Abstract
Stroke is a brain damage caused by a loss of blood supply to a portion of the brain, which requires prompt and effective treatment. The current pharmacotherapy for ischemic stroke primarily relies on thrombolysis using recombinant tissue plasminogen activators (rt-PAs) to breakdown blood clots. Neuroprotective agents that inhibit excitatory neurotransmitters are also used to treat ischemic stroke but have failed to translate into clinical benefits. This poses a major challenge in biomedical research to understand what causes the progressive brain cell death after stroke and how to develop an effective pharmacotherapy for stroke. This brief review analyzes the fate of about 430 potentially useful stroke medications over the period 1995–2015 and describes in detail those that successfully reached the market. Hopefully, the information from this analysis will shed light on how future stroke research can improve stroke drug discovery.
Abbreviations: ADP, adenosine diphosphate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ASIC1a, acid-sensing ion channel 1a; BDNF, brain-derived neurotrophic factor; CFDA, the China Food and Drug Administration; CNTF, ciliary neurotrophic factor; GDNF, glial cell line–derived neurotrophic factor; iGluRs, ionotropic glutamate receptors; MHRA, Medicine and Healthcare Products Regulatory Agency; NBP, butylphthalide/3-n-butylphthalide; NGF, nerve growth factor; NMDA, N-methyl-D-aspartate; rt-Pas, recombinant tissue plasminogen activators; TCM, traditional Chinese medicine; TRP, transient receptor potential; TRPC, transient receptor potential canonical; TRPM, transient receptor potential melastatin; TRPV, transient receptor potential vanilloid
KEY WORDS: Thrombosis, Neuroprotective agent, Ischemic stroke, Traditional Chinese medicine, Non-NMDA mechanism, Ion channel
Graphical abstract
The fate of 430 drugs evaluated in treating ischemic stroke over the period 1995–2015 are is analyzed. Only 4% of them, classified as thrombolytics and neuroprotective agents, successfully reached the market whereas all N-methyl-D-aspartate (NMDA) receptor antagonists failed in clinical trials. This analysis reveals that targeting non-NMDA-mediated calcium overload may be a promising strategy for improving the pharmacotherapy of ischemic stroke.
1. Introduction
Stroke is the leading cause of death and long-term disability in China with a population of stroke patients over 7 million1, 2. With the rise in prevalence and trend towards younger patients, stroke prevalence in China varies from 260 to 719 per 100,000 people for all ages3. According to the Report on Chinese Stroke Prevention released in 2015, approximately 15% of people over 40 are at high risk of stroke with a comprehensive standardized prevalence of stroke between 2011 and 2013 of about 2% and an 8.1% rise in prevalence1, 4. The prevalence of stroke patients in urban areas is higher than in rural areas and people in the North of China have a higher prevalence than those in the South1, 5. On the basis of World Bank Data, China will have 31.77 million stroke patients by 2030 costing the country as much as $40.0 billion per year.
Stroke is classified into either ischemic stroke affecting approximately 87% of patients or hemorrhagic stroke affecting the other approximately 13%6. Ischemic stroke is caused by a reduction in cerebral blood supply, whereas hemorrhagic stroke results from the rupture of a blood vessel in the brain causing bleeding into the brain or subarachnoid space. For hemorrhagic stroke, neurosurgery is required to treat the bleeding. For ischemic stroke, current interventional treatment regimens mainly include blood pressure management, catheter-based interventions, thrombolytic therapy, anticoagulation therapy, defibrinogen therapy and pharmacotherapy to improve cerebral blood circulation. This review is limited to drug therapy of ischemic stroke, which aims to restore blood flow.
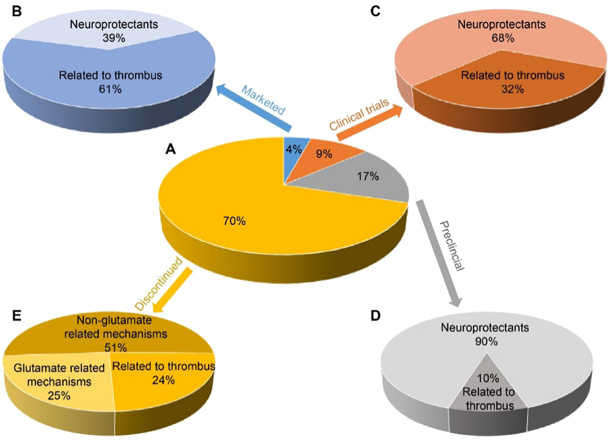
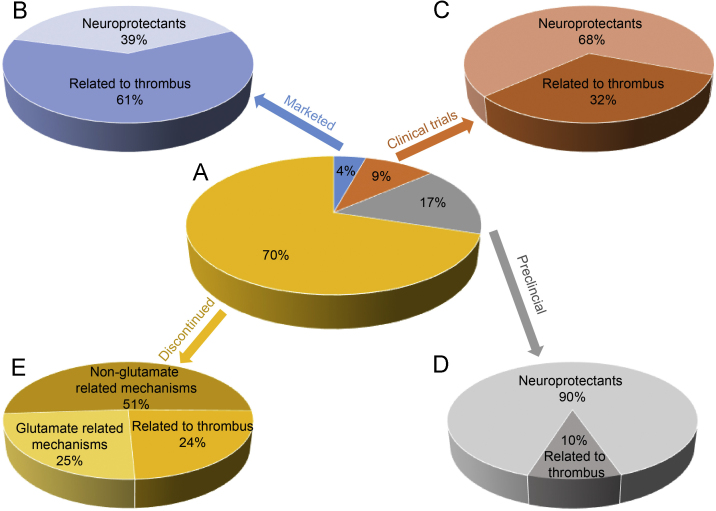
In an effort to gain insight into what makes a stroke drug succeed in reaching the market, we have examined the fate of about 430 potential stroke drugs evaluated over the past twenty years (1995–2015). The analysis was based on data reported from the Addis Insight Database deposited by December 25, 2015. We found that approximately 300 drug candidates (70%) were discontinued, about 70 (17%) are undergoing preclinical assessment, 40 (9%) are in various phases of clinical trials and only 19 (4%) have reached the market (Fig. 1).
Figure 1.
The landscape of ischemic stroke drug discovery and therapy over the period 1995–2015. (A) The classification of 430 drug candidates by pie chart based on the mechanism of action: (B) the 4% of drugs on the market; (C) the 9% undergoing clinical trials; (D) the 17% undergoing preclinical evaluation and (E) the 70% of failed drug candidates.
The 430 drug candidates can be divided into two main categories based on their mechanism of action: thrombolytics and neuroprotective agents. Thrombolytic therapy aims at removing the thrombus blockage and includes drugs that act as plasminogen activators, antithrombotic agents and platelet aggregation inhibitors. Neuroprotective agents aim to halt the ischemic cascade and prevent secondary injuries or at least decrease the loss of vulnerable neurons in the ischemic penumbra7, 8. Neuroprotective agents act by various mechanisms including as antioxidants, neuron stimulants, calcium channel antagonists and free radical scavengers.
2. Drugs discontinued in clinical trials
Analyzing the 300 drug candidates that did not reach the market reveals several interesting aspects. First, 72 (24%) drugs were thrombolytics and included inhibitors of thrombin, factor Xa, platelet aggregation and serine endopeptidase (Fig. 1). Of the remaining 228 failed drugs, 74 (32%) were central nervous system neuroprotective agents with putative ability to either limit the toxicity of the major excitatory neurotransmitter glutamate9 or act as calcium channel antagonists. An example of the latter is the atypical analgesic ziconotide (trade name Prialt), derived from the toxin of the cone snail as the synthetic form of an ω-conotoxin peptide. It was originally launched by Elan Corporation for the treatment of severe chronic pain where it acts as a selective N-type voltage-gated calcium channel blocker10. Phase III clinical trials for the prevention of ischemic brain damage following stroke and severe head trauma have been carried out in the United States, Europe and Japan11.
The classical neuronal excitotoxicity theory is based on the observation that cerebral ischemic injury causes the release of L-glutamate. This then activates ionotropic glutamate receptors (iGluRs) and leads to an overload of Ca2+ influx that ultimately aggravates ischemic injury and neuronal apoptosis12. It has been hypothesized that the excitotoxicity is mediated by three main glutamate receptor subtypes viz. the N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors. Activation of these receptors is known to result in neuronal cell death by excessive calcium influx, and that antagonistic action at iGluRs may promote the survival of neurons13. The competitive NMDA antagonist selfotel14, 15, 16 and the noncompetitive NMDA antagonist aptiganel17, 18 were effective in preclinical studies but ineffective in clinical use16, 19. Of the AMPA antagonists ZK 20077520, 21 and YM872, the first was discontinued in a phase II clinical trial for stroke in Europe in 1999 and the second even never reached clinical trial evaluation because of its severe side effects22.
Over the past twenty years, all putative NMDA receptor antagonists aimed at stroke treatment have failed in clinical trials23. This strongly suggests that the neuronal excitotoxicity is mediated by a non-NMDA mechanism, which could involve proton-sensitive ion channels/exchangers. These include the acid-sensing ion channel 1a (ASIC1a)24, proton-sensitive cation channels, ion exchangers25 and transient receptor potential (TRP) channels. The latter include transient receptor potential canonical (TRPC) 3/4/6 channels, transient receptor potential melastatin (TRPM) 2/4/7 channels and transient receptor potential vanilloid (TRPV) 1/3/4 channels26, 27, 28, 29, 30. Thus drugs that act through non-glutamate–related mechanisms may represent an alternative strategy for stroke drug discovery.
3. Drugs currently undergoing clinical trials
There are about 40 drug candidates currently in clinical development, of which 14 (35%) are in phase I, 15 (38%) in phase II and 11 (27%) in phase III clinical trials. In terms of their mechanism of action, the drugs include 13 (32%) thrombus-related candidates and 27 neuroprotective agents (68%). The thrombus-related drug candidates include inhibitors of carboxypeptidase U, factor Xa and thrombin, whereas the neuroprotective agents act mainly as cell replacements, antioxidants and potassium channel antagonists.
Edaravone, first developed by the Mitsubishi Corporation, is a free radical scavenger which protects against cerebrovascular damage. Borneol, described in the Bencao Gangmu (Compendium of Materia Medica), is a terpene bicyclic organic compound used in traditional Chinese medicine (TCM) as moxa. Simcere, a Shanghai-based company, introduced the new idea of using edaravone combined with borneol to treat acute ischemic stroke. The novelty is that borneol enhances the ability of edaravone to scavenge free radicals through accelerating its distribution. From 2013 to 2015, Simcere enrolled 400 acute ischemic stroke patients in a phase II clinical trial to determine the efficacy of the combination (NCT01929096; SIM-23-01)31. Subsequently, Simcere initiated a phase III trial to evaluate the safety and efficacy of the combination (NCT02430350; SIM-23-02)32 intending to enroll about 1200 patients. This interesting combination raises the possibility that combining a natural TCM with a small molecule medicine is a useful strategy for the development of effective stroke pharmacotherapy.
4. Drugs currently in clinical use
As shown in Table 1, there are 19 stroke drugs currently marketed in various countries. Among them, 11 are thrombus-dissolving agents divided into three categories, namely rt-PAs, antithrombotic agents and platelet aggregation inhibitors. The remaining 8 drugs are neuroprotective agents that include antioxidants, TCMs, calcium channel antagonists and neurostimulants. As regards their route of administration, 14 are used orally and 5 by intravenous injection, suggesting that most drugs are used to prevent stroke and its reoccurrence.
Table 1.
Current stroke drugs in the market.
| Name | Chemical class | Mechanism | Administration | Market time | Location | Organization | |
|---|---|---|---|---|---|---|---|
| A, rt-PAs thrombolysis | |||||||
| 1 | Alteplase | Recombinant proteins | Plasminogen activators | Treatment IV | 1997/12/1 | World | Boehringer Ingelheim |
| B, Antithrombotic agents | |||||||
| 2 | Argatroban | Pipecolic acids; small molecules | Thrombin inhibitors | Treatment | 1996/8/2 | Japan | Mitsubishi Chemical Daiichi Sankyo Company |
| IV; infusion | |||||||
| 3 | Rivaroxaban | Amides; morpholines; oxazolidinones; small molecules; thiophenes | Factor Xa inhibitors | Prevention | 2013/1/1 | Africa, Asia, Canada, European Union, Japan, Latin America, Middle East, USA, United Kingdom | Bayer; Johnson & Johnson Pharmaceutical; McMaster University |
| PO | |||||||
| 4 | Apixaban | Two-ring heterocyclic compounds; amides; lactams; phenyl ethers pyrazoles; pyridones; small molecules | Factor Xa inhibitors | Prevention | 2014/12/31 | Argentina, Australia, Brazil, Canada, European Union, Hong Kong, Iceland, Israel, Japan, Mexico, Norway, Turkey, USA | Bristol-Myers Squibb; Pfizer |
| PO | |||||||
| 5 | Dabigatran etexilate | Benzimidazoles; pyridines; small molecules | Thrombin inhibitors | Prevention | 2015/3/17 | Australia, Canada, China, European Union, Hong Kong, Japan, Malaysia, New Zealand, Philippines, Singapore, South Africa, South Korea, Taiwan, Thailand, Turkey, USA | Boehringer Ingelheim |
| PO | |||||||
| 6 | Edoxaban | Small molecules | Factor Xa inhibitors | Prevention | 2015/7/9 | China, Japan, USA, United Kingdom | Daiichi Sankyo Company |
| PO | |||||||
| C, Antiplatelet drugs | |||||||
| 7 | Cilostazol | Small molecules; tetrazoles | Platelet aggregation inhibitors; type 3 cyclic nucleotide phosphodiesterase inhibitors | Prevention | 2012/1/25 | China, Japan | Otsuka Pharmaceutical; Pfizer |
| PO | |||||||
| 8 | Clopidogrel | Two-ring heterocyclic compounds; chlorobenzenes; esters; pyridines; small molecules; thienopyridines | Platelet ADP receptor antagonists; platelet aggregation inhibitors; purinergic P2 receptor antagonists | Treatment | 2006/5/5 | Brazil, Chile, China, Colombia, Croatia, European Union, Japan, Mexico, New Zealand, Norway, Philippines, Puerto Rico, Russia, South Korea, Switzerland, Turkey, USA | Bristol-Myers Squibb; Sanofi |
| PO; Intracoronary | |||||||
| 9 | Clopidogrel (Hanmi Pharmaceutical) | Treatment | 2015/3/13 | Cyprus, Germany, Italy, Netherlands, Portugal, South Korea, Spain, United Kingdom | Hanmi Pharmaceuticals | ||
| PO | |||||||
| 10 | Aspirin controlled release (New Haven Pharmaceuticals) | Salicylic acids; small molecules | Cyclooxygenase inhibitors; nitric oxide stimulants; platelet aggregation inhibitors | Prevention | 2015/12/15 | USA | New Haven Pharmaceuticals |
| PO | |||||||
| 11 | Aspirin/dipyridamole extended release | Salicylates; small molecules; vasodilators | Platelet aggregation inhibitors | Prevention | 2008/5/19 | Australia, Canada, Europe, South Africa, South America, USA | Boehringer Ingelheim |
| PO | |||||||
| 12 | Hydrochlorothiazide/atenolol/ramipril/simvastatin/aspirin or Polycap | Benzothiadiazines; heterocyclic bicyclo compounds; naphthalenes; propanolamines; salicylates; small molecules | Antihyperlipidaemics; antihypertensives; platelet aggregation inhibitors; ACE inhibitors; β1 adrenergic receptor antagonists; cyclooxygenase inhibitors; HMG-CoA reductase inhibitors; thiazide diuretics | Prevention | 2009/8/1 | India | Cadila Pharmaceuticals |
| PO | |||||||
| D, Neuroprotective agents | |||||||
| 13 | Gingko mihuan | Flavonoids; glycosides; terpenes; traditional Chinese medicine | Antioxidants; platelet activating factor inhibitors | Treatment | 2012/7/17 | China | Tianyin Pharmaceutical |
| PO | |||||||
| 14 | Lumbricus rubellus extract | Alternative medicine; enzymes; thrombolytics; traditional Chinese medicine | Fibrin inhibitors; fibrinolytic agents; Janus kinase 1 inhibitors; matrix metalloproteinase 9 inhibitors; NF-κB inhibitors; platelet aggregation inhibitors; STAT1 transcription factor inhibitors; tumor necrosis factor α inhibitors | Treatment | 2012/8/8 | Indonesia | Dexa Medica |
| PO | |||||||
| 15 | Fasudil (QR Science and Technology) | Amines; isoquinolines; small molecules; sulfonamides | Calcium channel antagonists; protein kinase C inhibitors; Rho-associated kinase inhibitors | Treatment | 2013/1/1 | China | QR Science and Technology |
| Parenteral | |||||||
| 16 | Cerebrolysin | Nootropics; peptides | Neuron stimulants | Treatment IV | 2000/3/22 | Austria, Germany | EVER Neuro Pharma |
| 17 | Citicolinea | Pyrimidine nucleotides; small molecules; trimethyl ammonium compounds | Hydroxy radical formation inhibitors; phosphatidylcholine stimulants | Treatment | 2013/7/31 | Argentina, Austria, Chile, Indonesia, Mexico, Portugal, Thailand, Venezuela | Ferrer; Takeda |
| PO; IM; IV | |||||||
| 18 | Kallidinogenase (Techpool Bio-Pharma) | Pipecolic acids; small molecules | Enzyme replacements | Treatment | 2005/12/1 | China | Techpool Bio-Pharma |
| Parenteral | |||||||
| 19 | Edaravoneb | Pyrazolones; small molecules | Free radical scavengers | Treatment IV | 2001/5/23 injection; 2010/5/31 infusion | Japan | Mitsubishi Pharma Corporation |
IM, intramuscular; IV, intravenous; PO, per oral.
Oral Citicoline syrup first was launched in more than 20 countries around the world for the treatment of ischemic stroke on January 1, 1998. And its treatment of stroke in USA and Canada was discontinued on April 17, 2009.
The indication of Edaravone refers to cerebral infarction. And its treatment of acute ischemic stroke in Europe has been discontinued in May 2012.
Considering where the drugs were launched, the United States ranks number 1 for the number of drugs launched followed by Europe and then Asia with China ranked in the 10th place. The top three organizations for the development of stroke drugs are Boehringer Ingelheim, Mitsubishi Tanabe Pharma Corporation and Pfizer.
The 11 drugs for thrombolysis can be divided into three categories: rt-PAs, antithrombotic agents, and platelet aggregation inhibitors.
4.1. Recombinant tissue plasminogen activators (rt-PAs)
The rt-PAs for the treatment of acute ischemic stroke include alteplase, reteplase, and tenecteplase33. Alteplase developed by Boehringer Ingelheim and approved in 1996 is a recombinant tissue-type plasminogen activator/serine protease that catalyzes the conversion of plasminogen to plasmin resulting in clot breakdown. It is used clinically to treat embolic or thrombotic stroke but not hemorrhagic stroke or head trauma. Reteplase is more convenient to administer and produces a more rapid thrombolytic effect than alteplase. Tenecteplase gives rise to fewer bleeding complications but has a similar mortality rate after one-year treatment to that of alteplase. Other rt-PAs such as desmoteplase are still in clinical development.
In July 2015, the Medicines and Healthcare Products Regulatory Agency (MHRA) reconfirmed that the benefits of alteplase outweigh the risks of acute ischemic stroke34. This positive conclusion was based on evidence and reviews of clinical trials35, 36, 37. Nevertheless, due to its narrow therapeutic window of 4.5 h and the high risk of intracranial hemorrhage, only about 5% of stroke patients benefit from alteplase treatment25.
4.2. Antithrombotic agents
There are currently five antithrombotic agents in clinical use including argatroban, rivaroxaban, apixaban, dabigatran etexilate, and edoxaban38, 39. Argatroban is a potent, reversible thrombin inhibitor used in Japan since 1996. Rivaroxaban is the first orally available direct factor Xa inhibitor launched in 2013. Apixaban, another oral pyrazole-based factor Xa inhibitor, was launched in 14 countries in late 2014 (December 31). It provides greater thrombin inhibition and a better tolerability profile than conventional antithrombotic drugs such as warfarin40, 41, 42. Dabigatran etexilate, the oral double prodrug of dabigatran, is a new generation of oral anticoagulant that has been marketed by Boehringer Ingelheim in 14 countries since 2015 for stroke prophylaxis. Edoxaban, a small molecule compared with anticoagulants like heparin and warfarin, is expected to have better oral absorption and a lower risk of bleeding. Daiichi Sankyo launched the product in 2015 in China, Japan, the United States and United Kingdom.
4.3. Antiplatelet drugs
Antiplatelet drugs such as cilostazol, clopidogrel and aspirin decrease platelet aggregation and interfere with thrombus formation. They are effective in improving arterial circulation which is largely unaffected by anticoagulants. Cilostazol is a 2-oxoquinoline derivative used in the alleviation of intermittent claudication in individuals with peripheral vascular disease. It is a phosphodiesterase inhibitor that induces direct arterial vasodilation and was launched in China and Japan for stroke prevention by Otsuka Pharmaceuticals in January of 2012. Clopidogrel is an orally available thienopyridine derivative co-developed by Sanofi and Bristol-Myers Squibb which acts to selectively inhibit adenosine diphosphate (ADP)-induced platelet aggregation by antagonizing platelet ADP and purinergic P2 receptors. Hanmi Pharmaceuticals launched a safer, more effective and convenient formulation of clopidogrel incorporating the napadisilate salt in March of 2015.
Aspirin is an irreversible cyclooxygenase inhibitor undergoing development as a once-daily controlled release formulation for secondary stroke prevention. A combination of aspirin and dipyridamole has been developed to reduce the risk of ischemic stroke recurrence. Another combination medication for stroke prevention is the so-called Polycap, a “five-in-one” capsule launched in India by Cadila Pharmaceuticals Ltd. in August of 2009. The Polycap contains atenolol, ramipril, hydrochlorothiazide, aspirin and simvastatin43, 44, each of which has been previously shown to reduce cardiovascular events. The advantage of Polycap is its potential to prevent stroke in high risk patients with hypertension, diabetes, dyslipidemia, and smoking.
4.4. Neuroprotective agents
There are several neuroprotective agents available for the treatment of ischemic stroke, including natural products, ion channel regulators and endogenous metabolites. Their primary aim is to alleviate excitotoxicity, calcium dysregulation, mitochondrial dysfunction, oxidative and nitrosative stress and inflammation. In addition, they act to relieve tissue acidosis, blood-brain barrier disruption, and neuronal apoptosis, necrosis and autophagy45.
4.4.1. Natural products
Gingko mihuan is a TCM launched as a proprietary prescription oral liquid by Tianyin Pharmaceuticals in 2012. The product contains flavonoid and terpenoid gingko extracts. Flavonoids have been shown to possess potent antioxidant activity and are useful in reducing capillary fragility. Terpenoids are reported to inhibit platelet activating factor and reduce blood viscosity associated with certain cardiovascular disorders. Gingko Mihuan Oral Liquid was approved by the China Food and Drug Administration (CFDA) in 2012 and is commonly prescribed for the treatment of stroke. Tianyin Pharmaceuticals also plans to develop capsule and tablet formulations of Gingko mihuan.
In August 2012, Dexa Medica, Indonesia introduced an oral tablet containing a bioactive protein fraction extracted from Lumbricus rubellus (red earthworm) as an anticoagulant for the treatment of ischemic stroke. The extract contains eight under 100 kDa proteins, one of which is a serine protease enzyme with thrombolytic and fibrinolytic effects similar to those of lumbrokinase46. In November 2014, Dexa Medica began phase II/III trials to assess the effects of the medication in patients with acute ischemic stroke (NCT0179099747; NCT0213352148).
Butylphthalide (3-n-butylphthalide, NBP), isolated from the seeds of celery, is another natural product found to protect against ischemic brain injury by increasing blood flow49, 50. In 2005, NBP was approved by the CFDA to undergo clinical trials for the treatment of cerebral ischemia. Currently, a phase IV clinical trial is ongoing51.
4.4.2. Ion channel regulators
Ion channels are membrane proteins that mediate ionic homeostasis in neurovascular units during ischemic stroke. An excess of sodium influx to neurons induces nerve cell swelling and leads to cytotoxic edema. Intracellular calcium overload can trigger a series of pathological events that ultimately result in neuronal apoptosis as well as necrotic death. An unchecked outflow of potassium changes neuronal polarization and excitability. Therefore, modulation of ion channel function is a novel approach to the treatment of stroke.
Fasudil mesylate, developed by QR Science and Technology, is an injectable formulation approved by the CFDA in 2013 for the treatment of cerebral infarction. The mechanism of action includes inhibiting a rho-kinase52, vasodilation53 and influencing intracellular calcium. Fasudil has been shown to enhance motor recovery after ischemic stroke caused by middle cerebral artery occlusion in rats54. It was also shown to reduce ASIC1a expression and calcium currents in the treatment group. This indicates that fasudil influences intracellular calcium levels through regulating the ASIC1a channel55.
4.4.3. Other neuroprotective agents
Nootropics, also called smart drugs or cognitive enhancers, are supplements or endogenous substances that improve cognitive function and behavioral outcome in healthy individuals and patients probably by promoting neurogenesis or decreasing neuronal death. Supplementing endogenous substances, such as neurotrophic factors, intermediates of phosphatidylcholine and urinary kallidinogenase, may constitute an effective approach for alleviating stroke symptoms.
Cerebrolysin is a mixture of low molecular weight peptides and amino acids derived from pig brain that has been shown to facilitate neural growth and survival of cholinergic neurons. The drug mainly consists of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), and ciliary neurotrophic factor (CNTF)56. It improves neurological outcome through neurogenesis induced by proliferation, differentiation, and migration of adult subventricular zone neural progenitor cells through the PI3K/Akt pathway57. Cerebrolysin was approved in March 2000 in Austria and Germany for the treatment of stroke. However, at the present time, there is insufficient evidence to evaluate the effects of cerebrolysin on survival and dependency in people with acute ischemic stroke. This will require a high-quality, large-scale, randomized clinical trial58, 59.
Citicoline, a derivative of choline and cytidine, is a psychostimulant or nootropic. It is an intermediate in the biosynthesis of phosphatidylcholine, an important component of the neural cell membrane that is degraded to free fatty acids and free radicals during cerebral ischemia. Its neuroprotective action may involve increasing phosphatidylcholine synthesis, stimulating glutathione synthesis to scavenge hydroxy radicals, and reducing the elevated levels of arachidonic acid that occur after an ischemic stroke60. An oral syrup of citicholine was launched in more than 20 countries around the world for the treatment of ischemic stroke in 1998. Subsequently, intravenous and intramuscular formulations of the drug were approved in Argentina, Austria, Chile, Indonesia, Mexico, Portugal, Thailand and Venezuela in 2013. However, in some placebo-controlled studies, citicoline was found to be ineffective in the treatment of moderate-to-severe acute ischemic stroke61, 62.
Kailikang is an injectable formulation of urinary kallidinogenase developed by Techpool Bio-Pharma, Guangzhou, China. It is a proteolytic enzyme extracted from human urine that converts kininogen to kinin and kallidin in the treatment of thrombotic cerebral infarction. The product has been shown to expand arteries in vitro, inhibit platelet aggregation, promote deformability and oxygen separation ability of haemocytes, and inhibit apoptosis and inflammation63. Due to its efficacy, the product was launched in China in 200564.
5. Concluding remarks
Analyzing the fate of 430 drugs evaluated for the treatment of ischemic stroke over the period 1995–2015 reveals that only 19 drugs (4%) were successful in reaching the market. Among them, 11 drugs are antithrombotic and 8 are used for stroke prevention. This indicates thrombolytic therapy remains the dominant form of pharmacotherapy for ischemic stroke.
Based on the mechanism of action of the failed candidates, it appears that drugs targeting non-NMDA-mediated calcium transport represent a promising strategy for stroke drug discovery. For example, fasudil mesylate improves neurological deficit and neuronal damage in rats by inhibiting ASIC1a. The cocktail of Polycap that combines five different medications in a single, once-a-day pill is also used for preventing strokes, suggesting combined medications may be worth further exploration. One such combination containing edaravone and borneol is currently undergoing a phase III clinical trial, the outcome of which certainly deserves attention.
It is also noteworthy that natural products, such as Gingko mihuan and Lumbricus rubellus extract, have been approved for stroke protection and treatment. This suggests that drugs with multiple targets have potential for neuronal protection and effective pharmacotherapy of ischemic stroke.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Wang L.D. Report on the Chinese stroke prevention. Pecking Union Medical College Press; Beijing: 2015. [Google Scholar]
- 2.Liu L., Wang D., Wong K.S., Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42:3651–3654. doi: 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- 3.Liu M., Wu B., Wang W.Z., Lee L.M., Zhang S.H., Kong L.Z. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–464. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y.L., Zhao X.Q., Jiang Y., Li H., Wang L.M., Johnston S.C. Prevalence, knowledge, and treatment of transient ischemic attacks in China. Neurology. 2015;84:2354–2361. doi: 10.1212/WNL.0000000000001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X.M., Zhu B., Fu L.Y., Wang H.L., Zhou B., Zou S.F. Prevalence, incidence, and mortality of stroke in the chinese island populations: a systematic review. PLoS One. 2013;8:e78629. doi: 10.1371/journal.pone.0078629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M. Heart disease and stroke statistics—2016 update: a report from the american heart association. Circulation. 2016;133:e38–e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 7.Casson R.J., Chidlow G., Ebneter A., Wood J.P., Crowston J., Goldberg I. Translational neuroprotection research in glaucoma: a review of definitions and principles. Clin Exp Ophthalmol. 2012;40:350–357. doi: 10.1111/j.1442-9071.2011.02563.x. [DOI] [PubMed] [Google Scholar]
- 8.Seidl S.E., Potashkin J.A. The promise of neuroprotective agents in Parkinson׳s disease. Front Neurol. 2011;2:68. doi: 10.3389/fneur.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyot L.L., Diaz F.G., O׳Regan M.H., McLeod S., Park H., Phillis J.W. Real-time measurement of glutamate release from the ischemic penumbra of the rat cerebral cortex using a focal middle cerebral artery occlusion model. Neurosci Lett. 2001;299:37–40. doi: 10.1016/s0304-3940(01)01510-5. [DOI] [PubMed] [Google Scholar]
- 10.Miljanich G.P. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem. 2004;11:3029–3040. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- 11.Heading C.E. Ziconotide (Elan Pharmaceuticals) IDrugs. 2001;4:339–350. [PubMed] [Google Scholar]
- 12.Lipton S.A., Rosenberg P.A. Excitatory amino acids as a final common pathway for neurologic disorders. New Eng J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 13.Muir K.W., Lees K.R. Clinical experience with excitatory amino acid antagonist drugs. Stroke. 1995;26:503–513. doi: 10.1161/01.str.26.3.503. [DOI] [PubMed] [Google Scholar]
- 14.Grotta J., Clark W., Coull B., Pettigrew L.C., Mackay B., Goldstein L.B. Safety and tolerability of the glutamate antagonist CGS 19755 (Selfotel) in patients with acute ischemic stroke. Results of a phase IIa randomized trial. Stroke. 1995;26:602–605. doi: 10.1161/01.str.26.4.602. [DOI] [PubMed] [Google Scholar]
- 15.Stewart L., Bullock R., Teasdale G.M., Wagstaff A. First observations of the safety and tolerability of a competitive antagonist to the glutamate NMDA receptor (CGS 19755) in patients with severe head injury. J Neurotrauma. 1999;16:843–850. doi: 10.1089/neu.1999.16.843. [DOI] [PubMed] [Google Scholar]
- 16.Davis S.M., Lees K.R., Albers G.W., Diener H.C., Markabi S., Karlsson G. Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke. 2000;31:347–354. doi: 10.1161/01.str.31.2.347. [DOI] [PubMed] [Google Scholar]
- 17.Chan P.H. The N-methyl-D-aspartate antagonist CNS 1102 protects cerebral gray and white matter from ischemic injury following temporary focal ischemia in rats. Editorial Comment. Stroke. 2000;31:1714. doi: 10.1161/01.str.31.7.1709. [DOI] [PubMed] [Google Scholar]
- 18.Schabitz W.R., Li F.H., Fisher M. The N-methyl-D-aspartate antagonist CNS 1102 protects cerebral gray and white matter from ischemic injury following temporary focal ischemia in rats. Stroke. 2000;31:1709–1714. doi: 10.1161/01.str.31.7.1709. [DOI] [PubMed] [Google Scholar]
- 19.Albers G.W., Goldstein L.B., Hall D., Lesko L.M. Aptiganel Acute Stroke Investigators. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. J Am Med Assoc. 2001;286:2673–2682. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- 20.Turski L., Huth A., Sheardown M., McDonald F., Neuhaus R., Schneider H.H. ZK200775: a phosphonate quinoxalinedione AMPA antagonist for neuroprotection in stroke and trauma. Proc Natl Acad Sci U S A. 1998;95:10960–10965. doi: 10.1073/pnas.95.18.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters M.R., Kaste M., Lees K.R., Diener H.C., Hommel M., De Keyser J. The AMPA antagonist ZK 200775 in patients with acute ischaemic stroke: a double-blind, multicentre, placebo-controlled safety and tolerability study. Cerebrovasc Dis. 2005;20:304–309. doi: 10.1159/000087929. [DOI] [PubMed] [Google Scholar]
- 22.Terai K., Suzuki M., Sasamata M., Yatsugi S.I., Yamaguchi T., Miyata K. Effect of AMPA receptor antagonist YM872 on cerebral hematoma size and neurological recovery in the intracerebral hemorrhage rat model. Eur J Pharmacol. 2003;467:95–101. doi: 10.1016/s0014-2999(03)01572-3. [DOI] [PubMed] [Google Scholar]
- 23.Ikonomidou C., Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lan Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 24.O׳Bryant Z., Vann K.T., Xiong Z.G. Translational strategies for neuroprotection in ischemic stroke—focusing on acid-sensing ion channel 1a. Transl Stroke Res. 2014;5:59–68. doi: 10.1007/s12975-013-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leng T.D., Shi Y.J., Xiong Z.G., Sun D.D. Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke? Prog Neurobiol. 2014;115:189–209. doi: 10.1016/j.pneurobio.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang E., Liao P. Brain transient receptor potential channels and stroke. J Neurosci Res. 2015;93:1165–1183. doi: 10.1002/jnr.23529. [DOI] [PubMed] [Google Scholar]
- 27.Romero J.R., Ridker P.M., Zee R.Y. Gene variation of the transient receptor potential cation channel, subfamily M, member 7 (TRPM7), and risk of incident ischemic stroke: prospective, nested, case-control study. Stroke. 2009;40:2965–2968. doi: 10.1161/STROKEAHA.109.558346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelderblom M., Melzer N., Schattling B., Göb E., Hicking G., Arunachalam P. Transient receptor potential melastatin subfamily member 2 cation channel regulates detrimental immune cell invasion in ischemic stroke. Stroke. 2014;45:3395–3402. doi: 10.1161/STROKEAHA.114.005836. [DOI] [PubMed] [Google Scholar]
- 29.Xu X.S., Wang P.J., Zhao Z.G., Cao T.B., He H.B., Luo Z.D. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke. 2011;42:3245–3251. doi: 10.1161/STROKEAHA.111.618306. [DOI] [PubMed] [Google Scholar]
- 30.Simard J.M., Tarasov K.V., Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta. 2007;1772:947–957. doi: 10.1016/j.bbadis.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ClinicalTrials. gov [Internet]. China: compound edaravone injection for acute ischemic stroke. A multi-center, randomized, double-blind, multi-dose, parallel, and controlled phase II trial. [updated 01.07.15]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT01929096?term=NCT01929096&rank=1〉.
- 32.ClinicalTrials. gov [Internet]. China: compound edaravone injection for acute ischemic stroke. A multi-center, randomized, double-blind, parallel, and active-controlled phase III trial. [updated 19.04.16]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT02430350?term=NCT02430350&rank=1〉.
- 33.Verstraete M. Third-generation thrombolytic drugs. Am J Med. 2000;109:52–58. doi: 10.1016/s0002-9343(00)00380-6. [DOI] [PubMed] [Google Scholar]
- 34.Hudson I. Alteplase for ischaemic stroke–responses. Lancet. 2014;384:662–663. doi: 10.1016/S0140-6736(14)61388-X. [DOI] [PubMed] [Google Scholar]
- 35.Emberson J., Lees K.R., Lyden P., Blackwell L., Albers G., Bluhmki E. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandercock P., Wardlaw J.M., Lindley R.I., Dennis M., Cohen G., Murray G. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardlaw J., Dennis M., Cohen G., Murray G., Innes K., Whiteley M. Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third International Stroke Trial [IST-3]): 18-month follow-up of a randomised controlled trial. Lancet Neurol. 2013;12:768–776. doi: 10.1016/S1474-4422(13)70130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisløff T., Hagen G., Klemp M. Economic evaluation of warfarin, dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation. Pharmacoeconomics. 2014;32:601–612. doi: 10.1007/s40273-014-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rognoni C., Marchetti M., Quaglini S., Liberato N.L. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig. 2014;34:9–17. doi: 10.1007/s40261-013-0144-3. [DOI] [PubMed] [Google Scholar]
- 40.Halvorsen S., Atar D., Yang H.Q., De Caterina R., Erol C., Garcia D. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35:1864–1872. doi: 10.1093/eurheartj/ehu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goto S., Zhu J., Liu L.S., Oh B.H., Wojdyla D.M., Aylward P. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Am Heart J. 2014;168:303–309. doi: 10.1016/j.ahj.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Wallentin L., Lopes R.D., Hanna M., Thomas L., Hellkamp A., Nepal S. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127:2166–2176. doi: 10.1161/CIRCULATIONAHA.112.142158. [DOI] [PubMed] [Google Scholar]
- 43.Urquhart J. The indian polycap study (TIPS) Lancet. 2009;374:781–782. doi: 10.1016/S0140-6736(09)61586-5. [DOI] [PubMed] [Google Scholar]
- 44.Indian Polycap Study, Yusuf S., Pais P., Afzal R., Xavier D., Teo K. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet. 2009;373:1341–1351. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
- 45.Jeyaseelan K., Lim K.Y., Armugam A. Neuroprotectants in stroke therapy. Expert Opin Pharmacother. 2008;9:887–900. doi: 10.1517/14656566.9.6.887. [DOI] [PubMed] [Google Scholar]
- 46.Trisina J., Sunardi F., Suhartono M.T., Tjandrawinata R.R. DLBS1033, a protein extract from Lumbricus rubellus, possesses antithrombotic and thrombolytic activities. J Biomed Biotechnol. 2011;2011:519652. doi: 10.1155/2011/519652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ClinicalTrials. gov [Internet]. Indonesia: the role of DLBS1033 in evaluating bleeding profile and clinical outcome in patients with acute ischemic stroke: comparison with aspirin and clopidogrel. [updated 03.06.14]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT01790997?term=NCT01790997&rank=1〉.
- 48.ClinicalTrials. gov [Internet]. Indonesia: addition of DLBS1033 to standard therapy for acute ischemic stroke patients; [updated 13.06.16]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT02133521?term=NCT02133521&rank=1〉.
- 49.Zhang T., Yan W.H., Li Q., Fu J.L., Liu K.Y., Jia W.P. 3-n-Butylphthalide (NBP) attenuated neuronal autophagy and amyloid-β expression in diabetic mice subjected to brain ischemia. Neurol Res. 2011;33:396–404. doi: 10.1179/1743132810Y.0000000006. [DOI] [PubMed] [Google Scholar]
- 50.Ji X.C., Zhao W.H., Cao D.X., Shi Q.Q., Wang X.L. Novel neuroprotectant chiral 3-n-butylphthalide inhibits tandem-pore-domain potassium channel TREK-1. Acta Pharmacol Sin. 2011;32:182–187. doi: 10.1038/aps.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ClinicalTrials. gov [Internet]. China: the impact of NBP on the collateral circulation in ICAM1 occlusion (INCIMO). [updated 17.04.16]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT02594995?term=NBP&rank=1〉.
- 52.Ding J., Li Q.Y., Yu J.Z., Wang X., Sun C.H., Lu C.Z. Fasudil, a Rho kinase inhibitor, drives mobilization of adult neural stem cells after hypoxia/reoxygenation injury in mice. Mol Cell Neurosci. 2010;43:201–208. doi: 10.1016/j.mcn.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Okamura N., Saito M., Mori A., Sakamoto K., Kametaka S., Nakahara T. Vasodilator effects of fasudil, a Rho-kinase inhibitor, on retinal arterioles in stroke-prone spontaneously hypertensive rats. J Ocul Pharmacol Ther. 2007;23:207–212. doi: 10.1089/jop.2006.128. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y.H., Zhao Y., Huang F.Z., Chen Y.H., Wang H.X., Bonney E. Combination of early constraint–induced movement therapy and fasudil enhances motor recovery after ischemic stroke in rats. Int J Neurosci. 2016;126:168–173. doi: 10.3109/00207454.2014.998759. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Wen C.Y., Cui C.C., Xing Y. Effect of activation of the Ca2+-permeable acid-sensing ion channel 1a on focal cerebral ischemia in diabetic rats. Int J Clin Exp Pathol. 2015;8:13255–13260. [PMC free article] [PubMed] [Google Scholar]
- 56.Menon P.K., Muresanu D.F., Sharma A., Mössler H., Sharma H.S. Cerebrolysin, a mixture of neurotrophic factors induces marked neuroprotection in spinal cord injury following intoxication of engineered nanoparticles from metals. CNS Neurol Disord Drug Targets. 2012;11:40–49. doi: 10.2174/187152712799960781. [DOI] [PubMed] [Google Scholar]
- 57.Zhang C.L., Chopp M., Cui Y.S., Wang L., Zhang R.L., Zhang L. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J Neurosci Res. 2010;88:3275–3281. doi: 10.1002/jnr.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziganshina L.E., Abakumova T. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2015:6. doi: 10.1002/14651858.CD007026.pub3. CD007026. [DOI] [PubMed] [Google Scholar]
- 59.Ziganshina L.E., Abakumova T., Kuchaeva A. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2010:14. doi: 10.1002/14651858.CD007026.pub2. CD007026. [DOI] [PubMed] [Google Scholar]
- 60.Rao A.M., Hatcher J.F., Dempsey R.J. CDP-choline: neuroprotection in transient forebrain ischemia of gerbils. J Neurosci Res. 1999;58:697–705. [PubMed] [Google Scholar]
- 61.Dávalos A., Alvarez-Sabín J., Castillo J., Díez-Tejedor E., Ferro J., Martínez-Vila E. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380:349–357. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- 62.Alberts M.J. ACP journal club. Citicoline did not improve recovery at 90 days after moderate-to-severe acute ischemic stroke. Ann Intern Med. 2012;157:JC3–13. doi: 10.7326/0003-4819-157-6-201209180-02013. [DOI] [PubMed] [Google Scholar]
- 63.Xia C.F., Yin H., Yao Y.Y., Borlongan C.V., Chao L., Chao J.L. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum Gene Ther. 2006;17:206–219. doi: 10.1089/hum.2006.17.206. [DOI] [PubMed] [Google Scholar]
- 64.Zhang C.F., Tao W.D., Liu M., Wang D.R. Efficacy and safety of human urinary kallidinogenase injection for acute ischemic stroke: a systematic review. J Evid Based Med. 2012;5:31–39. doi: 10.1111/j.1756-5391.2012.01167.x. [DOI] [PubMed] [Google Scholar]