Abstract
Purpose
Triptan medications are serotonin agonists used to treat migraine, a chronic pain condition highly prevalent in women of reproductive age. Data on the safety of triptans during pregnancy are scant. We sought to quantify the association of prenatal triptan exposure on neurodevelopment in three-year-old children.
Methods
Using data from the Norwegian Mother and Child Cohort Study, we used propensity score matching to examine associations between prenatal triptan exposure and psychomotor function, communication, and temperament. We used an external validation study to perform propensity calibration to adjust effect estimates for confounders unmeasured in the main study (migraine severity, type, and maternal attitudes towards medication use).
Results
We identified 4,204 women who reported migraine headache at baseline, of which 375 (8.9%) reported using a triptan ≥ once during pregnancy. Children with prenatal triptan exposure had 1.37-fold greater unadjusted odds of fine motor problems (95% CI: 1.06-1.77), which decreased after propensity score matching (OR: 1.29, 95% CI 0.97-1.73) and was further attenuated after calibration (OR: 1.25, 95%CI 0.89- 1.74). We observed no increased risk for gross motor or communication problems, and no differences in temperament. Adjustment for migraine severity using propensity score calibration had a moderate impact on effect estimates, with percent changes ranging from 2.4% to 50%.
Conclusions
Prenatal triptan exposure was not associated with psychomotor function, communication problems, or temperament in three-year-old children. Adjustment for migraine severity reduced effect estimates, and should be considered in future studies of the safety of triptans during pregnancy.
Keywords: pregnancy medication, migraine, child neurodevelopment, propensity score calibration
Introduction
Migraine headache is a relapsing-remitting pain condition with a prevalence in women of reproductive age of 16-18%.1,2 The frequency and severity of migraines changes during pregnancy: 60-70% of women with migraine experience improvement of symptoms during pregnancy, with 20% of these reporting complete remission. Migraine course during pregnancy tends to be better in women with menstrual migraine as well as in women who have migraines without aura.1 However, if symptoms do not improve during the first trimester, migraines are likely to continue throughout pregnancy.1 Given the high prevalence of migraine headache in women of childbearing age, lack of migraine resolution until after first trimester, and high rates of unplanned pregnancy,3 the potential for early-pregnancy exposure to medications used to treat migraines is high.
Triptans are the most frequently-used prescription antimigraine medications;4 they are 5-HT1B, 5-HT1D and 5-HT1F receptor agonists that act on the trigeminocervical complex and smooth muscle. Ten studies (including >6,000 exposed infants) have examined the safety of triptan use during pregnancy on pregnancy outcomes, and noted no increased risk for congenital malformations,5-7 although some evidence suggests that triptan use may be associated with pre-eclampsia or preterm birth.7 A recent review of treatment options for pregnant women with migraine concluded that if acetaminophen was not effective, limited use of triptans could be considered.8
While several studies focus on immediate pregnancy outcome following exposure to triptans, long-term outcomes have received limited attention. Triptans cross the placenta and the blood-brain barrier and bind to 5-HT1B and 5-HT1D receptors which are found in fetal brain,9 and have a biologically-plausible mechanism for effects on the developing brain. Previously, we evaluated prenatal triptan exposure as a risk factor for internalizing and externalizing behaviors, and found that three-year-old children exposed prenatally to triptans had a 40% increased risk for externalizing problems.10 This was the first study on long-term safety of triptans; however, it is unknown whether prenatal exposure to triptans or migraine in itself may increase the risk of other neurodevelopmental outcomes like psychomotor development, temperament and communication. Building from our previous work, the aim of the current study was to evaluate the effect of prenatal exposure to triptans on these outcomes, using validated, parent-reported screening tools. Because several potentially-important confounders, migraine severity and type, were unmeasured in our main study, we used propensity score calibration using an external validation study to adjust our effect estimates for migraine severity and type.
We hypothesized that children born to women who took a triptan during pregnancy would have higher rates of psychomotor and communication problems and more extreme temperaments, particularly in the activity and emotionality domains, at three years of age than children unexposed to triptans, and that if these problems were due to the underlying disorder, adjustment for migraine severity would be necessary.
Methods
Norwegian Mother and Child Cohort Study (MoBa) Sample
Between 1999 and 2008, the Norwegian Institute of Public Health invited women to participate in the Norwegian Mother and Child Cohort Study (MoBa). Women were invited prior to their first routine ultrasound appointment (gestational week 13-17). A total of 108,841 women consented to participate (participation rate 40.6%), with 84.8% of the participants completing the six month post-partum questionnaire and 60.2% completing the 36 month post-partum questionnaire.11,12 Informed consent was obtained from all participants, and the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate approved the study; this analysis was granted an exemption from the University of Massachusetts Medical School Institutional Review Board. Data were taken from Data Version 6, released by MoBa in 2012 and includes all children born before 2009 for whom the age three years questionnaire was received by May 4, 2011; these data were linked to the Medical Birth Registry of Norway (MBRN) using participants’ 11-digit personal identification numbers. Figure 1 shows the exclusion criteria and the development of the analysis sample (n=4,204).
Fig 1.
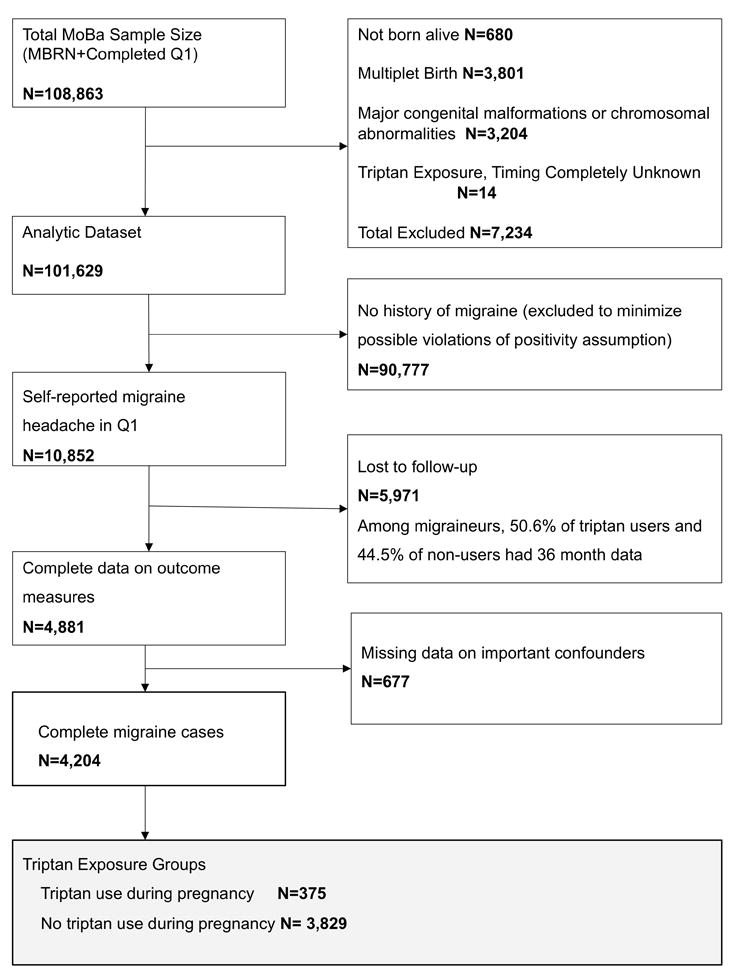
“Inclusion and exclusion criteria”.
Ascertainment of triptan exposure
Information on exposure to medications was gathered prospectively from two prenatal (Q1-gestational week17, Q3-gestational week 30) and one postpartum questionnaire (Q4-6 months post partum). Q2 is concerned with diet during pregnancy, and was not used in this analysis. Women were asked to indicate when they had taken a medication (during the six months before pregnancy, during weeks 0-4, 5-8, 9-12, and/or 13 or later for Q1, during weeks 13-16, 17-20, 21-24, 25-28, and/or week 29 or later for Q3, and from week 30 until birth for Q4), and to write the name of the medication in a text box. Women who indicated multiple medications in a single text box were assumed to have been exposed to all listed medications in all time periods. No information was available on formulation or dose. Exposure was categorized into two indicator variables: triptan use during pregnancy and prior to pregnancy (yes or no); we categorized pregnancy exposure in this way, rather than by trimester, because the entire pregnancy can be considered a vulnerable period for neurodevelopment.13
Ascertainment of outcome
Emotionality, Activity, and Shyness Temperament Questionnaire (EAS)
The Emotionality, Activity, and Shyness Temperament Questionnaire measures four domains (emotionality, shyness, sociability, and activity). A substantial body of literature has linked early childhood temperament to later life depression and other psychiatric diagnoses.14,15 The shortened version of the EAS used in the MoBa study, was developed with Norwegian social norms in mind, and includes 12 descriptions (e.g. “Your child likes to be with people;” “Your child cries easily.”), and parents are asked to rate how well each statement applies to their child.16 We calculated z-scores for each domain; higher z-scores indicate greater endorsement of each trait (e.g. more shy) relative to reports of other children in the sample.
Ages and Stages Questionnaire (ASQ)
The Ages and Stages Questionnaire is a parent-completed questionnaire appropriate for children aged four months to five years. Deficits detected by the ASQ predict school difficulties in older children,17 and fine motor skills predict later academic achievement.18 The abbreviated ASQ used in MoBa includes questions about developmental milestone attainment in three major categories: gross motor (two items), fine motor (two items), and communication (six items); this shortened version, adapted from the ASQ:SE-2, has been validated in a Norwegian population.19 We categorized the ASQ to indicate children who had not yet acquired skills in particular domains, identified by a parent reporting “Not yet” (vs. “Yes” or “Sometimes”).
Concomitant medication use
We examined other pain medications and psychotropic medications as ever vs. never used prior to and during pregnancy; details are available in the supplemental material
Potential confounders
Maternal age, pre-pregnancy body mass index (BMI) (underweight or <18.5 kg/m2, normal weight or 18.5-25 kg/m2, or overweight, >25 kg/m2 according to WHO guidelines), education (primary or secondary vs. university or higher), marital status (married or cohabiting vs. other), parity (multiparous vs. primiparous) and depression history (yes or no) were all ascertained by self-report. Smoking and alcohol use (ever during pregnancy vs. not during pregnancy) were ascertained by combining information from self-report and linkage to the Medical Birth Registry of Norway.
MIMEGA Migraine sample description
Women with migraine who were ≥18 years of age and who were currently pregnant or had a child <18 months old were invited to participate in the MIMEGA migraine study20 from October 1 2013-December 9 2014. Recruitment was done by the Oslo Migraine Clinic and the Norwegian Migraine Association, in addition to advertisements on websites and the study’s Facebook page. Women filled out an anonymous electronic questionnaire (http://www.nettskjema.uio.no/). The Regional Committee for Medical and Health Research Ethics (Region North) approved the study, and informed consent was obtained (n=380).
Women self-reported their age, education (primary or secondary vs university or higher), marital status (married or cohabiting vs. other), parity (multiparous vs. primiparous) smoking and alcohol use during pregnancy (use after recognition of pregnancy vs. no use), and use of other medications (triptans, acetaminophen, NSAIDs, and opioids).
Women were asked about the severity of migraine attacks during pregnancy using the Migraine Severity Scale 21, a four item questionnaire that assessed pain severity, tolerance, nausea, and impairment of daily activity due to migraine. Scores range from 0 to 15, and higher scores indicate greater severity. The Beliefs About Medications Questionnaire (BMQ)22,23 includes 10 statements to assess patients’ concerns about medications and views on medication necessity, divided into two subscales: the BMQ-Necessity and the BMQ-Concerns, each with five items; respondents indicate their agreement with each item (1=strongly disagree, 5=strongly agree), and items are summed to produce a total score (range 5-25), with higher scores indicating greater belief in the concepts represented by the subscale.
Statistical Analysis
We began analysis by estimating the prevalence of triptan exposure during pregnancy, and then obtaining descriptive statistics for the sample (collectively and by prenatal triptan exposure). Four modeling approaches were used to provide: 1) multivariable-adjusted; 2) propensity score matched; 3) propensity score adjusted; and 4) propensity score calibration estimates.
We modeled the EAS temperament outcomes by fitting general linear models and the ASQ problems outcomes by fitting logistic models, beginning with an unadjusted model and adding potential confounders into the model singly to assess the extent to which each confounder changed the measure of association between triptan use in pregnancy and the outcome variable. The final set of confounders used in all models included: age, pre-pregnancy BMI, marital status, education, smoking or alcohol use during pregnancy, depression, use of triptans prior to pregnancy, and concomitant medication use during pregnancy.
To create the propensity score, we used logistic regression in which triptan exposure (operationalized as any exposure during pregnancy) was the outcome variable and the variables previously selected as material or theoretical confounders during multivariable model building were predictors. We implemented propensity score matching without replacement using psmatch_multi, a SAS macro that uses optimal local matching to retain matches for the exposed units with the least number of possible matches first;24 we performed a 1:2 match, with non-replacement and a caliper of 0.10. We then compared the outcome measures (EAS and ASQ subscales) using general linear models for the continuous EAS outcomes and logistic regression for the categorical ASQ outcomes. These analyses included covariates that remained unbalanced after propensity matching. We also used propensity score as a covariate in outcome models fit in the full sample. Further details on propensity methods are included in the supplemental file.
Sensitivity analysis: propensity score calibration
The MoBa study did not include information on several potentially-important confounders, including migraine type and severity and medication beliefs. We implemented a method first described by Sturmer et al 25, which combines propensity score adjustment with regression calibration using an external validation study. We first estimated an “error-prone” propensity score in the MIMEGA sample using only the confounders available in both the MoBa sample and the MIMEGA sample: age, education, marital status, parity, smoking or alcohol use during pregnancy, history of depression, pre-pregnancy triptan use, and concomitant medication use during pregnancy. Next, we estimated a “gold standard” propensity score in the MIMEGA study using predictors available in MoBa and predictors available in our external validation sample, including migraine type, severity, and medication attitudes. The c-statistics for the error-prone propensity score model and gold-standard propensity score model were 0.81 and 0.87, respectively, indicating that the addition of information from the new predictors improved the gold-standard PS. Because regression calibration may fail in the presence of extreme measurement error (in this case, if the error-prone PS and gold standard PS widely diverged), we estimated the correlation between the two propensity scores and found it to be acceptable (r=0.81-0.89). Finally, we used a SAS macro (available at http://www.hsph.harvard.edu/donna-spiegelman/software/blinplus-macro/) to calibrate the error-prone PS estimated in MoBa using regression parameters obtained in the external validation study, and calculated the percent change (calculated as [ (PSCadjusted − PSadjusted) / PSCadjusted ] *100) from the PS-adjusted models to the PS-adjusted and calibrated models, to understand the magnitude of the impact that confounding by migraine type, severity, and medication beliefs may have had on effect estimates.
Results
MoBa Sample Description
Of the 4,204 women included in this study, 25.4% reported use of triptans prior to pregnancy and 8.9% used a triptan at least once during pregnancy. Among prenatal triptan users, 77.4% used during the first trimester, 34.1% during the second and third trimesters, and 11.5% reported triptan use during pregnancy but did not report timing of exposure. Women who took a triptan during pregnancy more often had a history of triptan use prior to pregnancy, and during pregnancy, were more likely to have used opioids, acetaminophen, and NSAIDs. Rates of concomitant medication use were higher among women who used triptans during pregnancy, while women with no triptan use prior to pregnancy more often discontinued other medications use. After propensity score matching, balance improved between exposed and unexposed groups on pre-pregnancy use of medications triptans and opioids, and alcohol use during pregnancy; use of opioids and NSAIDs during pregnancy remained somewhat unbalanced after matching (Table 1).
Table 1.
Maternal characteristics and medication use among women with history of migraine headache in the Norwegian Mother and Child Cohort Study (MoBa), before and after propensity score matching
| Full Sample | Propensity-Matched Sample | |||
|---|---|---|---|---|
| Triptans In Pregnancy | No Triptans In Pregnancy | Triptans In Pregnancy | No Triptans In Pregnancy | |
|
| ||||
| N=375 | N=3,829 | N=365 | N=730 | |
| Age in years (Mean, SD) | 30.9(4.3) | 30.3(4.4) | 30.8(4.3) | 30.8(4.1) |
| BMI (kg/m2) | ||||
| <18.5 | 2.71 | 3.5 | 2.7 | 2.6 |
| 18.5-25 | 60.3 | 61.6 | 60.0 | 62.2 |
| >25 | 37.1 | 34.9 | 37.3 | 35.2 |
| Multiparous | 47.7 | 51.8 | 48.0 | 47.5 |
| Married or cohabitating | 95.5 | 97.2 | 95.9 | 97.0 |
| Mother Education | ||||
| Primary or secondary | 30.1 | 35.6 | 30.4 | 29.6 |
| University or higher | 69.9 | 64.4 | 69.6 | 70.4 |
| Smoking during pregnancy | 11.7 | 12.7 | 11.8 | 11.9 |
| Alcohol during pregnancy | 20.3 | 15.2 | 19.5 | 18.0 |
| Folate Supplementation | 60.8 | 58.8 | 60.8 | 62.1 |
| Medications Taken In Pregnancy | ||||
| Opioids | 12.8 | 4.8 | 10.4 | 6.1 |
| Acetaminophen | 76.0 | 65.0 | 75.3 | 73.6 |
| NSAIDs | 22.4 | 12.1 | 20.8 | 13.2 |
| Anticonvulsants | 0.3 | 0.3 | 0.3 | 0.1 |
| Antidepressants | 1.6 | 1.5 | 1.6 | 1.2 |
| Benzodiazepines | 1.6 | 0.5 | 1.4 | 0.7 |
| Medications Before Pregnancy | ||||
| Triptans | 84.0 | 19.7 | 83.6 | 83.4 |
| Opioids | 7.7 | 15.1 | 6.9 | 6.4 |
| Acetaminophen | 46.1 | 45.4 | 45.8 | 48.1 |
| NSAIDs | 21.9 | 23.8 | 20.8 | 25.5 |
| Anticonvulsants | 0.5 | 0.3 | 0.6 | 0.3 |
| Antidepressants | 5.1 | 3.9 | 5.2 | 4.7 |
| Benzodiazepines | 1.3 | 1.0 | 1.6 | 1.5 |
| Maternal Depression | 12.3 | 12.2 | 12.3 | 12.6 |
Values reported are column percents; age is reported as mean(standard deviation)
MIMEGA Calibration Sample Description
Among women in the MIMEGA study (n=380), 12.1%, reported triptan use during pregnancy. Triptan users were more likely than non-users to report depression, were more likely to have taken triptans prior to pregnancy, and had higher rates of use of opioids, acetaminophen and NSAIDs during pregnancy. Women who reported triptan use during pregnancy reported higher migraine severity, were more likely to believe migraine medications were necessary, and showed higher levels of concern about using migraine medications than women who did not take triptans during pregnancy (Table 2).
Table 2.
Maternal characteristics and medication use among women with history of migraine headache, full sample and calibration sample
| MoBa Study | MIMEGA Study | |||
|---|---|---|---|---|
| Triptans In Pregnancy | No Triptans In Pregnancy | Triptans In Pregnancy | No Triptans In Pregnancy | |
|
| ||||
| N=375 | N=3,829 | N=46 | N=334 | |
| Age in years (Mean, SD) | 30.9(4.3) | 30.3(4.4) | 31.5(5.9) | 30.7(4.9) |
| BMI (kg/m2) | ||||
| <18.5 | 2.7 | 3.5 | -- | -- |
| 18.5-25 | 60.3 | 61.6 | -- | -- |
| >25 | 37.1 | 34.9 | -- | -- |
| Multiparous | 47.7 | 51.8 | 69.6 | 82.6 |
| Married or cohabitating | 95.5 | 97.2 | 93.5 | 95.2 |
| Mother Education | ||||
| Primary or secondary | 30.1 | 35.6 | 67.4 | 75.5 |
| University or higher | 69.9 | 64.4 | 32.6 | 24.6 |
| Smoking during pregnancy | 11.7 | 12.7 | 8.7 | 6.6 |
| Alcohol during pregnancy | 20.3 | 15.2 | 8.7 | 1.8 |
| Folate Supplementation | 60.8 | 58.8 | 89.1 | 86.5 |
| Medications Taken In Pregnancy | ||||
| Opioids | 12.8 | 4.8 | 23.9 | 9.3 |
| Acetaminophen | 76.0 | 65.0 | 78.3 | 65.3 |
| NSAIDs | 22.4 | 12.1 | 23.9 | 6.0 |
| Anticonvulsants | 0.3 | 0.3 | -- | -- |
| Antidepressants | 1.6 | 1.5 | -- | -- |
| Benzodiazepines | 1.6 | 0.5 | -- | -- |
| Medications Before Pregnancy | ||||
| Triptans | 84.0 | 19.7 | 97.8 | 61.7 |
| Opioids | 7.7 | 5.1 | 21.7 | 18.3 |
| Acetaminophen | 46.1 | 45.4 | 73.9 | 65.0 |
| NSAIDs | 21.9 | 23.8 | 60.9 | 58.1 |
| Anticonvulsants | 0.5 | 0.3 | -- | -- |
| Antidepressants | 5.1 | 3.9 | -- | -- |
| Benzodiazepines | 1.3 | 1.0 | -- | -- |
| Maternal Depression | 12.3 | 12.2 | 8.7 | 4.2 |
| Migraine Type | ||||
| With Aura | -- | -- | 37.0 | 42.8 |
| Without Aura | -- | -- | 43.5 | 31.7 |
| Other/Do not know | -- | -- | 19.6 | 25.5 |
| Migraine Severity Score (MIGSEV) (Mean, SD) | -- | -- | 12.0(2.6) | 9.1(4.7) |
| Beliefs About Medicines Questionnaire (BMQ): Necessity Subscale (Mean, SD) | -- | -- | 18.1(4.0) | 13.9(5.0) |
| Beliefs About Medicines Questionnaire (BMQ): Concern Subscale (Mean, SD) | -- | -- | 15.4(4.2) | 13.5(4.5) |
Values reported are column percents; age, Migraine Severity Score, and Beliefs About Medicines Questionnaire are reported as mean (standard deviation)
Comparison of MoBa and MIMEGA samples
Comparing the characteristics of the MoBa and MIMEGA samples revealed several differences, particularly in education, rates of cigarette and alcohol use in pregnancy, rate of folate supplementation, and rate of other medication use (Table 2). However, when we examined the associations between individual confounders and use of triptans during pregnancy for the MoBa and calibration studies, we found that maternal age and use of opioids, acetaminophen, and NSAIDs during pregnancy were similarly associated with triptan use during pregnancy, as was pre-pregnancy triptan and opioid use. Migraine severity was associated with triptan use in pregnancy, as were beliefs about medication necessity and concerns about medication use; migraine type was not associated with triptan use (Table 3).
Table 3.
Propensity for use of triptans in pregnancy for the main (“MoBa”) study and the external validation study (“MIMEGA”)
| MoBa Study | MIMEGA Study | |||
|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | |
| Age in years1 | 1.0 | 1.0 to 1.1 | 1.0 | 1.0 to 1.1 |
| BMI (kg/m2) | ||||
| <18.5 | 0.8 | 0.4 to 1.5 | -- | -- |
| 18.5-25 | REF | -- | -- | |
| >25 | 1.1 | 0.9 to 1.4 | -- | -- |
| Multiparous | 0.9 | 0.7 to 1.1 | 0.5 | 0.2 to 1.0 |
| Married or cohabitating | 0.6 | 0.4 to 1.0 | 0.7 | 0.2 to 2.6 |
| Mother Education | ||||
| University or higher vs. other categories | 0.8 | 0.6 to 1.0 | 1.5 | 0.8 to 2.9 |
| Smoking during pregnancy | 0.9 | 0.7 to 1.3 | 1.4 | 0.4 to 4.1 |
| Alcohol during pregnancy | 1.4 | 1.1 to 1.9 | 5.2 | 1.4 to 19.2 |
| Folate Supplementation | 1.1 | 0.9 to 1.4 | 1.3 | 0.5 to 3.4 |
| Medications Taken In Pregnancy | ||||
| Opioids | 2.9 | 2.1 to 4.1 | 3.1 | 1.4 to 6.6 |
| Acetaminophen | 1.7 | 1.3 to 2.2 | 1.9 | 0.9 to 4.0 |
| NSAIDs | 2.1 | 1.6 to 2.7 | 4.9 | 2.2 to 11.1 |
| Anticonvulsants | 0.9 | 0.1 to 6.6 | -- | -- |
| Antidepressants | 1.1 | 0.5 to 2.6 | -- | -- |
| Benzodiazepines | 2.6 | 1.0 to 6.9 | -- | -- |
| Medications Before Pregnancy | ||||
| Triptans | 21.4 | 16.1 to 28.5 | 27.9 | 3.8 to 204.9 |
| Opioids | 1.5 | 1.0 to 2.3 | 1.2 | 0.6 to 2.6 |
| Acetaminophen | 1.0 | 0.8 to 1.3 | 1.5 | 0.8 to 3.1 |
| NSAIDs | 0.9 | 0.7 to 1.2 | 1.1 | 0.6 to 2.1 |
| Anticonvulsants | 1.9 | 0.4 to 8.4 | -- | -- |
| Antidepressants | 1.3 | 0.8 to 2.1 | -- | -- |
| Benzodiazepines | 1.6 | 0.7 to 3.8 | -- | -- |
| Maternal Depression | 1.0 | 0.7 to 1.4 | 2.2 | 0.7 to 6.9 |
| Migraine Type | ||||
| With Aura vs Other/Don’t know | -- | -- | 1.1 | 0.5 to 2.6 |
| Without Aura vs Other/Don’t know | -- | -- | 1.8 | 0.8 to 4.1 |
| Migraine Severity Score (MIGSEV) 1 | -- | -- | 1.2 | 1.1 to 1.4 |
| Beliefs About Medicines Questionnaire (BMQ): Necessity Subscale 1 | -- | -- | 1.2 | 1.1 to 1.3 |
| Beliefs About Medicines Questionnaire (BMQ): Concern Subscale 1 | -- | -- | 1.1 | 1.0 to 1.2 |
Odds of triptan use in pregnancy associated with a one-unit increase in age, MIGSEV score or BMQ score
Main Outcome Analysis
Ages and Stages Questionnaire
In the MoBa sample, 2.5% of children had not yet attained at least one gross motor skill, 18.0% had not yet attained at least one fine motor skill, and 1.3% had not yet attained at least two communication skills. Unadjusted estimates for fine motor skills suggested an increased risk associated with prenatal triptan exposure (Odds Ratio (OR) 1.37, 95% Confidence Interval (CI): 1.06 to 1.77), which was reduced after propensity matching (OR 1.29, 95% CI: 0.94 to 1.76); multivariable and propensity adjustment attenuated this risk. No association was observed between prenatal triptan exposure and gross motor or communication skills (Table 4).
Table 4.
Comparison of associations between prenatal triptan exposure and neurodevelopmental outcome observed with multivariable adjusted, propensity adjusted, and propensity matched models
| β1 | 95% Confidence Interval | |
|---|---|---|
|
| ||
| Emotionality, Activity, and Shyness Questionnaire (EAS) | ||
| EAS Sociability | ||
| Unadjusted (MoBa study only) | 0.04 | -0.07 to 0.15 |
| Multivariable-adjusted (MoBa study only)2 | 0.02 | -0.10 to 0.14 |
| PS-adjusted (MoBa study only)3 | 0.02 | -0.10 to 0.14 |
| PS-matched (MoBa study only)4 | 0.02 | -0.11 to 0.15 |
| EAS Emotionality | ||
| Unadjusted (MoBa study only) | -0.02 | -0.14 to 0.10 |
| Multivariable-adjusted (MoBa study only)2 | 0.00 | -0.13 to 0.13 |
| PS-adjusted (MoBa study only)3 | -0.01 | -0.14 to 0.12 |
| PS-matched (MoBa study only)4 | -0.04 | -0.18 to 0.09 |
| EAS Activity | ||
| Unadjusted (MoBa study only) | 0.06 | -0.06 to 0.17 |
| Multivariable-adjusted (MoBa study only)2 | 0.03 | -0.09 to 0.16 |
| PS-adjusted (MoBa study only)3 | 0.03 | -0.09 to 0.16 |
| PS-matched (MoBa study only)4 | 0.05 | -0.09 to 0.18 |
| EAS Shyness | ||
| Unadjusted (MoBa study only) | 0.08 | -0.04 to 0.19 |
| Multivariable-adjusted (MoBa study only)2 | 0.05 | -0.07 to 0.18 |
| PS-adjusted (MoBa study only)3 | 0.05 | -0.07 to 0.18 |
| PS-matched (MoBa study only)4 | 0.09 | -0.04 to 0.22 |
|
| ||
| Ages and Stages Questionnaire (ASQ) | Odds Ratio1 | 95% Confidence Interval |
|
| ||
| ASQ Gross Motor | ||
| Unadjusted (MoBa study only) | 0.44 | 0.16 to 1.20 |
| Multivariable-adjusted (MoBa study only)2 | 0.42 | 0.15 to 1.18 |
| PS-adjusted (MoBa study only)3 | 0.46 | 0.16 to 1.31 |
| PS-matched (MoBa study only)4 | 0.54 | 0.18 to 1.61 |
| ASQ Fine Motor | ||
| Unadjusted (MoBa study only) | 1.37 | 1.06 to 1.77 |
| Multivariable-adjusted (MoBa study only)2 | 1.29 | 0.97 to 1.72 |
| PS-adjusted (MoBa study only)3 | 1.29 | 0.97 to 1.73 |
| PS-matched (MoBa study only)4 | 1.29 | 0.94 to 1.76 |
| ASQ Communication | ||
| Unadjusted (MoBa study only) | 1.22 | 0.52 to 2.88 |
| Multivariable-adjusted (MoBa study only)2 | 0.96 | 0.37 to 2.46 |
| PS-adjusted (MoBa study only)3 | 1.02 | 0.41 to 2.53 |
| PS-matched (MoBa study only)4 | 0.78 | 0.28 to 2.14 |
β is the change in standard deviation units associated with triptan exposure during fetal development, compared to no triptan exposure; Odds Ratio is the odds of not yet having acquired one or more skill in each domain associated with triptan exposure, relative to no exposure.
Adjusted for maternal age, pre-pregnancy BMI, reproductive history, marital status, maternal education, smoking or alcohol use during pregnancy, presence of depressive symptoms, pre-pregnancy triptan use, and co-medication use during pregnancy (acetaminophen, opioids, NSAIDs, antidepressants)
Adjusted for the propensity for taking triptans during pregnancy, conditional on maternal age, pre-pregnancy BMI, reproductive history, marital status, maternal education, smoking or alcohol use during pregnancy, presence of depressive symptoms, pre-pregnancy triptan use, and co-medication use during pregnancy (acetaminophen, opioids, NSAIDs, antidepressants)
Each exposed triptan user was matched to two non-users based on propensity score; models were further adjusted for factors that remained unbalanced after matching (pregnancy use of opioids and NSAIDs)
Emotionality, Activity, and Shyness Temperament Questionnaire
Before adjustment, children exposed to triptans during fetal development had slightly increased mean shyness (β 0.08, 95%CI -0.04 to 0.19); adjustment for propensity for triptan use, however, moved estimates closer to the null (β 0.05, 95%CI -0.07 to 0.18). No association was observed between triptan exposure and sociability, emotionality, or activity (Table 4).
Sensitivity Analysis: Propensity score calibration
To consider confounding by migraine type, severity, and medication attitudes, we performed propensity-score calibrations. In most cases, PS-calibrations moved point estimates closer to zero, with percent differences ranging from 8.8-50% relative to PS-adjusted models (EAS Sociability, Activity, and Shyness Scales; ASQ Fine Motor and Communication skills), suggesting that some unmeasured confounding by migraine type, severity, and medication attitudes was present in the traditional PS-adjusted effect estimates (Table 5).
Table 5.
Sensitivity analysis: propensity calibration to adjust for migraine type, severity, and medication beliefs
| β | 95% Confidence Interval | Percent Change3 from Main PS-adjusted to PSC-adjusted models | |
|---|---|---|---|
|
| |||
| Emotionality, Activity, and Shyness Questionnaire (EAS) | |||
| EAS Sociability | |||
| PS-adjusted (MoBa study only)1 | 0.02 | -0.10 to 0.14 | |
| PSC-adjusted (MoBa and Internet Migraine studies)2 | 0.01 | -0.13 to 0.14 | -50.0% |
| EAS Emotionality | |||
| PS-adjusted (MoBa study only)1 | -0.01 | -0.14 to 0.12 | |
| PSC-adjusted (MoBa and Internet Migraine studies)2 | -0.01 | -0.13 to 0.11 | 0.0% |
| EAS Activity | |||
| PS-adjusted (MoBa study only)1 | 0.04 | -0.08 to 0.17 | |
| PSC-adjusted (MoBa and Internet Migraine studies)2 | 0.03 | -0.11 to 0.17 | -25.0% |
| EAS Shyness | |||
| PS-adjusted (MoBa study only)1 | 0.06 | -0.07 to 0.19 | |
| PSC-adjusted (MoBa and Internet Migraine studies)2 | 0.04 | -0.06 to 0.18 | -33.3% |
|
| |||
| Ages and Stages Questionnaire (ASQ) | OR | 95% Confidence Interval | Percent Change3 from Main PS-adjusted to PSC-adjusted models |
|
| |||
| ASQ Gross Motor | |||
| PS-adjusted (MoBa study only)1 | 0.43 | 0.15 to 1.22 | |
| PSC-adjusted (MoBa and Internet Migraine studies)2 | 0.45 | 0.14 to 1.42 | -4.7% |
| ASQ Fine Motor | |||
| PS-adjusted (MoBa study only)1 | 1.28 | 0.96 to 1.71 | |
| PSC-adjusted (MoBa and Internet Migraine studies)2 | 1.25 | 0.89 to 1.74 | -2.4% |
| ASQ Communication | |||
| PS-adjusted (MoBa study only)1 | 1.02 | 0.42 to 2.48 | |
| PSC-adjusted (MoBa and Internet Migraine studies)2 | 0.93 | 0.31 to 2.81 | 9.7% |
Adjusted for the propensity for taking triptans during pregnancy, conditional on maternal age, pre-pregnancy BMI, reproductive history, marital status, maternal education, smoking or alcohol use during pregnancy, presence of depressive symptoms, pre-pregnancy triptan use, and co-medication use during pregnancy (acetaminophen, opioids, NSAIDs, antidepressants). PS-adjusted estimates are not IPC-weighted, and so differ slightly from those reported in Table 4.
Adjusted for the propensity for taking triptans during pregnancy (estimate from Table 3) and calibrated for migraine severity, migraine type, and attitude towards medication (BMQ Necessity and Concern scores), not including BMI and antidepressant exposure [inclusion of BMI and antidepressant use in PS did not result in substantial change in β estimates]
Percent change calculated as [ (PSCadjusted − PSadjusted) / PSCadjusted ] *100
Conclusion
Within the 10.9% of women in the MoBa study who reported migraine at baseline, 8.9% used a triptan medication at least once during pregnancy, which underscores the need for more information about the safety of these medications. Our study found no association between prenatal exposure to triptans and temperament or motor or communication skills, after adjustment for measured confounders and calibration for unmeasured confounders.
We hypothesized that migraine characteristics, including severity, would be associated with a woman’s likelihood of taking a triptan during pregnancy. Migraine itself is associated with adverse pregnancy outcomes, including hypertensive disorders, preterm birth26,27 and low birth weight,26 and increased risk of neurodevelopmental problems in children exposed in utero.28 This study provides evidence that migraine severity and medication beliefs were associated with triptan use in pregnancy. Independent of medication use, migraine severity in particular may also be associated with child neurodevelopment: chronic pain is related to increased stress, which in turn is associated with neurodevelopmental problems in children.29 We found that adjusting for migraine severity did influence our observed effect estimates, even within the context of a null study. Failing to consider these facets of the underlying indication for which the triptan medication is used could lead to overestimating the risk of prenatal triptan exposure.
Our study had several limitations. The MoBa study did not collect information on migraine type or severity, and results from our propensity calibration analysis suggest that adjustment for these factors tended to move effect estimates towards the null. The method we used to adjust for these factors, propensity score calibration, depends on an assumption of surrogacy, which states that the error-prone propensity score in the main MoBa study is independent of the outcome given the gold-standard propensity score in the validation study.30 Neurodevelopmental outcomes were not measured in the MIMEGA study, and so the surrogacy assumption is not verifiable. However, we hypothesized that the direction of confounding was similar for the variables measured in the MIMEGA and MoBa studies, and so should not result in a violation of surrogacy.31 In addition, lack of information on dose and formulation, as well as inadequate power to investigate specific triptans, may have resulted in residual confounding.
Despite these limitations, this study makes several important contributions. First, the study is set in the Norwegian Mother and Child Cohort Study, a prospective birth cohort in which information on medication exposure was gathered prior to observing any neurodevelopmental outcomes and therefore minimizing the potential for recall bias. We applied multiple methodological techniques to minimize, assess, and where possible, reduce bias due to measured and unmeasured confounders. Women who took triptans during pregnancy were different from those who did not in several ways, including the number of concomitant medications taken, history of depression, alcohol use during pregnancy, migraine severity and attitudes towards medication use: failure to adequately consider these confounders could have led us to attribute harmful effects to triptans that might be properly attributed to underlying disease.
Our prior work identified increased risks of externalizing behaviors associated with prenatal triptan exposure; the current study, focusing on temperament, psychomotor skills, and communication skills revealed no differences between children with and without prenatal triptan exposure. Taken together, the evidence so far is not sufficient to recommend changes to guidelines for use of triptans during pregnancy, which currently suggests that these medications may be used conservatively. Future studies should take into account migraine severity when considering the possible effects of prenatal exposure to triptan medications.
Supplementary Material
Key points.
Triptan exposure was not associated with motor skills, communication problems, or temperament differences in 36-month-old children.
Propensity calibration using an external validation study resulted in further attenuation of effect estimates; future studies should consider the role of migraine characteristics as potential confounders.
Acknowledgments
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1). We are grateful to all the participating families in Norway who take part in this on-going cohort study.
Dr. Lapane has received research support from Glaxo Smith Kline for research relating to lupus and from Janssen Connect for work relating to schizophrenia.
Dr. Frazier has received research support from Alcobra, Glaxo Smith Kline, Pfizer Inc., Neuren Roche, Seaside Therapeutics, and SyneRX International, and also serves on the Data Safety Monitoring Board for the clinical trial for adolescent depression sponsored by Forest Pharmaceuticals.
Footnotes
A version of this manuscript was included in Dr. Wood’s doctoral dissertation.
Conflicts of interest summary:
Drs Wood and Nordeng have no conflicts of interest.
Part of this work was presented at the 2015 International Society for Pharmacoepidemiology's Mid-Year Meeting in Bordeaux, France.
References
- 1.MacGregor EA. Headache in pregnancy. Neurol Cinics. 2012;30(3):835–66. doi: 10.1016/j.ncl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 3.Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu MK, Buse DC, Bigal ME, Serrano D, Lipton RB. Factors Associated With Triptan Use in Episodic Migraine: Results From the American Migraine Prevalence and Prevention Study. Headache J Head Face Pain. 2012;52(2):213–223. doi: 10.1111/j.1526-4610.2011.02032.x. [DOI] [PubMed] [Google Scholar]
- 5.Nezvalová-Henriksen K, Spigset O, Nordeng H. Triptan exposure during pregnancy and the risk of major congenital malformations and adverse pregnancy outcomes: results from the Norwegian Mother and Child Cohort Study. Headache. 2010;50(4):563–75. doi: 10.1111/j.1526-4610.2010.01619.x. [DOI] [PubMed] [Google Scholar]
- 6.Duong S, Nordeng H, Einarson A. Motherisk Update Safety of triptans for migraine headaches during pregnancy and breastfeeding. Can Fam Physician. 2010;56:537–539. [PMC free article] [PubMed] [Google Scholar]
- 7.Källén B, Nilsson E, Olausson PO. Delivery Outcome after Maternal Use of Drugs for Migraine. Drug Saf. 2011;34(8):691–703. doi: 10.2165/11590370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Amundsen S, Nordeng H, Nezvalova-Henriksen K, Stovner L, Spigset O. Pharmacological treatment of migraine during pregnancy and breastfeeding - current evidence and practical recommendations. Nat Rev Neurol. doi: 10.1038/nrneurol.2015.29. [DOI] [PubMed] [Google Scholar]
- 9.Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood ME, Lapane K, Frazier JA, Ystrom E, Mick E, Nordeng H. Prenatal triptan exposure increases externalizing behaviors at three years: results from the Norwegian Mother and Child Cohort Study. doi: 10.1111/ppe.12253. Manuscr under Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnus P. The Norwegian Mother and Child Cohort Study (MoBa) – new research possibilities. Nor Epidemiol. 2007;17(2):107–110. [Google Scholar]
- 12.Stoltenberg C, Schjølberg S, Bresnahan M, et al. The Autism Birth Cohort (ABC): a paradigm for gene-environment-timing research. Mol Psychiatry. 2010;15(7):676–680. doi: 10.1038/mp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10(4):303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annu Rev Clin Psychol. 2011;7:269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Althoff RR, Rettew DC, Faraone SV, Boomsma DI, Hudziak JJ. Latent class analysis shows strong heritability of the child behavior checklist-juvenile bipolar phenotype. Biol Psychiatry. 2006;60(9):903–911. doi: 10.1016/j.biopsych.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Mathiesen KS, Tambs K. The EAS Temperament Questionnaire--factor structure, age trends, reliability, and stability in a Norwegian sample. J Child Psychol Psychiatry. 1999;40:431–439. [PubMed] [Google Scholar]
- 17.Halbwachs M, Muller J-B, Tich SN, et al. Predictive Value of the Parent-Completed ASQ for School Difficulties in Preterm-Born Children < 35 Weeks’ GA at Five Years of Age. Neonatology. 2014;106:311–316. doi: 10.1159/000363216. [DOI] [PubMed] [Google Scholar]
- 18.Grissmer D, Grimm KJ, Aiyer SM, Murrah WM, Steele JS. Fine motor skills and early comprehension of the world: two new school readiness indicators. Dev Psychol. 2010;46(5):1008–1017. doi: 10.1037/a0020104. [DOI] [PubMed] [Google Scholar]
- 19.Richter J, Janson H. A validation study of the Norwegian version of the Ages and Stages Questionnaires. Acta Paediatr. 2007;96(5):748–52. doi: 10.1111/j.1651-2227.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 20.Amundsen S, Skretteberg AM, Gudmestad T, Buckley Poole AC, Nordeng H. Patients with migraine in pregnancy: pharmacological treatment, perceived disease control and information needs. under Rev. [Google Scholar]
- 21.El Hasnaoui A, Vray M, Richard A, Nachit-Ouinekh F, Boureau F Group M. Assessing the Severity of Migraine: Development of the MIGSEV Scale. Headache J Head Face Pain. 2003;43:628–635. doi: 10.1046/j.1526-4610.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 22.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. [Google Scholar]
- 23.Jónsdóttir H, Friis S, Horne R, Pettersen KI, Reikvam Å. Andreassen O a. Beliefs about medications: Measurement and relationship to adherence in patients with severe mental disorders. Acta Psychiatr Scand. 2009;119(4):78–84. doi: 10.1111/j.1600-0447.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 24.Fraeman KH. An introduction to implementing propensity score matching with SAS. NESUG. 2010:1–12. [Google Scholar]
- 25.Stürmer T, Schneeweiss S, Avorn J, Glynn RJ. Adjusting effect estimates for unmeasured confounding with validation data using propensity score calibration. Am J Epidemiol. 2005;162(3):279–89. doi: 10.1093/aje/kwi192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Facchinetti F, Allais G, Nappi RE, et al. Migraine is a risk factor for hypertensive disorders in pregnancy: a prospective cohort study. Cephalalgia. 2009;29(3):286–92. doi: 10.1111/j.1468-2982.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 27.Marozio L, Facchinetti F, Allais G, et al. Headache and adverse pregnancy outcomes: a prospective study. Eur J Obstet Gynecol Reprod Biol. 2012;161(2):140–3. doi: 10.1016/j.ejogrb.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Arruda Ma, Bigal ME. Migraine and behavior in children: influence of maternal headache frequency. J Headache Pain. 2012;13(5):395–400. doi: 10.1007/s10194-012-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31(4):285–92. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 30.Lunt M, Glynn RJ, Rothman KJ, Avorn J, Stürmer T. Propensity score calibration in the absence of surrogacy. Am J Epidemiol. 2012;175(12):1294–302. doi: 10.1093/aje/kwr463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stürmer T, Schneeweiss S, Rothman KJ, Avorn J, Glynn RJ. Performance of propensity score calibration- a simulation study. Am J Epidemiol. 2007;165(10):1110–1118. doi: 10.1093/aje/kwm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


