Abstract
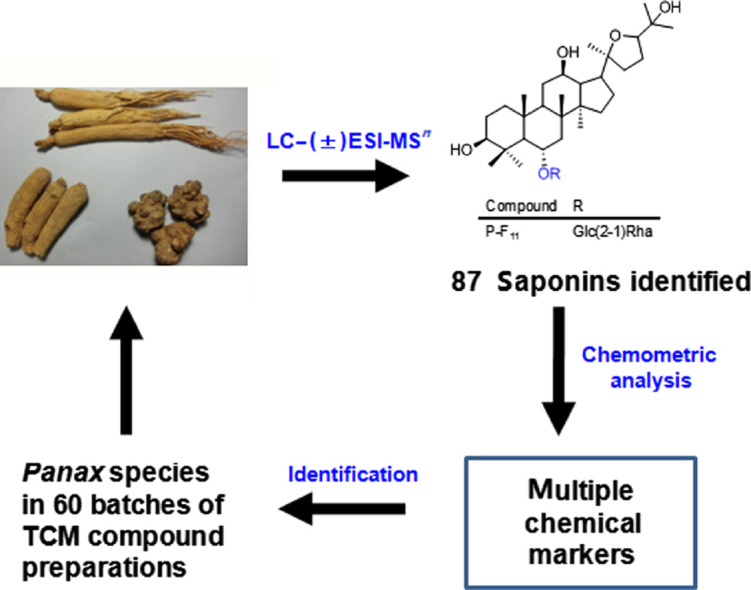
To differentiate traditional Chinese medicines (TCM) derived from congeneric species in TCM compound preparations is usually challenging. The roots of Panax ginseng (PG), Panax quinquefolium (PQ) and Panax notoginseng (PN) are used as popular TCM. They contain similar triterpenoid saponins (ginsenosides) as the major bioactive constituents. Thus far, only a few chemical markers have been discovered to differentiate these three species. Herein we present a multiple marker detection approach to effectively differentiate the three Panax species, and to identify them in compound preparations. Firstly, 85 batches of crude drug samples (including 32 PG, 30 PQ, and 23 PN) were analyzed by monitoring 40 major ginsenosides in the extracted ion chromatograms (EICs) using a validated LC–MS fingerprinting method. Secondly, the samples were clustered into different groups by pattern recognition chemometric approaches using PLS-DA and OPLS-DA models, and 17 diagnostic chemical markers were discovered. Aside from the previously known Rf and p-F11, ginsenoside Rs1 could be a new marker to differentiate PG from PQ. Finally, the above multiple chemical markers were used to identify the Panax species in 60 batches of TCM compound preparations.
KEY WORDS: Panax species, Ginsenoside, LC–MS fingerprinting, Chemical marker, TCM compound preparation
Graphical abstract
The roots of Panax ginseng (PG), Panax quinquefolium (PQ) and Panax notoginseng (PN) derive from congeneric species, and are used as different herbal medicines. In this study, we employed LC–MS analyses and chemometric approaches to discover 17 marker ginsenosides to differentiate these three species. These markers were further used to identify PG, PQ, and PN in 60 batches of traditional Chinese medicine compound preparations.
1. Introduction
The roots of Panax ginseng (PG), Panax quinquefolium (PQ), and Panax notoginseng (PN) are used as the popular traditional Chinese medicine (TCM) Ren-Shen, Xiyang-Shen, and San-Qi, respectively1. Chemical compositions of the three species are very similar. Nonetheless, PG, PQ and PN are considered to possess different properties in TCM theory and thus exhibit different therapeutic functions. PG has the “warm” property and is a good invigorator; PQ is “cool” and is thus capable of heat-clearing and refreshing;2 PN is mainly used to dispel stasis and stop bleeding. These functional varieties may originate from the difference in chemical composition, particularly in the bioactive triterpenoid saponins, popularly known as ginsenosides1. However, chemical difference among the three Panax species has not been fully clarified thus far. In addition, the market prices differ remarkably among the Panax species (for instance, between PG and PQ), and among the same species of different production areas (for instance, PQ cultivated in China and North America). Taken together, there is great demand to establish a reliable analytical method to differentiate the Panax species, and to identify their raw materials in TCM compound preparations.
Many analytical approaches have been used to identify Panax species, including DNA barcoding3, Raman or infrared spectrophotometry4, 5, 6, NMR spectroscopy7, 8, and LC–MS9, 10, 11. Among these approaches, LC–MS appears to be the most promising one. Wang et al.10 reported the potential significance of two pairs of ginsenosides (Rg1/Rf and Rc/Rb2) in the differentiation between PG and PQ by LC/MS/MS analysis. Chan et al.11 later reported the chemical markers ginsenoside Rf and 24(R)-pseudoginsenoside F11 together with the intensity ratio of ginsenosides Rg1/Re for species differentiation of PG and PQ. However, a limited number of markers may not be able to fully depict the chemical differences between the three species. The results could be more definitive by monitoring multiple markers.
LC–MS-based fingerprinting followed by chemometric analysis has been increasingly used for TCM analysis, which enables species differentiation of congeneric plant species12. Direct infusion mass spectrometry combined with chemometric analysis has been reported to differentiate Panax species13, 14. Our previous study has revealed the potential taxonomic significance of certain ginsenosides (oleanolic acid type, octillol type, malonylated, and peroxidized ginsenosides) in differentiating PG, PQ, and PN15. In this work, we present a new approach which integrates LC–MS based fingerprinting and pattern recognition chemometrics to discover more marker ginsenosides to differentiate these three species. These markers were further used in the identification of PG, PQ, and PN in 60 batches of TCM compound preparations.
2. Materials and methods
2.1. Chemical reagents and reference standards
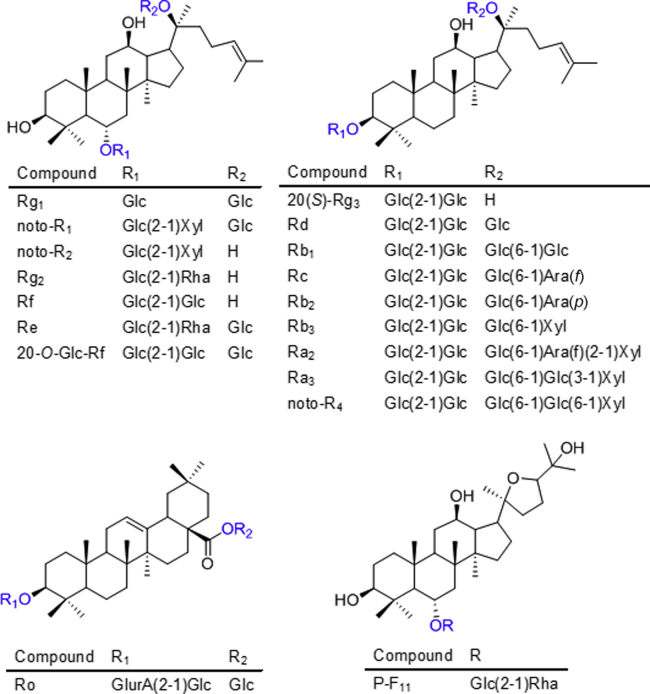
Ginsenosides Ro, Ra2, Ra3, Rb1, Rb2, Rc, Rd, Re, Rg1, Rg2, Rf, 20-O-glc-Rf, and notoginsenosides R1, R2, R4 were isolated from the roots of PG by the authors. Their structures were fully identified by NMR analysis15. 20(S)-Ginsenosides Rg3, Rb3, and 24(R)-pseudoginsenoside F11 were purchased from Nanjing Zelang Medical Technology Co., Ltd. (Nanjing, China). Their structures are shown in Fig. 1. The purities were >95% by LC–MS analysis. HPLC grade ammonium acetate (Fluka, Sigma–Aldrich, Netherland), formic acid, methanol, acetonitrile (J.T. Baker, Phillipsburg, NJ, USA) and ultra-pure water prepared using a Milli-Q water purification system (Millipore, MA, USA) were used for HPLC analysis. Analytical grade methanol and n-butanol were purchased from Damao Chemical Reagent Factory (Tianjin, China). The OASIS HLB Cartridge SPE columns were from Waters Corporation (Milford, MA, USA).
Figure 1.
Structures for 18 ginsenoside reference compounds. Glc, β-d-glucopyranosyl; Rha, α-l-rhamnopyranosyl; Xyl, β-d-xylopyranosyl; Ara (f), α-l-arabinofuranosyl; Ara (p), α-l-arabinopyranosyl, GlurA, β-d-glucuronopyranosyl.
2.2. Plant materials
Crude drug samples of PG were collected from Northeast China or local Tong-ren-tang drugstores (Beijing, China). PN samples were collected from Wenshan County, Yunnan Province, China. PQ samples were purchased from Xushi Yangshen Specialty Co,. Ltd. (Nanjing, China). Detailed information for the 85 batches of samples is given in Supplementary Table 1. In addition, 60 batches of TCM compound preparations which contain PG, PQ or PN were purchased from local drugstores. Their information is given in Supplementary Table 2. Voucher specimens are deposited at the author׳s laboratory, School of Pharmaceutical Sciences, Peking University (Beijing, China).
2.3. LC–MS conditions
The LC–MS fingerprints were recorded on a Surveyor HPLC instrument coupled with a TSQ triple-quadruple tandem mass spectrometer via ESI interface (Thermo Fisher, San Jose, CA, USA). The samples were separated on a YMC-Pack ODS-A column (250 mm × 4.6 mm, 5 μm) equipped with an Agilent Zorbax SB-C18 guard column (12.5 mm × 4.6 mm, 5 μm). The column temperature was maintained at 35 °C. A three-component mobile phase was used, composed of acetonitrile (A), methanol (B), and water containing 1 mmol/L ammonium acetate (C). The following gradient elution program was applied: 0 min: 12% A, 35% B, 53% C; 9 min: 20% A, 30% B, 50% C; 22 min: 35% A, 15% B, 50% C; 35 min: 50% A, 50% C; 45 min: 60% A, 40% C; 50 min: 90% A, 10% C; 55 min: 90% A, 10% C; 58 min: 12% A, 35% B, 53% C. The flow rate was 1 mL/min. For MS detection, the ESI source was operated in the negative ion mode. The LC eluant was introduced into the mass spectrometer at a post-column splitting ratio of 5:1. Ultra-high purity helium (He) and high purity nitrogen (N2) were used as the collision gas and nebulizing gas, respectively. The analyzer scanned over m/z 400–1500 in the full scan mode. An optimal source fragmentation voltage of 20 V was applied to suppress the adducted precursor ions. Using ginsenosides Re (protopanaxatriol type) and Rb2 (protopanaxadiol type) as reference compounds, the capillary voltage and tube lens offset voltage were optimized as –22 and –60 V, respectively. Ionspray voltage, 4.5 kV; sheath gas (N2), 45 arbitrary units; auxiliary gas (N2), 10 units; capillary temperature, 320 °C. To further identify the peaks in the LC–MS fingerprints, the samples were analyzed by LC–ESI-MSn and LC–qTOF-MS, as described in Supplementary Information 1, 2.
2.4. Sample preparation
Ultrasound-assisted extraction was used to prepare the herbal extract samples. An aliquot of 0.2 g finely ground dry powder was soaked in 10 mL of 50% aqueous methanol (v/v) for 30 min before extraction for 40 min at 40 °C. The extract was centrifuged at 4000 rpm for 15 min (Thermo Multifuge 1S-R, Thermo Fisher Scientific, MA, USA), and the supernatant was filtered through a 0.22-μm microporous membrane to obtain the test solution. An aliquot of 10 µL of the test solution was injected for analysis. The test solutions of TCM compound preparations were obtained in a similar manner, as described in Supplementary Information Section 3.
2.5. Multivariate data analysis
The peak areas for 40 major ginsenosides (marked in blue in Fig. 2) in the extracted ion chromatograms (EICs) were used as variables for multivariate data analysis. The peak areas were normalized to their sum values to minimize the deviation caused by system instability or different drug concentration. The normalized peak areas of 40 peaks in the 85 batches of crude drug samples were used to generate a 2D data lattice, which was subsequently imported into SIMCA-P 13.0 (Umetrics AB. Umeå, Sweden) for chemometric analysis. PLS-DA and OPLS-DA models were used for pattern recognition. The variables were pareto-scaled prior to automatic fitting. The variable importance in projection (VIP) plot, which directly reflects the contribution of each variable, together with a two-tailed t-test, were used to identify potential marker compounds. Characteristic markers were defined for those only detectable in one unique species, whereas the significantly differential markers should exhibit top-5 VIP values and statistical significance between two groups (P < 0.05).
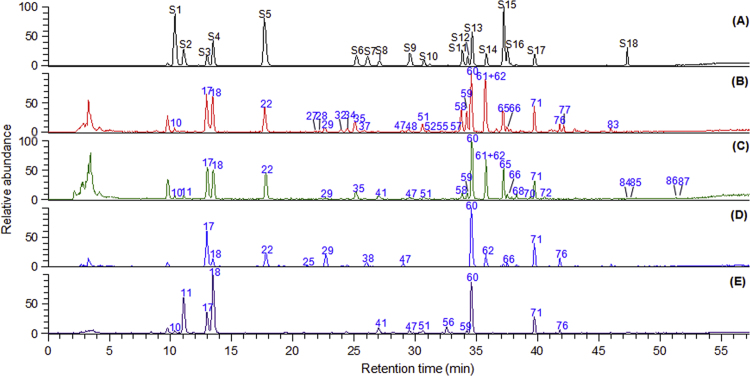
Figure 2.
The LC–(–)ESI-MS TIC chromatograms for reference standards (A), P. ginseng (B), steamed P. ginseng (red ginseng, C), P. quinquefolium (D), and P. notoginseng (E). The peak areas for 40 ginsenosides (10, 11, 17, 18, 22, 25, 27–29, 32, 34, 35, 37, 38, 41, 47, 48, 51, 52, 55, 56–62, 65, 66, 68, 70–72, 76, 77, 83–87) were used as variables for multivariate data analysis. S1: 20-O-glc-Rf (10), S2: noto-R1 (11), S3: Re (17), S4: Rg1 (18), S5: Ro (22), S6: Rf (35), S7: p-F11 (38), S8: noto-R2 (41), S9: Rg2 (48), S10: noto-R4 (51), S11: Ra2 (58), S12: Ra3 (59), S13: Rb1 (60), S14: Rc (62), S15: Rb2 (65), S16: Rb3 (66), S17: Rd (71), S18: 20(S)-Rg3 (84).
3. Results and discussion
3.1. Optimization of LC–MS conditions
An LC–MS-based fingerprinting method was established to analyze the chemical constituents of PG, PQ, and PN. The sample preparation procedure, chromatographic conditions, and MS detection parameters were optimized to achieve baseline separation of similar ginsenosides and sensitive detection of minor compounds. The method was validated in terms of inter-day and intra-day variation and reproducibility. The details were described in Supplementary Information Sections 4 and 5.
3.2. Identification of chromatographic peaks
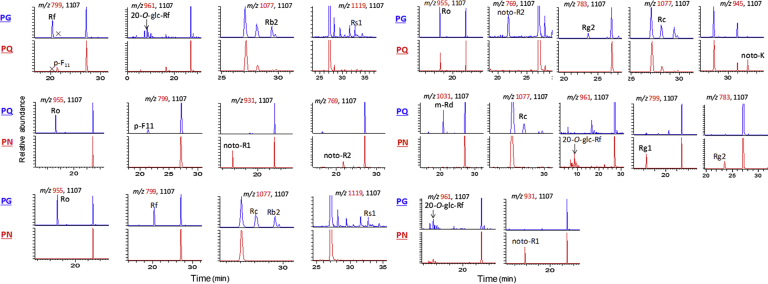
Based on the fragmentation pathways of 18 reference ginsenosides derived from an ion-trap mass spectrometer, a total of 87 chromatographic peaks were identified or putatively characterized. The fragmentation pathways were described in Supplementary Information Section 6, and the fragments for representative 20(S)-protopanaxadiol (PPD) type (Ra3), 20(S)-protopanaxatriol (PPT) type (20-O-glucosyl-Rf), and oleanolic acid (OA) type (Ro) ginsenosides were illustrated in Supplementary Fig. 1. Detailed information of identified ginsenosides is given in Supplementary Table 3. Among the 87 chromatographic peaks, peaks 10, 11, 17, 18, 22, 35, 38, 41, 48, 51, 58, 59, 60, 62, 65, 66, 71, and 84 were unambiguously identified by comparing the retention time (tR), MS and MS/MS product ions with those obtained by reference compounds. Here we take peaks 25, 68, 70 and 72 as examples to clarify the identification process. Other ginsenosides were identified following the same manner (Supplementary Table 3).
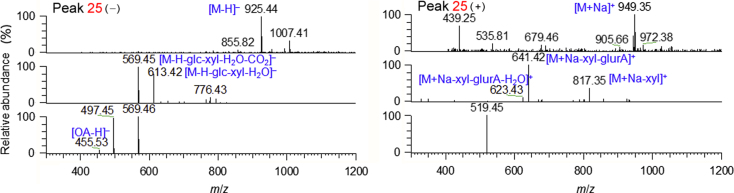
Peak 25 (tR 21.37 min), a common peak for PG, PQ and PN, had a molecular formula of C47H74O18 (m/z 925.4803 for [C47H73O18]–, mass error 0.11 ppm). The deprotonated precursor ion (m/z 925) fragmented into m/z 613 ([M–H–Glc–Xyl–H2O]–) and 569 ([M–H–Glc–Xyl–H2O–CO2]–) (Fig. 3). The product ion m/z 455 ([OA–H]–), dissociated from m/z 569, suggested a possible OA sapogenin. In the positive ion mode, CID of the sodium-adduct precursor m/z 949 generated the same Y0β+ (m/z 641 for [M+Na–Xyl–GlurA]+) and Z0β+ (m/z 623 for [M+Na–Xyl–GlurA–H2O]+) product ions as those of Ro (Supplementary Fig. 1), which indicated the presence of a 3-GlurA-Xyl disaccharide chain. The structure difference between peak 25 and Ro was a pentose instead of a hexose (Glc) at the terminal of 3-sugar chain. Moreover, peak 25 was eluted later than Ro (tR 21.37 versus 18.05 min), in agreement with its relatively lower polarity than Ro. These data supported the identification of 25 as chikusetsusaponin IV (OA-28-Glc-3-GlurA-Xyl)16.
Figure 3.
ESI-MSn spectra in negative and positive ion modes for chikusetsusaponin IV (OA-28-Glc-3-GlurA-Xyl, 25).
Among the three acetyl-substituted ginsenosides (68, 70, and 72), peak 68 (tR 37.97 min) was characterized as PPD-20-GlcGlc-3-GlcGlc-acetyl, while peaks 70 (tR 39.14 min) and 72 (tR 40.30 min) were both PPD-20-GlcXyl-3-GlcGlc-acetyl. In the extracted ion chromatograms, both the elution order and relative abundance of these three peaks were in agreement with those for Rb1, Rc, and Rb2 (Supplementary Fig. 2). Therefore, peaks 68, 70, and 72 were characterized as Ac-Rb1 (quinquenoside R1), Ac-Rc (ginsenoside Rs2), and Ac-Rb2 (ginsenoside Rs1), respectively17, 18. For all the ginsenosides characterized in PG, PQ and PN, the acetyl, malonyl or butenoyl group was substituted at 3-OH sugar chain for PPD type compounds or 6-OH sugar chain for PPT type, except for peak 2.
3.3. Discovery of marker compounds to differentiate among PG, PQ, and PN
3.3.1. Differentiation between PG and PQ
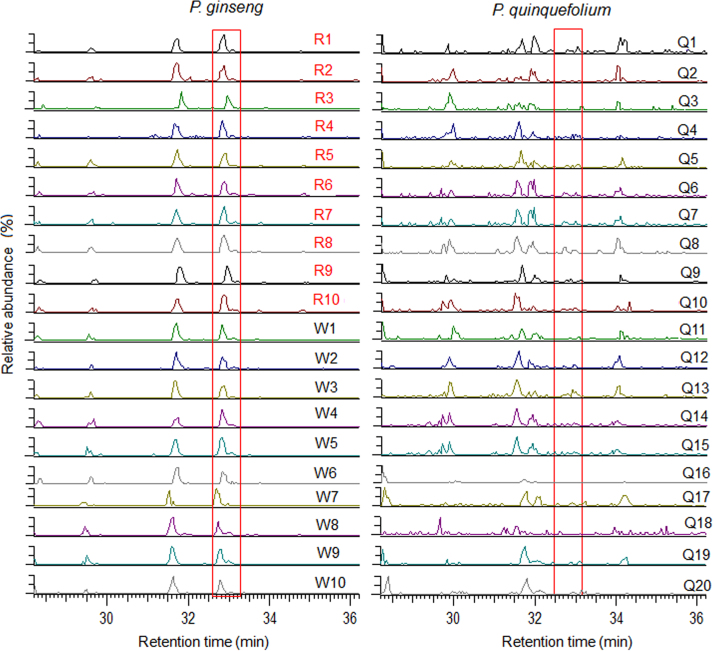
Several studies have compared the chemical composition of PG and PQ. The two major marker compounds that could discriminate the two species were ginsenoside Rf and 24(R)-pseudoginsenoside-F11 (p-F11).19, 20 Our results were consistent with these studies, where Rf (35) and p-F11 (38) were only detected in PG and PQ, respectively. In addition, a potential new marker compound, tentatively identified as ginsenoside Rs1 (72, Ac-Rb2), was found characteristic for PG. Fig. 4 exhibits the remarkably differential content of Rs1 between PG and PQ. Therefore, Rf and Rs1 were considered as the characteristic markers for PG, whereas p-F11 was characteristic for PQ.
Figure 4.
Extracted ion chromatograms for ginsenoside Rs1 ([M–H]−, m/z 1119.5), a potential characteristic marker to differentiate P. ginseng and P. quinquefolium. The chromatograms for 20 batches of P. ginseng and 20 batches of P. quinquefolium samples are shown. W and R refer to air-dried and steamed P. ginseng (white and red ginseng), respectively; Q refers to P. quinquefolium.
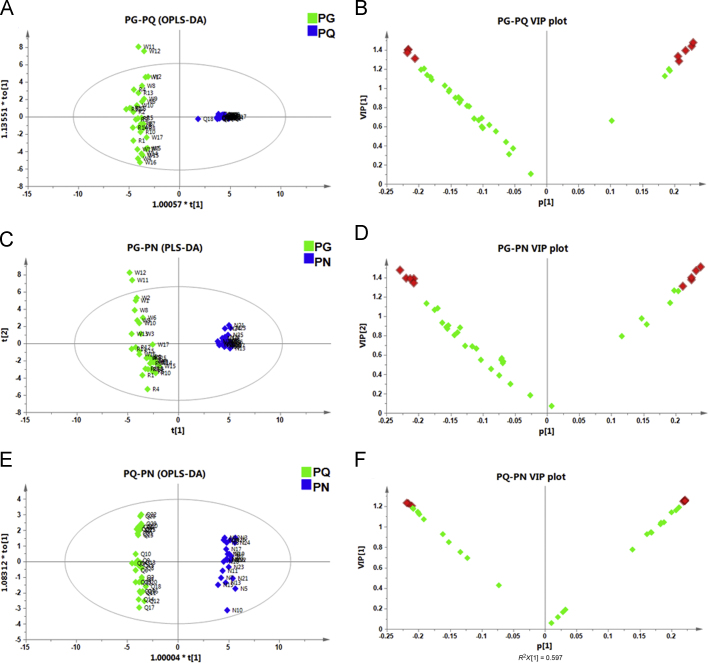
Chemometric analysis was then applied to identify ginsenosides capable of differentiating PG and PQ. The established OPLS-DA model, with good fitness (R2X 0.575, R2Y 0.983) and predictability (Q2 0.979), enabled good separation of PG and PQ groups (Fig. 5A). Two outliers (W11 and W12) were observed, but still segregated from the PQ group. The VIP plot could directly reflect the contribution level of the variables to group classification21, 22. A cutoff VIP value of 1.3 identified nine major potential marker ginsenosides: p-F11 (38), chikusetsusaponin IV (25), 20-O-glc-Rf (10), Rf (35), Re (17), Rb2 (65), Rb1 (60), Rg2 (48), and m-Rd (47) (Fig. 5B). The fact that Rs1 was not ranked among top-9 variables could be due to its low content in both PG and PQ. All the nine variables showed statistical significance between PG and PQ by a two-tailed t-test (type 3). Finally, we identified three characteristic markers (Rf, p-F11, and Rs1) and five significantly differential markers (chikusetsusaponin IV, 20-O-glc-Rf, Re, Rb2, and Rb1) to differentiate PG and PQ.
Figure 5.
Statistic analyses and discovery of potential markers to differentiate PG/PQ, PG/PN, and PQ/PN. PG, P. ginseng; PQ, P. quinquefolium, PN, P. notoginseng. (A) OPLS-DA score plot of PG and PQ; (B) VIP plot of PG and PQ showing 9 significantly differential components while VIP cutoff was set at 1.3; (C) PLS-DA score plot of PG and PN; (D) VIP plot of PG and PN showing 10 significantly differential components with VIP values higher than 1.3; (E) OPLS-DA score plot of PQ and PN; (F) VIP plot of PQ and PN showing 7 significantly differential components while VIP cutoff was set at 1.2.
3.3.2. Differentiation between PG and PN
The same procedure was employed to discover potential markers to discriminate PG from PN. Ginsenosides Ro (22), m-Rc (32) and m-Rb2 (37) were detected as common peaks for all PG samples, but not detected in PN. However, no characteristic peak was found for PN. Using the 40 ginsenosides as variables for multivariate data analysis, p-F11 was not detected in either PG or PN samples, and was thus excluded from the dataset. Here we applied PLS-DA for model fitting since over-fitting was observed when using the OPLS-DA model (Supplementary Fig. 3). The PLS-DA model was efficient to separate the samples into two different groups with good fits to the underlying models (R2X 0.606; R2Y 0.984) and excellent predictability (Q2 0.982) (Fig. 5C). However, W11 and W12 were also outliers, which was consistent with PG and PQ. Ten variables displayed VIP values of higher than 1.3 in the VIP plot (Fig. 5D), including noto-R1 (11), Rc (62), Rg1 (18), Rd (71), Rf (35), Rb2 (65), Ro (22), Rb1 (60), Rb3 (66), and noto-K (76). All these ten variables showed significant difference between PG and PN (P < 0.001). The characteristic components, Ro, m-Rc, and m-Rb2, and five most significantly differential components (noto-R1, Rc, Rg1, Rd, and Rf) were selected as markers to discriminate PG and PN.
3.3.3. Differentiation between PQ and PN
Relatively obvious differences were observed between PQ and PN (Fig. 2). Based on the analysis of EIC for 30 PQ and 23 PN samples, we found that Ro (22) and p-F11 (38) were characteristic markers for PQ, whereas Rf (35) and Ra3 (59) were characteristic for PN. Compounds m-Ra2 (27), m-Ra3 (28), m-Ra1 (34), Rs1 (72), Ra2 (58), peaks 52, 57, and 77 were not present or very low in both PQ and PN. Thus, the other 32 compounds were analyzed as variables using the OPLS-DA model. As shown in Fig. 5E, two obvious groups were separated. When the VIP boundary was set as 1.2, seven compounds were discovered, corresponding to noto-R1 (11), noto-R2 (41), Rg1 (18), 20-O-glc-Rf (10), p-F11 (38), Ro (22), and Re (17) (Fig. 5F). They all exhibited significant difference between PQ and PN. Four characteristic markers for PQ (Ro and p-F11) and PN (Rf and Ra3), together with five significantly differential markers (noto-R1, noto-R2, Rg1, 20-O-glc-Rf, and Re), were finally identified to differentiate PQ and PN.
Based on the above analyses, we were able to summarize the following points to rapidly differentiate the three Panax species (Fig. 6, Supplementary Table 3): (1) Ginsenoside Rs1 is unique for PG, while p-F11 is characteristic for PQ; (2) The presence of Ro, Rf, Ra3, Rs1, m-Rc, m-Rb2, and the absence of p-F11 are diagnostic for PG; the presence of p-F11, Ro, and the absence of Rf, Rs1, Ra3 could allow the identification of PQ. PN contains Ra3, but not p-F11, Rs1, Ro, m-Rc, and m-Rb2; (3) Ginsenosides 20-O-glc-Rf, Re, Rg1, Rc, Rb2, and Rd are rich in PG; Re and Rd are abundant in PQ; noto-R1, Rg1, and Rd are abundant in PN.
Figure 6.
Extracted ion chromatograms of the 17 diagnostic chemical markers to differentiate PG, PN and PQ crude drugs.
3.4. Identification of the three Panax species in TCM compound preparations
In total 17 diagnostic chemical marker compounds, including 7 characteristic markers (Rf, Rs1, p-F11, Ro, m-Rc, m-Rb2, and Ra3, species specific) and 10 significantly differential markers (chikusetsusaponin IV, 20-O-glc-Rf, Re, Rb2, Rb1, Rc, Rg1, Rd, noto-R1, and noto-R2, showing significant abundance variance between species) were discovered. These markers were used to identify PG, PQ, and PN in 40 different TCM compound preparations (60 batches).
All 60 batches of samples were analyzed by LC–MS, and the peak areas for the markers were obtained by extracting the [M–H]– ions. For the 7 characteristic markers, their presence or absence was used to identify the Panax species. For the 10 significantly differential markers, the relative peak area ratios for each compound in the extracted ion chromatograms against that of Rb1 were calculated. The results are shown in Supplementary Table 4.
By monitoring multiple diagnostic chemical markers, the Panax species used for the manufacturing of TCM compound preparations could be identified. Among the 40 preparations we analyzed, the identification results were consistent with the specified species for 37 preparations. The only two exceptions were Wu-ji-bai-feng Pills (A6) and Sheng-mai-yin (B1). Although their chemical patterns were mostly similar to P. ginseng (which was the specified species), they showed very weak chromatographic peaks, and several marker compounds were not detected. Sample A8 (Yi-nian-jin) could be identified to contain P. ginseng according to its multi-marker chemical pattern. Two characteristic markers for PG (m-Rc and m-Rb2) were not detected, probably due to poor thermostability of malonyl ginsenosides23. Similarly, C13 (Wei-kai-ling Capsules) and C14 (Jin-kang Capsules) could be identified to contain PN, by analyzing their multi-marker patterns, though the signals for ginsenoside Rf were very weak. In traditional Chinese medicine, the roots of P. ginseng could be used as white ginseng (dried after collection) or red ginseng (processed by steaming). By using the multiple chemical markers discovered in this study, both white and red ginseng could be correctly identified as P. ginseng. However, these two types could not be differentiated from each other.
4. Conclusions
To effectively differentiate the three Panax species, PG, PQ and PN, an LC–MS-based fingerprinting method coupled with multivariate data analysis was established. The peak areas for 40 ginsenosides were used for pattern recognition chemometric analysis by PLS-DA and OPLS-DA. A total of 17 diagnostic chemical marker compounds, including 7 characteristic markers (Rf, p-F11, Ro, Rs1, Ra3, m-Rc, and m-Rb2, species specific) and 10 significantly differential markers (20-O-glc-Rf, chikusetsusaponin IV, Re, Rg1, Rd, Rc, Rb2, noto-R1, noto-R2, and Rb1, showing significant abundance variance between species) were discovered to differentiate PG, PQ, and PN. Ginsenoside Rs1 could be a new marker to differentiate PG and PQ. By monitoring the above multiple diagnostic markers, the Panax species in 60 batches of TCM compound preparations could be effectively identified.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81222054).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.apsb.2016.05.005.
Contributor Information
De-an Guo, Email: daguo@simm.ac.cn.
Min Ye, Email: yemin@bjmu.edu.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Qi L.W., Wang C.Z., Yuan C.S. Isolation and analysis of ginseng: advances and challenges. Natl Prod Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C.F., Chiou W.F., Zhang J.T. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen X.C., Liao B.S., Song J.Y., Pang X.H., Han J.P., Chen S.L. A fast SNP identification and analysis of intraspecific variation in the medicinal Panax species based on DNA barcoding. Gene. 2013;530:39–43. doi: 10.1016/j.gene.2013.07.097. [DOI] [PubMed] [Google Scholar]
- 4.Chen P., Luthria D., Harrington P.B., Harnly J.M. Discrimination among Panax species using spectral fingerprinting. J AOAC Int. 2011;94:1411–1421. doi: 10.5740/jaoacint.10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang C., Qu H.B. A comparative study of using in-line near-infrared spectra, ultraviolet spectra and fused spectra to monitor Panax notoginseng adsorption process. J Pharm Biomed Anal. 2015;102:78–84. doi: 10.1016/j.jpba.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Edwards H.G.M., Munshi T., Page K. Analytical discrimination between sources of ginseng using Raman spectroscopy. Anal Bioanal Chem. 2007;389:2203–2215. doi: 10.1007/s00216-007-1605-4. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen H.T., Lee D.K., Choi Y.G., Min J.E., Yoon S.J., Yu Y.H. A 1H NMR–based metabolomics approach to evaluate the geographical authenticity of herbal medicine and its application in building a model effectively assessing the mixing proportion of intentional admixtures: a case study of Panax ginseng: metabolomics for the authenticity of herbal medicine. J Pharm Biomed Anal. 2016;124:120–128. doi: 10.1016/j.jpba.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Yuk J., McIntyre K.L., Fischer C., Hicks J., Colson K.L., Lui E. Distinguishing Ontario ginseng landraces and ginseng species using NMR-based metabolomics. Anal Bioanal Chem. 2013;405:4499–4509. doi: 10.1007/s00216-012-6582-6. [DOI] [PubMed] [Google Scholar]
- 9.Sun J.H., Chen P. Differentiation of Panax quinquefolius grown in the USA and China using LC/MS-based chromatographic fingerprinting and chemometric approaches. Anal Bioanal Chem. 2011;399:1877–1889. doi: 10.1007/s00216-010-4586-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang X.M., Sakuma T., Asafu-Adjaye E., Shiu G.K. Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal Chem. 1999;71:1579–1584. doi: 10.1021/ac980890p. [DOI] [PubMed] [Google Scholar]
- 11.Chan T.W.D., But P.P.H., Cheng S.W., Kwok I.M.Y., Lau F.W., Xu H.X. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Anal Chem. 2000;72:1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- 12.Li T., Zhuang S.X., Wang Y.W., Wang Y.L., Wang W.H., Zhang H.H. Flavonoid profiling of a traditional Chinese medicine formula of Huangqin Tang using high performance liquid chromatography. Acta Pharm Sin B. 2016;6:148–157. doi: 10.1016/j.apsb.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai Y.H., So P.K., Lo S.C.L., Ng E.W.Y., Poon T.C.W., Yao Z.P. Rapid differentiation of Panax ginseng and Panax quinquefolius by matrix-assisted laser desorption/ionization mass spectrometry. Anal Chim Acta. 2012;753:73–81. doi: 10.1016/j.aca.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 14.Chen P., Harnly J.M., Harrington P.B. Flow injection mass spectroscopic fingerprinting and multivariate analysis for differentiation of three Panax species. J AOAC Int. 2011;94:90–99. [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W.Z., Ye M., Qiao X., Liu C.F., Miao W.J., Bo T. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal Chim Acta. 2012;739:56–66. doi: 10.1016/j.aca.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Li Y.J., Wei H.L., Qi L.W., Chen J., Ren M.T., Li P. Characterization and identification of saponins in Achyranthes bidentata by rapid-resolution liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:2975–2985. doi: 10.1002/rcm.4728. [DOI] [PubMed] [Google Scholar]
- 17.Besso H., Kasai R., Wei J.X., Wang J.F., Saruwatari Y.I., Fuwa T. Further studies on dammarane-saponins of American ginseng, roots of Panax quinquefolium L. Chem Pharm Bull. 1982;30:4534–4538. [Google Scholar]
- 18.Kasai R., Besso H., Tanaka O., Saruwatari Y.I., Fuwa T. Saponins of red ginseng. Chem Pharm Bull. 1983;31:2120–2125. [Google Scholar]
- 19.Li W.K., Gu C.G., Zhang H.J., DVC Awang, Fitzloff J.F., HHS Fong. Use of high-peghormance liquid chromatography–tandem mass spectrometry to distinguish Panax ginseng C. A. Meyer (Asian ginseng) and Panax quinquefolius L. (North American ginseng) Anal Chem. 2000;72:5417–5422. doi: 10.1021/ac000650l. [DOI] [PubMed] [Google Scholar]
- 20.Li L., Luo G.A., Liang Q.L., Hu P., Wang Y.M. Rapid qualitative and quantitative analyses of Asian ginseng in adulterated American ginseng preparations by UPLC/Q-TOF-MS. J Pharm Biomed Anal. 2010;52:66–72. doi: 10.1016/j.jpba.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Sun H., Ni B., Zhang A.H., Wang M., Dong H., Wang X.J. Metabolomics study on Fuzi and its processed products using ultra-performance liquid-chromatography/electrospray-ionization synapt high-definition mass spectrometry coupled with pattern recognition analysis. Analyst. 2012;137:170–185. doi: 10.1039/c1an15833c. [DOI] [PubMed] [Google Scholar]
- 22.Liang J., Wu W.Y., Sun G.X., Wang D.D., Hou J.J., Yang W.Z. A dynamic multiple reaction monitoring method for the multiple components quantification of complex traditional Chinese medicine preparations: Niuhuang Shangqing pill as an example. J Chromatogr A. 2013;1294:58–69. doi: 10.1016/j.chroma.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y.T., You J.Y., Yu Y., Qu C.L., Zhang H.R., Ding L. Analysis of ginsenosides in Panax ginseng in high pressure microwave-assisted extraction. Food Chem. 2008;110:161–167. doi: 10.1016/j.foodchem.2008.01.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material