Abstract
Background and Purpose
Essential tremor (ET) is a neurological disorder with unknown aetiology. Its symptoms include cerebellar motor disturbances, cognitive and personality changes, hearing and olfactory deficits. Hyperactivity of excitotoxic cerebellar climbing fibres may underlie essential tremor and has been induced in rodents by systemic harmaline administration. Cannabinoid (CB) receptor agonists can cause motor disturbances; although, there are also anecdotal reports of therapeutic benefits of cannabis in motor disorders. We set out to establish the effects of CB receptor agonism and antagonism on an established rodent model of ET using a battery of accepted behaviour assays in order to determine the risk and therapeutic potential of modulating the endocannabinoid system in ET.
Experimental Approach
Behavioural effects of systemic treatment with a CB receptor agonist (0.1, 0.5 and 1 mg kg−1 WIN55, 212–2) or two CB1 receptor antagonists (1 mg kg−1 AM251 and 10 mg kg−1 rimonabant) on tremor induced in rats by harmaline (30 mg kg−1; i.p.), were assessed using tremor scoring, open field, rotarod, grip and gait tests.
Key Results
Overall, harmaline induced robust tremor that was typically worsened across the measured behavioural domains by CB receptor agonism but ameliorated by CB1 receptor antagonism.
Conclusions and Implications
These results provide the first evidence of the effects of modulating the endocannabinoid system on motor function in the harmaline model of ET. Our data suggest that CB1 receptor manipulation warrants clinical investigation as a therapeutic approach to protection against behavioural disturbances associated with ET.
Abbreviations
- ET
essential tremor
- MS
multiple sclerosis
- PC
Purkinje cell
Tables of Links
| TARGETS |
|---|
| GPCRs a |
| CB1 receptors |
| Ligand‐gated ion channels b |
| Glycine receptors |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
Simple essential tremor (ET) is a neurological disorder of unknown aetiology (prevalence: 0.4–3.9%), typically affecting the upper limbs and, less commonly, the head, jaw, tongue, trunk and lower limbs. Although a syndrome of tremor in posture and movement, cerebellar motor disturbances, cognitive and personality changes, hearing and olfactory deficits are also associated with ET (Deuschl and Elble, 2009). Interest in ET remains prominent due to its relatively high prevalence, adverse effect upon quality of life (Schmouth et al., 2014) and apparently increasing prevalence in diseases like multiple sclerosis (MS) (approximately 25%) (Fox et al., 2004). Treatment of ET includes pharmacotherapy with β adrenoceptor antagonists, anticonvulsants, neuroleptics and antidepressants; although, surgical treatments are required in approximately 50% of cases due to pharmacoresistance (Chopra et al., 2013), demonstrating a significant unmet clinical need (Koller and Vetere‐Overfield, 1989).
Hyperactivity of the excitotoxic climbing fibres has been suggested as one possible cause of ET and can be induced in laboratory animal species by i.p. administration of harmaline, a β carboline derivative of harmala alkaloids from Peganum harmala (Syrian Rue) seeds. Harmaline produces an 8–16 Hz tremor in mice and rats and, in rats, is associated with Purkinje cell (PC) loss (Handforth, 2012).
Recent studies have revealed a role for endocannabinoids in tremor disorders (Glass, 2001; Howard et al., 2013; Arjmand et al., 2015). Cannabinoid (CB) receptors and their endogenous ligands, the endocannabinoids, are abundant in brain areas that manage motor function where they play a neuromodulatory role (Rodriguez de Fonseca et al., 1998). The abundant expression of cerebellar CB1 receptors, particularly on PC inputs from interneurons and excitatory climbing fibres arising from granule cells and PC synapses, emphasises the importance of endocannbinoid signalling in the cerebellum where it modulates classical cerebellar neurotransmission via activity‐induced inhibition of presynaptic neurotransmitter release through inhibition of presynaptic Ca2 + influx, mediated by K+ channel activation (Daniel et al., 2004).
Although specific changes to cannabinergic signalling in motor diseases remain unclear and significant gaps in our understanding of cannabinergic influences on motor pathways remain, patients have claimed therapeutic benefits of medical cannabis in tremor‐associated diseases (Clifford, 1983). Reduced tremor and spasticity in animal models of MS have been reported following treatment with Δ9‐tetrahydrocannabinol (THC), a psychoactive plant cannabinoid (Baker et al., 2000; Koch et al., 2007), and numerous but unsubstantiated patient claims for benefits of cannabis use in ET have been made (Tudge et al., 2015). Interestingly, there are several publications showing dose‐dependent effects of the CB receptor partial agonist, Δ9‐tetrahydrocannabinol (Δ9‐THC), in this regard (Frederickson et al., 1976; Kujtan et al., 1983; Stanford and Fowler, 1998; Freedland et al., 2002). Most notably, a systematic review revealed that Δ9‐THC was probably ineffective for easing MS‐related tremors (Koppel et al., 2014) while, conversely, sustained use of Δ9‐THC‐rich extracts reduced tremor and spasticity in MS (Buccellato et al., 2011). Thus, there are confusing reports of cannabinoid effects upon tremor in MS, and, to date, no studies have investigated the effects of cannabinoids in ET, a discrete disorder. Here, we report the effects of a CB receptor agonist and two CB1 receptor antagonists on harmaline‐induced tremor in rats, using behavioural measures to determine whether endocannabinoid modulation represents a plausible therapeutic strategy for the treatment of ET. In addition, we have assessed the potential risks associated with therapeutic or recreational use of cannabinoid preparations by ET patients.
Methods
All animal care and experimental procedures were in accordance with National Institutes of Health guidelines and approved by the Kerman University of Medical Sciences. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Male Wistar Kyoto rats (40–60 g;P 24–28), provided by the Kerman Neuroscience Research Center, were group housed (2–3 animals per cage) in conventional laboratory rodent cages (Razirad Co., Iran) of dimensions 26.5 (W) × 15 (H) × 42 (L) cm and maintained on a 12 h light–dark cycle at a 23 ± 2°C with access to food and water ad libitum. Experiments were conducted during the light phase (08:00–16:00 h).
Three experiments (see Experimental design below) were undertaken, each of which employed five behavioural tasks: tremor scoring, open field test, rotarod test, grip strength test and gait analysis test (Vaziri et al., 2015). Tests were administered sequentially. Pilot studies (n = 16) revealed that 30 mg kg−1 harmaline induced stable tremor in this population for the duration of the testing period (2.5–3 h). Previous studies have revealed that harmaline produces tremor at doses of 9–50 mg kg−1 in laboratory rodent species (Al‐Deeb et al., 2002; Handforth, 2012; Shourmasti et al., 2014).
Behavioural assays
Tremor scoring
Tremor was rated by two observers blinded to treatment. Intra‐observer and inter‐observer reliability was assessed via the kappa coefficient (acceptance criterion: >80%). Tremor data were acquired during the open field test and quantitatively scored as follows: 0: no tremor; 1: occasional tremor affecting only the head and neck; 2: intermittent (occasional tremor affecting all body parts); 3: persistent (persistent tremor affecting all body parts and tail); and 4: severe (persistent tremor rendering the animal unable to stand and/or walk) (Al‐Deeb et al., 2002). Number of rearing events (standing on hind paws with a body‐floor angle >45°) (Lamprea et al., 2008) (a measure of vertical and explorative activity related to locomotor behaviour) and number of grooming events, i.e, coordinated, patterned and obsessive motor action of front paws or mouth on the fur (Komorowska and Pellis, 2004; Kalueff et al., 2007), per session were also recorded.
Open‐field test assessing locomotor behaviour
A Plexiglas arena [90 (W) × 90 (L) × 30 (H) cm] was used. Each animal was placed in the centre of the arena and horizontal activity recorded for 5 min with subsequent offline analysis (Ethovision 7.1, Noldus Information Technology, The Netherlands) that assessed total distance moved, duration of mobility and speed. The chamber was cleaned with 70% ethanol and dried between sessions (Vaziri et al., 2015).
Rotarod test
Motor and balance performance were evaluated by accelerating rotarod device (Hugo Sachs, Germany). Prior to placing an animal on the apparatus, rod rotation was set to 10 rpm. At test start, the animal was placed on the rod which was linearly accelerated at 10 rpm min−1 to a maximum of 60 rpm. Each animal undertook three trials with a 30 min inter‐trial rest interval. The duration for which each animal remained in the apparatus was recorded and the mean for all trials per animal calculated (Vaziri et al., 2015).
Wire grip test
The wire grip test assesses muscle strength and balance (Marks et al., 2009). Each animal was suspended by both forepaws from a horizontal steel wire (80 cm long, 7 mm diameter) suspended 45 cm from the ground. Each animal was held in a vertical position when its front paws were placed in contact with the wire. When the animal grasped the wire, it was released and latency to fall recorded with a stopwatch. Each animal undertook three trials with a 5 min inter‐trial rest interval.
Gait analysis test
The gait analysis test assesses animal walking patterns and gait kinematics. The hind paws of each animal were marked with a non‐toxic ink and the animal allowed to traverse a clear Plexiglas tunnel [100 cm (L) × 10 cm (H) × 10 cm (W)] lined with white absorbent paper (100 × 10 cm) and ending in a darkened cage. The resulting tracks provide the spatial relationship of consecutive footfalls from which animal stride length and width were measured. Animals were habituated to the runway for three training runs before testing. Hind paw stride lengths were measured by distance (cm) between the respective paw prints to the successive ipsilateral prints to assess unilateral or bilateral effects of treatment upon gait. Hind paw stride widths were measured by distance between the centers of the respective paw prints to the corresponding contralateral stride length measurements at a right angle. Footprints at the beginning and end of each run were not considered in the analysis (Wecker et al., 2013).
Experimental design
The present study comprised three discrete experiments. Experiment 1 assessed the effects of harmaline in the behavioural tests described. Here, two groups of animals were employed, one of which received harmaline (30 mg kg−1; i.p.) and the other harmaline vehicle (dH2O; i.p.), each 15 min before behavioural testing began. Experiment 2 assessed the effect of CB receptor agonism upon harmaline‐induced symptoms. Here, four groups of animals were used where one received WIN55, 212–2 vehicle (i.p.; administered 30 min before harmaline) plus harmaline (30 mg kg−1; i.p.; 15 min before behavioural testing), and three received WIN55, 212–2 at doses of 0.1, 0.5 and 1 mg kg−1 (i.p.; administered 30 min before harmaline) plus harmaline (30 mg kg−1; i.p.; 15 min before behavioural testing). Finally, Experiment 3 examined the effects of CB1 receptor antagonism upon harmaline‐induced symptoms. Here, three groups of animals were used where one received AM251 or rimonabant vehicle (i.p.; administered 30 min before harmaline) plus harmaline (30 mg kg−1; i.p.; 15 min before behavioural testing) and two received either AM251 (1 mg kg−1; i.p.; administered 30 min before harmaline) or rimonabant (10 mg kg−1; i.p.; administered 30 min before harmaline) plus harmaline (30 mg kg−1; i.p.; 15 min before behavioural testing).
In vitro, AM251 exhibits greater affinity for CB1 receptors (3–10‐fold; dependent on assay) and exerts greater inhibition of agonist effects at CB1 receptors (6–10‐fold difference in IC50; dependent on assay and agonist) (Pertwee (2005). Therefore, AM251 and rimonabant were employed at doses of 1 and 10 mg kg−1 respectively. CB receptor agonists and antagonists employed in the present study were also examined for effects in the tasks described when administered in the absence of harmaline (doses as stated above; i.p.; 45 min before testing began; See Supplemental Results; Figures S3A and S4A vs other supplemental Figures [Link], [Link]). Briefly, when administered in the absence of harmaline, only rimonabant treatment affected any measure where a decrease in rearing events and time on rotarod were observed.
Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). On entry into the study, 192 animals were randomized, using an online tool (http://graphpad.com/quickcalcs/randomize1/; seeded using the time of day) into 16 groups of 12 animals as described. Where animals failed to complete a task and provide valid data, no value was included for analysis. Reasons for task failure included the following: failure to habituate to handling, failure to habituate to equipment, technical (e.g. equipment) failure or data provided not amenable to robust analysis (e.g. indistinguishable footprints in the gait task). The number of animals per group per assay that contributed data for quantitative analysis is shown in parentheses in each figure. Group size was determined by sample size calculation to provide statistical power of ≥80% to detect effect sizes consistent with relevant comparators previously described for this animal model (Handforth, 2012) at the 5% level of significance with the intention to establish differences between control and study drug groups.
Experimental data were collected by researchers blinded to drug treatment and analysed by an independent researcher blinded to group identity. Data were unblinded prior to pairwise statistical comparisons (see below) in order to allow specification of the comparator control group. SPSS (IBM, USA), Origin (OriginLab Co., MA, USA) and GraphPad Prism 6 (GraphPad Software, USA) were used for statistical analysis of data and figure production. Prior to the conduct of comparative statistics, the presence or not of outlier data points pooled by task was assessed using the ROUT method as implemented in GraphPad Prism 6 (Motulsky and Brown, 2006). These data were excluded from the statistical analysis and comprised 6/1648 (approximately 0.3%) data points across all groups and all assays. Data were then assessed for normality using a Kolmogorov–Smirnov test. Results found to be normally distributed (P > 0.05 in K–S test) were expressed as mean ± SEM and analysed using either a paired Student's t‐test or a one‐way ANOVA test. Where a main effect was seen in ANOVA tests, pairwise comparisons between control and each drug treated group were then made using Tukey's post hoc tests. Results that were not normally distributed (P < 0.05 in K–S test) were expressed as median and interquartile range [expressed as median (interquartile range)] and analysed using either a Mann–Whitney test or a Kruskal–Wallis test. Where a main effect was seen in Kruskal–Wallis tests, pairwise comparisons between control and each drug treated group were then made using Dunn's multiple comparisons test. In each case, P < 0.05 was considered statistically significant.
Materials
The non‐selective CB receptor agonist, WIN55, 212–2 (Sigma, USA), and the CB1 receptor selective antagonists, AM251 (Sigma) and rimonabant (Cayman, USA), were first dissolved in DMSO before further dilution in distilled water (maximum final DMSO concentration, 1%v/v). Harmaline hydrochloride dihydrate (Sigma) was dissolved in distilled water. Drugs were administered i.p. in a maximum total injection volume of 1 mL.
Results
Experiment 1 assessed the effects of harmaline versus a single, distilled water‐treated control group. Harmaline reliably induced a significant and persistent tremor that affected all body parts (Figure 1A) and also significantly reduced rearing Figure 1B)] and grooming events (Figure 1C)]. In the open field test, harmaline significantly decreased total distance moved (Figure 1D) while mean mobility duration (Figure 1E and median speed (Figure 1F) were also significantly decreased by treatment. In the rotarod test, median time on the apparatus was significantly decreased by harmaline treatment (Figure 2A), and, similarly, treatment significantly decreased median gripping time in the grip strength test (Figure 2B). When animal gait was assessed, harmaline significantly increased mean gait width (Figure 2C) and reduced mean right (Figure 2D) and left (Figure 2E) stride length. These results demonstrate that treatment with 30 mg kg−1 harmaline reliably and reproducibly induced severe tremor associated with significant functional deficits that were detected and assessed using the tasks employed.
Figure 1.
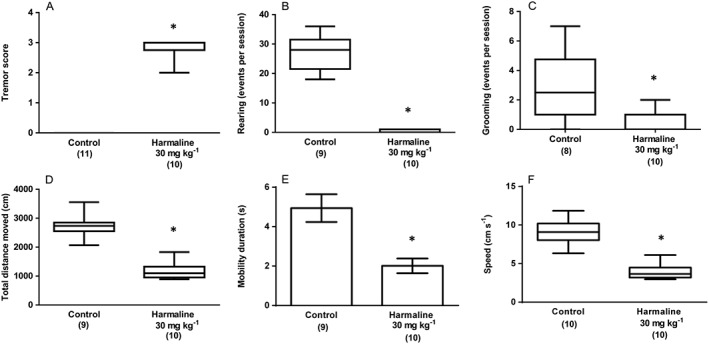
Experiment 1: The effect of harmaline (30 mg kg−1; i.p.) on (A) tremor score, (B) rearing events per session and (C) grooming events per session. Results from the same treatment in the open field test are shown as (D) total distance moved (cm), (E) mobility duration (s) and (F) movement speed (cm s−1). Data describing mobility duration exhibited a normal distribution and are represented as mean ± SEM. Data describing tremor score, rearing events, grooming events, total distance moved and movement speed were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Figure 2.
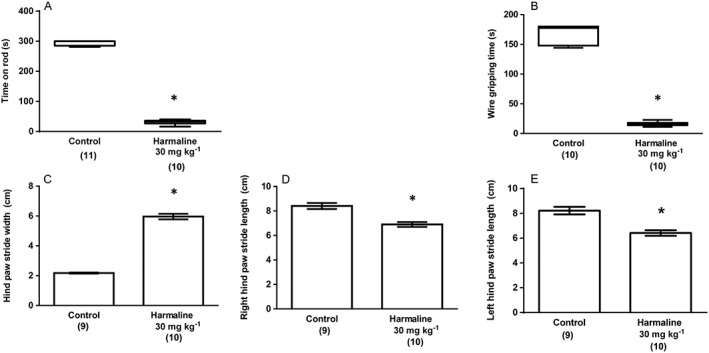
Experiment 1: The effect of harmaline (30 mg kg−1; i.p.) upon (A) time spent on rotarod apparatus and (B) gripping time in the wire grip test. Results from the same treatment in the gait analysis test are shown as (C) hind paw stride width (cm), (D) right hind paw stride length (cm) and (F) left hind paw stride length (cm). Data describing measures from the gait analysis exhibited a normal distribution and are represented as mean ± SEM. Data describing time on the rotarod apparatus and gripping time in the wire grip test were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Experiment 2 assessed the effect of CB receptor agonism upon the harmaline‐induced symptoms described in Experiment 1. The CB receptor agonist, WIN55, 212–2 (0.1, 0.5 and 1 mg kg−1) or vehicle was administered 30 min before harmaline (30 mg kg−1) and effects assessed behaviourally as previously described. Here, an overall effect of treatment upon median harmaline‐induced tremor [H(3) = 12.1, P < 0.05; Figure 3A], median rearing events [H(3) = 13.47, P < 0.05; Figure 3B] and median grooming events was seen [H(3) = 18.01, P < 0.05; Figure 3C], although subsequent pairwise comparisons only revealed significant effects of higher doses (0.5, 1 mg kg−1) of WIN55, 212–2 upon grooming events (Figure 3C).
Figure 3.
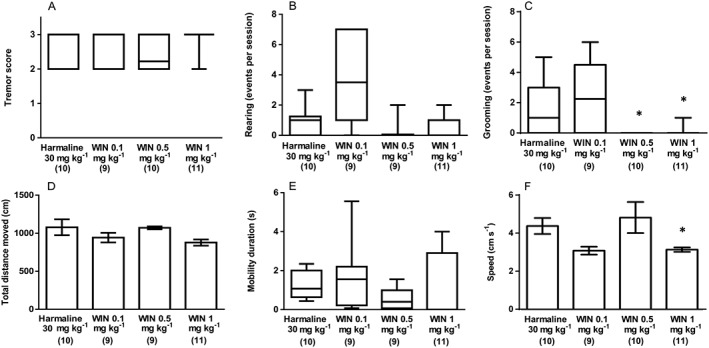
Experiment 2: The effect of CB receptor agonist (WIN55212–2 0.1, 0.5 and 1 mg kg‐1 ; i.p.) treatment upon harmaline (30 mg kg−1; i.p.) induced symptoms. (A) Tremor score, (B) rearing events per session and (C) grooming events per session. Results from the same treatment in the open field test are shown as (D) total distance moved (cm), (E) mobility duration (s) and (F) movement speed (cm s−1). Data describing total distance moved and movement speed exhibited a normal distribution and are represented as mean ± SEM. Data describing tremor score, rearing events, grooming events and mobility duration were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the harmaline only group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
In the open field test, no overall effects of treatment upon mean total distance moved (F3, 41 = 2.270, P > 0.05; Figure 3D) or median mobility duration [H(3) = 4.509, P > 0.05; Figure 3E] were seen; although, mean movement speed (F3, 37 = 4.688, P < 0.05; Figure 3F) was affected where post hoc tests revealed that WIN55, 212–2 1 mg kg−1 significantly reduced movement speed. In the rotarod test, a main effect of treatment upon median time on the rotarod apparatus [H(3) = 14.21, P < 0.05] was seen where WIN55, 212–2 caused a dose‐dependent exacerbation of harmaline effects on this measure (Figure 4A). Furthermore, treatment significantly affected median grip strength [H(3) = 20.28, P < 0.05]; although, post hoc comparisons revealed that only WIN55, 212–2 0.5 mg kg−1 significantly reduced gripping time (Figure 4B). Finally, when animal gait was assessed, significant effects of treatment upon median gait width [H(3) = 13.32, P < 0.05; Figure 4C] and median stride length [right stride: H(3) = 17.35, P < 0.05; left stride: H(3) = 9.703, P < 0.05; Figure 4D,E] were seen. Post hoc comparisons with harmaline plus WIN55, 212–2 vehicle‐treated controls tests revealed that the lowest dose of WIN 55212–2 (0.1 mg kg−1) decreased the harmaline‐induced increase in gait width, although the highest dose of WIN 55212–2 (1 mg kg−1) exacerbated the harmaline‐induced decrease in right, but not left, stride length.
Figure 4.
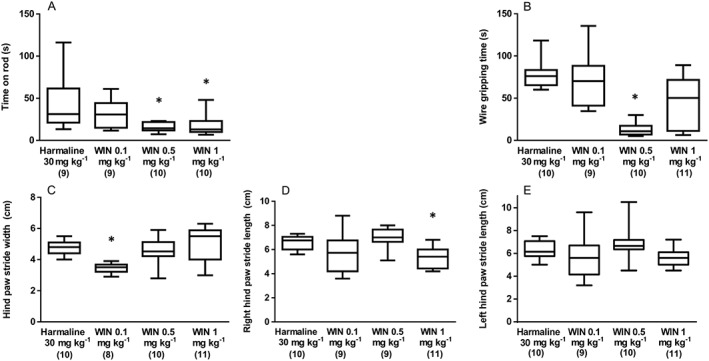
Experiment 2: The effect of CB receptor agonist (WIN55–212,2 0.1, 0.5 and 1 mg kg−1; i.p.) treatment upon harmaline (30 mg kg−1; i.p.) induced symptoms. (A) Time spent on rotarod apparatus and (B) gripping time in the wire grip test. Results from the same treatment in the gait analysis test are shown as (C) hind paw stride width (cm), (D) right hind paw stride length (cm) and (F) left hind paw stride length (cm). Data for all measures in this experiment were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the harmaline only group. Numbers in parentheses indicate group sizes. 3/199 data points were detected as outliers and excluded from the presented analyses.
Experiment 3 assessed the effects of CB1 receptor antagonism upon harmaline‐induced symptoms by examining the effects of the CB1 receptor selective antagonists AM251 (1 mg kg−1) and rimonabant (10 mg kg−1) when administered 30 min before harmaline (30 mg kg−1) in our battery of behavioural tasks. A significant effect of drug treatment [H(2) = 17.02, P < 0.05] on median tremor score was seen, and post hoc tests revealed that AM251 and rimonabant (Figure 5A) significantly reduced tremor scores when compared with harmaline plus vehicle controls. When rearing events were assessed, a main effect of treatment was detected [H(2) = 12.86, P < 0.05] and revealed that rimonabant significantly increased rearing events when compared with harmaline plus vehicle (Figure 5B). A significant effect of treatment upon grooming events was also seen [H(2) = 19.88, P < 0.05] where both antagonists produced significant increases when compared with harmaline plus vehicle (Figure 5C). In the open field test, significant effects of treatment were seen on the median total distance moved [H(2) = 17.51, P < 0.05], mean mobility duration (F2, 27 = 10.84, P < 0.05) and mean movement speed (F2, 27 = 3.792, P < 0.05). Here, when comparisons were made versus the harmaline plus vehicle group, post hoc tests revealed that both AM251 and rimonabant significantly increased total distance moved (Figure 5D) and mobility duration (Figure 5E), but only rimonabant significantly increased movement speed (Figure 5F).
Figure 5.
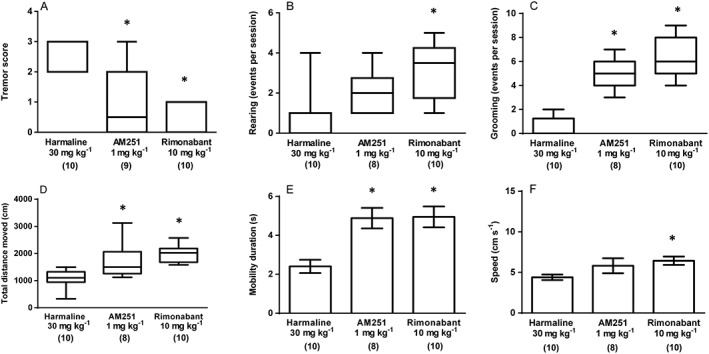
Experiment 3: The effect of the CB1 receptor antagonists (AM251 1 mg kg−1 and rimonabant 10 mg kg−1; both i.p.) treatment upon harmaline (30 mg kg−1; i.p.) induced symptoms. (A) Tremor score, (B) rearing events per session and (C) grooming events per session. Results from the same treatment in the open field test are shown as (D) total distance moved (cm), (E) mobility duration (s) and (F) movement speed (cm s−1). Data describing mobility duration and movement speed exhibited a normal distribution and are represented as mean ± SEM. Data describing tremor score, rearing events, grooming events and total distance moved were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the harmaline only group. Numbers in parentheses indicate group sizes. 3/172 data points were detected as outliers and excluded from the presented analyses.
In the rotarod test, a main effect of treatment upon mean time on the apparatus was seen (F2, 23 = 47.21, P < 0.05) that revealed CB1 receptor antagonist treatment to significantly increase times on the rod when compared with harmaline plus vehicle controls (Figure 6A). In the grip strength test, a similar effect was seen where the main effect of treatment (F2, 24 = 24.04, P < 0.05) arose from significant effects of CB1 receptor antagonism to increase mean grip time (Figure 6B). Finally, in our analysis of gait, a significant effect of treatment was seen upon median stride width [H(2) = 14.71, P < 0.05; Figure 6C] but not mean stride length (right: F2, 25 = 1.559, P > 0.05and left: F2, 25 = 2.685, P > 0.05; Figure 6D,E) where post hoc tests revealed that CB1 receptor antagonism reduced stride width, when compared with harmaline plus vehicle controls.
Figure 6.
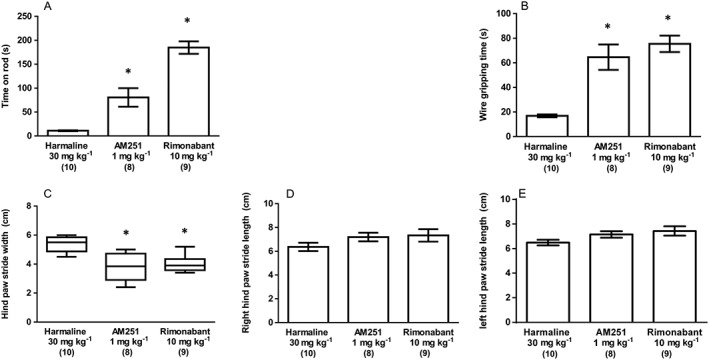
Experiment 3: The effect of CB1 antagonist (AM251 1 mg kg−1 and rimonabant 10 mg kg−1; both i.p.) treatment upon harmaline (30 mg kg−1; i.p.) induced symptoms. (A) Time spent on rotarod apparatus and (B) gripping time in the wire grip test. Results from the same treatment in the gait analysis test are shown as (C) hind paw stride width (cm), (D) right hind paw stride length (cm) and (F) left hind paw stride length (cm). Data for time on rotarod apparatus, gripping time in the wire grip test and right and left hind paw stride lengths were normally distributed and are represented as mean ± SEM. Hind paw stride width data were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the harmaline only group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Discussion
Essential tremor is the most common movement disorder (Louis et al., 1998), has unmet clinical need (approximately 50% of the cases of ET are resistant to pharmacotherapy) and is most frequently cerebellar in origin. The endocannabinoid system plays an important role in cerebellar function, and CB1 receptor expression is at its most abundant in mammalian cerebellum (Miller and Devi, 2011). However, while behavioural effects of CB1 receptor agonism in healthy laboratory species are well established (Little et al., 1988), CB1 receptor modulation in ET has never been examined. Such a study is important and timely since the endocannabinoid system may represent an unexploited target for ET pharmacotherapy. Moreover, recreational and medical use of cannabis are increasing, raising the risk of exposure in ET patients. Finally, recreational abuse of synthetic cannabinoids (typically CB1 receptor agonists) is also increasing, presenting additional risks within the ET patient population (Fox et al., 2004; Gilman et al., 2014; Tudge et al., 2015). We therefore assessed the effects of a CB receptor agonist and two CB1 receptor antagonists in a murine ET model using five conventional behavioural assessments.
In our first experiment, and consistent with the published reports, harmaline reliably induced tremor (Martin et al., 2005) which was manifested as marked deficits in performance, in all of the behavioural tasks employed. These deficits were shown by significant reductions in rearing and grooming events, distance moved by animals in the open field test, mobility duration, movement speed, time on rotarod, grip strength, bilateral gait width and stride length. Harmaline produces tremor, the severity of which is reliably dose‐dependent and species‐specific (Miwa et al., 2006). Notably, studies seeking to detect the effects of agents that are hypothesized to potentiate ET, such as caffeine (Al‐Deeb et al., 2002), most commonly employ a lower dose of harmaline, about 10 mg kg−1, while those exploring the potential therapeutic utility of novel agents to treat ET symptoms, will most often employ higher harmaline doses, about 30 mg kg−1 (Shourmasti et al., 2014). Here, since a severe tremor state was required, upon which only potent ameliorating or exacerbating pharmacological effects of the cannabinoids studied would be revealed, a harmaline dose of 30 mg kg−1 was employed. The primary cause of harmaline‐induced tremor is via alteration of synchronous activation of climbing fibres from the inferior olive projecting to cerebellar PC (Kolasiewicz et al., 2009), most likely via repetitive discharge generation in inferior olivary nucleus neurons through potentiation of CaV3.1 calcium channels responsible for intrinsic oscillatory activity in this neuronal population (Miwa and Kondo, 2011).
One of the most frequently reported effects of cannabis in a survey of MS patients was tremor relief (Koch et al., 2007). However, other studies have reported that cannabis does not improve MS‐associated tremor (Fox et al., 2004; Koppel et al., 2014), and static ataxia can be reliably induced by CB1 receptor agonism in dogs and mice (Dewey et al., 1972). In our second experiment, we examined the consequences of CB receptor activation upon harmaline‐induced behavioural deficits in rat. Here, the CB receptor agonist largely exacerbated harmaline‐induced symptoms, as demonstrated by reduced grooming events, movement speed and time spent on the rotarod, consistent with CB1 receptor agonist effects in healthy animals (Little et al., 1988). While these effects occurred only at the higher doses of WIN55212–2 and suggested a possible dose‐dependent effect, CB receptor agonism also exerted conflicting and apparently dose‐independent effects upon features of gait. Here, only the lowest dose of WIN55212–2 partly reversed harmaline‐induced changes in stride width, yet the highest dose exacerbated right, but not left stride length. Similarly, only the middle dose of WIN55212–2 exacerbated the harmaline‐induced decrease in grip strength which was unaffected by either the lowest or highest doses.
WIN55212–2 is an agonist that acts at both CB1 and CB2 receptors. While the presence and functional relevance of central CB2 receptors remains controversial (Morgan et al., 2009; Xi et al., 2011), the potential for some of the effects of WIN55212–2 reported here to have been mediated, wholly or in part, via CB2 receptor activation cannot be ruled out. Overall, CB receptor agonism typically worsened harmaline‐induced symptoms as assessed using the behavioural measures employed. While some conflicting results were found in more nuanced tests of motor function (e.g. gait), they did not appear to be dose‐dependent, and no indication of potential therapeutic benefit was seen in tests which assessed fundamental features of the model (e.g. tremor).
Our previous in vitro studies have suggested that CB1 receptor antagonism may be beneficial in movement disorders by reducing CB1 receptor‐mediated inhibition of GABA release (Ma et al., 2008). In the present study, we have shown that CB1 receptor antagonists ameliorated severe ET symptoms and such data represent the first behavioural evidence of clinical potential in an established and relevant animal model. Importantly, the two CB1 receptor antagonists tested both significantly decreased harmaline‐induced tremor score, showing beneficial effects on the primary behavioural deficit exhibited in this model. Moreover, while not reaching magnitudes comparable with control animal behaviours, both AM251 and rimonabant increased grooming events when compared with animals only treated with harmaline, while rimonabant alone increased rearing events, largely consistent with previous reports (Zavatti et al., 2011). CB1 receptor antagonism also exerted beneficial effects in the open field test where both antagonists tested ameliorated harmaline‐induced behavioural deficits in all measured domains (with the exception of AM251 in movement speed). Similarly, both antagonists exerted beneficial effects upon harmaline‐induced adverse effects in the rotarod and grip strength tasks in addition to ameliorating harmaline effects upon stride width but not stride length. Thus, blockade of endocannabinergic tone exerts intrinsic therapeutic benefit in this rodent model of severe ET. Harmaline treatment evokes rhythmic burst‐firing activity in the medial and dorsal accessory inferior olivary nuclei that is propagated via climbing fibres to PCs, before further transmission to deep cerebellar nuclei, brainstem and spinal cord, consistent with our previous observation (Ma et al., 2008) that CB1 receptor antagonism inhibits PC firing via blockade of endocannabinergic inhibition of GABA release. However, the involvement of other, additional, endocannabinoid‐mediated processes cannot yet be eliminated.
While a reversal by CB1 receptor antagonism of harmaline effects upon simple motor functions or their exacerbation by CB1 receptor agonists most likely arise predominantly from central CB1 receptor‐mediated effects, some CB1 receptor antagonists exert off‐target effects. Therefore, and particularly with regard to results where a clear dose‐related response was not evident, further investigation is warranted to determine potential interplay between such signalling systems. In vitro, rimonabant and AM251 can allosterically potentiate GABAA receptors at nanomolar concentrations; although, their site of action is distinct from other allosteric modulators of this receptor (Baur et al., 2012; Battistella et al., 2014). Moreover, glycine receptors are involved in a number of movement disorders (Yang et al., 2008) and exhibit a distinct pharmacological profiles for several cannabinoid compounds and CB receptor ligands and so establish glycine receptors as novel targets for endogenous and exogenous cannabinoids (Yang et al., 2008).
As found in the cerebellum and ET, CB1 receptor expression is also abundant in the cerebral ganglia (Pacher and Steffens, 2009) and has been studied in a primate model of dyskinesia. Here, while rimonabant reduced dyskinetic symptoms (van der Stelt et al., 2005), another CB1 receptor antagonist, 1‐[7‐(2‐chlorophenyl)‐8‐(4‐chlorophenyl)‐2‐methylpyrazolo(1,5‐a)‐[1,3,5] triazin‐4‐yl]‐3‐ethylaminoazetidine‐3‐carboxylic acid amide benzenesulfonate, failed to affect dyskinetic symptoms (Cao et al., 2007). Moreover, the CB receptor partial agonist, nabilone, also alleviated symptoms in the same model (Fox et al., 2002) and in a small clinical pilot (Sieradzan et al., 2001), but these results were not replicated in a randomized–controlled clinical trial (Carroll et al., 2004). Thus, as found in the present study with respect to CB1 receptor modulation of ET symptoms, other dyskinesias appear either improved or unaffected by CB1 receptor antagonism but paradoxically alleviated and exacerbated by CB receptor agonists. This contradiction, exemplified by our own results and those describing therapeutic benefits of CB1 receptor agonists in animal models of chronic tremor (Baker et al., 2000; Koch et al., 2007), may suggest that overall effects are determined by the aetiology of the disorder model. Thus, in a chronic encephalomyelitis modelling MS (Baker et al., 2000) where widespread demyelination and axon loss occur, CB receptor agonism can be of use while, in acute tremor arising from cerebellar hyperexcitability (e.g. harmaline treatment) to model idiopathic ET, CB1 receptor antagonism is beneficial.
In conclusion, our results demonstrated that acute CB1 receptor antagonism improved severe ET symptoms and so demonstrated their therapeutic potential for ET. Rimonabant was previously licensed for weight loss although this drug was withdrawn in 2008 following reports of psychiatric side effects in a trial population where higher doses were employed (Moreira and Crippa, 2009). However, adverse reactions of this nature do not necessarily preclude the use of a treatment, as in the case of suicidal ideation associated with SSRIs (Ghaziuddin et al., 2014) and so should not hinder drug development, if warranted by unmet clinical needs. Moreover, rimonabant has since been shown to act as an inverse agonist at CB1 receptors (Landsman et al., 1997) and so making the investigation of neutral CB1 receptor antagonists, such as ∆9‐tetrahydrocannabidavarin (Tudge et al., 2015), in ET even more necessary, because it is already known to modulate PC firing in vitro (Ma et al., 2008).
Our study reinforces the pivotal role of the endocannabinoid system in motor function and highlights its therapeutic potential in the treatment of ET symptoms. Our novel findings justify further study of the basic neuronal circuits that subserve CB1 receptor antagonist therapies for ET alongside further in vivo studies to elucidate mechanisms of CB1 receptor antagonist effects on harmaline symptoms (e.g. central microdialysis). Moreover, while harmaline‐induced tremor is a valuable first line model used to inform prioritisation of candidate ET treatments for subsequent investigation, it is necessarily limited as a result of its acute nature. Harmaline‐induced tremor is predictive of clinical efficacy in ET in approximately 50% of cases (Handforth, 2012), and so the findings presented here strongly support further preclinical study of repeated CB1 receptor antagonist treatment in animal models of disease, in comparison with models of acute symptoms, as used here, and subsequent clinical development.
Author contributions
H.A. executed the research project, statistical analysis, manuscript preparation. B.J.W. was responsible for the conception, organization, review and critique of research, statistical analysis and manuscript preparation. V.S. carried out the organization of the research project. M.S. took part in the conception and organization of the research project, the statistical analysis and manuscript preparation.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 The effect of WIN55–212,2 (0.1, 0.5 and 1 mg kg−1; i.p.) upon (A) rearing events per session and (B) grooming events per session. Results from the same treatment in the open field test are shown as (C) total distance moved (cm), (D) mobility duration (s) and (E) movement speed (cm s−1). Data describing rearing events and total distance moved exhibited a normal distribution and are represented as mean ± SEM. Data describing grooming events, mobility duration and movement speed were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Figure S2 The effect of WIN55–212,2 (0.1, 0.5 and 1 mg kg−1; i.p.) upon (A) time spent on rotarod apparatus and (B) gripping time in the wire grip test. Results from the same treatment in the gait analysis test are shown as (C) hind paw stride width (cm), (D) right hind paw stride length (cm) and (F) left hind paw stride length (cm). Data describing measures from the gait analysis exhibited a normal distribution and are represented as mean ± SEM. Data describing time on the rotarod apparatus and gripping time in the wire grip test were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Figure S3 The effect of AM251 (1 mg kg−1; i.p.) and rimonabant (10 mg kg−1; i.p.) upon (A) rearing events per session and (B) grooming events per session. Results from the same treatment in the open field test are shown as (C) total distance moved (cm), (D) mobility duration (s) and (E) movement speed (cm s−1). Data describing rearing and grooming events and total distance moved exhibited a normal distribution and are represented as mean ± SEM. Data describing mobility duration and movement speed were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Figure S4 The effect of AM251 (1 mg kg−1; i.p.) and rimonabant (10 mg kg−1; i.p.) upon (A) time spent on rotarod apparatus and (B) gripping time in the wire grip test. Results from the same treatment in the gait analysis test are shown as (C) hind paw stride width (cm), (D) right hind paw stride length (cm) and (F) left hind paw stride length (cm). Data describing measures from the gait analysis exhibited a normal distribution and are represented as mean ± SEM. Data describing time on the rotarod apparatus and gripping time in the wire grip test were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
We thank Dr Hosseinzade (Mashhad University of Medical Sciences) for advising upon harmaline doses and providing harmaline hydrochloride. We thank Ms Vaziri for support to behavioural tests. Funding for this study was provided by Kerman University of Medical Sciences as a grant for the PhD research of Hassan Abbassian.
Abbassian, H. , Whalley, B. J. , Sheibani, V. , and Shabani, M. (2016) Cannabinoid type 1 receptor antagonism ameliorates harmaline‐induced essential tremor in rat. British Journal of Pharmacology, 173: 3196–3207. doi: 10.1111/bph.13581.
Contributor Information
Benjamin J Whalley, Email: b.j.whalley@reading.ac.uk.
Mohammad Shabani, Email: shabani@kmu.ac.ir, Email: shabanimoh@yahoo.com.
References
- Al‐Deeb S, Al‐Moutaery K, Arshaduddin M, Biary N, Tariq M (2002). Effect of acute caffeine on severity of harmaline‐induced tremor in rats. Neurosci Lett 325: 216–218. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjmand S, Vaziri Z, Behzadi M, Abbassian H, Stephens GJ, Shabani M (2015). Cannabinoids and Tremor Induced by Motor‐related Disorders: Friend or Foe? Neurotherapeutics 12: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW et al. (2000). Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404: 84–87. [DOI] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni J‐M, Chtioui H, Dao K, Fabritius M et al. (2014). Long‐Term Effects of Cannabis on Brain Structure. Neuropsychopharmacology 39: 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Gertsch J, Sigel E (2012). The cannabinoid CB1 receptor antagonists rimonabant (SR141716) and AM251 directly potentiate GABA(A) receptors. Br J Pharmacol 165: 2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccellato E, Carretta D, Utan A, Cavina C, Speroni E, Grassi G et al. (2011). Acute and chronic cannabinoid extracts administration affects motor function in a CREAE model of multiple sclerosis. J Ethnopharmacol 133: 1033–1038. [DOI] [PubMed] [Google Scholar]
- Cao X, Liang L, Hadcock JR, Iredale PA, Griffith DA, Menniti FS et al. (2007). Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐treated rhesus monkeys. J Pharmacol Exp Ther 323: 318–326. [DOI] [PubMed] [Google Scholar]
- Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C et al. (2004). Cannabis for dyskinesia in Parkinson disease: a randomized double‐blind crossover study. Neurology 63: 1245–1250. [DOI] [PubMed] [Google Scholar]
- Chopra A, Klassen BT, Stead M (2013). Current clinical application of deep‐brain stimulation for essential tremor. Neuropsychiatr Dis Treat 9: 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB (1983). Tetrahydrocannabinol for tremor in multiple sclerosis. Ann Neurol 13: 669–671. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H, Rancillac A, Crepel F (2004). Mechanisms underlying cannabinoid inhibition of presynaptic Ca2+ influx at parallel fibre synapses of the rat cerebellum. J Physiol 557: 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Elble R (2009). Essential tremor‐‐neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord 24: 2033–2041. [DOI] [PubMed] [Google Scholar]
- Dewey WL, Jenkins J, O'Rourke T, Harris LS (1972). The effects of chronic administration of trans‐ 9 ‐tetrahydrocannabinol on behavior and the cardiovascular system of dogs. Arch Int Pharmacodyn Ther 198: 118–131. [PubMed] [Google Scholar]
- Fox SH, Henry B, Hill M, Crossman A, Brotchie J (2002). Stimulation of cannabinoid receptors reduces levodopa‐induced dyskinesia in the MPTP‐lesioned nonhuman primate model of Parkinson's disease. Mov Disord 17: 1180–1187. [DOI] [PubMed] [Google Scholar]
- Fox P, Bain PG, Glickman S, Carroll C, Zajicek J (2004). The effect of cannabis on tremor in patients with multiple sclerosis. Neurology 62: 1105–1109. [DOI] [PubMed] [Google Scholar]
- Frederickson RC, Hewes CR, Aiken JW (1976). Correlation between the in vivo and an in vitro expression of opiate withdrawal precipitated by naloxone: their antagonism by l‐(−)‐delta9‐tetrahydrocannabinol. J Pharmacol Exp Ther 199: 375–384. [PubMed] [Google Scholar]
- Freedland CS, Whitlow CT, Miller MD, Porrino LJ (2002). Dose‐dependent effects of Delta9‐tetrahydrocannabinol on rates of local cerebral glucose utilization in rat. Synapse 45: 134–142. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin N, Merchant C, Dopp R, King C (2014). A naturalistic study of suicidal adolescents treated with an SSRI: suicidal ideation and behavior during 3‐month post‐hospitalization period. Asian J Psychiatr 11: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N et al. (2014). Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci 34: 5529–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M (2001). The role of cannabinoids in neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry 25: 743–765. [DOI] [PubMed] [Google Scholar]
- Handforth A (2012). Harmaline Tremor: Underlying Mechanisms in a Potential Animal Model of Essential Tremor. Tremor Other Hyperkinet Mov 2: 02‐92‐769‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard P, Twycross R, Shuster J, Mihalyo M, Wilcock A (2013). Cannabinoids. J Pain Symptom Manage 46: 142–149. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P (2007). Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc 2: 2538–2544. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Mostert J, Heersema D, De Keyser J (2007). Tremor in multiple sclerosis. J Neurol 254: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasiewicz W, Kuter K, Wardas J, Ossowska K (2009). Role of the metabotropic glutamate receptor subtype 1 in the harmaline‐induced tremor in rats. J Neural Transm 116: 1059–1063. [DOI] [PubMed] [Google Scholar]
- Koller WC, Vetere‐Overfield B (1989). Acute and chronic effects of propranolol and primidone in essential tremor. Neurology 39: 1587–1588. [DOI] [PubMed] [Google Scholar]
- Komorowska J, Pellis SM (2004). Regulatory mechanisms underlying novelty‐induced grooming in the laboratory rat. Behav Processes 67: 287–293. [DOI] [PubMed] [Google Scholar]
- Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G et al. (2014). Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 82: 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujtan PW, Carlen PL, Kapur BM (1983). delta 9‐Tetrahydrocannabinol and cannabidiol: dose‐dependent effects on evoked potentials in the hippocampal slice. Can J Physiol Pharmacol 61: 420–426. [DOI] [PubMed] [Google Scholar]
- Lamprea MR, Cardenas FP, Setem J, Morato S (2008). Thigmotactic responses in an open‐field. Braz J Med Biol Res 41: 135–140. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI (1997). SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol 334: R1–R2. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR (1988). Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther 247: 1046–1051. [PubMed] [Google Scholar]
- Louis ED, Ottman R, Hauser WA (1998). How common is the most common adult movement disorder? estimates of the prevalence of essential tremor throughout the world. Mov Disord 13: 5–10. [DOI] [PubMed] [Google Scholar]
- Ma YL, Weston SE, Whalley BJ, Stephens GJ (2008). The phytocannabinoid Delta(9)‐tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. Br J Pharmacol 154: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks W, Fournier NM, Kalynchuk LE (2009). Repeated exposure to corticosterone increases depression‐like behavior in two different versions of the forced swim test without altering nonspecific locomotor activity or muscle strength. Physiol Behav 98: 67–72. [DOI] [PubMed] [Google Scholar]
- Martin FC, Thu Le A, Handforth A (2005). Harmaline‐induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord 20: 298–305. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines onreporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LK, Devi LA (2011). The highs and lows of cannabinoid receptor expression in disease: mechanisms and their therapeutic implications. Pharmacol Rev 63: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Kondo T (2011). T‐type calcium channel as a new therapeutic target for tremor. Cerebellum 10: 563–569. [DOI] [PubMed] [Google Scholar]
- Miwa H, Kubo T, Suzuki A, Kihira T, Kondo T (2006). A species‐specific difference in the effects of harmaline on the rodent olivocerebellar system. Brain Res 1068: 94–101. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Crippa JA (2009). The psychiatric side‐effects of rimonabant. Rev Bras Psiquiatr 31: 145–153. [DOI] [PubMed] [Google Scholar]
- Morgan NH, Stanford IM, Woodhall GL (2009). Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 57: 356–368. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Brown RE (2006). Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Steffens S (2009). The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol 31: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2005). Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76: 1307. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Del Arco I, Martin‐Calderon JL, Gorriti MA, Navarro M (1998). Role of the endogenous cannabinoid system in the regulation of motor activity. Neurobiol Dis 5: 483–501. [DOI] [PubMed] [Google Scholar]
- Schmouth JF, Dion PA, Rouleau GA (2014). Genetics of essential tremor: from phenotype to genes, insights from both human and mouse studies. Prog Neurobiol 119‐120: 1–19. [DOI] [PubMed] [Google Scholar]
- Shourmasti FR, Goudarzi I, Abrari K, Salmani ME, Laskarbolouki T (2014). Riluzole ameliorates harmaline‐induced tremor in rat. Basic Clin Neurosci 5: 138–143. [PMC free article] [PubMed] [Google Scholar]
- Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM (2001). Cannabinoids reduce levodopa‐induced dyskinesia in Parkinson's disease: a pilot study. Neurology 57: 2108–2111. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JA, Fowler SC (1998). At low doses, harmaline increases forelimb tremor in the rat. Neurosci Lett 241: 41–44. [DOI] [PubMed] [Google Scholar]
- Tudge L, Williams C, Cowen PJ, McCabe C (2015). Neural effects of cannabinoid CB1 neutral antagonist tetrahydrocannabivarin on food reward and aversion in healthy volunteers. Int J Neuropsychopharmacol 18: pii: pyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V et al. (2005). A role for endocannabinoids in the generation of parkinsonism and levodopa‐induced dyskinesia in MPTP‐lesioned non‐human primate models of Parkinson's disease. FASEB J 19: 1140–1142. [DOI] [PubMed] [Google Scholar]
- Vaziri Z, Abbassian H, Sheibani V, Haghani M, Nazeri M, Aghaei I et al. (2015). The therapeutic potential of Berberine chloride hydrate against harmaline‐induced motor impairments in a rat model of tremor. Neurosci Lett 590: 84–90. [DOI] [PubMed] [Google Scholar]
- Wecker L, Engberg ME, Philpot RM, Lambert CS, Kang CW, Antilla JC et al. (2013). Neuronal nicotinic receptor agonists improve gait and balance in olivocerebellar ataxia. Neuropharmacology 73: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z‐X, Peng X‐Q, Li X, Song R, Zhang H‐Y, Liu Q‐R et al. (2011). Brain cannabinoid CB2 receptors modulate cocaine's actions in mice. Nat Neurosci 14: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Aubrey KR, Alroy I, Harvey RJ, Vandenberg RJ, Lynch JW (2008). Subunit‐specific modulation of glycine receptors by cannabinoids and N‐arachidonyl‐glycine. Biochem Pharmacol 76: 1014–1023. [DOI] [PubMed] [Google Scholar]
- Zavatti M, Carnevale G, Benelli A, Zanoli P (2011). Effects of the cannabinoid antagonist SR 141716 on sexual and motor behaviour in receptive female rats. Clin Exp Pharmacol Physiol 38: 771–775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The effect of WIN55–212,2 (0.1, 0.5 and 1 mg kg−1; i.p.) upon (A) rearing events per session and (B) grooming events per session. Results from the same treatment in the open field test are shown as (C) total distance moved (cm), (D) mobility duration (s) and (E) movement speed (cm s−1). Data describing rearing events and total distance moved exhibited a normal distribution and are represented as mean ± SEM. Data describing grooming events, mobility duration and movement speed were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Figure S2 The effect of WIN55–212,2 (0.1, 0.5 and 1 mg kg−1; i.p.) upon (A) time spent on rotarod apparatus and (B) gripping time in the wire grip test. Results from the same treatment in the gait analysis test are shown as (C) hind paw stride width (cm), (D) right hind paw stride length (cm) and (F) left hind paw stride length (cm). Data describing measures from the gait analysis exhibited a normal distribution and are represented as mean ± SEM. Data describing time on the rotarod apparatus and gripping time in the wire grip test were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Figure S3 The effect of AM251 (1 mg kg−1; i.p.) and rimonabant (10 mg kg−1; i.p.) upon (A) rearing events per session and (B) grooming events per session. Results from the same treatment in the open field test are shown as (C) total distance moved (cm), (D) mobility duration (s) and (E) movement speed (cm s−1). Data describing rearing and grooming events and total distance moved exhibited a normal distribution and are represented as mean ± SEM. Data describing mobility duration and movement speed were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Figure S4 The effect of AM251 (1 mg kg−1; i.p.) and rimonabant (10 mg kg−1; i.p.) upon (A) time spent on rotarod apparatus and (B) gripping time in the wire grip test. Results from the same treatment in the gait analysis test are shown as (C) hind paw stride width (cm), (D) right hind paw stride length (cm) and (F) left hind paw stride length (cm). Data describing measures from the gait analysis exhibited a normal distribution and are represented as mean ± SEM. Data describing time on the rotarod apparatus and gripping time in the wire grip test were not normally distributed and are represented as medians with interquartile ranges as a box and maxima/minima as whiskers. * P < 0.05, significantly different from the vehicle (distilled water; i.p.) treated group. Numbers in parentheses indicate group sizes. No data points were excluded as outliers in the presented analyses.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


