Abstract
Two new phenylpropanoid glycosides named cuneataside E (1) and cuneataside F (2), were isolated from the aerial parts of Lespedeza cuneata (Dum. Cours.) G. Don, whose structures were E and Z isomer, respectively. Their structures were elucidated on the basis of comprehensive spectroscopic analysis (UV, IR, HR-ESI-MS, 1D and 2D NMR). In in vitro bioassays at 10 μmol/L, compound 1 showed moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity in HeG2 cells.
KEY WORDS: Lespedeza cuneata, Phenylpropanoid glycosides, Extraction and isolation, Hepatoprotective activity, Cuneataside E, Cuneataside F
Graphical abstract
Two new phenylpropanoid glycosides, cuneataside E (1) and cuneataside F (2), were isolated from the aerial parts of Lespedeza cuneate (Dum. Cours.) G. Don, as E and Z isomer, respectively. At the concentration of 10 μmol/L, compound 1 showed moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity in HepG2 cells.

1. Introduction
Lespedeza cuneata (Dum. Cours.) G. Don, an annual herbaceous plant, is distributed in China, Korea, India, Australia and USA1, which named “ye guan men” in Chinese, is a very important traditional medicine, and has been used in the treatment of diabetes2, hematuria, insomnia and malnutrition3. Previous phytochemical studies have revealed flavonoids, sterols, triterpenoids4, 5, 6 and phenylpropanoid glycosides7 as chemical constituents of the plant, which showed antioxidant effects8, 9, 10, 11, 12, anti-inflammatory effects13 and antibacterial avtivities14. Among them, flavonoids were the main components of L. cuneata. In our continuing effort in studying constituents from this important medicinal plant, two new phenylpropanoid glycosides (Fig. 1) were isolated. Their structures were elucidated by various spectroscopic methods (UV, IR, HR-ESI-MS, 1D and 2D NMR). The isolation and structural elucidation of the new compounds were described in this paper.
Figure 1.
Structures of compounds 1 and 2.
2. Results and discussion
Compound 1 was obtained as a white amorphous powder. The UV spectrum showed absorption maximums at 210, 228 and 314 nm. In the IR spectrum, absorption bands at 3375, 2901, 1604, 1515, and 1449 cm−1 were observed. These data indicated the presence of hydroxyl, benzene, and carbonyl groups in 1. The molecular formula was determined to be C22H30O13 on the basis of HR-ESI-MS m/z 525.1588 [M+Na]+ (Calcd. for C22H30O13Na 525.1579). In the 1H NMR spectrum of 1, a set of AB-type signals at δH7.56 (2H, d, J=8.4 Hz, H-2″, 6″) and δH 6.57 (2H, d, J=8.4 Hz, H-3″, 5″) were observed, which suggested the existence of a 1,4-disubstituted benzene ring. Additionally, a methoxy signal at δH 3.35 (3H, s, OMe) and two anomeric proton signals at δH 4.25 (1H, d, J=8.0 Hz), δH 4.16 (1H, d, J=8.0 Hz) with large coupling constants suggested β-glucosidic linkages. From the hydrolysate of 1, a neutral residue containing sugars was obtained by extraction and evaporation. The sugar residue and authentic d-glucose were separately allowed to react with l-cycteine methyl ester and N-trimethylsilylimidazole (Section 4.4). Subsequent GC analysis indicated that two sugar derivatives from the sugar residue had retention time (tR) identical to that of authentic d-glucose. This verified that both glycosyl units in 1 possessed the d-configuration. We can also find trans-disubstituted double bond at δH 7.55 (1H, d, J=16.0 Hz) and δH 6.43 (1H, d, J=16.0 Hz), which suggests that the compound is E isomer. The 13C NMR spectrum showed 22 carbon signals. An α,β-unsaturated carbonyl group was demonstrated at δC 166.5. These spectroscopic data indicates that 1 has a trans-p-coumaroyl and two β-glucopyranosyl groups, for which the structure was further elucidated by 2D NMR data analysis.
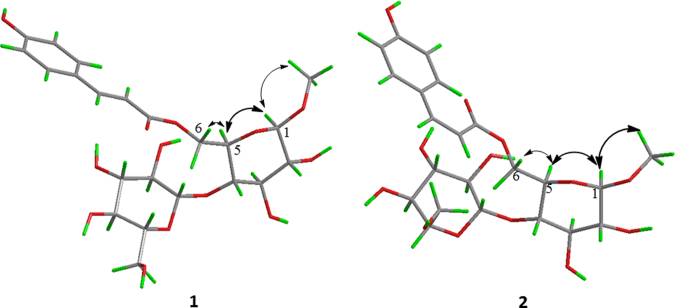
The proton-bearing carbon signals in the NMR spectra were assigned by cross-peaks in the HSQC spectra. HMBC correlations from H-1 to C-3, C-5, C–OCH3; from H-6 to C-5, C-4, and C-9″ (Fig. 2); together with their chemical shifts, revealed the presence of a methoxy group at C-1 and a trans-p-coumaroyl group at C-6. In the NOE spectra (Fig. 3), an enhancement of the proton signal at the H-OCH3/H-5 on irradiation of the H-1, and at the H-5 on irradiation of the H-6 revealed that H-OCH3 and the coumaroyl groups are linked on the same glucopyranosyl moiety. The HMBC correlations from H-1′ to C-4 demonstrated that two β-glucopyranosyl groups were connected through a 1,4-linkage. Therefore, the structure of 1 was elucidated as methyl-6-O-[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]-4-O-β-d-glucopyranoside-β-d-glucopyranoside, and named cuneataside E.
Figure 2.
Key HMBC (arrows, from 1H NMR to 13C NMR) correlations of compounds 1 and 2.
Figure 3.
The NOE enhancements induced by irradiation of H-1 and H-6 for compounds 1 and 2.
Compound 2 was obtained as a white powder, whose molecular formula was determined to be C22H30O13 on the basis of HR-ESI-MS. The UV spectrum showed absorption maximums at 210, 227 and 313 nm. The IR spectrum indicated absorption bands for hydroxyl group, carbonyl groups and a benzene ring. In the 1H NMR spectrum of 2, a set of AB-type signals, one methoxy signal and two aromatic proton signals were also observed. The 13C NMR spectrum showed 22 carbon signals (12 aromatic carbon signals, 12 saccharide moiety carbons, one methoxy signals and an α,β-unsaturated carbonyl group). These NMR spectroscopic data suggested compound 2 shares the same skeleton as compound 1. The only difference between 1 and 2 is that 2 has a cis-disubstituted double bond with two olefinic protons showing at δH 6.86 (1H, d, J=13.2 Hz) and δH 5.80 (1H, d, J=13.2 Hz), which suggest that the compound is Z isomer. The structure was further elucidated by HSQC, HMBC and NOE data analysis (Figure 2, Figure 3). Thus, the structure of 2 was concluded to be methyl-6-O-[(Z)-3-(4-hydroxyphenyl)prop-2-enoyl]-4-O-β-d-glucopyranoside-β-d-glucopyranoside, and named cuneataside F.
Compounds 1 and 2 were tested for hepatoprotective activity in the N-acetyl-p-aminophenol (APAP)-induced toxicity model in HepG2 (human hepatocellular liver carcinoma cell line) cells, using the hepatoprotective drug bicyclol as the positive control15. As shown in Table 2, compound 1 exhibited moderate hepatoprotective activity.
Table 2.
Hepatoprotective effects of compounds 1 and 2 (1×10−5 mol/L) against N-acetyl-p-aminophenol (APAP)-induced toxicity in HepG2 cells.
| Compd. | OD (Mean±SD) | Cell survival rate (percentage of normal) |
|---|---|---|
| Control | 2.228±0.067 | 100.00 |
| APAP (8 mmol/L) | 1.257±0.024* | 56.42 |
| 1 | 1.366±0.049## | 61.31 |
| 2 | 1.314±0.030# | 58.96 |
| Bicyclol | 1.343±0.045## | 60.27 |
#P<0.05, ##P<0.01, compared with APAP-induced model.
P<0.001, compared with control.
3. Conclusions
The plant L. cuneate (Ye guan men) is a known traditional Chinese medicine. Previous phytochemical studies have shown that ligan glycosides7 are considered as the characteristic constituents for the plant L. cuneata. As a part of the ongoing research program for the discovery of hepatoprotective compounds from L. cuneata, two new phenylpropanoid glucosides (1 and 2) were isolated from the aerial parts of this plant. The findings could provide some insight into the chemotaxonomic diversity of natural products in the genus Lespedeza. In an in vitro assay, compound 1 showed moderate hepatoprotective activity.
4. Experimental
4.1. General experimental procedures
Optical rotations were measured on a JASCO P2000 automatic digital polarimeter. UV spectra were recorded on a JASCO V-650 spectrophotometer. IR spectra were recorded on a Nicolet 5700 spectrometer using an FT-IR microscope transmission method. NMR spectra were acquired with Bruker AVIIIHD 600, VNS-600, or Mercury-400 spectrometers in DMSO-d6. HRESIMS spectra were collected on an Agilent 1100 series LC/MSD ion trap mass spectrometer. MPLC system was composed of two C-605 pumps (Büchi), a C-635 UV detector (Büchi), a C-660 fraction collector (Büchi), and an ODS column (450 mm×60 mm, 50 μm, 400 g; YMC). Semi-preparative HPLC was conducted using a Shimadzu LC-6AD instrument with an SPD-20A detector and a Daicel Chiralpak AD-H column (250 mm×10 mm, 5 μm). Preparative HPLC was also performed on a Shimadzu LC-6AD instrument with a YMC-Pack ODS-A column (250 mm×20 mm, 5 μm). Column chromatography (CC) was performed with silica gel (200–300 mesh, Qingdao Haiyang Chemical Inc., Qingdao, China), SF-PRP 512 A (100–200 mesh, Beijing Sunflower and Technology Development Co., Beijing, China), ODS (50 μm, YMC, Japan), and Sephadex LH-20 (GE, Sweden). TLC was carried out on glass precoated silica gel GF254 plates. Spots were visualized under UV light or by spraying with 10% sulfuric acid in EtOH followed by heating. GC analyses were obtained using an Agilent Technologies 7890A instrument.
4.2. Plant material
L. cuneata was collected in October 2013 in Xinyang City, Henan Province, China. The plant material was identified by Professor Ceming Tan (JiuJiang Forest Institute). A voucher specimen is deposited at the Herbarium of the Institute of Material Medical, Chinese Academy of Medical Sciences and Peking Union Medical College, China (No. 22276).
4.3. Extraction and isolation
L. cuneata (21 kg) were extracted with 70% EtOH under reflux for three times. After the solvent was evaporated under reduced pressure, the residue (3.68 kg) was subjected to a diatomite column, eluting with ether, CHCl3, EtOAc, CH3COCH3, 95% EtOH and 70% EtOH, to afford six corresponding fractions (Frs. 1–6). Fr. 4 (148 g) was subjected to CC over polyamide resin eluted with H2O and EtOH–H2O (30%, 60%, and 95%, v/v) to produce four major fractions (A, B, C and D). Fr. A (77.2 g) was then divided into four subfractions (A1–A4) via D101 macroporous adsorption resin CC eluted with H2O and EtOH–H2O (30%, 60%, and 95%, v/v). Fr. A3 (6.7 g) was fractionated by sephadex LH-20 CC eluted with MeOH to furnish six fractions (A3-1–A3-6). Fraction A3-3 (2.1 g) was further separated by MPLC with MeOH–H2O (20–50%, v/v, 6 h) to yield nineteen fractions (A3-3-1–A3-3-19). Separation of Fr. A3-3-5 (71 mg) was purified by semi-preparative HPLC (3.0 mL/min, 25% MeOH–H2O (v/v) isocratic elution, detected at 210 nm, tR=31.6 and 36.4 min) to yield A3-3-5-1 (15 mg) and A3-3-5-2 (47 mg), respectively. A3-3-5-2 was purified by semi-preparative HPLC (3.0 mL/min, 13% MeCN-H2O isocratic elution, detected at 210 nm, tR=26.8 and 33.6 min) to yield compounds 1 (31 mg) and 2 (7 mg), respectively.
Cuneataside E (1) White amorphous powder, [α]D20 −22.6 (c 0.15, MeOH); UV (MeOH) λmax (logε): 210 (4.26), 228 (4.27), 314(4.59) nm; IR (KBr) νmax: 3375, 2901, 1701, 1632, 1604, 1515, 1449, 1328, 1279, 1170, 1026 cm−1; For 1H and 13C NMR data, see Table 1. HR-ESI-MS m/z 525.1588 [M+Na]+ (Calcd. for C22H30O13Na, 525.1579).
Table 1.
1H NMR and 13C NMR spectral data (δ) of compounds in DMSO-d6 (δ in ppm, J in Hz).
| No. |
1a |
2b |
||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 4.16 d (8.0) | 103.3 | 4.15 d (7.8) | 103.4 |
| 2 | 3.09 m | 72.9 | 3.04 m | 72.9 |
| 3 | 3.37 m | 74.8 | 3.36 m | 74.7 |
| 4 | 3.39 m | 80.6 | 3.34 m | 80.7 |
| 5 | 3.62 m | 71.8 | 3.59 m | 71.7 |
| 6 | 4.50 dd (12.0, 2.0) | 63.0 | 4.48 d (11.4) | 62.9 |
| 4.34 dd (12.0, 5.6) | 4.34 dd (11.4, 5.4) | |||
| 1′ | 4.25 d (8.0) | 103.4 | 4.22 d (7.8) | 103.4 |
| 2′ | 3.00 m | 73.2 | 2.99 m | 73.2 |
| 3′ | 3.20 m | 76.9 | 3.19 m | 76.8 |
| 4′ | 3.03 m | 70.0 | 3.04 m | 70.0 |
| 5′ | 3.13 m | 76.5 | 3.13 m | 76.4 |
| 6′ | 3.69 m | 61.1 | 3.69 m | 61.0 |
| 3.41 m | 3.39 m | |||
| 1″ | 125.1 | 125.3 | ||
| 2″, 6″ | 7.56 d (8.4) | 130.4 | 7.66 d (8.4) | 132.5 |
| 3″, 5″ | 6.57 d (8.4) | 115.8 | 6.76 d (8.4) | 115.2 |
| 4″ | 159.9 | 158.8 | ||
| 7″ | 7.55 d (16.0) | 144.8 | 6.86 d (13.2) | 143.2 |
| 8″ | 6.43 d (16.0) | 114.1 | 5.80 d (13.2) | 114.9 |
| 9″ | 166.5 | 165.8 | ||
| OCH3 | 3.35 s | 56.0 | 3.33 s | 55.9 |
Data was measured at 400 MHz for 1H NMR and at 100 MHz for 13C NMR.
Data was measured at 600 MHz for 1H NMR and at 150 MHz for 13C NMR.
Cuneataside F (2) White amorphous powder, [α]D20 −20.2 (c 0.11, MeOH); UV (MeOH) λmax (logε): 210 (4.34), 227 (4.32), 313 (4.63) nm; IR (KBr) νmax: 3394, 2921, 1699, 1645, 1604, 1514, 1449, 1419, 1277, 1168, 1050 cm−1; For 1H and 13C NMR data, see Table 1. HR-ESI-MS m/z 525.1589 [M+Na]+ (Calcd. for C22H30O13Na, 525.1579).
4.4. Determination of absolute configurations of the sugar moieties in 1 and 2
Compounds 1 and 2 (2.0 mg) were separately dissolved in 2 mol/L HCl–H2O (2 mL) and heated at 70 °C for 12 h. After extraction with EtOAc (3×2 mL) to remove the aglycone, the aqueous layer was evaporated to afford a neutral residue. The dried sugar residue was diluted in anhydrous pyridine (1 mL), to which l-cysteine methyl ester hydrochloride (2 mg) was added. The mixture was stirred at 60 °C for 2 h, and then treated with N-trimethylsilylimidazole (0.2 mL). The mixture was then heated to dryness at 60 °C for another 2 h. The dried reactant was partitioned between n-hexane (2 mL) and H2O (2 mL) three times. The n-hexane layer was concentrated (1 mL) and subjected to GC analysis (column: HP-5, 60 m×0.25 mm×0.25 μm, Dikma; detector: FID; detector temperature: 280 °C; injector temperature: 250 °C; carrier: N2; temperature-programmed: from 200 to 280 °C in 2 min and maintain the final temperature 30 min).
4.5. Cell viability assay
HepG2 cells were cultured in DMEM medium supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The cells were then passaged by treatment with 0.25% trypsin in 0.02% EDTA. The MTT assay was used to assess the cytotoxicity of test samples. The cells were seeded in 96-well multiplates. After an overnight incubation at 37 °C with 5% CO2, 10 μmol/L test samples and APAP (final concentration of 8 mmol/L) were added into the wells and incubated for another 48 h. Then 100 μL of 0.5 mg/mL MTT was added to each well after the withdrawal of the culture medium and incubated for an additional 4 h. The resulting formazan was dissolved in 150 μL of DMSO after aspiration of the culture medium. The plates were placed on a plate shaker for 30 min and read immediately at 570 nm using a microplate reader.
Acknowledgments
This project was financially supported by the National Mega-project for Innovative Drugs (No. 2012ZX09301002-002) and National Natural Science Foundation of China (Nos. 81560632 and 81202546).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.apsb.2016.05.009.
Appendix A. Supplementary material
Supplementary material
References
- 1.Jiangsu New Medical College. Dictionary of Chinese herbal medicines. Shanghai: Shanghai Science and Technology Publishing House; 1979.
- 2.Su S.H. Lespedeza cuneata for II type diabetes. J Med Theory Pract. 1999;12:333–334. [Google Scholar]
- 3.Xu P.H. Curative effect observation of 62 cases of glomerular hematuria treated with Lespedeza cuneata. Chin J Tradit Med Sci Technol. 2010;17:43. [Google Scholar]
- 4.Kwon D.J., Bae Y.S. Flavonoids from the aerial parts of Lespedeza cuneata. Biochem Syst Ecol. 2009;37:46–48. [Google Scholar]
- 5.Atsushi N., Hokimopa K., Yamaguchi H. C-glycosyl flavones in Lespedeza cuneata. Chem Pharm Bull. 1980;28:964–965. [Google Scholar]
- 6.Deng F., Chang J., Zhang J.S. New flavonoids and other constituents from Lespedeza cuneata. J Asian Nat Prod Res. 2007;9:655–658. doi: 10.1080/10286020600979894. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J., Li C.J., Yang J.Z., Ma J., Wu L.Q., Wang W.J. Phenylpropanoid and lignan glycosides from the aerial parts of Lespedeza cuneata. Phytochemistry. 2016;121:58–64. doi: 10.1016/j.phytochem.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim J.S., Kim M.J. In vitro antioxidant activity of Lespedeza cuneata methanolic extracts. J Med Plants Res. 2010;4:674–679. [Google Scholar]
- 9.Kim Y.H., Ryu S.N. Antioxidant activity of methanol extract from aerial parts in Lespedeza cuneata G. Don. Korean J Crop Sci. 2008;53:121–123. [Google Scholar]
- 10.Cho E.J., Lee S.G., Kim D.O. The effect of Lespedeza cuneata extract for antioxidative and whitening effect. J Life Resour Sci Res. 2009;28:34–38. [Google Scholar]
- 11.Kim S.J., Kim D.W. Antioxidative activity of hot water and ethanol extracts of Lespedeaza cuneata. Korean J Food Preserv. 2007;14:332–335. [Google Scholar]
- 12.Cho E.J., Chu H.M., Jung C.H., Eom S.H., Hur H.J., Kim D.R. Effect of phenolic extract of dry leaves of Lespedeza cuneata G. Don on antioxidant capacity and tyrosinase inhibition. Korean J Hort Sci Technol. 2011;29:358–365. [Google Scholar]
- 13.Lee H., Jung J.Y., Hwangbo M., Ku S.K., Kim Y.W., Jee S.Y. Anti-inflammatory effects of Lespedeza cuneata in vivo and in vitro. Korean J Herb. 2013;28:83–92. [Google Scholar]
- 14.Jin L.H., Lim G.N., Min A.P., Park S.N. Antibacterial and antioxidative activity of Lespedeza cuneata G. Don extracts. Korean J Micro Biotechnol. 2011;39:63–69. [Google Scholar]
- 15.Hao Z.Y., Liang D., Luo H., Liu Y.F., Ni G., Yu D.Q. Bioactive sesquiterpenoids from the rhizomes of Acoruscalamus. J Nat Prod. 2012;75:1083–1089. doi: 10.1021/np300095c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material