Autophagy is usually a pro-survival catabolic process, which provides energy and nutrients to the cell by recycling cytoplasmic material. In starving cells, this process is further stimulated above basal rates. During autophagy, the phagophore (autophagic membrane) encircles cytoplasmic material to form the autophagosome (AP) (Figure 1). To actually degrade these substrates, the autophagosome needs to fuse with one or more lysosomes, which provides the hydrolytic enzymes in an acidic environment. This structure is referred to as the autolysosome (AL) (Figure 1). Autophagosomes can also fuse with endosomes before autolysosome formation to form an amphisome (AM). The least selective form of autophagy is known as macroautophagy, whereas forms of autophagy that degrade selective substrates such as ribosomes, pathogens and damaged mitochondria, are referred to as ribophagy, xenophagy and mitophagy, respectively.
Figure 1.
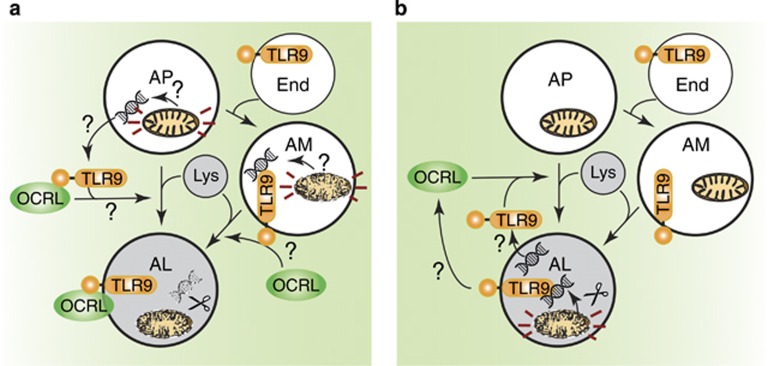
Potential models of TLR-mediated autophagic flux. (a) TLR9 is activated by mitochondrial (mt)DNA from damaged mitochondria in the AP and/or AM, which recruits OCRL and induces AL formation. (b) Intact mitochondria are surrounded in the AP and degraded in the AL to expose mtDNA. The free mtDNA then activates TLR9 and induces a positive feedback loop to increase autophagosome–lysosome fusion and autophagic flux
The fusion of the autophagosome to the lysosome to form the autolysosome is mediated by several different classes of proteins, including Rab GTPases, SNAREs (such as STX17), UVRAG and UVRAG's binding-partner VPS16, which is a component of the endosome–autophagosome tethering complex HOPS.1 Which specific proteins are utilised depends the type of autophagy and cell type. In Nature Cell Biology, De Leo et al.2 recently published that the cargo of the autophagosome may actually regulate fusion to the lysosome, and therefore regulate autophagic flux (the rate of autophagic degradation). Specifically, they demonstrate that TLR9 is activated during starvation and drives autolysosome formation (using the RFP-GFP-LC3 reporter) and by extension autophagic flux (as also measured by LC3B-II and p62 turnover assays) (Figure 1). TLR9 is a toll-like receptor found on the endoplasmic reticulum and endosomes that has an important role in innate immunity. TLR9 is activated by unmethylated CpG dinucleotides found in both bacterial and mitochondrial (mt)DNA. Indeed, De Leo et al.2 show that eliminating mtDNA inhibits translocation of several proteins on the lysosome during starvation, whereas induction of mitophagy increases this effect. One protein that translocated to lysosomes was OCRL, a protein mutated in Lowe syndrome (Figure 1a). Indeed, they go on to demonstrate that depletion of OCRL causes an increase in autophagosomes (presumably due to an inhibition of autophagic flux) and that activation of it's binding-partner MCOLN1 may rescue this defect in OCRL-depleted and Lowe syndrome patient samples. Some papers resolve old problems, whereas others raise lots of interesting follow-up questions. I believe this paper falls in the latter category, and how mtDNA might regulate autophagic flux by activating TLR9 will be the focus of this News & Views piece.
For instance, if whole mitochondria are engulfed and the mitochondrial inner and outer membranes protect the mtDNA, how does the mtDNA become available to bind TLR9? If TLR9 instigates autolysosome formation, as De Leo et al. suggest, then the mitochondria will not yet be in the presence of lysosomal hydrolases to degrade them (Figure 1a). Indeed, the lysosomal deoxyribonuclease (DNase) II may need to process CpG-containing DNA before it can be a suitable ligand for TLR9.3, 4 On the other hand, it has previously been reported by Otsu and colleagues5 that mtDNA from damaged mitochondria activates TLR9 from the autolysosome when mtDNA is unable to be properly degraded, such as when the DNase II is genetically ablated. In the experimental conditions of De Leo et al.2, DNAse II is expected to be active, but when it was inhibited, OCRL translocation to lysosomes was reduced. It would be interesting to formally demonstrate if OCRL can localise to autolysosomes as well as lysosomes, for instance with co-localisation assays between OCRL and LC3/LAMPs under starvation conditions. A model that fits data from both the De Leo and Otsu papers is that autolysosomes (made independently of mtDNA-driven TLR9 activation) expose mtDNA by degrading the mitochondrial membrane, which is then recognised by TLR9 in the autolysosome.2, 5 This would then cause a positive feedback loop via TLR9-dependent to increase autophagic flux, but would mean that TLR9 is not strictly required for autolysosome formation under these circumstances (Figure 1b). Another possibility is that TLR9 is brought to the cargo via endosome–autophagosome fusion (Figure 1a). It is conceivable that mitochondria could at least partially be degraded to expose mtDNA to the receptor at this stage.
Another question raised by the work of De Leo et al. is whether mitochondria consumed during starvation are healthy or damaged. Starvation with Hank's balanced salt solution is usually considered to induce relatively non-selective autophagy (macroautophagy), and this was the main autophagy stimulus used by De Leo et al. Although it has been previously shown that starvation-induced autophagy engulfs mitochondria, this appears to be to a minimal extent initially but increases after extended periods of starvation (24 h).6 The most efficient form of mitochondrial degradation via autophagy is mitophagy, which specifically removes damaged mitochondria and is canonically dependent on Parkin and PINK1. How are mitochondria being engulfed via macroautophagy or mitophagy during starvation when TLR9 is regulating autophagic flux? Are mitochondria damaged by starvation before they are engulfed? What would be the benefit, if any, to the cell to degrade healthy mitochondria during nutrient starvation? Future experiments using mitochondrial-targeted dyes, such as MitoTracker, and PicoGreen to stain mitochondrial DNA to formally demonstrate if both mitochondria and mtDNA are present in autophagosomes (e.g., co-localise with LC3) and/or autolysosomes (e.g., co-localise with LC3 and LAMP1) after starvation would help to answer many of these questions.
On the other hand, free mtDNA could potentially be accessible to TLR9 before autolysosome formation (Figure 1a). For instance, starvation can induce activation of the mitochondrial apoptosis effector proteins Bax and Bak, which compromise the mitochondrial membrane and could release mtDNA into the cytoplasm/autophagosome. However, if the mitochondrial membrane is permeabilized in the cytoplasm, caspases would also be activated and apoptosis would quickly occur. Indeed, Kile and colleagues7 recently demonstrated that mtDNA released into the cytoplasm during apoptosis can induce type I interferon production, similar to what was seen by De Leo and colleagues, but via the STING pathway, not TLR9. Why mtDNA induces proinflammatory cytokines by different mechanisms is still unclear, but it may be due to different localisation of the mtDNA.
The general idea of cargo-modulated autophagy is certainly intriguing. What happens in cell types, which express no or low levels of TLR9 when mtDNA is consumed? What other cargo may have the ability to affect autophagic flux? Would different cargo get recognised by different proteins involved in fusion? Does this differ in different cell types? What is the physiological advantage of a cargo-mediated regulation of autolysosome formation? Indeed, it is possible that autophagosome–lysosome fusion is a more sophisticated regulation point than we once thought.
Acknowledgments
I thank Drs Eric Baehrecke, James Vince, Grant Dewson and Dominic De Nardo for thought-provoking discussions, as always. LML holds an NHMRC Peter Doherty Early Career Fellowship (1035502). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS and from support form the Australian Cancer Research Fund.
The authors declare no conflict of interest.
References
- Ganley IG. Essays Biochem 2013; 55: 65–78. [DOI] [PubMed]
- De Leo MG et al. Nat Cell Biol 2016; 18: 839–850. [DOI] [PMC free article] [PubMed]
- Chan MP et al. Nature Commun 2015; 6: 5853. [DOI] [PubMed]
- Pawaria S et al. J Immunology 2015; 194: 1403–1407. [DOI] [PMC free article] [PubMed]
- Oka T et al. Nature 2012; 485: 251–255. [DOI] [PMC free article] [PubMed]
- Kristensen AR et al. Mol Cell Proteomics 2008; 7: 2419–2428. [DOI] [PubMed]
- White MJ et al. Cell 2014; 159: 1549–1562. [DOI] [PMC free article] [PubMed]