluor 488 conjugated antibody, respectively. Treatment with hyaluronidase was performed before Mks staining to confirm the presence of intracellular HA. Scale Bar=10μm. Hoechst 33258 was used to highlight nuclei (blue).
HA receptor expression in primary human megakaryocytes
HA exerts its main biological functions through CD44 and RHAMM engagement. Therefore, we evaluated the expression and localization of these receptors during Mk differentiation from HSCs. Western blotting analysis showed that differentiating HSCs at day 7 of TPO stimulation displayed higher levels of CD44 receptor with respect to 10 days or 13 days maturing Mks (Figure 2A, upper panel). On the contrary, as shown in Figure 2A (lower panel), similar levels of RHAMM expression in immature and mature Mks were detected by western blotting.
Figure 2. Hyaluronan receptors are differently expressed during hematopoietic stem cell differentiation into mature Mks.

A) Western blotting analysis of CD44 and RHAMM expression in Mk lysates at day 7, 10 and 13 of differentiation. Actin was revealed to correct minor changes in protein loading. B) Immunofluorescence staining of RHAMM (red) in permeabilized (upper panels) and non permeabilized CD61 (green) positive Mks (lower panels). Scale Bar=30μm. C) FACS analysis of RHAMM staining in permeabilized (left panel) and non permeabilized (right panel) CD61 positive Mks. 2×105 cells were harvested from cultures and analyzed by FACS. D) Representative FACS analysis of CD44 and RHAMM in mature CD61+ Mks. Percentages of CD44/CD61 or RHAMM/CD61 double positive cells are shown. E) Quantification of CD61/CD44 and CD61/RHAMM double positive cells percentages at day 13 of differentiation by FACS. At least three independent experiments were performed. Scale Bar=30μm.
However, with respect to CD44, RHAMM localization seemed to be mainly intracellular as staining was achieved exclusively after cell permeabilization by triton X-100 by both immunofluorescence and FACS analysis (Figure 2B-C). Moreover, CD44 and RHAMM expression were further explored by FACS analysis at the end of Mk maturation (Figure 2D). We estimated a median of 18±2% of CD61+/CD44+ cells (Figure 2D/Left panel and Figure 2E) while 73±6% of mature Mks expressed both RHAMM and CD61 receptors (Figure 2D/Right Panel and Figure 2E). Overall these data showed that HA receptors are differently modulated during human Mk differentiation with CD44 down regulated in HSC-Mk transition and intracellular RHAMM maintained during all the steps of Mk maturation.
Effects of exogenous high molecular weight hyaluronan on human thrombopoiesis
We next analyzed the effects of HMW HA on Mk differentiation. Cord blood-HSCs were stimulated with three different doses of 10, 50 and 100 μg/mL HA for the entire duration of the culture (13 days). High molecular weight HA (average of 5000 kDa) was added to Mks during their maturation. Analysis of the Mk marker CD61 by FACS revealed no significant differences in terms of differentiation at the end of the cultures (Figure 3A). Maturational stages of Mks were also evaluated by FACS analysis of cell ploidy (10) and similar fractions of immature 2N, 4N and poliployd 8N, 16N, 32N Mks were detected in replicated cultures (Figure 3B). Further, Mk function was not affected by HA treatment. 1×105 Mks at day 13 of differentiation were seeded onto fibrinogen coated glass coverslips for 16 hours in the presence or absence of soluble HA and Mks extending proplatelets were detected by α-tubulin staining and counted. Comparable percentages of Mk extended proplatelets in untreated, 10, 50 or 100 μg/mL HA stimulated conditions were measured (Figure 3C). Consistently, western blotting analysis demonstrated that the key signaling pathways underlying TPO stimulation, such as AKT and ERK 1/2 kinases, were activated to a similar extent in control and Mks treated with the highest HA concentration (100μg/mL) (Figure 3D). Densitometry analysis of AKT and ERK 1/2 activation in three independent experiments demonstrated similar levels of phosphorylation of these proteins in untreated or HA treated Mks (Figure 3E). In summary, these experiments revealed that high molecular weight HA does not seem to exert measurable effects on human megakaryopoiesis and platelet production.
Figure 3. Hyaluronan does not influence Mk differentiation, maturation and function.
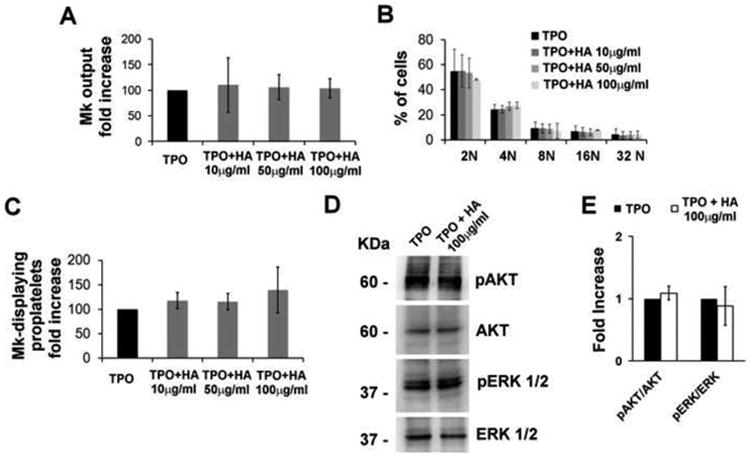
A) Mks were differentiated for 13 days in the presence of TPO (10 ng/mL) and three different concentration of high molecular weight HA (average 5000 kDa), in particular 10, 50 and 100 μg/mL. Mk output is defined as the normalized percentage of CD61 positive cells at the end of cultures with respect to TPO treated cells. At least three independent experiments were performed. B) Quantification of HA effects on Mk ploidy. 2×105 untreated (Ctrl) or HA treated Mks were collected at day 13 of differentiation and analyzed by FACS after propidium iodide staining. At least three independent experiments were performed. C) Quantification of in vitro Mk-forming proplatelets in TPO and HA stimulated Mks. 1×105 Mks were collected at day 13 of differentiation and seeded onto fibrinogen coated glass coverslips in the presence of TPO or TPO plus HA at different concentrations. After 16 hours, adherent cells were fixed, permeabilized and stained with α-tubulin antibody. Results are expressed as the normalized percentage of Mk-displaying proplatelets with respect to TPO treated cultures. D) Representative western blotting analysis of AKT and ERK 1/2 phosphorylation levels in TPO and TPO plus HA (100μg/ml) treated Mks. CD34+ HSC were purified from cord blood and cultured for 13 days in the presence of TPO (Ctrl) or TPO plus 100μg/ml of HA. Cells at day 13 were collected and lysed. Total AKT, ERK 1/2 were revealed to ensure equal protein loading. E) Densitometry analysis of pAKT/AKT and pERK/ERK ratios in TPO or TPO plus HA treated cultures of at least three independent experiments.
Hyaluronan hydrogels represent a suitable 3D model for the analysis and imaging of megakaryocyte-ECM interaction
The absence of a direct HA modulation on Mk differentiation and function led us to explore the possibility to exploit the biochemical properties of HA as a suitable scaffold for the assessment of Mk behavior in a 3D model. Therefore, we produced HA scaffold in which HA was methacrylated (MeHA) and photocross-linked to form hydrogels (Figure 4A, Upper and Bottom Panels). Importantly, biocompatibility and low toxicity of MeHA hydrogels after cell entrapment was confirmed by confocal analysis of Mk viability using live/dead kit that revealed at least 95% of live cells after 16 hours after cell seeding (Figure 4B). Distribution of Mks in hydrogels was analyzed by confocal microscopy after CD61 staining and cells were homogenously distributed in the cross sections of z-stacks over a 200μm distance (Figure 4C, Supplemental Video 1). Hydrogels were then “functionalized” through the incorporation of fixed amount of fibrous BM ECM components (5μg each), such as type I collagen, type IV collagen and fibronectin, that were previously reported to exert opposing effects on the process of proplatelet formation by differentiated Mks in 2D cultures (Figure 4D). Viability did not vary in the presence of fibrous ECM (Supplemental Figure 1). Further, Mks entrapped into MeHA or type I collagen enriched hydrogel did not display Mk-forming pro-platelets (Figure 4E-F). On the contrary, MeHA scaffolds containing type IV collagen or fibronectin allowed the extension of proplatelets in the 3D environment after 16 hours of culture (p value < 0.001) (Figure 4E-F).
Figure 4. Hyaluronan hydrogel-based scaffolds represent an ideal model for studying 3D megakaryocyte interactions with other fibrous extracellular matrix proteins.
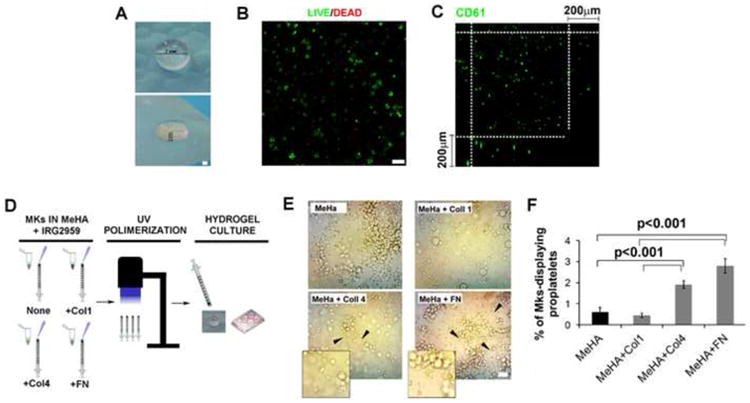
A) Front (upper panel) and side (bottom panel) photomicrographs of hyaluronan hydrogels. HA was methacrylated and photocrosslinked using Irgacure 2959. Scale bar=7mm and 2mm, respectively. B) Mks were entrapped into methacrylated HA (MeHA) and hydrogels were seeded in culture media with TPO for 16 hours. Cell viability was evaluated by confocal microscopy after cell staining with a live (green)/dead (red) cell staining kit. Scale Bar=50μm. C) Cross section analysis of Z-stack of Mks encapsulated into methacrylated HA hydrogels as measured by confocal microscopy. Scale bar=200μm. D) Cartoon showing strategy adopted for the generation of MeHA hydrogels containing ECM components, such as type I and IV collagens and fibronectin (5 μg each). E) Phase contrast evaluation of proplatelet formation by human Mks in MeHA and MeHA plus ECM components hydrogels after 16 hours of culture. Scale Bar=20μm. F) Quantification of percentages of Mk-displaying proplatelets with respect to total Mks in MeHA hydrogels containing ECM components. At least 100 Mks per conditions were counted and three independent experiments were performed. p value<0.001.
Discussion
The interaction of Mks with the ECM components is a fundamental step for proper blood platelet supply within BM (15). Integrins and glycoproteins involved in the recognition of fibrous ECM have been extensively studied in both Mks and platelets (48). However, the tridimensional features of BM have hampered the ex vivo analysis of Mk interaction with the surrounding environment. Glycosamminoglycans partecipate to the establishment of proteoglycan hydrogels that fill the interstitial space in most tissues. In particular, HA differs from other GAGs in that it is a high molecular polymer not sulfated and not linked to core protein.
Although HA connects isles of hematopoietic cells and localizes to sinusoids endothelial cells within BM (36), its thrombopoietic features have not been studied so far.
Biological processes following HA binding are strictly dependent on HA engagement of specific receptors. Among the receptors involved in HA signaling, the CD44 is present in almost all human cells and activates signaling cascades that includes PI3K/PDK1/AKT pathway as well as Ras phosphorylation cascade involving RAF1, MEK and ERK1/2 (50, 51, 52). RHAMM receptor was described in tumor cell line (53) and in several cell types, including endothelial cells (54), and reported to trigger several cellular signaling including Ras, focal adhesion kinase (FAK), ERK1/2, protein kinase C (PKC) and phosphatidylinositol 3-kinase (PI3K) (55-56).
In this study we showed that primary human Mks express HA and isoenzymes required for its biosynthesis such as HAS2 and HAS3. Furthermore, our data demonstrated that differentiation of HSCs into mature Mks is characterized by a progressive decrease in CD44 expression while RHAMM expression is maintained during megakaryopoiesis, but limited to cytoplasm compartment. Consistently, stimulation of Mk precursors with increasing concentrations of HA did not affect the maturational profile of Mks nor their ability to undergo the process of proplatelet formation, the distinctive process of cytoplasm conversion into long-branching protusions required for platelet assembly and release (3). Biochemical and molecular mechanisms regulating these events are not completely understood. A balanced activation of the pathways underlying thrombopoietin (TPO) receptor c-Mpl in Mks, such as PI3K-AKT axis and ERK MAPK kinases, seems to be fundamental for platelet release triggering (57, 58). Interestingly, those signaling pathways were not affected by Mk stimulation with different doses of HA in our experimental conditions.
The low thrombopoietic features of HA, emerged from our data, prompted us to exploit the biochemical and biomechanical properties of this polymer by producing HA hydrogel-based scaffold to study Mk function in a more physiological 3D environment mimicking BM tissue. Scaffolds with methacrylated and photocross-linked HA were previously reported (59, 60, 61, 62). Hydrogels were further modified through the incorporation of BM fibrous ECM components, previously described as important determinants of Mk function in 2D culture models (10, 13), such as type I collagen, type IV collagen and fibronectin. We demonstrated the high biocompatibility of HA hydrogels and that addition of fibronectin and type IV collagen enhanced proplatelet formation. Interestingly, the inhibitory function of type I collagen on platelet release was also confirmed. Recently, physical features of crosslinked HA based hydrogels were demonstrated to influence cell morphology and phenotype. In particular, cells embedded in tridimensional homogeneous HA hydrogels were demonstrated to constrain cells to spherically symmetric shapes while, the presence of an additional matrix increased the number of cells with elongated cells shape through myosin-dependent mechanisms (63, 64). Therefore, it's conceivable that in our model the addition of ECM components to homogenous HA hydrogels might change physical and biochemical properties of HA hydrogels, thus differently modulating Mk function. In conclusion, we demonstrated that, due to its inactivity on Mk function, HA is an ideal candidate for the production of hydrogel scaffolds for direct visualization and study of Mk-ECM component interaction in a more physiological 3D environment.
Supplementary Material
Highlights.
Evidences that Megakaryocytes (Mks) contain hyaluronan (HA)
HA does not influence Mk differentiation, ploidy and function in vitro.
HA hydrogels constitute a suitable 3D model for studying Mk-ECM interactions
Acknowledgments
We are grateful to Dr. Giancluca Viarengo (IRCCS San Matteo Foundation, Pavia, Italy) for helping us with FACS analysis and to Dr. Patrizia Vaghi (Centro Grandi Strumenti, University of Pavia, Pavia, Italy) for technical assistance with confocal microscopy analysis. This paper was supported by Cariplo Foundation (2010-0807 and 2013.0717), US National Institutes of Health (grant EB016041-01), Italian Ministry of Health (grant RF-2009-1550218), Italian Ministry of University and Research FIRB (grant RBFR1299KO).
Footnotes
Conflicts of Interests: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 2.Klein G. The extracellular matrix of the hematopoietic microenvironment. Experientia. 1995;51:914–926. doi: 10.1007/BF01921741. [DOI] [PubMed] [Google Scholar]
- 3.Thon JN, Montalvo A, Patel-Hett S, Devine MT, Richardson JL, Ehrlicher A, Larson MK, Hoffmeister K, Hartwig JH, Italiano JE., Jr Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191(4):861–74. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Jr, Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 5.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10(1):64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 6.Machlus KR, Italiano JE., Jr The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201(6):785–96. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61(2):198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339(1):237–46. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson SK, Debatis ME, Dooner MS, Madri JA, Quesenberry PJ, Becker PS. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem. 1998;46(3):371–7. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- 10.Malara A, Currao M, Gruppi C, Celesti G, Viarengo G, Buracchi C, Laghi L, Kaplan DL, Balduini A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells. 2014;32(4):926–37. doi: 10.1002/stem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Naveiras O, Balduini A, Mammoto A, Conti MA, Adelstein RS, Ingber D, Daley GQ, Shivdasani RA. The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood. 2007;110(1):171–9. doi: 10.1182/blood-2007-02-071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang Y, Auradé F, Larbret F, Zhang Y, Le Couedic JP, Momeux L, Larghero J, Bertoglio J, Louache F, Cramer E, Vainchenker W, Debili N. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109(10):4229–36. doi: 10.1182/blood-2006-04-020024. [DOI] [PubMed] [Google Scholar]
- 13.Balduini A, Pallotta I, Malara A, Lova P, Pecci A, Viarengo G, Balduini CL, Torti M. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6(11):1900–7. doi: 10.1111/j.1538-7836.2008.03132.x. [DOI] [PubMed] [Google Scholar]
- 14.Shin JW, Swift J, Spinler KR, Discher DE. Myosin-II inhibition and soft 2D matrix maximize multinucleation and cellular projections typical of platelet-producing megakaryocytes. Proc Natl Acad Sci U S A. 2011;108(28):11458–63. doi: 10.1073/pnas.1017474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malara A, Gruppi C, Pallotta I, Spedden E, Tenni R, Raspanti M, Kaplan D, Tira ME, Staii C, Balduini A. Extracellular matrix structure and nano-mechanics determine megakaryocyte function. Blood. 2011;118(16):4449–53. doi: 10.1182/blood-2011-04-345876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen XD, Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280(34):30481–9. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 17.Maurer AM, Gezer A. Promoting Effects of Heparin on ex vivo Expansion of Megakaryocytopoiesis from Human Cord Blood CD34+ Cells. Transfus Med Hemother. 2013;40(5):344–50. doi: 10.1159/000355519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiwakura I, Teramachi T, Kakizaki I, Takagi Y, Takahashi TA, Takagaki K. The effects of glycosaminoglycans on thrombopoietin-induced megakaryocytopoiesis. Haematologica. 2006;91(4):445–51. [PubMed] [Google Scholar]
- 19.Hunt P, Hokom MM, Wiemann B, Leven RM, Arakawa T. Megakaryocyte proplatelet-like process formation in vitro is inhibited by serum prothrombin, a process which is blocked by matrix-bound glycosaminoglycans. Exp Hematol. 1993;21(2):372–81. [PubMed] [Google Scholar]
- 20.Shen ZX, Basara N, Xi XD, Caen J, Maffrand JP, Pascal M, Petitou M, Lormeau JC, Han ZC. Fraxiparin, a low-molecular-weight heparin, stimulates megakaryocytopoiesis in vitro and in vivo in mice. Br J Haematol. 1994;88(3):608–12. doi: 10.1111/j.1365-2141.1994.tb05080.x. [DOI] [PubMed] [Google Scholar]
- 21.Han ZC, Bellucci S, Shen ZX, Maffrand JP, Pascal M, Petitou M, Lormeau J, Caen JP. Glycosaminoglycans enhance megakaryocytopoiesis by modifying the activities of hematopoietic growth regulators. J Cell Physiol. 1996;168(1):97–104. doi: 10.1002/(SICI)1097-4652(199607)168:1<97::AID-JCP12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Tajika K, Ikebuchi K, Dan K, Asano S. A role of GAGs in ECM on morphogenesis of megakaryocytes. Br J Haematol. 1996;94(1):34–9. doi: 10.1046/j.1365-2141.1996.d01-1781.x. [DOI] [PubMed] [Google Scholar]
- 23.Strassel C, Eckly A, Léon C, Moog S, Cazenave JP, Gachet C, Lanza F. Hirudin and heparin enable efficient megakaryocyte differentiation of mouse bone marrow progenitors. Exp Cell Res. 2012;318(1):25–32. doi: 10.1016/j.yexcr.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 25.McDonald JA, Camenisch TD. Hyaluronan: genetic insights into the complex biology of a simple polysaccharide. Glycoconj J. 2002;19:331–339. doi: 10.1023/A:1025369004783. [DOI] [PubMed] [Google Scholar]
- 26.Vigetti D, Ori M, Viola M, Genasetti A, Karousou E, Rizzi M, Pallotti F, Nardi I, Hascall VC, De Luca G, Passi A. Molecular cloning and characterization of UDP-glucose dehydrogenase from the amphibian Xenopus laevis and its involvement in hyaluronan synthesis. J Biol Chem. 2006;281:8254–8263. doi: 10.1074/jbc.M508516200. [DOI] [PubMed] [Google Scholar]
- 27.Vigetti D, Genasetti A, Karousou E, Viola M, Clerici M, Bartolini B, Moretto P, De Luca G, Hascall VC, Passi A. Modulation of hyaluronan synthase activity in cellular membrane fractions. J Biol Chem. 2009;284:30684–30694. doi: 10.1074/jbc.M109.040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 29.Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008;18:275–280. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Greenwald RA, Moy WW. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980;23:455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- 31.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 32.Culty M, Miyake K, Kincade PW, Sikorski E, Butcher EC, Underhill C. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990;111(6 Pt 1):2765–74. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardwick C, Hoare K, Owens R, Hohn HP, Hook M, Moore D, Cripps V, Austen L, Nance DM, Turley EA. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol. 1992;117(6):1343–50. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26(1):58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 35.Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A. Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta. 2014;1840(8):2452–9. doi: 10.1016/j.bbagen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103(8):2981–9. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 37.Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, Bertoncello I, Nilsson SK. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118(6):1516–24. doi: 10.1182/blood-2010-08-303800. [DOI] [PubMed] [Google Scholar]
- 38.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Advanced Materials. 2011;23(12):H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurski LA, Jha AK, Zhang C, Jia X, Farach-Carson MC. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials. 2009;30(30):6076–6085. doi: 10.1016/j.biomaterials.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan L, Ren Y, Cui F, Xu Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. Journal of Neuroscience Research. 2009;87(14):3207–3220. doi: 10.1002/jnr.22142. [DOI] [PubMed] [Google Scholar]
- 41.Perng CK, Wang YJ, Tsi CH, Ma H. In vivo angiogenesis effect of porous collagen scaffold with hyaluronic acid oligosaccharides. Journal of Surgical Research. 2011;168(1):9–15. doi: 10.1016/j.jss.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 42.Takeda K, Sakai N, Shiba H, Nagahara T, Fujita T, Kajiya M, et al. Characteristics of high-molecular-weight hyaluronic acid as a brain-derived neurotrophic factor scaffold in periodontal tissue regeneration. Tissue Engineering Part A. 2011;17(7–8):955–967. doi: 10.1089/ten.TEA.2010.0070. [DOI] [PubMed] [Google Scholar]
- 43.Allison DD, Grande-Allen KJ. Hyaluronan: a powerful tissue engineering tool. Tissue Engineering. 2006;12(8):2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 44.Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balduini A, Di Buduo CA, Malara A, Lecchi A, Rebuzzini P, Currao M, Pallotta I, Jakubowski JA, Cattaneo M. Constitutively released adenosine diphosphate regulates proplatelet formation by human megakaryocytes. Haematologica. 2012 Nov;97(11):1657–65. doi: 10.3324/haematol.2011.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nettles DL, Parker Vail T, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004 Mar;32(3):391–7. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 47.Khetan S, Burdick JA. Cellular encapsulation in 3D Hydrogel for tissue engineering. J Vis Exp. 2009;(32) doi: 10.3791/1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesengial cells in response to hyperglicemia induce monocyte adhesion. J Biol Chem. 2004;279(11):10279–85. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- 49.Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. J Thromb Haemost. 2009;7(Suppl 1):200–5. doi: 10.1111/j.1538-7836.2009.03378.x. [DOI] [PubMed] [Google Scholar]
- 50.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 51.Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994;6:726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 52.Vigetti D, Viola M, Karousou E, Rizzi M, Moretto P, Genasetti A, Clerici M, Hascall VC, De Luca G, Passi A. Hyaluronan–CD44–ERK1/2 regulate human aortic smooth muscle cell motility during aging. J Biol Chem. 2008;283:4448–4458. doi: 10.1074/jbc.M709051200. [DOI] [PubMed] [Google Scholar]
- 53.Yang B, Zhang L, Turley EA. Identification of two hyaluronan-binding domains in the hyaluronan receptor RHAMM. J Biol Chem. 1993;268:8617–8623. [PubMed] [Google Scholar]
- 54.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 55.Nikitovic D, Kouvidi K, Karamanos NK, Tzanakakis GN. The roles of hyaluronan/RHAMM/CD44 and their respective interactions along the insidious pathways of fibrosarcoma progression. Biomed Res Int. 2013;2013:929531. doi: 10.1155/2013/929531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kouvidi K, Berdiaki A, Nikitovic D, Katonis P, Afratis N, Hascall VC, Karamanos NK, Tzanakakis GN. Role of receptor for hyaluronic acid-mediated motility (RHAMM) in low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell adhesion. J Biol Chem. 2011;286:38509–38520. doi: 10.1074/jbc.M111.275875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blauteau D, Balduini A, Balayn N, Currao M, Nurden P, Deswarte C, Leverger G, Noris P, Perrotta S, Solary E, Vainchenker W, Debili N, Favier R, Raslova H. Thrombocytopenia-associated mutations in the ANKRD26 regulatory region induce MAPK hyperactivation. J Clin Invest. 2014 Feb 3;124(2):580–91. doi: 10.1172/JCI71861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Currao M, Balduini CL, Balduini A. High doses of romiplostim induce proliferation and reduce proplatelet formation by human megakaryocytes. PLoS One. 2013;8(1):e54723. doi: 10.1371/journal.pone.0054723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leach J, Bivens KA, Patrick CW, Schmidt JC. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnology and Bioengineering. 2003;82(5):578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 60.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 61.Seidlits SK, Drinnan CT, Petersen RR, Shear JB, Suggs LJ, Schmidt CE. Fibronectin–hyaluronic acid composite hydrogels for three dimensional endothelial cell culture. Acta Biomater. 2011;7(6):2401–2409. doi: 10.1016/j.actbio.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 62.Skardal A, Jianxing Z, McCoard L, Xu X, Siam O, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Engineering Part A. 2010;16(8):2675–2685. doi: 10.1089/ten.tea.2009.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin JW, Buxboim A, Spinler KR, Swift J, Christian DA, Hunter CA, Léon C, Gachet C, Dingal PC, Ivanovska IL, Rehfeldt F, Chasis JA, Discher DE. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014 Jan 2;14(1):81–93. doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rehfeldt F, Brown AE, Raab M, Cai S, Zajac AL, Zemel A, Discher DE. Hyaluronic acid matrices show matrix stiffness in 2D and 3D dictates cytoskeletal order and myosin-II phosphorylation within stem cells. Integr Biol (Camb) 2012 Apr;4(4):422–30. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


