Abstract
Biological sample pretreatment is an important step in biological sample analysis. Due to the diversity of biological matrices, the analysis of target substances in these samples presents significant challenges to sample processing. To meet these emerging demands on biopharmaceutical analysis, this paper summarizes several new techniques of on-line biological sample processing: solid phase extraction, solid phase micro-extraction, column switching, limited intake filler, molecularly imprinted solid phase extraction, tubular column, and micro-dialysis. We describe new developments, principles, and characteristics of these techniques, and the application of liquid chromatography–mass spectrometry (LC–MS) in biopharmaceutical analysis with these new techniques in on-line biological sample processing.
KEY WORDS: Biological sample pretreatment, Solid phase micro-extraction, Column, Turbulent flow chromatography, Restricted access material, Molecular imprinting solid phase extraction
Graphical abstract
This paper summarizes several new techniques of on-line biological samples processing in recent years (such as solid phase extraction, solid phase micro-extraction, column switching technique, limit intake filler technique, molecular imprinted solid phase extraction technique, tubular column technique and micro-dialysis technique), and describes their developments, principles, characteristics and application matched with liquid chromatography–mass spectrometry (LC–MS) in biopharmaceutical analysis in on-line biological sample processing field.
1. Introduction
Biological sample pretreatment is an important part of biopharmaceutical analysis and the most troublesome aspect in the entire process of sample separation and analysis. Compared with the raw materials needed for pharmaceutical preparations, biological samples are more complicated: a drug, often in trace quantities, can distribute in complex biological media, and a sample may contain a large number of endogenous substances including the metabolites of the assayed drug1, 2. Thus, most biological samples, such as organs, tissues, plasma and urine, must be properly processed for separation, purification, enrichment and chemical modification to meet the requirements of the analytical instruments, such as high performance liquid chromatography (HPLC) and mass spectrometry (MS).
An ideal sample pretreatment technique should maximally remove the interfering substances and be suitable to a wide range of samples. Conventional sample-handling techniques, including liquid–liquid extraction (LLE), distillation, crystallization, filtration, pre-sedimentation, centrifugation, adsorption, and Soxhlet extraction separation have many shortcomings, such as voluminous organic solvent usage, complex and time-consuming operations, and lengthy and complex sample processing. All of these shortcomings limit the development of biopharmaceutical analysis with regard to miniaturization, high flux and automation. In recent years, on-line processing techniques for biological samples combined with HPLC–MS have improved sample flow-through, overcome previous shortcomings and deficiencies of the traditional analysis, and allowed the analysis of target compounds on-line automatically in a rapid and efficient way3, 4. This paper summarizes these new on-line sample processing techniques that are matched with LC–MS in the field of biopharmaceutical analysis.
2. Development of new techniques of on-line biological sample processing matched with LC–MS
In biopharmaceutical analysis, compositions of biological samples can be complex. Samples often contain a large number of interfering substances in addition to the analytes, including endogenous substances, metabolites, and coexisting drugs. As the content of the target analytes is often at picogram and nanogram levels or lower, most existing separation and analysis technologies cannot directly detect drugs in these complex matrices. Biological samples must be isolated, purified, enriched, and target analytes may need to be chemically modified for detection. Thus, sample pretreatment is one of the most tedious steps in the process of separation and analysis and is particularly critical. Biological sample pretreatment has been a relatively neglected part of the biopharmaceutical analysis field.
The traditional means of biological sample pretreatment, such as protein precipitation, centrifugation and filtration, and LLE are still widely used, but these methods have many shortcomings, including a requirement for a large number of reagents, high testing costs, complicated sample processing steps, which can be time- and labor-consuming, and have low recovery and precision. In addition, these methods are not conducive to on-line processing and automation, and may be detrimental to the environment and hazardous to the technician.
In fact, these traditional off-line sample processing methods are gradually becoming a limiting bottleneck in the development of biopharmaceutical analysis. Therefore, biological sample processing has attracted increasing attention and recognition, and the demands of new methods for on-line automatic analysis are becoming increasingly urgent in recent years. There have emerged a series of sample preparation techniques, such as solid phase extraction (SPE), solid phase micro-extraction technology (SPME), column switching technology, restricted access material (RAM) technique, turbulent-flow chromatography (TFC), molecularly imprinted SPE technology, micro-dialysis (MD), and other new technologies which can overcome flaws and shortcomings of the traditional sample preparation techniques and greatly improve the speed and accuracy of sample analysis.
These new techniques of on-line sample processing combined with the LC–MS have greatly promoted the development of biopharmaceutical analysis. On the one hand, in biological samples matrix compositions are complex, interfering substances are multiple, and pharmaceutical ingredients are small; so biological sample must be pretreated for analyte purification and enrichment. Pretreatment can improve the accuracy and precision of the analysis results, extend column life, improve selectivity and component testability, and improve chromatographic behavior. On the other hand, LC–MS technology has become one of the most important contemporary isolation and identification methods. It is a comprehensive analysis technique based on HPLC for the separation and MS for the detection, combines the high separation capacity of LC with the high sensitivity of MS5, 6.
LC–MS has a high degree of specificity that makes detection more sensitive and quantitation faster than other analytical methods, making up for the deficiencies of traditional LC detector. Undoubtedly, LC–MS has become the core analysis techniques in pharmacokinetic (PK) and metabolism studies. Therefore, the development of on-line sample preparation techniques combined with LC–MS directs biopharmaceutical analysis towards miniaturization, high selectivity, high-throughput, and automation. The development, principles, characteristics and the application of LC–MS in biopharmaceutical analysis with the various new technologies in on-line biological sample processing field will be described as follows.
3. New technology of on-line biological sample processing
3.1. SPE technique
SPE technology is a separation and purification method based on liquid—solid phase chromatography theory. In 1971, Broich et al.7, used the technique to extract urine samples of drug abusing persons for the first time. The equipment was considered a revolution of sample pretreatment techniques; it could integrate separation and concentration, and had the advantages of high extraction efficiency, good selectivity, wide application range, briefness and ease of automation. SPE realizes the separation and purification of the sample based on the liquid chromatographic separation principle of selective adsorption and selective elution.
Depending upon the type of solvent, SPE methods can be divided into three categories: normal phase, reversed phase and ion exchange SPE. Reversed phase and ion exchange SPE are commonly used for biopharmaceutical analysis. SPE mainly utilizes a non-polar mechanism (dispersion effect), polarity effects (hydrogen bonding, dipole-induced moment and dipole moment, etc.), and ionic and covalent mechanisms. Samples pass the extraction column that is filled with the adsorbent, analytes and the impurities are retained in the absorption column, and solvents are used to selectively remove impurities and elute analytes to achieve separation.
Compared with conventional LLE, SPE does not require emulsification which can affect the reproducibility of the result; another drawback of LLE is that the level of recovery depends largely on the operator so that the results obtained by different operators may vary greatly by the same method. The SPE method is based on interactions between functional groups of analytes and the stationary phase to extracts the analytes, and this method has good reproducibility and is conducive to standardization. Ferreiro-Veraa et al.8 used SPE–LC–MS/MS hyphenated technique to analyze prostaglandin levels in human serum, and the results showed that this method had good accuracy, precision and recovery. Compared with protein precipitation and LLE, this method overcomes some shortcomings such as metabolites that are readily biodegradable and target analytes that are easily degraded.
The technique of on-line SPE combined with LC–MS realizes the optimal combination between sample preparation and separation, especially as SPE–LC–MS becomes a technique of biological sample on-line processing. SPE and LC–MS have their own pump systems independently and combined through the conversion of a six-way valve. The adsorption column is connected to the LC–MS at the position of the sampling tube on the six-way valve, the targets are adsorbed and retained in the adsorption column and effluent is discharged to the waste reservoir bottle. The mobile phase is used to wash the analytes into chromatographic column for the next separation and determination. With on-line operation, all the analytes enriched by the SPE column are directly accessed by the LC–MS system. Compared with traditional protein precipitation and LLE, it greatly improves the extraction efficiency, reduces opportunities of contact with toxic reagents, and shortens the analysis time. On-line analysis not only greatly improves the recovery, accuracy and the detection limit, but also allows real-time automatic continuous biological sample testing.
Naxing et al.9 described an automated procedure using monolithic-phase based on-line SPE combined with HPLC–MS/MS for determination of amprenavir (APV) and atazanavir (AZV) in human plasma. This method combines a rugged on-line high-flow extraction method based on monolithic material with the narrow-bore analytical column for efficient separation and high sensitivity. In an evaluation of over 450 plasma injections, reproducible and reliable quantitative data were obtained for multiple analytes using same monolithic extraction cartridge. The precision and accuracy of the batch anaylses performed by this approach satisfied the requirements for GLP bio-analysis and the results were comparable with those obtained by LLE. The monolithic-phase on-line extraction approach demonstrated very low carry-over, high recovery, and was matrix-independent.
Wang et al.10 developed a highly precise, automatic and rapid method for quantification of puerarin in canine and human plasma using an on-line SPE column switching LC–MS. This system involves direct plasma injection into a liquid chromatograph and column switching, which was automated by software control and required only 6.5 min for a single analysis. The method yielded good characteristics of specificity, linearity, sensitivity and precision, which allowed for numerous samples to be processed in PK studies of puerarin in canine plasma. Furthermore, this method was successfully applied to quantification of puerarin in human plasma. Thus, it should be a powerful method for preclinical and clinical PK study of puerarin and other isoflavonoids drugs. Currently, the on-line SPE–LC–MS technique has been widely used in analysis of complex biological matrices, such as plasma11, 12, serum13 and tissue homogenates14, etc.
3.2. SPME technique
SPME is a relatively new type of sample pretreatment technique, first founded in 1990 by the Pawliszyn research15 from University of Waterloo (Canada). In 1993, the Supelco company (USA) launched the commercialization of an SPME device, which has been widely used in environmental analysis and showed great impact in the field of analytical chemistry. In recent years, many studies combined SPME with LC–MS for biopharmaceutical analysis of low volatile or non-volatile compounds of high polarity. The principle of SPME technique is based on the liquid—solid adsorption equilibrium and utilizes the adsorption affinity between analytes and the active solid surface to realize separation and enrichment.
Biological samples, such as blood plasma, whole blood, urine and tissue, contain thousands of complex components such as salts, proteins, cells, endogenous and exogenous small organic molecules (lipids, amino acids, etc). Thus, the determination of analytes in a complex matrix without an appropriate treatment may not be possible even with powerful modern analytical instruments like LC–MS. Traditional off-line biological sample processing techniques, such as LLE and SPE, mainly rely on using a large number of extraction agents to make sure that analytes will be separated from the sample matrix. In contrast, SPME is a balanced sample preparation technique; a certain percentage of the analytes can be separated from the biological matrix with a small volume of extraction agent. It demonstrates fast and efficient analysis, low cost of analysis, and does not need pumps or a power supply.
For example, PK studies on terephthalic diazepine drugs comparing conventional plasma protein precipitation method to SPME showed that SPME had better precision16, 17. Sampling and analyzing the target analyte in green leaves with SPME and MD technique was also demonstrated18. The results showed that the fully automated SPME procedure offered several advantages, including high-throughput and more efficient sampling, less labor intensity, and a capability for batch analysis compared to MD. In addition, the SPME technique had an additional advantage: it promoted the development of analysis of endogenous and exogenous compounds in biological samples, focused on the determination of free concentrations and binding constants, and provided rapid sample preparation both in the laboratory and on site; it allowed combination of samples into a single one, and even for complex biological samples19.
SPME was used for automatic analysis of volatile and semi-volatile substances beginning in 1992, with the analysis of benzene, toluene, ethyl benzene and xylene with a Varian Model 8100 auto sampler20. Subsequently, many automated SPME techniques were developed. In 1997, automatic SPME-LC was first used to analyze semi-volatile and non-volatile substances by Eisert21. This method requires an improved HPLC automatic sampler that contains adsorption material, samples adsorb and de-adsorb on the adsorption material, and analyte is eluted by mobile phase to the analysis column for separation and further analysis.
A typical example is that of Kataoka et al.22 who used this method to simultaneously determine amphetamine and β-blockers in biological matrices. This method was also used with complex biological matrices such as serum23, urine24, homogenized tissue25 and saliva26. Yang et al.27 determined the concentration of cannabinoid in biological samples with the SPME membrane technique combined with LC–MS. SPME is a holistic method with sampling, extraction and sample enrichment, combined with the advantages of SPME and membrane separation technique. In this study, a kind of polyamide and Tenax compound membrane material was applied to SPME, testing the adsorption ability of the membrane, studying and optimizing the membrane extraction conditions, such as adsorption time, desorption solvent, desorption time and auxiliary methods.
SPME–LC–MS is less widely used than SPME–GC–MS in the field of biopharmaceutical analysis for the following reasons: firstly, in biological analysis, analyte extraction and separation from SPME is often limited, and most of the analytes are high-protein conjugates. Therefore, high sensitivity instrumentation must be used to extend the limit and develop SPME. If this limitation can be effectively addressed, LOD and LOQ of analytes can achieve pg/mL or ng/mL sensitivity by MS.
Secondly, due to the low sample throughput and lack of commercial instruments for all SPME steps, the SPME–LC/MS application and even the use of SPME to analyze large samples in the laboratory are limited. In addition, equipment and packing required by SPME–LC are still not commercial, with the experimenter having to prepare packing materials themselves to meet experimental requirements.
Finally, in order to reach the acceptable precision and accuracy, users need to master the basic theory of SPME and appropriate calibration methods to control all the necessary parameters in the extraction process properly. Subtle changes of temperature, pH value, ionic strength, extraction time and other factors will have a significant impact on the SPME extraction results, and therefore these factors must be strictly controlled and properly addressed to develop the method. As it may lead to poor reproducibility of the method if not handled well, this is also the reason why poor precision is occasionally reported with the SPME technique.
3.3. Column switching technique
In 1970, column switching terminology was first proposed by Snyder28 and also called sequential chromatography, multi-column chromatography, coupled chromatographic column chromatography, and shunt chromatography. The column-switching technique actually refers to the technique that uses a valve to change the mobile phase system, such that eluent can go from the pre-column into the analytical column consecutively.
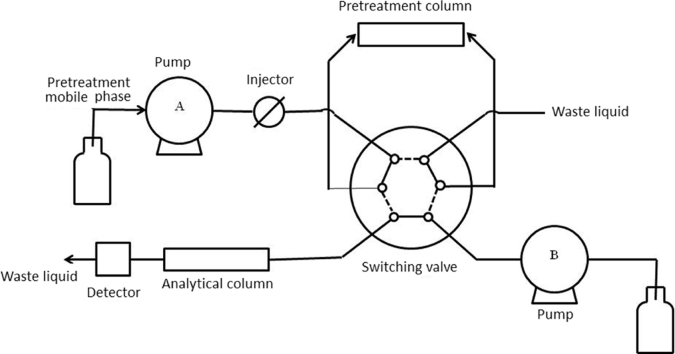
The switching valve and the flow path unit shown in Fig. 1 29. This connection of pre-column and the analytical column is called “on-line” connection. The switching valve (a 6- or 10-way valve) is usually controlled by a computer. At first, fluid samples that have been simply pretreated are injected through the injection valve while procedure is starting; samples arrive in the pretreatment column with the pretreatment mobile phase, the drug remains in the pretreatment column, and proteins and interfering substances flow out with the waste. Then at the end of preprocessing, the switching valve automatically changes the mobile phase flow channel to the analysis position, therefore, pretreatment mobile phase moves in the same direction with preconditioning flow or against the preconditioning flow with analytical mobile phase instead of going through pre-column. Finally, the components that concentrated in the pretreatment column are brought to the analytical column for the next elution and separation. Eventually, analytes enter the detector for detection. The switching valve and pre-column is washed with pretreatment mobile phase for the next sample injection.
Figure 1.
Sketch of column switching. Macromolecular interfering substances and analytes in biological fluids are separated in the pretreatment column, and the analytes are transferred from the pre-column to the analytical column chromatography through a valve to complete the analysis. Reprinted from Ref. 29 with permission of the copyright holder, Chinese chemical society.
Column switching technology has developed rapidly in recent years, and is becoming an increasingly popular method for high-throughput analysis of biological samples. It is widely used in various fields, such as environmental protection, pesticide residue monitoring, food inspection, biochemical analysis, and biopharmaceutical analysis. Of all those applications, column switching is most widely used in biopharmaceutical analysis, for example: (1) on-line deproteinization: protein-rich fluids (such as plasma and serum) samples cannot be directly injected into HPLC instrument because of the chemical reactivity of protein and organic solvents, which could lead to the chromatographic column being blocked and chemically modified. With the column switching technique a large volume of sample can directly go into the pre-column under the guidance of the water-based pre-treatment mobile phase. (2) Direct injection of whole blood: the key to whole blood injection is that the pretreatment column must use solid filler of coarse particle size (such as 50–90 μm) and column plates of larger aperture (e.g., 40 μm), so that blood cells can easily pass through the pretreatment column.
For lipophilic drugs, ODS can be used as pretreatment column packing, water can be used as the flushing fluid, the appropriate solution can be used for purification after blood cells and proteins are washed, and then the analytical mobile phase can be used to wash the testing components from the pre-column to the analytical column. Lastly, 0.5% SDS and methanol are available for washing the pre-column and removing residual macromolecules and endogenous impurities. As a rapid and stable method, column-switching technology simplifies sample processing and avoids the shortcomings of conventional treatment techniques. It has better chromatographic resolution and higher selectivity; it can enrich the trace components and achieve many separation objectives in a chromatographic network, and utilize on-line derivatization to improve the detection sensitivity and reproducibility of derivatization. Finally, on-line purification can be automated.
The technique of the column switching technique combined with LC–MS makes biopharmaceutical analysis automatic, rapid, and efficient, and it has been widely used in complex biological matrix analysis with serum30, blood31, plasma32, urine33, and other matrices.
Borrey et al.34 used the technique of column switching combined with LC–MS/MS for the quantitative determination of testosterone in human serum. Testosterone was extracted from methyl tert-butyl ether in 200 μL serum after the oxime derivatization. The matrix components were eliminated through on-line column switching HPLC methods. The instrument was a API4000 tandem mass spectrometer equipped with an Agilent 1312A Series binary pump and Agilent Series 1311A quaternary pump, and analysis total time was 3 min. The linear concentration range of the standard curve was 0.035 nmol/L (0.01 μg/L)–6.92 nmol/L (2 μg/L).
The correlation between this method and internal standards (solvent–extraction radio immunoassay (RIA)) showed R2 = 0.920. This indicates that the column-switching technique provides a simple, fast, economical way for testosterone analysis in human serum. This operation required only a small sample size and is very suitable for quantitative analysis of testosterone in serum.
Ansermot et al.35 used column switching liquid chromatography–electrospray ionization mass spectrometry (LC–ESI-MS) for quantitative analysis of cyclosporine A (CsA) in peripheral blood mononuclear cells (PBMC). This sensitive and selective analysis method was fully validated in the range of 5–400 ng/mL. This allowed the measurement of very small CsA amounts present in cells up to 0.5 fg/PBMC in clinical samples. Accuracy (95.0%–113.2%), repeatability (5.1%–9.9%) and intermediate precision (7.0%–14.7%) were found to be satisfactory. This method represents a new potential tool for therapeutic drug monitoring of CsA and could be used in clinical conditions if the utility of intracellular measurements is confirmed in prospective clinical trials.
3.4. RAM technique
RAM, is also called restricted access to the stationary phase, a concept was first proposed by Desilets et al.36 in 1991. It is a new class of fixed-phase LC developed at the beginning of the 1990s. The development of such materials aimed at establishing a direct injection on-line analytical method. RAM is a kind of porous exclusion chromatography packing material dedicated to the removal of endogenous macromolecules in biological matrices. It is based on the size exclusion principle and is suitable for bio-based analysis of small molecules.
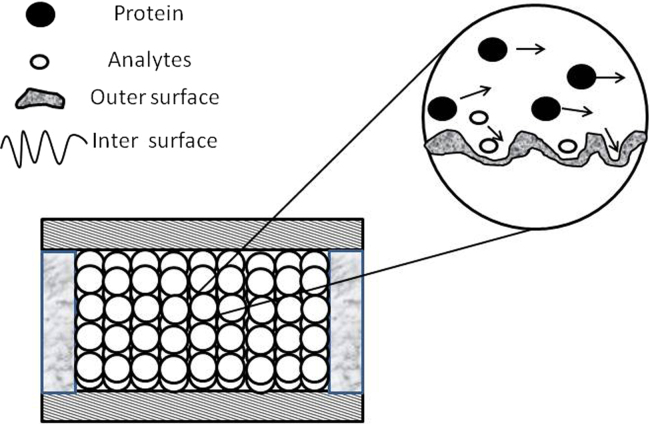
According to the structure and properties of adsorbents, RAM can be classified into five types37: the inner surface of the reverse phase packing material (ISRP), semi-permeable surface side filler (SPS), shielding filler hydrophobic phase (SHP), protein-coated silica filler C18 (ODS) and mixed functional filler. The structure is shown in Fig. 238. Using traditional manual techniques (LLE and SPE), it is often extremely difficult and time-consuming to remove proteins and avoids the loss of analytes; working with a large series of samples is almost impossible. RAMs as extraction pre-columns in a column-switching mode for HPLC offer the best prospects for the future, as this allows direct injection of previously unprepared biological material into the HPLC system. Compared to traditional SPE sorbents, RAM sorbents have many advantages, including longer lifetime, higher separation efficiency, higher analyte recovery, reduced analyte losses, lower organic waste production, lower total costs per analysis, and lower risk of error by laboratory staff. Table 1 lists several kinds of new techniques with on-line biological sample processing from recent literature reports.
Figure 2.
Sketch of restricted access material material (RAM). Small molecules penetrate into the inner surface and are retained because of the hydrophobic effect, and thus matrix components of biological macromolecules are removed while the analytes are retained. Reprinted from Ref. 38 with permission of the copyright holder, Chinese Chemical Society.
Table 1.
Features of new techniques of on-line biological sample processing.
| Pretreatment technique | Mechanism | Application | Characteristic | Ref |
|---|---|---|---|---|
| SPE | Non-polar effect, polar effects, ionic and covalent effect | Firstly extraction of urine samples of drug abuse; analyzing prostaglandin level in human serum | Good accuracy, precision and recovery; no emulsification and damage compared with conventional LLE | 7, 8 |
| SPME | Evolved from SPE technique; a fused silica fiber surface coated with adsorbed material | Extremely complex mixture such as blood plasma, whole blood, urine and tissue | Small volume of solvent compared with LLE and SPM; promoting the development for the analysis of endogenous and exogenous compounds | 19 |
| Column switching technique | Change through a valve to the mobile phase system, then eluent go from the pre-column into the analytical column | Complex biological matrices such as serum, blood, plasma, urine; environmental protection, pesticide residue monitoring, food inspection | Good resolution and higher selectivity compared with traditional method | 30, 31, 32, 33 |
| RAM | Based on the size exclusion principle | Removal of endogenous macromolecules in biological matrices | Longer lifetime, higher efficiency, higher analyte recovery, lower organic waste, lower total costs compared to traditional SPE sorbents | 37 |
| TFC | Column filled with adsorption material of large particle size | Removal of macromolecular proteins | A high flow rate to extract and remove the interference in the matrix | 43 |
| MIP | Synthesizing materials with specific molecular recognition properties | Identification and determination of low concentration compounds in complex matrices | High mechanical, thermal and pressure stability, but influenced by sample solvent | 56 |
| MD | Perfuse MD probe under the non-equilibrium conditions | Monitoring physiological levels of the active substances in the animal and human | A novel miniaturized sample pretreatment technique; consuming no solvent and achieving good sensitivity compared with SPE and LLE. | 64 |
LLE, liquid–liquid extraction; MD, micro-dialysis; MIP, molecularly imprinted polymers; RAM, restricted access material; SPE, solid phase extraction; SPME, solid phase micro-extraction; TFC, turbulent-flow chromatography.
The traditional methods of biological samples processing, like protein precipitation, LLE, and SPE are often time- and labor-consuming and the relative reproducibility is poor. The tedious processing of a biological sample has become a bottleneck in biological samples analysis, and prompted the development of high-throughput analysis39.
Considerable effort has gone into developing automation and high-throughput sample processing procedures. The RAM technique coupled with HPLC and column-switching can realize automatic analysis, and this method has been widely and successfully applied for the analysis of drug molecules in complex biological matrices. However, for target trace analytes in biological matrices, it requires a more sensitive and accurate analytical method. In early 1990s, the introduction of mass spectrometer with atmospheric pressure ionization techniques (such as electrical ionization and atmospheric pressure chemical ionization), with the RAM technique combined with LC facilitated the development of RAM in the direction of high-throughput analysis.
The RAM technique combined with LC—MS allows rapid on-line and sensitive analysis of the target analytes in biological samples. It has become a promising method for the rapid and sensitive determination of targets in biological sample matrices. Zhang et al.40, studied the on-line enrichment ability of the RAM column coupled with HPLC by column switching technique for benazepril hydrochloride in plasma. The enrichment ability of RAM-HPLC system was satisfactory. The system was used for the separation and detection of the trace benazepril hydrochloride in rat plasma after its administration. The sensitivity of HPLC can be improved by RAM pre-enrichment. It is a simple and economic measurement method. Song et al.41, prepared novel hydrophilic microparticles containing β-cyclodextrin via one-pot synthesis using reversible addition-fragmentation chain-transfer precipitation polymerization, a “controlled/living” radical polymerization technique. As chiral RAM, the hydrophilic microparticles can be used for determination of enantiomers in biological samples with direct injection via HPLC analysis. An overview of new techniques of on-line biological sample processing matched with LC–MS applied in the field of biopharmaceutical analysis is listed in Table 2.
Table 2.
An overview of new techniques of on-line biological sample processing matched with LC–MS applied in the field of biopharmaceutical analysis.
| Combination technique | Application | Characteristic | Ref |
|---|---|---|---|
| SPE–LC–MS | APV and AZV determination in human plasma | Combined high-flow online extraction method based on the monolithic material with a narrow-bore analytical column; low carry-over, high recovery, and was matrix-independent compared with LLE | 9 |
| Quantification of puerarin in canine and human plasma | Process controlled by software to realize automation; good characteristics of specificity, linearity, sensitivity and precision | 10 | |
| SPME–LC–MS | Cannabinoid concentration determination in biological samples | A holistic method combined with the advantages of SPE and membrane separation technique; more simple, convenient and environmentally friendly compared with the traditional LLE and SPE extraction | 27 |
| Column switching LC–MS | Testosterone quantitative determination in human serum | Providing a simple, fast, economical way for testosterone analysis | 34 |
| Cyclosporine A quantitative analysis in peripheral blood mononuclear cells | Satisfactory trueness, repeatability and intermediate precision; a new potential therapeutic drug monitoring method for cyclosporine A | 35 | |
| RAM–HPLC | Benazepril hydrochloride analysis in plasma | The enrichment ability of RAM–HPLC system was satisfactory | 40 |
| TFC-LC–MS | Simultaneously TCM ingredient determination in rat plasma | Fast, sensitive, and feasible method for PK study of TCM; it can automatical analysis; no need of complicated and time-consuming sample preparation compared with conventional LLE and SPE | 51 |
| MIP–LC–MS | Verapamil and its metabolite levels determination in human urine and plasma | Good selectivity and extraction efficiency; applied to selective screening of verapamil metabolites for the first time | 62 |
| NNAL analysis in human urine | Matrix effects evaluated and resolved; the method validated according to the FDA bioanalytical method validation guidance | 63 | |
| MD–LC–MS | PK study in awake animal after multiple dosing of Danshen | Stable, reliable, and simple method | 75 |
| Determination of l-THP in the rat striatum | Sensitive method with good linearity; all the validation within the required limits, such as accuracy, precision, and inter-day repeatability | 76 |
APV, amprenavir; AZV, atazanavir; LLE, liquid–liquid extraction; MD, micro-dialysis; MIP, molecularly imprinted polymers; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; PK, pharmacokinetic; RAM, restricted access material; SPE, solid phase extraction; SPME, solid phase micro-extraction; TCM, traditional Chinese medicine; TFC, turbulent-flow chromatography.
3.5. TFC technique
In 1997, Quinn and Takarewski42 developed a kind of filler of large particle size to allowed high-flow rate analysis, for which a new chromatographic analysis method called TFC was developed. The TFC column is filled with adsorption material of large particle size (such as hydrophilic–lipophilic balance (HLB) material). Since the size of this packing is large (30–50 μm) and generation of column pressure is small, the flow rate (usually in the range of 3–5 mL/min) of the sample load can be increased.
By using large packing materials in TFC, biological samples could be directly injected into a high-flow rate aqueous mobile-phase stream in which high-molecular-weight analytes (e.g., plasma proteins) are rapidly washed off and the low-molecular-weight analytes (targeted compounds for analysis) are retained on the stationary phase. The extracted analytes are then eluted via column-switching for MS or LC–MS analysis. TFC has the advantage of faster, stable and residual effects indistinguishable from results obtained under laminar-flow conditions. When coupling the TFC to HPLC for on-line sample pretreatment, the analysis throughput of biological samples can be significantly improved because fast HPLC can dramatically increase the analysis speed without decreasing the resolution and sensitivity43.
Currently, there are two main types of commercial TFC columns: one is using silica-bonded classic alkyl chain (e.g., C2, C8 or C18), phenyl and mixed polar/non-polar phase as the substrate; the other is using polymer matrix as the substrate, such as Oasis HLB column, launched by Waters Co., and using divinyl-phenyl-N-vinyl pyrrolidone copolymer as the filler. Compared with the ordinary C18 enrichment column, the TFC column has two distinct advantages in the preparation of the sample. Firstly, it adopts a single mobile phase to load and wash the sample at high flow rate, shortening extraction and cleaning time. Gjerde et al.44 utilized TFC to analyze tamoxifen and its metabolites in serum, using 0.05% formic acid (pH 2.8) to load samples at the rate of 4 mL/min. It took only 1 min for sample extraction and cleaning and the entire analysis time was 6 min so that 200 samples could be analyzed in a day. Ynddal et al.45 used a 5.0 mL/min flow rate to load and wash for 0.5 min, a mixture of methanol and 0.05% formic acid for gradient elution, and used MS to determine dextromethorphan and its metabolites. The lower LOQ and identification was 0.5 ng/mL, corresponding to 12.5 pg injected. For the first time, a fully automated assay including sample clean-up using TFC–HPLC–TOF-MS was demonstrated. Secondly, TFC has a high flow rate to extract and remove the interfering compounds such as protein in the matrix, increasing the life of analytical column. Ceglareka et al.46 established an on-line TFC–LC–MS analysis method for immunosuppressant cyclosporine A, tacrolimus and sirolimus. As many as 80 samples can be analyzed with this method in a day. The performance of turbulent-flow column in this system was unchanged after the analysis of 800 samples; the analytical column can be used to analyze over 2000 samples steadily.
The TFC and LC—MS coupling technique is a very promising technique, which directly analyzes biological samples in a rapid, sensitive and specific way. Utilizing the high flow rate mobile phase and large particle porous packing, on-line sample processing and quantitative analysis can be completed on an HPLC column. In recent years, there have been many reports on the application of on-line TFC–LC–MS coupling for analysis of a series of biological samples, and it has been widely used in the analysis of urine47, 48, serum49, 50, 51 and other biological substrates.
Xin et al.51 developed a method based on the on-line TFC and fast high-performance liquid chromatography—mass spectrometry (TFC–LC–MS) was for sensitive and high throughput PK study of traditional Chinese medicine (TCM). The method combines the speed and robustness of turbulent-flow extraction and the sensitivity and separation efficiency of fast HPLC–MS to analyze multiple and trace constituents of TCMs in plasma matrix. Each plasma sample was analyzed within 7 min. The method demonstrated good linearity with satisfactory accuracy, precision and LLOQ. These results indicate that the proposed method is fast, sensitive, and feasible for PK study of TCM. Compared with conventional LLE and SPE, TFC–LC–MS does not need complicated and time-consuming sample preparation procedures and can automatically analyze.
Helfer et al.47 established a fast on-line extraction using a Transcend TLX-II system based on TFC (Turbo Flow). The method was applied to the analysis of authentic urine samples containing amatoxins. In conclusion, this method allowed the determination of amatoxins using the novel pseudo quick elute mode (PQEM) in a faster, robust, and more reliable way than existing methods, making it suitable for daily routine and especially emergent toxicological analysis.
3.6. Molecularly imprinted SPE technique
Owing to the chemical, mechanical and thermal stability together with high selectivity for template molecules, molecularly imprinted polymers (MIPs) have been utilized for a wide variety of applications, including chromatography, protein separation, SPE, drug-controlled release and sensor devices. The imprinting technique is a well-established and simple technique for synthesizing materials with specific molecular recognition properties. Although the bulk MIP prepared by conventional methods exhibits high selectivity, some disadvantages were noted, such as the heterogeneous distribution of the binding sites, embedding of most binding sites, and poor site accessibility for template molecule52, 53, 54, 55.
In 1994, Sellergren et al.56 first combined an MIP with an SPE technique, forming a new sample preparation technique called molecularly imprinted SPE. Sellergren used special MIPs for pentamidine enrichment in urine, and MIPs allowed direct determination of the target compound during the desorption step without continuous chromatographic analysis with good selectivity. Since then, as a very promising method, the molecularly imprinted SPE technique is widely used in biological sample processing in the field of biopharmaceutical analysis, such as urine57, plasma58, serum59, and saliva60. MIP as a new type of packing of SPE, can fully integrate the advantages of molecularly imprinted and SPE technique, and overcome the cumbersome pretreatment and other unfavorable factors of the complex medical, biological and environmental sample analysis to achieved separation and purification. Thus, it is very well suited to selectively adsorb target molecules or a certain class of compounds with similar structures from complex samples. Compared with immunoadsorbents, MIPs have some advantages, such as high mechanical, thermal and pressure stability low cost, long shelf life, and good reproducibility.
3.6.1. Principle of molecularly imprinted technique
The molecularly imprinted technique uses a new type of polymer to match a template molecule binding site in a spatial structure. A typical imprinting system consists of a print molecule, at least one type of functional monomer and cross-linker, and a porogenic solvent. To induce radical polymerization an appropriate initiator is included as well.
The basic principle of molecularly imprinted technique is as follows61: in the first step, the template molecule and functional monomers form a stable mixture in solvent via reversible covalent or non-covalent interaction; in the second step, the cross-linker is added into solvent, the polymerization reaction is initiated through UV or heat to form a rigid polymer; in the third step, a three-dimensional hole is obtained in the copolymer by eluting the template molecule, the hole is completely matched with the template molecule and contains functional groups that can specifically bond template molecule. Due to MIP being prepared from different template molecules that have different three-dimensional forms, an MIP can only bond a template molecule, just like the relationship between “lock” and “key”, where “lock” is MIP and “key” represents the template molecule.
3.6.2. Molecularly imprinted SPE coupled to LC–MS
Molecularly imprinted SPE coupled to LC–MS greatly improves the selectivity and sensitivity of SPE, simplifies the sample preparation process and enhances the detection limits. Compared with other methods, this method has the advantages of predetermination (different fillers could be prepared according to different extraction purposes), recognition (specific spatial arrangement of the polymer could selectively bind the template molecule), and usability (simple preparation, chemical and mechanical stability, low cost and reusable usage).
In recent years this technique has been widely used for environmental sample analysis, biomedical sample analysis, pesticide residue testing, drug separation and purification, etc. Mullett et al.62 used molecularly imprinted SPE coupled with LC–MS to determine verapamil and its metabolite levels in human urine and plasma. The MIP material was coupled on-line to a RAM precolumn. The method enabled the direct injection of biological samples for the selective isolation, pre-concentration, identification and analysis of verapamil and its phase I metabolites.
Shah et al.63 used a molecularly imprinted SPE technique coupled to LC–MS/MS with direct injection of microfluids to analyze 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. The method was optimized and matrix effects were evaluated and resolved. The method enabled low sample volume (200 μL) and rapid analysis of urinary NNAL by direct injection onto the microfluidic column packed with molecularly imprinted beads engineered to NNAL. The method was validated according to the FDA bioanalytical method validation guidance.
3.7. MD technique
MD is an in vivo sampling technique for monitoring physiological levels of the active substances in the animal and human such as extracellular fluid, tissue, and brain. The technology was generated in the 1960s64. MD originally applied to study cerebrospinal chemical composition. Since the 1990s, MD had been increasingly used in the field of biopharmaceutical analysis. MD technique as a novel miniaturized sample pretreatment technique evolved from traditional SPE technique and can handle trace levels of biological samples. The simple and rapid MD technique consumes no solvent and can achieve good sensitivity compared with SPE and LLE.
Lafay et al.65 utilized MD and SPE/LLE to determine cotinine in urine. The results showed the total time of sample processing was 24 h and organic solvent consumption was large for SPE/LLE method, while for MD, sample processing time was just 5 min and consumed no solvent. Both methods had good linearity and precision and the same of LOD and LOQ. MD adsorbent could be used more than 200 times without loss of extraction capacity. Compared with SPME, the MD technique is more suitable for trace analysis of small samples.
Anizan et al.66 used MD and SPME combined GC–MS to analyze steroid metabolites in urine. The results showed that SPME fibers began to degrade after extracting urine samples 5 times, while the MD technique demonstrated to be rapid, reproducible and high-throughput. The extraction time and recovery for SPME was 60 min and 11%, respectively; while the extract time and recovery for MD technique was only 3 min and 60%– 98%, respectively. In short, compared with other sample handling techniques, MD offers sampling flexibly, ease of operation and allows on-line analysis; sampling can be finished continuously at one site and at one time from different sites with little damage to sampling site and no destruction to the body׳s integrity; in addition, the MD technique refines samples to obtain free drug that better reflect the relationship between concentration and pharmacodynamics (PD). Also, Gao et al.67 studied the feasibility of MD as a tool to determine the skin concentration of mometason furoate (MF), a lipophilic and highly protein-bound compound.
3.7.1. Principle of MD
MD is a technique that allows perfusion through an implanted probe in tissue under non-equilibrium conditions (i.e., concentration of compound to be tested in effluent dialysate is lower than it is in the sample matrix surrounding the probe membrane). Compounds in tissues to be tested go into the dialysate along a concentration gradient by counterdiffusion to allow sampling from the living tissue.
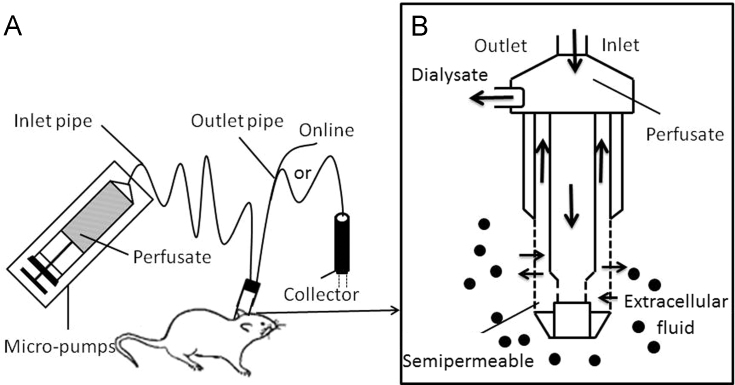
The MD sampling device consists of micro-pumps, an MD probe, collector, connecting tubes and ancillary equipment shown in Fig. 3A68. Firstly, the semipermeable membrane probe is implanted into organs and tissues or blood and a micro-perfusion pump control perfusate flow through the probe at a constant rate (general ranges from 0.5 to 5 μL/min). Analytes reach a dynamic equilibrium between the perfusate and extracellular fluid (ECF), as shown in Fig. 3B68. The analyte is constantly brought out by a continuous flow of perfusate in the MD tube and then stored in the sample collector. Concentration changes of the analytes in the ECF can be determined by analyzing the dialysate. The catheter can also be connected with HPLC, HPCE (high performance capillary electrophoresis), GC or other detection equipment to allow on-line detection directly. MD probes can be implanted into various tissues and organs in living body, including liver, heart, skin, blood, placenta, stomach and ears. The probe acts like blood vessels, transporting materials to specific parts or removing them from specific parts without fluid loss.
Figure 3.
Composition of micro dialysis sampling system (A) and the local amplification of probe sampling (B). Reprinted from Ref. 68 with permission of the copyright holder, Zhejiang University.
3.7.2. MD technique combined with LC–MS
MD can be combined with separation techniques such as HPLC and capillary electrophoresis (CE), but also with detection systems, such as MS, RIA, electrochemical detection (ECD), and fluorescence detection. When compared with other hyphenated techniques, the LC—MS technique is generally applicable with MD for separation and analysis. In recent years, MD–LC–MS has been widely used in the pharmacy, biology, clinical medicine and other fields, especially in the field of biopharmaceutical analysis analyzing complex biological matrices such as whole blood69, 70, plasma71, 72, and urine73, 74, etc.
MD–LC–MS as a new analytical technique has the advantages of high efficiency, less interference, and simple sample pretreatment. Zhu et al.75 used MD–LC–MS detection technology to carry out a PK study in awake animals after multiple dosing of Danshen (Salvia miltiorrhiza); this method was stable, reliable, and simple. Wang et al.76 developed a rapid and sensitive method based on MD–LC–MS for the determination of levo-tetrahydropalmatine (1-THP) in the rat striatum. The method was sensitive with a lower limit of quantitation (0.1 ng/mL) and good linearity (r2≥0.999) in the range of 0.1–1000 ng/mL. All the validation data, including accuracy, precision, and inter-day repeatability were within the required limits. This method was applied to a PK study of the l-THP in the rat striatum.
MD–LC–MS has a number of advantages, such as in vivo sampling, dynamic observation, on-line quantitative analysis, small sample volume, with little tissue damage. A large number of samples can be collected from the same animal for analysis. But it also has shortcomings, for example the quality of probe recovery has been the primary problem faced by MD technique. Thus, the MD technique still needs further study.
4. Conclusions
Biological sample pretreatment is typically the most complicated part of the process of separation and analysis. Biological sample pretreatment has been a relatively neglected part of the biopharmaceutical analysis field. However, in recent years, sample preparation techniques have been improved, generating a series of new methods and techniques of samples pretreatment, such as SPE, SPME, column switching, RAM, TFC, molecular imprinted SPE and MD. There are four main reasons for the development of sample preparation techniques: firstly, the view of sample pretreatment has changed—it is now generally recognized as a science; secondly, sample preparation has become a part of the entire analysis system, eliciting the concern and attention of researchers and developers; thirdly, sample pretreatment has tended to miniaturization to adapt to the development trend of biopharmaceutical analysis; fourthly, the technique of sample preparation combined with LC–MS allows on-line automatic high-throughput analysis.
The future focus of biopharmaceutical analysis will be on processing large number of samples, small volume samples, and trace analysis of complex biological matrices. To meet and adapt to the growing demand for biopharmaceutical analysis, sample preparation techniques need further optimization and development. On-line sample pretreatment techniques and LC–MS techniques play an important role in qualitative and quantitative analysis of complex biological matrices. However, the current enrichment columns are mainly based on hydrophobic interactions to retain analytes. With the wider used of trace analysis, higher selectivity requirements will be required for in-line enrichment material. In addition, achieving higher throughput analysis via switching between multi-columns will allow further applications. With the development of biopharmaceutical analysis, qualitative and quantitative analysis of unknown substances are bound to be further strengthened.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81102499), the Fundamental Research Funds for the Central Universities of Central South University and Hunan Science and Technology Project (No. 2011SK3261).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Peng Yu, Email: peng.yu@csu.edu.cn.
Lingli Mu, Email: mulingli@sina.com.
References
- 1.Han N.Y., Xu B.J. Determination of the matrix effect and accuracy in the analysis of biological samples. Chin J New Drugs. 2012;21 1607-10,1626. [Google Scholar]
- 2.Akimaru M., Okubo K., Hiruta Y., Kanazawa H. Temperature-responsive solid-phase extraction column for biological sample pretreatment. Anal Sci. 2015;31:881–886. doi: 10.2116/analsci.31.881. [DOI] [PubMed] [Google Scholar]
- 3.Yuan H., Feng W., Tu J., Peng W., Li H. Determination of lovastatin in human plasma by ultra-performance liquid chromatography–electrospray ionization tandem mass spectrometry and its application in a pharmacokinetic study. J Pharm Biomed Anal. 2008;46:808–813. doi: 10.1016/j.jpba.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Burdette C.Q., Marcus R.K. In-line desalting of proteins from buffer and synthetic urine solution prior to ESI–MS analysis via a capillary-channeled polymer fiber microcolumn. J Am Soc Mass Spectrom. 2013;24:975–978. doi: 10.1007/s13361-013-0593-1. [DOI] [PubMed] [Google Scholar]
- 5.Boos K.S., Wilmers B., Schlimme E., Sauerbrey R. On-line sample processing and analysis of diol compounds in biological fluids. J Chromatogr A. 1988;456:93–104. doi: 10.1016/0021-9673(86)80009-7. [DOI] [PubMed] [Google Scholar]
- 6.Miró M., Hansen E.H. On-line sample processing involving microextraction techniques as a front-end to atomic spectrometric detection for trace metal assays: a review. Anal Chim Acta. 2013;782:1–11. doi: 10.1016/j.aca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Broich J.R., Hoffman D.B., Goldner S.J., Andryauskas S., Umberger C.J. Liquid–solid extraction of lyophilized biological material for forensic analysis. I. Application to urine samples for detection of drugs of abuse. J Chromatogr A. 1971;63:309–312. doi: 10.1016/s0021-9673(01)85643-0. [DOI] [PubMed] [Google Scholar]
- 8.Ferreiro-Vera C., Mata-Granados J.M., Priego-Capote F., Luque de Castro M.D. Automated method for targeting analysis of prostanoids in human serum by on-line solid-phase extraction and liquid chromatography–mass spectrometry in selected reaction monitoring. J Chromatogr A. 2011;1218:2848–2855. doi: 10.1016/j.chroma.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Naxing X.R., Fan L.M., Kim G.E., El-Shourbagy T.A. A monolithic-phase based on-line extraction approach for determination of pharmaceutical components in human plasma by HPLC–MS/MS and a comparison with liquid–liquid extraction. J Pharm Biomed Anal. 2006;40:728–736. doi: 10.1016/j.jpba.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q.Q., Li X.S., Dai S.J., Ou L., Sun X., Zhu B.Z. Quantification of puerarin in plasma by on-line solid-phase extraction column switching liquid chromatography–tandem mass spectrometry and its applications to a pharmacokinetic study. J Chromatogr B. 2008;863:55–63. doi: 10.1016/j.jchromb.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K., Ikemura A., Tsuruta Y., Tsutsumiuchi K., Hino T., Oka H. On-line solid-phase extraction LC–MS/MS for the determination of Ac-SDKP peptide in human plasma from hemodialysis patients. Biomed Chromatogr. 2012;26:137–141. doi: 10.1002/bmc.1636. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y.J., Wang Q.Q., Yang Z.H., Yang J., Guan H., Zhou P.K. The development and validation of an on-line solid phase extraction HPLC–MS/MS method for quantification of syringaldehyde in biological matrix and its application in Sprague-Dawley rat pharmacokinetic study. Chin Pharm Bull. 2012;28:999–1004. [Google Scholar]
- 13.Hu L.H., Boos K.S., Ye M.L., Zou H.F. Analysis of the endogenous human serum peptides by on-line extraction with restricted-access material and HPLC–MS/MS identification. Talanta. 2014;127:191–195. doi: 10.1016/j.talanta.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu M., Uno T., Tamura H.O., Kanazawa H., Murakami I., Sugawara K. A developed determination of midazolam and 1′-hydroxymidazolam in plasma by liquid chromatography–mass spectrometry: application of human pharmacokinetic study for measurement of CYP3A activity. J Chromatogr B. 2007;847:275–281. doi: 10.1016/j.jchromb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Pawliszyn J. Elsevier; Amsterdam: 2009. Handbook of Solid Phase Microextraction. [Google Scholar]
- 16.Musteata F.M., Musteata M.L., Pawliszyn J. Fast in vivo microextraction: a new tool for clinical analysis. Clin Chem. 2006;52:708–715. doi: 10.1373/clinchem.2005.064758. [DOI] [PubMed] [Google Scholar]
- 17.Vuckovic D., de Lannoy I., Gien B., Yang Y.B., Musteata F.M., Shirey R. In vivo solid-phase microextraction for single rodent pharmacokinetics studies of carbamazepine and carbamazepine-10,11-epoxide in mice. J Chromatogr A. 2011;1218:3367–3375. doi: 10.1016/j.chroma.2010.07.060. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S.N., Ouyang G.F., Pawliszyn J. Comparison of microdialysis with solid-phase microextraction for in vitro and in vivo studies. J Chromatogr A. 2008;1196–1197:46–56. doi: 10.1016/j.chroma.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 19.Musteata F.M. 11-Ligand-receptor binding and determination of free concentrations. In: Pawliszyn J., editor. Handbook of solid phase microextraction. Elsevier; Oxford: 2012. pp. 383–397. [Google Scholar]
- 20.Arthur C.L., Killam L.M., Buchholz K.D., Pawliszyn J., Berg J.R. Automation and optimization of solid-phase microextraction. Anal Chem. 1992;64:1960–1966. [Google Scholar]
- 21.Eisert R., Pawliszyn J. Automated in-tube solid-phase microextraction coupled to high-performance liquid chromatography. Anal Chem. 1997;69:3140–3147. [Google Scholar]
- 22.Kataoka H., Lord H.L., Yamamoto S., Narimatsu S., Pawliszyn J. Development of automated in-tube SPME/LC/MS method for drug analysis. J Microcolumn Sep. 2000;12:493–500. [Google Scholar]
- 23.Nezhadali A., Ahmadi B.G., Nakhaei H. Electrosynthesis of polypyrrole on steel fiber for solid-phase microextraction of citalopram in serum. Anal Bioanal Chem. 2012;403:593–600. doi: 10.1007/s00216-012-5825-x. [DOI] [PubMed] [Google Scholar]
- 24.Saito K., Yagi K., Ishizaki A., Kataoka H. Determination of anabolic steroids in human urine by automated in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J Pharm Biomed Anal. 2010;52:727–733. doi: 10.1016/j.jpba.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Togunde O.P., Lord H., Oakes K.D., Servos M.R., Pawliszyn J. Development and evaluation of a new in vivo solid-phase microextraction sampler. J Sep Sci. 2013;36:219–223. doi: 10.1002/jssc.201200839. [DOI] [PubMed] [Google Scholar]
- 26.Ruiter A.F.C., Teeninga N., Nauta J., Endert E., Ackermans M.T. Determination of unbound prednisolone, prednisone and cortisol in human serum and saliva by on-line solid-phase extraction liquid chromatography tandem mass spectrometry and potential implications for drug monitoring of prednisolone and prednisone in saliva. Biomed Chromatogr. 2012;26:789–796. doi: 10.1002/bmc.1730. [DOI] [PubMed] [Google Scholar]
- 27.Yang R.Q., Xie W.L. Determination of cannabinoids in biological samples using a new solid phase micro-extraction membrane and liquid chromatography–mass spectrometry. Forensic Sci Int. 2006;162:135–139. doi: 10.1016/j.forsciint.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Snyder L.R. Comparisons of normal elution, coupled-columns, and solvent, flow or temperature programming in liquid chromatography. J Chromatogr Sci. 1970;8:692–706. [Google Scholar]
- 29.Wu X.Y., Wang R., Xie H., Wang X.F., Jia Z.P., Zhang Q. Rapid determination of propranolol enantiomers in rat plasma by column-switching–high performance liquid chromatography. Chin J Chromatogr. 2011;29:1205–1209. [PubMed] [Google Scholar]
- 30.Emara S., Kamal M., Hadad G., ZaaZaa H., Kawi M.A. Back-flush column-switching technique for on-line sample cleanup and enrichment to determine guaiphenesin in human serum. J Liq Chromatogr R T. 2012;35:15–27. [Google Scholar]
- 31.Wu X.Y., Wang R., Xie H., Jia Z.P., Li X.Y., Li W.B. Rapid monitoring of carbamazepine blood drug level in epileptics by column-switching high performance liquid chromatography. Chin J Pharm Anal. 2013;33:1715–1719. [Google Scholar]
- 32.Fagundes V.F., Leite C.P., Pianetti G.A., Fernandes C. Rapid and direct analysis of statins in human plasma by column-switching liquid chromatography with restricted-access material. J Chromatogr B. 2014;947–948:8–16. doi: 10.1016/j.jchromb.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Šatínský D., Havlíková L., Solich P. HPLC column-switching technique for sample preparation and fluorescence determination of propranolol in urine using fused-core columns in both dimensions. Anal Bioanal Chem. 2013;405:6583–6587. doi: 10.1007/s00216-013-7098-4. [DOI] [PubMed] [Google Scholar]
- 34.Borrey D., Moerman E., Cockx A., Engelrelst V., Langlois M.R. Column-switching LC–MS/MS analysis for quantitative determination in human serum. Clin Chim Acta. 2007;382:134–137. doi: 10.1016/j.cca.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Ansermot N., Fathi M., Veuthey J.L., Desmeules J., Hochstrasser D., Rudaz S. Quantification of cyclosporine A in peripheral blood mononuclear cells by liquid chromatography-electrospray mass spectrometry using a column-switching approach. J Chromatogr B. 2007;857:92–99. doi: 10.1016/j.jchromb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Desilets C.P., Rounds M.A., Regnier F.E. Semipermeable-surface reversed-phase media for high-performance liquid chromatography. J Chromatogr A. 1991;544:25–39. doi: 10.1016/s0021-9673(01)83976-5. [DOI] [PubMed] [Google Scholar]
- 37.Sadilek P., Šatínský D., Solich P. Using restricted-access materials and column switching in high-performance liquid chromatography for direct analysis of biologically-active compounds in complex matrices. Trac Trend Anal Chem. 2007;26:375–384. [Google Scholar]
- 38.Liu M., Zhao L., Guo B., Lin J. Development of on-line sample preparation coupled with liquid chromatography–mass spectrometry for analysis of small molecules in biofluids. Chin J Chromatogr. 2007;25:646–653. [PubMed] [Google Scholar]
- 39.Ehrlich M., Trittler R., Daschner F.D., Kümmerer K. A new and rapid method for monitoring the new oxazolidinone antibiotic linezolid in serum and urine by high performance liquid chromatography-integrated sample preparation. J Chromatogr B. 2001;755:373–377. doi: 10.1016/s0378-4347(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X.H., Wang R., Xie H., Yin Q., Li X.Y., Jia Z.P. Online enrichment ability of restricted-access column coupled with high performance liquid chromatography by column switching technique for benazepril hydrochloride. Chin J Chromatogr. 2013;31:451–455. doi: 10.3724/sp.j.1123.2012.11021. [DOI] [PubMed] [Google Scholar]
- 41.Song W.J., Wei J.P., Wang S.Y., Wang H.S. Restricted access chiral stationary phase synthesized via reversible addition-fragmentation chain-transfer polymerization for direct analysis of biological samples by high performance liquid chromatography. Anal Chim Acta. 2014;832:58–64. doi: 10.1016/j.aca.2014.04.063. [DOI] [PubMed] [Google Scholar]
- 42.Quinn HM, Takarewski JJ, inventors; High performance liquid chromatography method and apparatus. US Patent 5,919,368; 1997 May 9.
- 43.Zhou J.L., Qi L.W., Li P. Herbal medicine analysis by liquid chromatography/time-of-flight mass spectrometry. J Chromatogr A. 2009;1216:7582–7594. doi: 10.1016/j.chroma.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 44.Gjerde J., Kisanga E.R., Hauglid M., Holm P.I., Mellgren G., Lien E.A. Identification and quantification of tamoxifen and four metabolites in serum by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2005;1082:6–14. doi: 10.1016/j.chroma.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Ynddal L., Hansen S.H. On-line turbulent-flow chromatography–high-performance liquid chromatography–mass spectrometry for fast sample preparation and quantitation. J Chromatogr A. 2003;1020:59–67. doi: 10.1016/s0021-9673(03)00773-8. [DOI] [PubMed] [Google Scholar]
- 46.Ceglarek U., Lembcke J., Fiedler G.M., Werner M., Witzigmann H., Hauss J.P. Rapid simultaneous quantification of immunosuppressants in transplant patients by turbulent flow chromatography combined with tandem mass spectrometry. Clin Chim Acta. 2004;346:181–190. doi: 10.1016/j.cccn.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Helfer A.G., Meyer M.R., Michely J.A., Maurer H.H. Direct analysis of the mushroom poisons α- and β-amanitin in human urine using a novel on-line turbulent flow chromatography mode coupled to liquid chromatography–high resolution-mass spectrometry/mass spectrometry. J Chromatogr A. 2014;1325:92–98. doi: 10.1016/j.chroma.2013.11.054. [DOI] [PubMed] [Google Scholar]
- 48.Perez F., Llorca M., Farré M., Barceló D. Automated analysis of perfluorinated compounds in human hair and urine samples by turbulent flow chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem. 2012;402:2369–2378. doi: 10.1007/s00216-011-5660-5. [DOI] [PubMed] [Google Scholar]
- 49.Füzéry A.K., Breaud A.R., Emezienna N., Schools S., Clarke W.A. A rapid and reliable method for the quantitation of hydroxychloroquine in serum using turbulent flow liquid chromatography–tandem mass spectrometry. Clin Chim Acta. 2013;421:79–84. doi: 10.1016/j.cca.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Petrides A.K., Joshua M., Johnson-Davis K.L., Jannetto P.L., Langman L.J., Clarke W. The development and validation of a turbulent flow-liquid chromatography–tandem mass spectrometric method for the simultaneous quantification of citalopram, sertraline, bupropion and hydroxybupropion in serum. Clin Biochem. 2014;47:73–79. doi: 10.1016/j.clinbiochem.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Xin G.Z., Zhou J.L., Qi L.W., Li C.Y., Liu P., Li H.J. Turbulent-flow chromatography coupled on-line to fast high-performance liquid chromatography and mass spectrometry for simultaneous determination of verticine, verticinone and isoverticine in rat plasma. J Chromatogr B. 2010;878:435–441. doi: 10.1016/j.jchromb.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 52.Xiao D.L., Dramou P., Xiong N.Q., He H., Li H., Yuan D.H. Development of novel molecularly imprinted magnetic solid-phase extraction materials based on magnetic carbon nanotubes and their application for the determination of gatifloxacin in serum samples coupled with high performance liquid chromatography. J Chromatogr A. 2013;1274:44–53. doi: 10.1016/j.chroma.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Chen H.J., Zhang Z.H., Luo L.J., Yao S.Z. Surface-imprinted chitosan-coated magnetic nanoparticles modified multi-walled carbon nanotubes biosensor for detection of bovine serum albumin. Sens Actuat B-Chem. 2012;163:76–83. [Google Scholar]
- 54.Shi Y.F., Lv H.L., Lu X.F., Huang Y.X., Zhang Y., Xue W. Uniform molecularly imprinted poly(methacrylic acid) nanospheres prepared by precipitation polymerization: the control of particle features suitable for sustained release of gatifloxacin. J Mater Chem. 2012;22:3889–3898. [Google Scholar]
- 55.Zhang H., Dramou P., He H., Tan S.H., Pham-Huy C., Pan H.J. Molecularly imprinted stationary phase prepared by reverse micro-emulsion polymerization for selective recognition of gatifloxacin in aqueous media. J Chromatogr Sci. 2012;50:499–508. doi: 10.1093/chromsci/bms028. [DOI] [PubMed] [Google Scholar]
- 56.Sellergren B. Direct drug determination by selective sample enrichment on an imprinted polymer. Anal Chem. 1994;66:1578–1582. [Google Scholar]
- 57.Ambrosini S., Shinde S., De Lorenzi E., Sellergren B. Glucuronide directed molecularly imprinted solid-phase extraction: isolation of testosterone glucuronide from its parent drug in urine. Analyst. 2012;137:249–254. doi: 10.1039/c1an15606c. [DOI] [PubMed] [Google Scholar]
- 58.Meucci V., Minunni M., Vanni M., Sgorbini M., Corazza M., Intorre L. Selective and simultaneous determination of NSAIDs in equine plasma by HPLC with molecularly imprinted solid-phase extraction. Bioanalysis. 2014;6:2147–2158. doi: 10.4155/bio.14.79. [DOI] [PubMed] [Google Scholar]
- 59.Zohre N., Turghun M., Zorem M. Dummy-template molecularly imprinted polymer for selective solid-phase extraction of zidovudine in human serum. Chin J Anal Lab. 2013;32:6–10. [Google Scholar]
- 60.Alenus J., Ethirajan A., Horemans F., Weustenraed A., Csipai P., Gruber J. Molecularly imprinted polymers as synthetic receptors for the QCM-D-based detection of l-nicotine in diluted saliva and urine samples. Anal Bioanal Chem. 2013;405:6479–6487. doi: 10.1007/s00216-013-7080-1. [DOI] [PubMed] [Google Scholar]
- 61.Ye L., Mosbach K. Molecularly imprinted microspheres as antibody binding mimics. React Funct Polym. 2001;48:149–157. [Google Scholar]
- 62.Mullett W.M., Walles M., Levsen K., Borlak J., Pawliszyn J. Multidimensional on-line sample preparation of verapamil and its metabolites by a molecularly imprinted polymer coupled to liquid chromatography–mass spectrometry. J Chromatogr B. 2004;801:297–306. doi: 10.1016/j.jchromb.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 63.Shah K.A., Peoples M.C., Halquist M.S., Rutan S.C., Karnes H.T. Microfluidic direct injection method for analysis of urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) using molecularly imprinted polymers coupled on-line with LC–MS/MS. J Pharm Biomed. 2011;54:368–378. doi: 10.1016/j.jpba.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Bito L., Davson H., Levin E., Murray M., Snider N. The concentrations of free amino acids and other electrolytes in cerebrospinal fluid, in vivo dialysate of brain, and blood plasma of the dog. J Neurochem. 1966;13:1057–1067. doi: 10.1111/j.1471-4159.1966.tb04265.x. [DOI] [PubMed] [Google Scholar]
- 65.Lafay F., Vulliet E., Flament-Waton M. Contribution of microextraction in packed sorbent for the analysis of cotinine in human urine by GC–MS. Anal Bioanal Chem. 2010;396:937–941. doi: 10.1007/s00216-009-3236-4. [DOI] [PubMed] [Google Scholar]
- 66.Anizan S., Bichon E., Monteau F., Cesbron N., Antignac J.P., Le Bizec B. A new reliable sample preparation for high throughput focused steroid profiling by gas chromatography–mass spectrometry. J Chromatogr A. 2010;1217:6652–6660. doi: 10.1016/j.chroma.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 67.Gao Q.Z., Zhao Y., Yu J.K., Yang T.Z., Zhu Z.L., Ding P.T. Microdialysis as a tool to determine the skin concentration of mometason furoate in rats. Pharmazie. 2014;69:787–791. [PubMed] [Google Scholar]
- 68.Wang Y. Zhejiang University; Hangzhou: 2012. A novel evaluation method on drugs׳ absorption in intestine [dissertation] [Google Scholar]
- 69.Ma R.H., Yang J., Qi L.W., Xin G.Z., Wang C.Z., Yuan C.S. In vivo microdialysis with LC–MS for analysis of spinosin and its interaction with cyclosporin A in rat brain, blood and bile. J Pharm Biomed. 2012;61:22–29. doi: 10.1016/j.jpba.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Ling J.J., Wu X.J., Fu X. Pharmacokinetics of nicotine in blood and brain using microdialysis and stable labelled isotope. China J Chin Mater Med. 2012;37:104–108. [PubMed] [Google Scholar]
- 71.Wei J.B., Lai Q., Shumyak S.P., Xu L.F., Zhang C.X., Ling J.J. An LC/MS quantitative and microdialysis method for cyclovirobuxine D pharmacokinetics in rat plasma and brain: the pharmacokinetic comparison of three different drug delivery routes. J Chromatogr B. 2015;1002:185–193. doi: 10.1016/j.jchromb.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 72.Córcoles E.P., Boutelle M.G. Biosensors and invasive monitoring in clinical applications. Springer; Heidelberg: 2013. [Google Scholar]
- 73.Petersen L.J., Sørensen M.A., Codrea M.C., Zacho H.D., Bendixen E. Large pore dermal microdialysis and liquid chromatography–tandem mass spectroscopy shotgun proteomic analysis: a feasibility study. Skin Res Technol. 2013;19:424–431. doi: 10.1111/srt.12063. [DOI] [PubMed] [Google Scholar]
- 74.Müller M. Microdialysis in drug development. Springer; New York: 2013. Introduction to the microdialysis technology; pp. 3–12. [Google Scholar]
- 75.Zhu L.X., Zhang Y.F. Pharmacokinetic study on Danshen Decoction with multiple dosing to awake animal by blood microdialysis method combined with LC–MS. Chin Trad Herbal Drugs. 2014;45:2206–2209. [Google Scholar]
- 76.Wang C., Li S., Tang Y.J., Wang S.W., Zhang Y.L., Fan G.R. Microdialysis combined with liquid chromatography–tandem mass spectrometry for the determination of levo-tetrahydropalmatine in the rat striatum. J Pharm Biomed. 2012;64–65:1–7. doi: 10.1016/j.jpba.2012.01.016. [DOI] [PubMed] [Google Scholar]