Abstract
Caspase-1 cleaves and activates the pro-inflammatory cytokine interleukin-1 beta (IL-1β), yet the mechanism of IL-1β release and its dependence on cell death remains controversial. To address this issue, we generated a novel inflammasome independent system in which we directly activate caspase-1 by dimerization. In this system, caspase-1 dimerization induced the cleavage and secretion of IL-1β, which did not require processing of caspase-1 into its p20 and p10 subunits. Moreover, direct caspase-1 dimerization allowed caspase-1 activation of IL-1β to be separated from cell death. Specifically, we demonstrate at the single cell level that IL-1β can be released from live, metabolically active, cells following caspase-1 activation. In addition, we show that dimerized or endogenous caspase-8 can also directly cleave IL-1β into its biologically active form, in the absence of canonical inflammasome components. Therefore, cell death is not obligatory for the robust secretion of bioactive IL-1β.
Interleukin-1β (IL-1β) is a pro-inflammatory cytokine that is activated by proteolytic cleavage to a mature 17 kD form before it is released from the cell. After its release, IL-1β binds to the IL-1 receptor to coordinate immune responses that are important for host protection against microbial organisms, and regulate the wound-healing response following tissue damage.1, 2 In some circumstances the pathological production of IL-1β results in auto-inflammatory diseases, such as cryopyrin-associated periodic syndromes (CAPS), which are successfully treated by blocking IL-1β signaling.
Caspase-1 cleaves and activates the precursor of IL-1β (pro-IL-1β) within large cytosolic protein complexes termed inflammasomes. Inflammasome sensor proteins include NOD-like receptor (NLR) family members, such as NLRP1, NLRP3 and NLRC4, AIM2-like receptors and the tripartite motif family member pyrin. Of the NLRs, NLRP3 is the most widely studied as it responds to a diverse range of microbial products, host cell derived danger molecules and environmental irritants.3
Pyrin domain-containing inflammasome sensor proteins, such as NLRP3 and AIM2, drive caspase-1 recruitment and activation via the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD). Once activated, caspase-1 can process inflammatory cytokines, such as IL-1β and IL-18. Cleaved, mature IL-1β is rapidly released from the cell, often alongside processed caspase-1 and ASC. IL-1β lacks a conventional secretory signal sequence and hence is secreted in a non-conventional manner.4 How IL-1β is secreted from cells and whether other caspases can directly substitute for caspase-1 in this process remains of outstanding interest.
Recent research has highlighted cross-talk between inflammasome-associated caspase-1 and the death receptor apoptotic initiator caspase, caspase-8.5 For example, like caspase-1, caspase-8 can directly process pro-IL-1β into its bioactive form.6, 7, 8, 9 Caspase-8 has also been proposed to directly cleave caspase-1 following bacterial detection10, 11 or trigger caspase-1 by activating the NLRP3 inflammasome following toll-like receptor (TLR) or TNF ligation.12, 13 In addition, recent studies have reported that NLRP3 and AIM2 inflammasome-associated ASC can directly bind to caspase-8 to cause apoptotic cell death in the absence of caspase-1.14, 15, 16 Despite these reported novel roles for caspase-8, it remains unclear how caspase-8 activates IL-1β.
It has been suggested that IL-1β is released passively when a cell dies, and that cell death is required for IL-1β release. This idea stems from the observation that caspase-1 activation can trigger a lytic pro-inflammatory cell death program termed pyroptosis.17 Recent studies have postulated that most, if not all, inflammasome activators cause either necrosis or pyroptosis, thus allowing the passive release of active IL-1β.18, 19, 20 However, several groups have suggested that IL-1β secretion may occur prior to the loss of plasma membrane integrity in macrophages and dendritic cells.21, 22 Therefore, we sought to resolve whether caspase-1 must induce cell death for IL-1β to be secreted.
Results
Dimerization of a caspase-1-gyrase fusion protein leads to activation and secretion of IL-1β in mouse embryonic fibroblasts, in the absence of inflammasome machinery
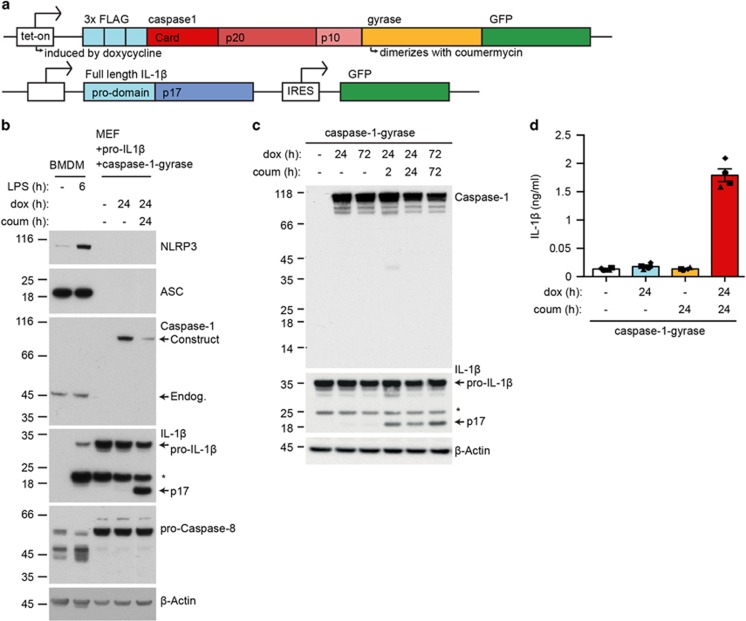
In order to study caspase-1-mediated activation of IL-1β directly, we designed a FLAG- and GFP-tagged dimerizable doxycycline (dox)-inducible caspase-1 construct, referred to as caspase-1-gyrase (Figure 1a). By expressing caspase-1 as a fusion protein with Escherichia coli gyrase, we can dimerize the protein using the divalent compound coumermycin.23, 24 Hence, this system allows induction of caspase-1 expression, which can be monitored at a single cell level by GFP fluorescence, followed by dimerization.
Figure 1.
Forced dimerization of caspase-1 causes IL-1β cleavage in the absence of an intact inflammasome pathway. (a) A schematic of the doxycycline inducible fusion protein FLAG-caspase-1-gyrase-GFP (caspase-1-gyrase) vector system and pro-IL-1β retroviral vector system. Doxycycline treatment induces expression of caspase-1-gyrase fusion protein, whereas coumermycin binds to the gyrase domain to cause caspase-1 dimerization. (b) MEFs lack expression of the inflammasome components NLRP3, ASC and caspase-1. WT BMDMs were treated with 100 ng/ml LPS for 6 h to induce NLRP3 expression. WT MEFs, previously infected with caspase-1-gyrase and a pro-IL-1β, were treated with 1 μg/ml doxycycline to induce caspase-1-gyrase expression and 700 nM coumermycin to dimerize caspase-1-gyrase for the indicated times. Cells lysates were analyzed by western blot for the indicated proteins. (c) Dimerization of caspase-1-gyrase with coumermycin caused rapid cleavage of pro-IL-1β. Western blot of lysates from MEFs containing caspase-1-gyrase and pro-IL-1β, which were treated with 1 μg/ml doxycycline and 700 nM coumermycin for the indicated times. Blot is representative of three independent experiments. (d) Dimerization of caspase-1-gyrase causes secretion of IL-1β. MEFs stably infected with caspase-1-gyrase vector and pro-IL-1β were treated with 1 μg/ml doxycycline and 700 nM coumermycin. Supernatants were analyzed by ELISA. n=4 independent experiments. Error bars represent the S.E.M. Asterisks denote non-specific bands in western blots
Using a lentiviral vector, the caspase-1-gyrase construct was stably infected into murine SV40 large T immortalized mouse embryonic fibroblasts (MEFs) bearing a second construct that constitutively expresses untagged mouse pro-IL-1β and GFP separately (Figure 1a). We took advantage of our observation that, unlike bone marrow-derived macrophages (BMDMs), MEFs do not endogenously express inflammasome components that could confound the interpretation of data, including the sensor NLRP3, adaptor protein ASC, caspase-1 and pro-IL-1β (Figure 1b). Induction of caspase-1-gyrase expression by dox alone did not cause significant amounts of pro-IL-1β cleavage. However, when caspase-1-gyrase was dimerized by co-treatment with coumermycin, cleavage of pro-IL-1β into the active p17 fragment was readily detected in cell lysates within 2 h (Figure 1c). Interestingly, we were not able to detect robust capsase-1-gyrase cleavage of itself, suggesting that processing of caspase-1 may not be required for it to cleave pro-IL-1β, as previously reported.25 Importantly, caspase-1 dimerization also induced secretion of IL-1β, as measured in supernatants by ELISA (Figure 1d).
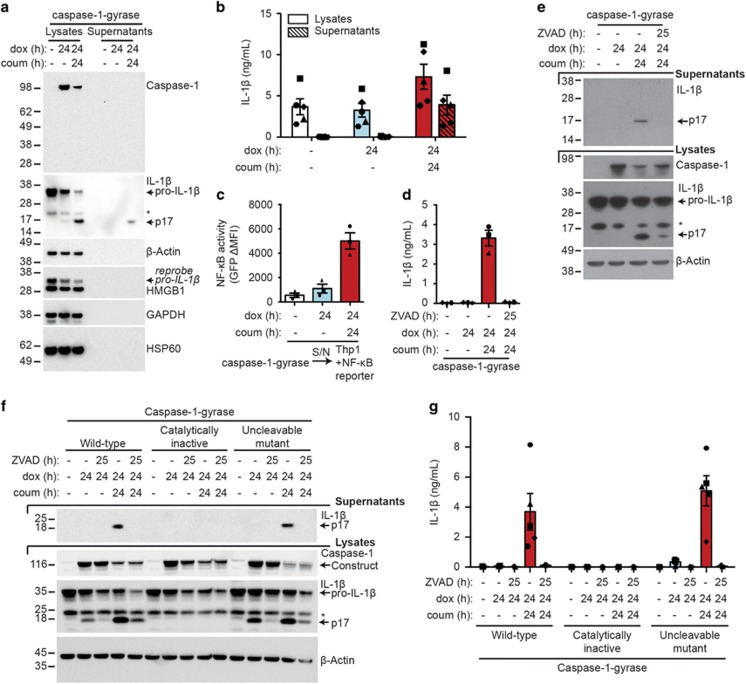
Western blot analysis confirmed that the IL-1β detected in the supernatants was cleaved to the mature bioactive p17 fragment (Figure 2a). As measured by densitometry from three independent experiments, 32.3% (±0.02 S.E.M.) of the processed IL-1β was released into the supernatant after 24 h. Measurements of IL-1β levels by ELISA (Mouse IL-1β/IL-F2 ELISA DY401, R&D) did not distinguish clearly between pro-IL-1β and the active p17 fragment, although they appeared to be more specific for cleaved IL-1β (Figure 2b). Despite this caveat, the ELISA results largely agreed with the western blot data, indicating that at least one-third of cleaved cellular IL-1β is released into the supernatant upon caspase-1-gyrase dimerization (Figure 2b). Notably, the mature p17 fragment of IL-1β, but not cellular proteins such as precursor IL-1β, caspase-1, HSP60, HMGB1, GAPDH or β-actin, were released into the supernatant upon caspase-1 dimerization (Figure 2a). The absence of these abundant intracellular components in the supernatant, other than cleaved IL-1β, implies that caspase-1-gyrase-induced secretion of mature IL-1β may occur prior to, or in the absence of, significant cell lysis.
Figure 2.
Biologically active IL-1β is secreted from MEFs following caspase-1 dimerization. (a and b) Dimerization of caspase-1-gyrase causes cleavage and secretion of IL-1β. MEFs stably infected with caspase-1-gyrase vector and pro-IL-1β were treated with 1 μg/ml doxycycline and 700 nM coumermycin. Lysates and supernatants were adjusted to be of equal volume when harvested. (a) Equal volumes of lysates and supernatants were analyzed by western blot. Blot is representative of three independent experiments. (b) One-third of cleaved cellular IL-1β is released into the supernatant. IL-1β detected from equal volumes of lysates and supernatants by ELISA. n=5 independent experiments. (c) IL-1β secreted from MEFs is biologically active. Supernatants (S/N) from MEFs stably infected with caspase-1-gyrase and pro-IL-1β, treated for 24 h with 1 μg/ml doxycycline and 700 nM coumermycin, were transferred onto Thp1 cells bearing an NF-κB GFP reporter construct. After 24 h incubation, GFP expression was quantified by flow cytometry. Data are expressed as the change in mean fluorescence intensity (MFI) relative to Thp1 cells in media alone. n=3 independent experiments. (d and e) Secretion of IL-1β following caspase-1 activation requires catalytic caspase activity. MEFs containing pro-IL-β and caspase-1-gyrase were treated with 1 μg/ml doxycycline, 700 nM coumermycin and 25 μM pan-caspase inhibitor Z-VAD-fmk for the indicated times. Supernatants were analyzed by (d) ELISA (n=3 independent experiments) and (e) western blot, whereas lysates were analyzed by (e) western blot. (f and g) Cleavage and secretion of IL-1β requires catalytic activity of caspase-1 but not auto-processing. MEFs stably infected with pro-IL-1β and wild-type caspase-1-gyrase, or a catalytically inactive mutant (C385G), or an uncleavable mutant (D296N, D300N, D304N, D308N, D313N, D314N) were treated with 1 μg/ml doxycycline, 700 nM coumermycin and 25 μM Z-VAD-fmk. (f) Lysates were analyzed by western blot, whereas supernatants were analyzed by (f) western blot and (g) ELISA. n=3-5 independent experiments. Error bars represent the S.E.M. in all graphs. Asterisks denote non-specific bands in western blots. See also Supplementary Figure S1 and S2
To formally demonstrate that the cleaved IL-1β detected in the supernatant by western blot was biologically active, we used Thp1 cells that express GFP specifically upon NF-κB activation.26 As expected, these Thp1 reporter cells exhibited robust GFP fluorescence, indicating NF-κB activation, when they were treated with recombinant mature IL-1β or LPS (Supplementary Figure S1a). Similar NF-κB activity was observed when the Thp1 reporter cells were treated with supernatants from dox- and coumermycin-treated caspase-1-gyrase MEFs, as indicated by strong GFP expression (Figure 2c, Supplementary Figure S1b). In contrast, supernatants prepared by freeze-thawing MEFs to release cytosolic proteins, such as monomeric caspase-1-gyrase, pro-IL-1β and β-actin, did not cause robust NF-κB reporter activity in Thp1 cells, unless caspase-1-gyrase cleavage of IL-1β was induced before freeze/thaw lysis (Supplementary Figure S1c, S1d and S1e). These data demonstrate that dimerization of caspase-1 is sufficient to cause cleavage and secretion of bioactive IL-1β; even in the absence of other inflammasome components or obvious cell lysis.
In order to demonstrate that the secretion of IL-1β was dependent on the proteolytic activity of caspase-1-gyrase, we used the broad-spectrum caspase inhibitor Z-VAD-fmk. Pre-treating MEFs for 1 h with Z-VAD-fmk, before inducing and activating caspase-1-gyrase, prevented IL-1β cleavage and secretion, as seen by both ELISA and western blot (Figures 2d and e). To confirm and extend this finding we generated caspase-1-gyrase constructs with mutations to render caspase-1 either catalytically inactive (caspase-1-gyrase catalytically inactive; C385G), or unable to be processed between the p10 and p20 domains (caspase-1-gyrase uncleavable; D296N, D300N, D304N, D308N, D313N, D314N) (Supplementary Figure S2a).27, 28, 29 As expected, the catalytically inactive caspase-1-gyrase did not cleave IL-1β (Figure 2f). On the other hand, we observed that uncleavable caspase-1-gyrase was still capable of cleaving IL-1β as efficiently, or even more so, than wild-type caspase-1-gyrase (Figures 2f and g). These data demonstrate that the dimerization of capase-1 is sufficient to induce catalytic activity and the cleavage of IL-1β in the absence of caspase-1 processing between the p20 and p10 subunits. This is in agreement with our results with the wild-type caspase-1-gyrase (Figure 1c) and a recent report indicating that some inflammasomes (that is, NLRP1b) activate caspase-1 directly, causing secretion of processed IL-1β in the absence of caspase-1 auto-processing.25
Dimerized caspase-1-gyrase activates IL-1β in the absence of cell death
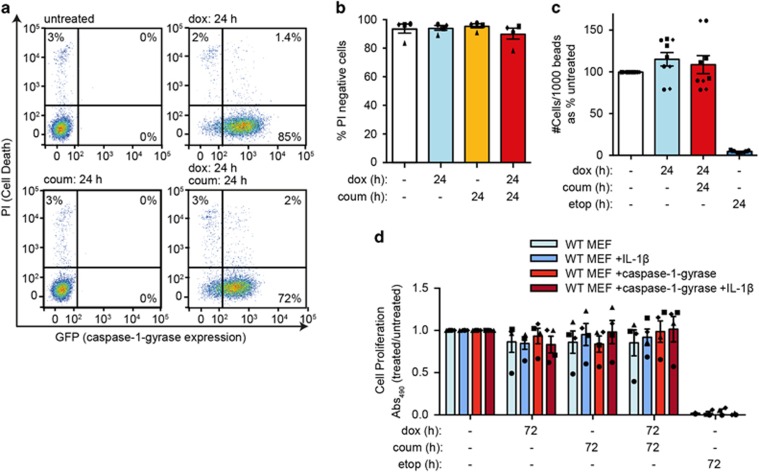
Caspase-1-gyrase-mediated cleavage of IL-1β and its secretion appeared to occur in the absence of cell death, as we could not detect other intracellular proteins (that is, HSP60, HMGB1, GAPDH, β-actin and caspase-1) in supernatants upon caspase-1-gyrase dimerization (Figure 2a). To confirm that IL-1β secretion could occur in the absence of cell death, we monitored individual cells. Flow cytometry was used to follow dox-induced expression of caspase-1-gyrase by measuring fluorescence of its GFP tag in cells that did not contain the GFP-expressing pro-IL-1β construct (Figure 3a). Despite caspase-1-gyrase being expressed in 72–85% of all cells, caspase-1 dimerization for 24 h did not cause cell death, as measured by propidium iodide (PI) uptake (Figures 3a and b). Nevertheless, at this time mature IL-1β was detected at high levels in the supernatant (Figures 1d and 2a).
Figure 3.
Dimerization of caspase-1-gyrase in MEFs does not induce cell death. (a and b) Caspase-1-gyrase was induced in MEFs with 1 μg/ml doxycycline and dimerized with 700 nM coumermycin for the indicated times. Cell viability was measured by PI uptake and flow cytometric analysis. (a) Representative flow cytometry data. (b) Quantification of cell viability from four independent experiments. (c) Dimerization of caspase-1-gyrase does not alter the total number of viable cells. MEFs containing caspase-1-gyrase were treated with 1 μg/ml doxycycline and 700 nM coumermycin, or 34 μM etoposide as specified for the indicated times and analyzed by flow cytometry. The absolute number of PI-negative cells was measured as a ratio to unstained flow cytometry beads. n=3 independent experiments, each performed in triplicate. (d) Cell proliferation and metabolism is not altered by caspase-1-gyrase dimerization. MEFs containing caspase-1-gyrase and pro-IL-1β constructs were treated as in (c) for the indicated times and cellular proliferation was measured by the MTT-PMS viability assay. n=4 independent experiments. Error bars represent the S.E.M. in all graphs
To determine whether cells were undergoing a complete cellular rupture that could, in theory, prevent PI staining of cells, we measured the absolute number of PI-negative cells using calibration beads. Etoposide treatment, which induces intrinsic apoptosis, reduced the number of viable cells by more than 95%. In contrast, the expression and dimerization of caspase-1-gyrase caused no reduction in the total number of viable cells (Figure 3c). Similar to the wild-type caspase-1-gyrase, the catalytically inactive mutant did not induce cell death, whereas the uncleavable mutant induced a small amount of cell death after 24 h of dimerization (Supplementary Figure S2b).
As an alternative readout for cellular health, we used the MTT-PMS assay to measure cell replication and metabolism. Consistent with our previous results, MEFs showed comparable colorimetric change after 72 h regardless of pro-IL-1β or caspase-1-gyrase expression and dimerization, in contrast to etoposide treatment (Figure 3d). Therefore, despite efficient caspase-1-gyrase expression at a single cell level, and high levels of IL-1β detectable in the supernatant, cells with dimerized caspase-1-gyrase are metabolic activity and viable.
Caspase-8-gyrase dimerization can induce cleavage and secretion of IL-1β
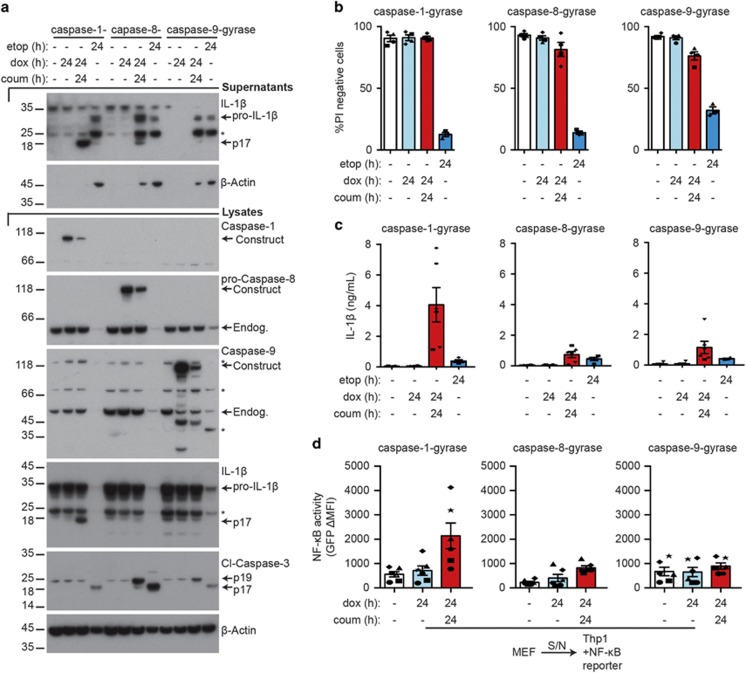
Recent studies have reported that caspase-8 may (i) process caspase-1,10, 11 (ii) activate NLRP3,12, 30 or (iii) cleave and activate IL-1β directly.6, 7, 8, 9 Therefore, to determine whether dimerization of caspase-8 can directly activate IL-1β and induce its secretion in the absence of other inflammasome components (that is NLRP3 and caspase-1), we generated IL-1β-expressing MEF lines bearing dox-inducible FLAG- and GFP- tagged dimerizable caspase-8 (caspase-8-gyrase) or control caspase-9 (caspase-9-gyrase). Direct comparison of caspase-8- and caspase-9-gyrase constructs to caspase-1-gyrase MEFs revealed that dimerized caspase-8, but not caspase-9, induced cleavage and secretion of IL-1β (Figure 4a). In agreement with our previous work,6 caspase-8 was less efficient than caspase-1.
Figure 4.
Caspase-8-gyrase dimerization can induce low levels of IL-1β secretion. (a) Caspase-8-gyrase dimerization causes IL-1β cleavage and secretion. Western blot analysis of lysates and supernatants from MEFs containing overexpressed pro-IL-1β and either caspase-1-gyrase, caspase-8-gyrase or caspase-9-gyrase treated as specified with 1 μg/ml doxycycline and 700 nM coumermycin, or 34 μM etoposide, for the indicated times. Asterisks denote non-specific bands. (b) Caspase-8-gyrase and caspase-9-gyrase dimerization induces small amounts of cell death. Cells were treated as in (a) and analyzed by flow cytometry for uptake of PI. n=4 independent experiments. (c) IL-1β is secreted upon dimerization and activation of caspases. MEFs were treated as in (a) and IL-1β released into the supernatant was measured by ELISA. n=4–6 independent experiments. (d) IL-1β secreted following caspase-1-gyrase or caspase-8-gyrase dimerization is biologically active. Thp1 cells bearing an NF-κB GFP reporter were incubated for 24 h with supernatants from caspase-1-gyrase, caspase-8-gyrase or caspase-9-gyrase MEFs treated for 24 h with 1 μg/ml doxycycline and 700 nM coumermycin. GFP expression indicative of NF-κB activation was quantified as the change in mean fluorescence intensity (MFI) relative to Thp1 cells in media alone. n=6 independent experiments. Error bars represent the S.E.M. in all graphs. Asterisks denote non-specific bands in western blots. Data for caspase-1, -8 and -9 were collected in parallel. See also Supplementary Figure S3
To determine whether in contrast to caspase-1-gyrase, caspase-8-gyrase or caspase-9-gyrase dimerization was capable of inducing cell death we measured the cleavage of the apoptotic effector caspase, caspase-3, as well as the release of cellular proteins into the supernatant by western blot. Intriguingly, in contrast to dimerized caspase-1-gyrase, we observed cleavage of caspase-3 to the active p19 fragment upon expression and dimerization of apoptotic caspase-8 and caspase-9 (Figure 4a). We confirmed apoptotic cell death occurred, albeit less efficiently than etoposide, by analyzing PI uptake after 24 h in dimerized caspase-8-gyrase and caspase-9-gyrase MEFs (Figure 4b). This cell death was clearly apoptotic in nature, as it was blocked by pre-treatment with the pan-caspase inhibitor Z-VAD-fmk (Supplementary Figure S3a). Consistent with this low level of caspase-dependent death upon caspase-8- and caspase-9-gyrase dimerization, both β-actin and pro-IL-1β were released into the supernatants (Figure 4a). This was in contrast to the absence of these proteins in the supernatants of cells expressing dimerized caspase-1-gyrase (Figures 2a and 4a,Supplementary Figure S1c).
Analysis of supernatants by ELISA revealed that IL-1β was released upon dox-induction and dimerization of all three constructs, as well as with etoposide treatment (Figure 4c). The release of IL-1β by the caspase-8-gyrase and caspase-9-gyrase was dependent on the catalytic activity of caspase-8 and caspase-9, respectively, as it was inhibited by Z-VAD-fmk (Supplementary Figure S3b). To establish whether, as with caspase-1, the caspase-8-gyrase construct was causing the release of biologically active IL-1β, as suggested by our western blot analysis (Figure 4a), we transferred supernatants from each of the caspase-gyrase constructs onto the NF-κB GFP reporter Thp1 cells. Notably, only supernatants from caspase-1-gyrase or caspase-8-gyrase transferred from MEFs treated with both dox and coumermycin were able to increase the activation of NF-κB above the level of untreated cells, as indicated by increased GFP fluorescence (Figure 4d). In contrast, despite releasing detectable amounts of IL-1β by ELISA (Figure 4c,Supplementary Figure S3b and S3c), supernatants from MEFs-expressing caspase-9-gyrase were not able to further activate NF-κB (Figure 4d). This is consistent with western blot results showing that dimerized caspase-1-gyrase induced the cleavage and release of the mature p17 form of IL-1β, dimerized caspase-8-gyrase stimulated the release of both the pro and cleaved forms of IL-1β, whereas caspase-9-gyrase and etoposide treatment only caused the release of inactive pro-IL-1β into the supernatant (Figure 4a).
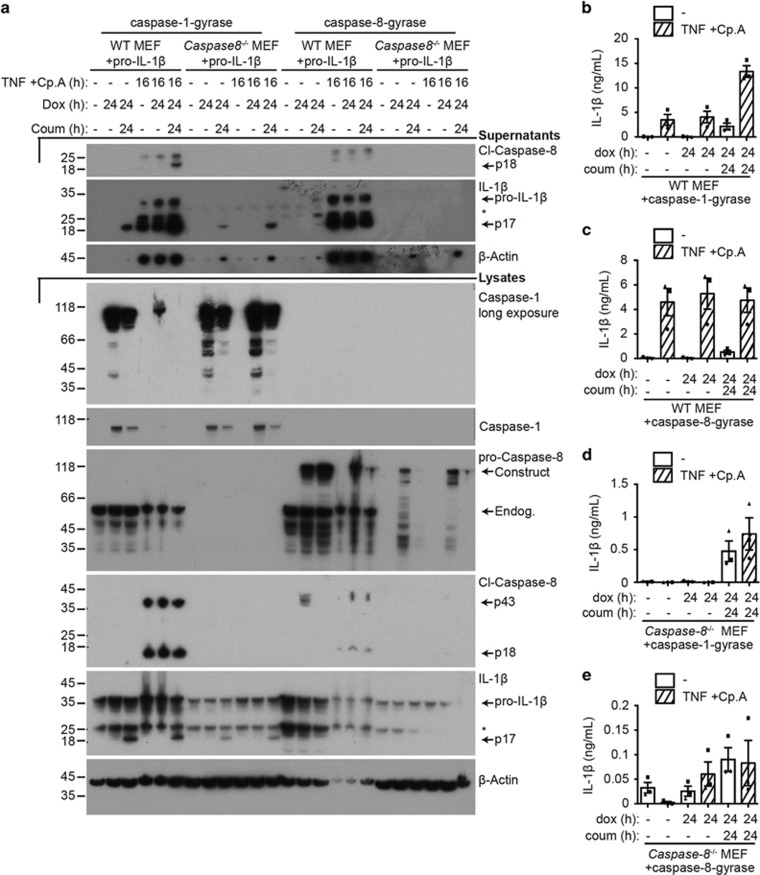
In order to determine whether the caspase-8-gyrase construct would be more efficient at killing cells via endogenous death receptor signaling, in comparison with dimerization-induced activation, we stimulated cells with TNF and induced the degradation/inhibition of inhibitor of apoptosis proteins using Smac-mimetic compound A (Cp.A).26 As expected based on our previous work,26, 31 TNF and Cp.A killed about half of the wild-type (WT) MEFs after 16 h of treatment (40–50% PI positive; Supplementary Figure S4a, S4b). Similarly, Caspase-8−/− MEFs were sensitive to TNF and Cp.A treatment when reconstituted with caspase-8-gyrase, either when induced with dox or dimerized with coumermycin (Supplementary Figure S4c). In contrast, Caspase-8−/− MEFs-expressing caspase-1-gyrase did not die following TNF and Cp.A treatment (Supplementary Figure S4d). Hence, the caspase-8-gyrase protein, but not the caspase-1-gyrase, can function in place of endogenous caspase-8 to kill cells when activated by death receptor signaling.
Given that forced dimerization of caspase-8 causes cleavage and secretion of IL-1β (Figures 4a and c), we asked if activated endogenous caspase-8 could do the same. In fact, endogenous caspase-8 exhibited greater efficiency in inducing IL-1β cleavage and secretion in WT MEFS treated with TNF and Cp.A, compared with caspase-8-gyrase dimerization (Figures 5a–c). Furthermore, as Caspase-8−/− MEFs lack both expression of caspase-8, as well as expression of endogenous inflammasome machinery, including caspase-1 (Figure 1b), cleavage and secretion of IL-1β could only occur if caspase-1-gyrase was exogenously expressed and dimerized (Figures 5a and d), or if caspase-8-gyrase was exogenously expressed and activated either with TNF and Cp.A or by dimerization (Figures 5a and e). Therefore, activated endogenous caspase-8, or dimerized overexpressed caspase-8, is capable of directly cleaving IL-1β and causing its secretion in the absence of other inflammasome components, including ASC and caspase-1.
Figure 5.
Both endogenous caspase-8 and caspase-8-gyrase can cleave IL-1β and cause its secretion. MEFs containing pro-IL-1β and either caspase-1-gyrase (a, b and d) or caspase-8-gyrase (a, c and e) vectors were treated with 1 μg/ml doxycycline and 700 nM coumermycin, as well as 100 ng/ml TNF and 1 μM Cp.A when indicated by cross-hatched columns. (a) Representative western blot of lysates and supernatants blotted for the indicated proteins. Asterisks denote non-specific bands. (b, c, d and e) Activation of endogenous caspase-8, caspase-8-gyrase or caspase-1-gyrase causes secretion of IL-1β. Supernatant of indicated cells was analyzed by IL-1β ELISA after treatment. Error bars represent S.E.M. n=3–5 independent experiments. See also Supplementary Figure S4
Collectively, these results demonstrate that endogenous caspase-8 activation, or the dimerization of caspase-8-gyrase or caspase-1-gyrase (but not caspase-9-gyrase) can cleave and activate IL-1β and induce its secretion. This may explain why some viral proteins, such as CrmA, have evolved to inhibit both caspase-1 and caspase-8.
IL-1β secretion occurs in viable cells
Recent studies propose that caspase-1 killing invariably coincides with IL-1β secretion, and/or that cell death is the dominant mechanism by which IL-1β is released.18, 19, 20 However, our data on populations of cells suggest that dimerized caspase-1 is capable of inducing active IL-1β secretion without loss of viability or impacting cell growth. To extend these results to a single cell level, we visualized dimerized caspase-1-gyrase-induced IL-1β secretion from individual living cells by ELISpot (Experimental schematic Supplementary Figure S5a).
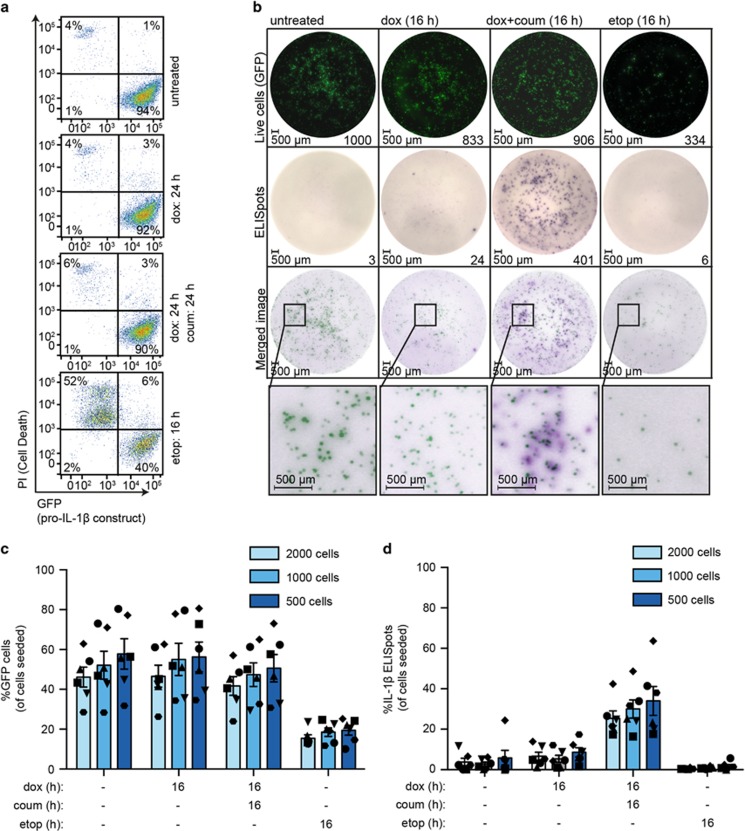
The vector that expresses pro-IL-1β is a bicistronic construct that also expresses GFP (Figure 1a). Comparably strong GFP expression from this construct was maintained whether caspase-1-gyrase was expressed or not, as seen both by flow cytometry (Figure 6a) and by single cell imaging on the ELISpot plate (Figure 6b). In contrast, when MEFs were killed with etoposide, GFP was no longer detectable in the PI-positive dead cells (Figure 6a), indicating that in this system GFP acts as marker of live cells. Following overnight incubation of caspase-1-gyrase MEFs on ELISpot plates and treatment with etoposide, or dox with or without coumermycin, live cells were imaged by GFP fluorescence (top row Figure 6b). As expected, there were 70% less GFP-positive cells detected when the cells were treated with 34 μM etoposide, indicating significant cell death. In contrast, whether the caspase-1-gyrase cells were untreated, treated with dox alone (caspase-1-gyrase expression), or treated with dox plus coumermycin (caspase-1-gyrase expression and dimerization), we observed no differences in the number of GFP-positive cells (top row Figure 6b,c), as we showed previously in viability assays (Figure 3).
Figure 6.
IL-1β is secreted from live MEFs when caspase-1-gyrase is dimerized. (a) GFP fluorescence is lost upon cell death. MEF cells containing pro-IL-1β and caspase-1-gyrase were treated with 1 μg/ml doxycycline and 700 nM coumermycin, or 34 μM etoposide for indicated times, and GFP fluorescence and PI uptake were analyzed by flow cytometry. (b, c and d) Caspase-1 activity causes secretion of IL-1β from live cells. MEF cells containing pro-IL-1β and caspase-1-gyrase constructs were seeded at three densities on the ELISpot plate. After 16 h treatment with 1 μg/ml doxycycline and 700 nM coumermycin, or 34 μM etoposide, GFP images were taken and then ELISPot staining was immediately performed. (b) Representative images of 2000 cells/well ELISpot experiment. The brightness and contrast of images were adjusted using Fiji (ImageJ) and Adobe Illustrator in a linear fashion, with settings applied equally to every image. GFP images were inverted in the merged image and pseudo-coloring was applied using Adobe Illustrator to allow overlay of ELISpots and GFP-positive cells. (c) Analysis of the number of GFP-positive cells as a ratio of total cells seeded. (d) Analysis of ELISpots represented as a percentage of cells seeded per well. Both GFP and ELISpot images were acquired and quantified using an AID ELISpot Reader. n=6 independent experiments. Error bars represent the S.E.M. See also Supplementary Figure S5
To ensure the ELISpot-seeded cells were not aggregating, which would make it difficult to define if individual GFP-positive live cells could secrete IL-1β, we plated duplicate experiments in parallel on tissue culture treated plates of the same well size. This allowed for simultaneous analysis of cell density by bright light microscopy and GFP fluorescence (Supplementary Figure S5b). These images confirmed that cells treated with dox and coumermycin maintain their GFP positivity equivalent to untreated or dox alone treated cells. Importantly, we failed to detect aggregated cell clusters; the cell densities used to seed the ELISpot plates resulted in clearly distinguishable individual GFP-positive cells. Moreover, dox and coumermycin-treated cells exhibited equivalent density and morphology to control treated wells (Supplementary Figure S5b).
Immediately after imaging the plates for GFP fluorescence, we developed the IL-1β ELISpots (second row Figure 6b). In wells where MEFs-expressing caspase-1-gyrase were dimerized, hundreds of discrete dark purple spots were detected, indicating IL-1β released from single cells (second row Figure 6b). All other conditions yielded ~10-fold fewer spots or only had background staining (Figure 6b). The position of the IL-1β spots typically corresponded to the position of live GFP cells imaged prior to ELISpot processing (Figure 6b, bottom two rows), identifying that live cells are capable of secreting IL-1β. Incubating ELISpot plates with mature recombinant IL-1β resulted in diffuse uniform purple staining across the entire well, with the staining intensity being dependent on the dose of recombinant IL-1β added (Supplementary Figure S5c). This demonstrates that IL-1β is quickly captured by the coating antibody after being released from a single cell, and that a small number of cells releasing diffuse IL-1β into the supernatant cannot explain the presence of ELISpots.
Quantitation of IL-1β ELISpots as a ratio to the number of cells seeded, indicated that 30–40% of caspase-1-gyrase-dimerized MEF cells secreted IL-1β (Figure 6d), despite no significant cell death occurring at the population level (Figure 3). To accurately quantify the per cent live (GFP-positive) cells secreting IL-1β, we evaluated the ELISpots as a ratio of the GFP-positive cells measured for each well. Importantly, when caspase-1-gyrase was dimerized, 60–70% of GFP-positive cells were also positive for IL-1β secretion (Supplementary Figure S5d). Taken together, these data provide strong evidence that caspase-1 can cleave and cause the secretion of IL-1β without simultaneously causing cell death.
Discussion
IL-1β can be activated and released from cells in a caspase-1 or caspase-8 dependent manner.1 However, because it lacks a canonical signal sequence IL-1β is not secreted via the conventional ER/Golgi secretory pathway.4 Considering caspase-1-dependent IL-1β release occurs simultaneously with pyroptosis or cellular necrosis, it has been proposed that these two caspase-1-depdendent functions cannot be separated and therefore that lysis of the plasma membrane is the mechanism by which mature IL-1β leaves the cell.18, 19, 20 However, recent studies have reported that active IL-1β can be released from cells without the release of cellular LDH, which occurs upon cell death.13, 21, 32, 33, 34, 35, 36 Using multiple experimental parameters we now demonstrate on both the population and single cell levels that cell death is not required for IL-1β secretion.
The dimerization of caspase-1 in MEFs resulted in IL-1β cleavage to the mature form within 2 h – an efficiency comparable to canonical NLRP3-caspase-1 inflammasome mediated IL-1β activation in myeloid cells. This active IL-1β was secreted from living cells whose plasma membranes resisted the uptake of PI, and maintained metabolic activity.
Given that caspase-1 oligomerization is sufficient to trigger pyroptosis, it remains unclear why our dimerized caspase-1 construct was unable to induce cell death. It is possible that the fused C-terminal gyrase-GFP on caspase-1 is inhibiting its pyroptotic functions.37 Alternatively, our immortalized MEFs may express reduced levels of important pyroptotic effector molecules required for efficient cell death downstream of inflammatory caspase activation, such as gasdermin D.38, 39 Regardless of the cause, our system has fortuitously allowed us to separate the cell death function of caspase-1 from its ability to cleave cytosolic IL-1β and cause its secretion.
Several different mechanisms for the secretion of IL-1β have been proposed, but none have been definitively proven.4 Several groups have provided evidence that active IL-1β is released together with other inflammatory cell components when the plasma membrane ruptures.40 Underlying this hypothesis is the observation that caspase-1 can trigger a lytic form of cell death termed pyroptosis.29 Reports of single cell studies have concluded that once caspase-1 activation passes a threshold, cells cannot escape death.16, 19 Experimentally this means activating caspase-1 results in both cleavage of IL-1β and its release, both of which often occur almost simultaneously with the death of the cell. By bypassing the need for upstream inflammasome components and activating stimuli, which have the capacity to trigger both pyroptosis and apoptosis, we have been able to demonstrate that membrane lysis is not necessary for the release of active IL-1β from cells following caspase-1 activation.
Previous work has implicated caspase-8 in the direct processing and activation of precursor IL-1β, following induction of TLR, death receptor or dectin-1 signaling.6, 7, 9, 41, 42, 43, 44 However, complicating matters are reports indicating that caspase-8 contributes to inflammasome priming,12, 45, 46 may process and activate caspase-1,10, 11 or can induce NLRP3 inflammasome formation.12, 13, 30 Here, we show in the absence of IL-1β-activating platforms, including ASC, caspase-1 and NLRP3, that the direct dimerization of caspase-8, or TNF and Smac-mimetic activation of endogenous caspase-8, is sufficient to cause IL-1β cleavage into a biologically active form and induce its secretion. Hence, dimerized active caspase-8 is likely to be a direct activator of IL-1β.
The literature suggests that pyroptosis has an important role in viral and bacterial responses, and sepsis.47 However, IL-1 blockade alone suffices to treat auto-inflammatory diseases such as CAPS. Our findings show that caspase-1 activation can cause IL-1β release in the absence of cell death. Hence, caspase-1 may perform distinct functions, depending on the disease context and pathogenesis.
Materials and Methods
Cell Lines and materials
WT and Caspase-8−/− MEF cells immortalized with SV40 large T antigen were maintained and are described elsewhere.26, 48 NF-κB reporter Thp1 monocytic cells were made by infection with the lentiviral reporter vector (pTRH1-NF-κB-dscGFP, TR503PA; System Bioscience, Palo Alto, CA, USA) and sorted for GFP expression by flow cytometry. BMDMs were generated by culturing bone marrow progenitors from C57BL/6 mice (purchased from WEHI Bioservices, Kew, Australia) as described elsewhere.12 The Walter and Eliza Hall Institute (WEHI) Animal Ethics Committee approved all animal experiments. Compounds and cytokines were obtained from the following sources: dox (Sigma-Aldrich, St. Louis, MO, USA), coumermycin (Sigma), Z-VAD-fmk (Sigma), etoposide (Clifford Hallam, Dandenong South, Victoria, Australia), human Fc-TNF (produced in house49) and Compound A (TetraLogic Pharmaceuticals, Malverna, PA, USA), and utilized as indicated in the figure legends.
Generation of caspase constructs
Mouse caspase-1 (wild-type, catalytically inactive (C385G), uncleavable mutant (D296N, D300N, D304N, D308N, D313N, D314N0)), caspase-8 and caspase-9 were cloned into the pFTRE 3G rtTA puro vector,50 with N-terminal FLAG tag, C-terminal fragment of E. coli gyrase B (residues 2–220) that dimerizes in response to the antibiotic coumermycin and C-terminal GFP as depicted in Figure 1a. Full-length mouse IL-1β was cloned into a pMIGR retroviral vector. All constructs were verified by DNA sequencing.
BMDM cell lysates
WT BMDMs were derived, treated and lysed as described.12 Ultrapure lipopolysaccharide from E. coli K12 strain (Invivogen, San Diego, CA, USA) was used as indicated in the figure legends.
Cell death analysis
MEFs were seeded at 1.5 × 105 cells per well in six-well plates and treated 24 h later as indicated in the figure legends. Adherent and non-adherent cells were harvested and incubated with 1 μg/ml PI in PBS prior to analysis by flow cytometry. Data were analyzed using FlowJo software version 7.6.5. MTS viability assays were conducted as described in.51
Quantification of cell viability using flow cytometry particles
Cells were seeded and treated 24 h later as indicated in the figure legends. Equal numbers of blank calibration beads (6.3 × 104 beads/well) (Spherotech, Lake Forest, IL, USA) were added to each culture well just before harvesting. Adherent and non-adherent cells were harvested and incubated with 1 μg/ml PI in PBS prior to analysis by flow cytometry. The number of PI-negative cells per 1000 beads was measured for each sample using a FACSCalibur (Becton Dickinson, Scoresby, Victoria, Australia).
Measurement of cytokines by ELISA
Cells were treated as indicated. Supernatants were removed from cultured cells and pelleted at 1 500 rpm to remove any debris. Lysates were made in DISC buffer (20 mM Tris pH 7.5, 150 mM NaCl, 2 mM EDTA, 10% Glycerin, 1% Triton X-100 (Sigma-Aldrich, T9284) including protease inhibitors (Roche, Basel, Switzerland, 11697498001)) or by subjecting cells to three rounds of freeze thawing and centrifuging lysates at 13 000 rpm to remove cellular debris. IL-1β ELISA (mouse IL-1β duoSet ELISA, R&D systems,Minneapolis, MN, USA; DY401) was used to quantify cytokine levels according to the manufacturer's instructions.
NF-κB Signaling
MEF cells were treated as indicated. Supernatants were removed from cultured cells and pelleted to remove any debris. Thp1 cells bearing the NF-κB reporter construct were cultured in the MEF supernatant for 24 h prior to measuring GFP expression of Thp1 cells relative to those in media alone using a FACSCalibur (Becton Dickinson).
Immunoblotting
Reduced and denatured cell lysates (lysed in Triton X-100 based ONYX buffer (20 mM Tris, pH 7.5, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 10% glycerol, 1% Triton X-100 (Sigma-Aldrich, T9284) including protease inhibitors (Roche, 11697498001)) and supernatants were separated on NuPAGE Novex 4-12% Bis-Tris gels (Life Technologies, Carlsbad, CA, USA) and transferred onto nitrocellulose (Amersham, Little Chalfont, Buckinghamshire, UK) or PVDF (Millipore, Billerica, MA, USA) membranes. In total, 20 μg of protein lysate was loaded unless otherwise stated. In total, 16 μl of supernatant samples was loaded without concentration. Blots were probed with antibodies against pro- and cleaved caspase-1 (Santa Cruz Biotechnology, Dallas, TX, USA; sc-514, Adipogen, San Diego, CA, USA, AG-20B-0042-C100), IL-1β (R&D; AF-401-NA), NLRP3 (Adipogen; AG-20B-0014-C100), ASC (Santa Cruz Biotechnology; sc-22514-R), pro-caspase-8 (in house), cleaved caspase-8 (Cell Signaling Technology, Danvers, MA, USA; 8592), caspase-9 (in house), cleaved caspase-3 (Cell signaling; 9661), β-actin (Sigma; A-1978) and HSP90 (Enzo, Farmingdale, NY, USA, ADI-SPA-835). All secondary antibodies used were conjugated to HRP and detected by ECL (Amersham or Millipore).
ELISpot
Capture antibody (mouse IL-1β duoSet ELISA, R&D systems; DY401) was incubated at 50 μg/ml in 50 μl PBS overnight on a 96-well filtration plate (Millipore, MAHAS4510). Plates were subsequently blocked with 100 μl Dulbecco's Modified Eagle's Medium supplemented with 8% FCS at 37 °C for 1 h. MEF cells bearing FLAG-caspase-1-gyrase-GFP and pro-IL-1β GFP constructs were then seeded on the plates as indicated and treated for 16 h. As a control media with recombinant IL-1β (standard from mouse IL-1β duoSet ELISA, R&D systems; DY401) at concentrations between 20 000 pg/ml to 1000 pg/ml was incubated on the plate for 16 h. An AID ELISpot Reader (Autoimmun Diagnostika GMBH, Strassberg, Germany) was used to take fluorescent images of GFP-positive live cells. Plates were immediately washed 3 × with PBS/0.05% Tween 20 then 3 × with PBS alone. Plates were incubated with biotin-conjugated detection antibody (mouse IL-1β duoSet ELISA, R&D systems; DY401) at 5 μg/ml in 50 μl PBS 1% FCS for 2 h at RT. After washing, streptavidin-ALP (Mabtech, Sweden; 3310) diluted 1/1000 in PBS/1% FCS was added to the plates for 1 h at room temperature. After washing, ELISpots were developed using 50 μl of BCIP/NBT-plus substrate (Mabtech; 3650-10). GFP-positive cells and ELISpot numbers were analyzed using the AID ELISpot software. The brightness and contrast of figure images were adjusted using Fiji (ImageJ) and Adobe Illustrator in a linear fashion, with settings applied equally to every image. Pseudo-coloring was applied to merged images using Adobe Illustrator to allow overlay of ELISpots and GFP-positive cells. Tissue culture plates were also seeded simultaneously to ELISpots to allow for microscopy imaging using the Opera Phenix, taken at 10 × with 2 × 2 binning.
Acknowledgments
We thank W Cook and J Silke (WEHI) for vectors, M Rashidi (WEHI) for NF-κB Thp1 reporter cells, K Schroder (IMB) for the pMIGR retroviral vector and R Lewis (WEHI) for cloning the IL-1β vector. This work was supported by National Health and Medical Research (Canberra, Australia) Project grants (1051210, 1101405), fellowships (JEV (1052598); LL (1035502); DLV (1020136)) and Program Grants (461221), and operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS (361646). We thank R Crawley for animal care; S Monard and staff for cell sorting; M Hardy for assistance with ELISpots; L Whitehead for assistance with image acquisition.
Glossary
- ASC
apoptosis-associated speck-like protein containing a CARD
- BMDM
bone marrow-derived macrophages
- CAPS
cryopyrin-associated periodic syndromes
- Cp.A
smac-mimetic compound A
- DME
Dulbecco's modified eagle's medium
- dox
doxycycline
- IAP
inhibitor of apoptosis protein
- IL-1β
interleukin-1β
- IL-1R
interleukin-1 receptor
- MEF
murine embryonic fibroblast
- NLR
NOD-like receptor
- NLRP3
NLR protein 3
- PI
propidium iodide
- pro-IL-1β
precursor interleukin-1β
- TLR
toll-like receptor
- WT
wild type
- TNF
tumor necrosis factor
- AIM2
absent in melanoma 2
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by A Ashkenazi
Supplementary Material
References
- Menu P, Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol 2011; 166: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE. When beauty is skin deep: regulation of the wound response by caspase-8, RIPK3, and the inflammasome. J Invest Dermatol 2015; 135: 1936–1939. [DOI] [PubMed] [Google Scholar]
- Lawlor KE, Vince JE. Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochim Biophys Acta 2014; 1840: 1433–1440. [DOI] [PubMed] [Google Scholar]
- Monteleone M, Stow JL, Schroder K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine 2015; 74: 213–218. [DOI] [PubMed] [Google Scholar]
- Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci 2016; 73: 2349–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 2012; 36: 215–227. [DOI] [PubMed] [Google Scholar]
- Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med 2008; 205: 1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 2012; 13: 246–254. [DOI] [PubMed] [Google Scholar]
- Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol 2012; 189: 5508–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc Natl Acad Sci USA 2014; 111: 7385–7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Kanneganti TD. Novel roles for caspase-8 in IL-1beta and inflammasome regulation. Am J Pathol 2015; 185: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun 2015; 6: 6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F et al. Human monocytes engage an alternative inflammasome pathway. Immunity 2016; 44: 833–846. [DOI] [PubMed] [Google Scholar]
- Antonopoulos C, Russo HM, El Sanadi C, Martin BN, Li X, Kaiser WJ et al. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem 2015; 290: 20167–20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 2013; 20: 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 2012; 19: 1709–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker BA, O'Donnell JA, Gerlic M. Pyroptotic death storms and cytopenia. Curr Opin Immuol 2014; 26: 128–137. [DOI] [PubMed] [Google Scholar]
- Cullen SP, Kearney CJ, Clancy DM, Martin SJ. Diverse activators of the NLRP3 inflammasome promote IL-1beta secretion by triggering necrosis. Cell Rep 15; 11: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Liu T, Yamaguchi Y, Shirasaki Y, Shikada K, Yamagishi M, Hoshino K et al. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Rep 2014; 8: 974–982. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y, Yamagishi M, Suzuki N, Izawa K, Nakahara A, Mizuno J et al. Real-time single-cell imaging of protein secretion. Sci Rep 2014; 4: 4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci 2007; 120(Pt 5): 772–781. [DOI] [PubMed] [Google Scholar]
- Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G et al. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 2012; 36: 388–400. [DOI] [PubMed] [Google Scholar]
- Farrar MA, Olson SH, Perlmutter RM. Coumermycin-induced dimerization of GyrB-containing fusion proteins. In: Jeremy Thorner SDE, John NA (eds). Methods in Enzymology, Volume 327 Academic Press: Amsterdam, Netherlands, 2000, pp 421–429. [DOI] [PubMed] [Google Scholar]
- Cook WD, Moujalled DM, Ralph TJ, Lock P, Young SN, Murphy JM et al. RIPK1- and RIPK3-induced cell death mode is determined by target availability. Cell Death Differ 2014; 21: 1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guey B, Bodnar M, Manie SN, Tardivel A, Petrilli V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci USA 2014; 111: 17254–17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 2007; 131: 682–693. [DOI] [PubMed] [Google Scholar]
- Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 2010; 8: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 1992; 356: 768–774. [DOI] [PubMed] [Google Scholar]
- Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 1993; 75: 653–660. [DOI] [PubMed] [Google Scholar]
- Kang S, Fernandes-Alnemri T, Rogers C, Mayes L, Wang Y, Dillon C et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun 2015; 6: 7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol 2008; 182: 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep 2014; 8: 570–582. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kenny S, Ge L, Xu K, Schekman R. Translocation of interleukin-1beta into a vesicle intermediate in autophagy-mediated secretion. Elife 2015; 4: e11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016; 352: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG et al. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J immunol 2015; 194: 1763–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sanchez F, Diamond C, Zeitler M, Gomez AI, Baroja-Mazo A, Bagnall J et al. Inflammasome-dependent IL-1beta release depends upon membrane permeabilisation. Cell Death Differ 2016; 23: 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann MC, Rabe S, Russ S, Kapplusch F, Schulze F, Stein R et al. Fluorescent tags influence the enzymatic activity and subcellular localization of procaspase-1. Clin Immunol 2015; 160: 172–179. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015; 526: 660–665. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. Nature 2015; 526: 666–671. [DOI] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1beta via caspase-8 in dendritic cells. J Immunol 2013; 191: 4789–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, Rathinam VA, Bossaller L, Army K, Kaiser WJ, Mocarski ES et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1beta production in response to beta-glucans and the fungal pathogen, Candida albicans. J Immunol 2014; 193: 2519–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Bertin J, Gough PJ, Chan FK. A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. J Immunol 2015; 194: 1938–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol 2014; 192: 2029–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allam R, Lawlor KE, Yu EC, Mildenhall AL, Moujalled DM, Lewis RS et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep 2014; 15: 982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 2014; 192: 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 2015; 265: 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moujalled DM, Cook WD, Lluis JM, Khan NR, Ahmed AU, Callus BA et al. In mouse embryonic fibroblasts, neither caspase-8 nor cellular FLICE-inhibitory protein (FLIP) is necessary for TNF to activate NF-kappaB, but caspase-8 is required for TNF to cause cell death, and induction of FLIP by NF-kappaB is required to prevent it. Cell Death Differ 2012; 19: 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem 2006; 281: 13964–13971. [DOI] [PubMed] [Google Scholar]
- Lindqvist LM, Heinlein M, Huang DC, Vaux DL. Prosurvival Bcl-2 family members affect autophagy only indirectly, by inhibiting Bax and Bak. Proc Natl Acad Sci USA 2014; 111: 8512–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard JA, Anderton H, Etemadi N, Nachbur U, Darding M, Peltzer N et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. Elife 2014; 3: e03464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.