Summary
Aims
Patients with Fabry disease (FD) characteristically develop peripheral neuropathy at an early age, with pain being a crucial symptom of underlying pathology. However, the diagnosis of pain is challenging due to the heterogeneous and nonspecific symptoms. Practical guidance on the diagnosis and management of pain in FD is needed.
Methods
In 2014, experts met to discuss recent advances on this topic and update clinical guidance.
Results
Emerging disease‐specific tools, including FabryScan, Fabry‐specific Pediatric Health and Pain Questionnaire, and Würzburg Fabry Pain Questionnaire, and more general tools like the Total Symptom Score can aid diagnosis, characterization, and monitoring of pain in patients with FD. These tools can be complemented by more objective and quantifiable sensory testing. In male and female patients of any age, pain related to FD can be an early indication to start disease‐specific enzyme replacement therapy before potentially irreversible organ damage to the kidneys, heart, or brain occurs.
Conclusion
To improve treatment outcomes, pain should be diagnosed early in unrecognized or newly identified FD patients. Treatment should include: (a) enzyme replacement therapy controlling the progression of underlying pathology; (b) adjunctive, symptomatic pain management with analgesics for chronic neuropathic and acute nociceptive, and inflammatory or mixed pain; and (c) lifestyle modifications.
Keywords: Diagnosis, Enzyme replacement therapy, Fabry disease, Pain, Peripheral nervous system
Introduction
Fabry disease (FD) is an X‐linked inherited lysosomal storage disorder, manifesting as a multisystemic disease. Due to mutations in the GLA gene encoding the enzyme α‐galactosidase A (abbreviated as α‐Gal), accumulation of glycolipids, mainly globotriaosylceramide (GL‐3), occurs in a wide range of cell types including vascular endothelial and smooth muscle cells 1. Elevated plasma levels of the deacylated metabolite of GL‐3, lyso‐GL‐3, is also a hallmark of FD 2. Glycolipid accumulation is often evident from a very early age, even in utero and in the umbilical cord 3, and may trigger a range of pathological processes that can ultimately lead to potentially fatal complications in the kidneys, heart, and cerebrovascular system 1. FD covers a wide spectrum of clinical presentations 4, including the classic severe phenotype, late‐onset variant phenotypes, and the oligosymptomatic presentations that can be observed in female patients 1, 5. Since 2001, enzyme replacement therapy (ERT) with recombinant agalsidase has been available for the treatment of FD.
Pain, one of the earliest clinical symptoms of FD reported by children and young adults 6, 7, 8, 9, has significant physical and social impacts on patient's quality of life 10, 11. Although long‐term improvement has been observed after ERT initiation, particularly in male patients, life‐impacting pain may still be present and require the use of adjunctive medications 11.
As pain may be an indicator of underlying FD pathology 12, any type of pain related to FD is an important symptom that can indicate the need to start ERT, regardless of patient age or gender 13.
Materials and Methods
This article aims to provide practical diagnostic and management guidelines regarding pain in FD. These recommendations are based on an FD Expert Panel that convened in Rome, Italy, in March 2014 and on subsequent discussions.
Results
Characteristics and Clinical Course of Pain in Classic Fabry Disease
Pain is a commonly reported early symptom in FD, affecting 60–70% of male and 40–60% of female patients 7, 14, 15, 16. A recent systematic literature review showed that the most frequently reported symptom in patients aged <5 years was pain and/or dysesthesias, which develop in children aged 2–4 years 9. The fingertips, palms, toes, and soles of the feet are the sites commonly affected by pain in FD (Figure 1) 16. However, pain may develop in any body region, such as the joints, teeth, or shoulders 16, some of which, like joint pain, can easily be overlooked or misdiagnosed. A recent survey helped characterize several types of pain in FD 16. Typically, pain in FD manifests as episodes of burning, stabbing, tingling, or shooting pain (previously misleadingly referred to as “acroparesthesias”) that begin in the distal extremities and radiate proximally 10, 16. Patients also commonly experience attacks of excruciating, incapacitating pain that may spread over the entire body, referred to as “pain crises”, as well as evoked pain comprising allodynia and hyperalgesia 16. The most important triggers of pain in FD are physical exercise, thermal stimuli, and fever 7, 12, 16, but some patients may also develop permanent pain of mostly mild‐to‐moderate intensity that manifests mainly at the hands and feet and persists even in the absence of identifiable triggers 16. Although pain manifests in early childhood, it seems that the timing of pain onset varies according to gender, starting earlier in males than in females 7, 9, 12.
Figure 1.
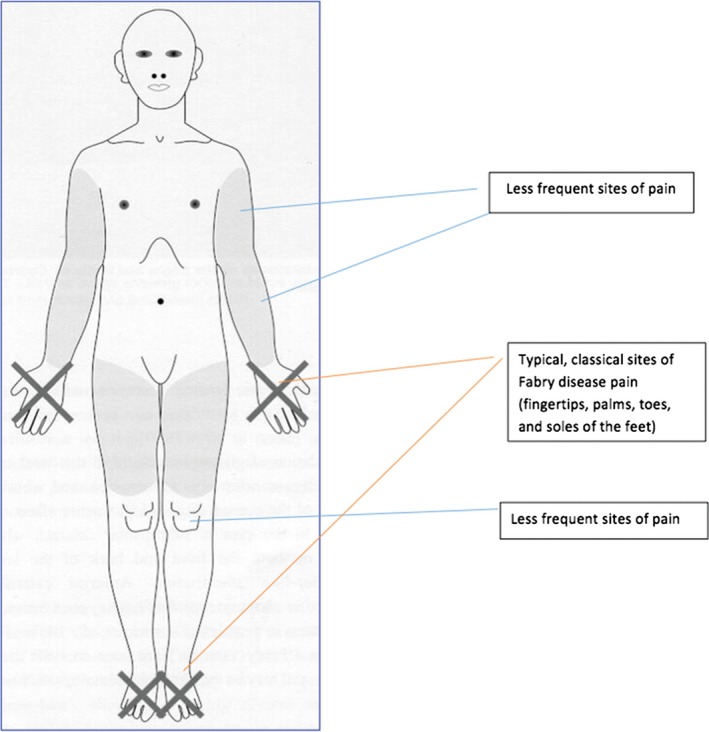
Potential locations of pain manifestations in patients with Fabry disease.
Interestingly, several patients with FD report a reduction in pain as they reach adulthood 16, but they can develop symptoms of large nerve fiber impairment, which mostly manifest as uremic polyneuropathy 17. Entrapment syndromes, such as carpal tunnel syndrome, have been reported in up to a quarter of patients with FD 18, 19. Another important issue is the high prevalence of depression in patients with FD that may be associated with pain 20, 21. It is of note that pain in FD may also manifest as gastrointestinal pain and other gastrointestinal symptoms 22. All of these indicate the variety of pain manifestations associated with FD.
Pain Mechanisms in Fabry Disease
The different types of pain reported by patients with FD may be sustained by diverse mechanisms, and their characterization is highly important for appropriate, individually targeted symptomatic pain treatment. Since describing all potential pathophysiological mechanisms of pain in patients with FD is beyond the scope of this review, focus is on the most relevant mechanisms with supporting evidence.
Pain in FD is assumed to be predominantly neuropathic, because of its frequently reported neuropathic characteristics and reports of GL‐3 deposits in the dorsal root ganglion neurons of patients with FD (Figure 2) which may cause the frequently experienced “shooting pains” 23, 24. Pain in FD is known as small fiber neuropathy as patients have a predominantly length‐dependent reduction in the density of small, thinly myelinated Aδ nerve fibers (mediating pricking pain, cold perception) and unmyelinated C nerve fibers (mediating “slow” pain, warmth, and heat perception) 25, 26. The functional impairment of small fiber conduction is particularly associated with increased heat and cold pain perception, suggesting damage to Aδ and C nerve fibers 25, 27. Pain in FD also presents with components that are typical of nociceptive or inflammatory pain, as evidenced by the efficacy of nonsteroidal anti‐inflammatory drugs (NSAIDs) in many patients 16. Recent experimental studies have suggested that some of the spontaneous types of pain in patients with FD may be explained by hyperexcitability of peripheral nociceptive neurons mediated by upregulation of sodium ion channels (Nav1.8) 28, transient receptor potential cation channel subfamily V member 1 (TRPV1) 28, or a lyso‐GL‐3‐dependent increase in calcium (Ca2+) influx 29.
Figure 2.
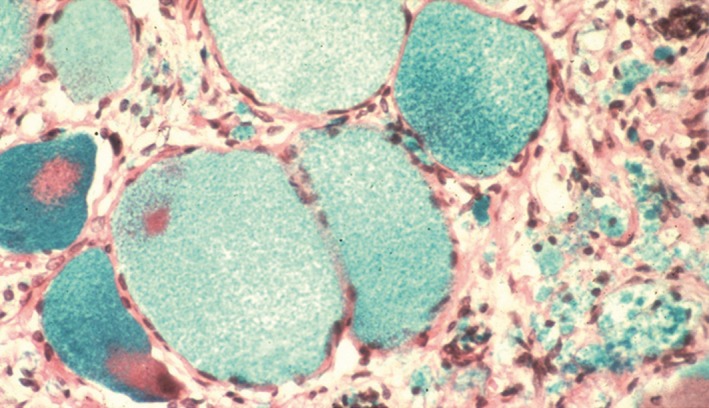
Dorsal root ganglion cells from a Fabry patient. The ganglion cells are swollen because of glycolipid accumulation. Image from Burlina et al. 17. Photo courtesy of E. Kaye.
Other mechanisms that may be responsible for pain in patients with FD include spontaneous ectopic firing, altered pain modulation, or central nervous system sensitization 17. Repeated bouts of peripheral neuropathic pain in epigenetically vulnerable individuals (i.e., those with genetic risk and multiple stressors) may sensitize the central neural pain matrix, such that all pain in the body becomes amplified. As there is overlap between central neural pain perception and neural connectivity related to memory, fatigue, sleep, and mood, it may be that central sensitization of pain may also contribute to fatigue, sleep disorders, and other problems in vulnerable patients with FD, as suggested for fibromyalgia 30.
Diagnosis of Pain in Fabry Disease
Differential Diagnosis
Fabry patients who present with chronic pain may receive incorrect diagnoses, due to the unspecific nature of pain in FD 1. These diagnoses represent primary and secondary causes of small fiber neuropathy 17, and can include diabetic polyneuropathy, postherpetic neuralgia, autoimmune neuropathy, fibromyalgia syndrome, rheumatism, amyloidosis, growing pains (essentially in preschool pediatric patients), radiculopathy, trauma, postoperative pain, complex regional pain syndrome, erythromelalgia, paroxysmal extreme pain disorder, idiopathic small fiber neuropathy, and celiac disease. Consequently, there may be more than a 10‐year delay in the diagnosis of FD 8.
Diagnostic Methods
Diagnosing “undiagnosed” patients
Diagnosing FD‐associated neuropathy requires careful assessment starting with, and based upon, a detailed medical history, a thorough physical examination, and comprehensive pain assessment. A detailed medical history is of paramount importance and should include a family history, the temporal course of pain, details of any autonomic symptoms, and any previous or current medication. Typical questions should include the following:
-
Does the patient have pain crises?
Although not common to all patients, pain crises are a diagnostic red flag that should raise suspicion of FD or other causes of small fiber neuropathy. As mentioned earlier, pain crises are characterized by excruciating, unbearable pain, often beginning in the distal part of the extremities and radiating proximally and to the rest of the body. They can last hours or days, and persist despite the elimination of the trigger (e.g., fever). Treatment of pain crises is very difficult.
Does the patient have, or recall having, any “burning” pain in hands or feet?
Is there any deterioration or spreading of the pain distribution with heat or cold exposure, physical effort (such as sports), stress, or fever?
Has the pain ever prevented, or does the pain currently prevent, the patient from participating in sports?
Does the patient sweat less than others or not at all during physical effort, or in a warm/hot environment (e.g., on a hot summer day)?
Are there any family members who have had or currently have similar complaints?
In addition to the most common differential diagnoses described above, the patient's medical history may suggest other possible causes of pain that should be considered.
If FD is suspected, the diagnosis must be confirmed by α‐Gal enzyme activity and GLA mutation analysis 31. This is particularly important in women, who often have normal levels of α‐Gal due to the heterozygous nature of their condition 5. Standardized and validated pain questionnaires, complemented with sensory testing, should then be used to characterize and evaluate the pain. This allows the longitudinal follow‐up of pain severity and its impact on daily life, and provides the opportunity to assess the effect of ERT and supportive pain‐management strategies.
Pain assessment tools
In recent years, FD‐specific pain questionnaires have been published for children and adults (Table 1). Pain during childhood can now be captured as part of the Fabry‐specific Pediatric Health and Pain Questionnaire 32 as well as with the well‐established general pain scales such as the Brief Pain Inventory (BPI), Short‐Form (SF) McGill Pain Questionnaire, or as part of the SF‐36 health‐related quality of life assessment. The first face‐to‐face questionnaire for adult patients with FD was published recently and a self‐administered version is now available 33, 34. The Würzburg Fabry Pain Questionnaire comprises 22 open questions assessing the four main pain phenotypes in childhood and adolescence, pain history prior to and during ERT, pain characters, pain triggers, and impairments of daily life due to pain.
Table 1.
Recommended approach to the assessment of the peripheral nervous system in the evaluation of patients with Fabry disease
| Neurologist evaluation | Specialist assessment | |
|---|---|---|
| General evaluations and pain | Medical history: especially family history | Würzburg Fabry Pain Questionnaire (adults) |
| Physical examination: including height, weight, heart rate, standing and supine blood pressure, and skin examination for angiokeratoma | Total Symptom Score | |
| Pain evaluation: temporal course of pain, localization of pain, characteristics of pain, and quantification of pain using general pain assessment scales (e.g., Brief Pain Inventory, Short‐Form McGill Pain Questionnaire, and Fabry‐specific [FabryScan]) | Fabry‐specific Pediatric Health and Pain Questionnaire (children/adolescents) | |
| Somatosensory evaluation | Thermal perception tests (thermal disks; hot‐, warm‐, cold‐water tubes) | Quantitative sensory testing, nociceptive evoked potentials, analysis of skin biopsies (intra‐epidermal nerve fiber density) |
| Cold perception tests (ice‐bucket test, cold‐water tubes) | ||
| Pinprick pain perception test | ||
| Touch perception (skin‐brushing test) | ||
| Vibration perception (e.g., 8/8 scaled vibrating 128 Hz Rydel‐Seiffer tuning fork held to medial malleolus as used in FabryScan) |
In addition to FD‐specific pain questionnaires, there are several other valuable pain assessment tools that can be used to evaluate different aspects of pain in FD. The most notable are the Neuropathic Pain Symptom Inventory (NPSI) 35, the Neuropathic Pain Questionnaire (NPQ) 36, the DN4 questionnaire 37, the painDETECT questionnaire 38, the LANSS Pain Scale 39, and the ID Pain questionnaire 40. The Total Symptom Score is a more general tool originally developed to assess neuropathic pain in patients with diabetes; it can provide a rapid assessment of pain type, severity, and frequency 27, 41.
FabryScan is a 15‐item questionnaire with three bedside sensory function tests that assesses small and large fiber function. It helps distinguish pain in FD from other forms of chronic neuropathic pain 42, and identify FD patients with chronic extremity pain.
Sensory testing
In addition to pain questionnaires, tests for thermal and pain perception deficits and touch and vibration perception deficits are important to determine the extent of peripheral nerve fiber involvement in patients with FD. Specialized centers are able to perform distinct tests, such as quantitative sensory testing (QST) 43 for the functional investigation of small nerve fibers and skin punch biopsies to quantify intra‐epidermal nerve fiber density (Table 1) 44.
If these tests are unavailable, there is a range of “bedside” sensory tests that can be carried out by general neurologists to characterize pain and sensory deficits in patients with FD (Table 1).
Small nerve fibers (Aδ and C) require special attention and need specific tests to determine their function and morphology. These include assessment of cold perception (Aδ function); warmth, heat pain, and pain perception (unmyelinated C fibers); and response to sharp pinprick (Aδ function). Thermal perception can be evaluated by: (a) assessing the ability to discriminate the temperature of glass tubes filled with warm or cold water; (b) devices with surfaces such as polyvinyl or metal disks which mediate warm or cold temperature sensation, respectively, when placed on the patient's hand or foot 45; or (c) using the so‐called “ice‐bucket” test. The ice‐bucket thermal challenge test can be particularly useful in centers with limited resources. It involves the patient plunging a leg or arm into iced water (up to mid‐thigh or elbow) for up to 30 seconds. Normal subjects would tolerate the cold challenge, but a patient with FD is likely to experience intense burning pain within 15–20 seconds 46.
Large nerve fiber abnormalities (particularly in Aβ fibers that mediate vibration perception and touch) usually present in more advanced stages of the disease, require evaluation as part of the neurological examination of patients with FD, and can be tested with light‐touch tests and vibration perception (Aβ function) 47, 48. Light‐touch tests assess proximal versus distal sensation loss and can be performed using a cotton swab (skin brushing). Vibration sensitivity should be evaluated against normative values for vibratory perception using a 128 Hz‐scaled Rydel‐Seiffer vibrating tuning fork 49. Pain perception can be tested by evaluating a patient's pinprick sensation, exerting just enough pressure with the point of a sharp pin to dent the skin. If the patient is unable to distinguish between the sharp pinprick and blunt pressure, small fiber neuropathy is suggested. Carpal tunnel syndrome can be investigated with nerve conduction studies 19.
Overall, there is no evidence for one test being better than another. This is partly because some assessment tools, such as the FabryScan and the Würzburg Fabry Pain Questionnaire, have been introduced only quite recently. These tools have been used to show changes in pain perception, and future clinical use of these tools will provide evidence of their value. Furthermore, some tests are available only in specific units devoted to the study of peripheral nerve pathology (e.g., QST, nociceptive evoked potentials, and skin biopsy). A recommended screening strategy should include the BPI, FabryScan, ice‐bucket test, and all the somatosensory evaluation tests listed in Table 1. Indeed, these tests are readily available and are usually performed by the neurologist; however, the specialist assessments may not always be available. QST and skin biopsies are the most widely used small nerve fiber assessment tools and are recommended to be performed, if available, together with the assessment of nociceptive evoked potentials.
Treatment of Pain in Fabry Disease
Pain, an early sign of peripheral nervous system involvement in FD, indicates nervous system damage 50 and, therefore, requires effective treatment. Treatment of pain in FD has two aims, each of which requires a different therapeutic approach: slowing progression of Fabry pathology and achieving pain control. ERT has been shown to slow disease progression and reduce renal or cardiac complications. Pain management may be achieved through disease‐specific treatment with ERT, supportive symptomatic treatment with analgesic drugs, and strategies to avoid the abovementioned pain triggers. In addition, nonpharmacologic approaches including psychological and physical treatments have been proven effective in the relief of pain and the treatment of comorbid disorders including anxiety and depression 51. Among the psychological treatments, cognitive‐behavioral therapies are most commonly implemented to treat chronic pain 51.
Enzyme Replacement Therapy
The underlying deficiency in α‐Gal can be treated with ERT: either agalsidase beta 1.0 mg/kg every 2 weeks (Fabrazyme; Sanofi Genzyme) or agalsidase alfa 0.2 mg/kg every 2 weeks (Replagal; Shire Human Genetic Therapies Inc.). Currently, both ERTs are available in the European Union and other countries worldwide, but only agalsidase beta is licensed in the USA. In 2014, a biosimilar agalsidase beta (Fabagal; ISU Abxis, ISU Global) was approved in South Korea.
Clinical evidence indicates that early initiation of ERT is associated with better clinical outcomes 52, reducing or delaying the development of irreversible damage to the kidneys and heart 53, 54. Considering that damage to small nerve fibers occurs early, prompt treatment is important in order to limit damage to the peripheral nervous system 26. As noted above, increased lyso‐GL‐3 levels are directly associated with pain in FD, hence timely initiation of ERT at adequate doses is a key component of pain‐reduction strategy 29. Clinical studies have shown that ERT may improve overall pain scores and pain intensity in patients with FD 11, 27, 55, 56; improvements in pain outcomes have been sustained during long‐term follow‐up, allowing many patients to reduce their use of pain medication 57, 58. Long‐term studies in children have also reported that ERT can reduce Fabry‐associated neuropathic and gastrointestinal pain 59, 60. In another pediatric study, FD patients with morphological evidence of early kidney damage also presented with high pain scores at baseline that improved during treatment with agalsidase beta 53. In terms of small nerve fiber sensory function, a study of 22 patients with FD revealed improved thermal perception and vibration detection thresholds with agalsidase beta 27. Although ERT reduces pain outcomes in a variety of Fabry‐related pain conditions, pain does not always completely resolve 55, 56, 57, 58, 59, 60, 61, 62, explaining the need for adjunctive medications.
The licensed doses are 1.0 mg/kg every 2 weeks for agalsidase beta and 0.2 mg/kg every 2 weeks for agalsidase alfa (5‐fold difference). These two ERT agents have not been directly compared in terms of pain outcomes. Data from the Canadian Fabry Disease Initiative, in which agalsidase alfa and agalsidase beta were used head‐to‐head, have shown no difference in clinical endpoints between patients treated with one or the other ERT. However, pain outcome data were not reported; the only data referring to peripheral nerve involvement present in the analysis were those related to hearing loss outcome; patients were not randomized for variables known to affect prognosis, leading to an uneven patient distribution; and the study was underpowered due to limited event rates and measures taken to account for temporary worldwide shortage of agalsidase beta 63. There is some indirect evidence from dose‐switching studies that suggests that ERT dose may be of relevance for pain outcomes, at least in the short term. At 1‐year follow‐up, there was improvement or stabilization of pain symptoms in patients who continued treatment with agalsidase beta, but an increase in pain when patients receiving agalsidase beta were transferred to treatment with agalsidase alfa 61. In a different study, patients who switched from agalsidase beta 1.0 mg/kg to a lower dose or to agalsidase alfa 0.2 mg/kg reported an increase in pain attacks, chronic pain, gastrointestinal pain, and diarrhea compared with those who continued receiving agalsidase beta 1.0 mg/kg after 1 year 62. At the 2‐year follow‐up of the same study, there was no significant difference in the frequency of pain attacks or permanent pain in patients who had switched or reduced their dose; however, the frequency of gastrointestinal pain increased, kidney function deteriorated, and overall disease severity increased 64. Considering the potential link between lyso‐GL‐3 and pain 29, lowering lyso‐GL‐3 levels, using early and adequately dosed ERT, may help decrease pain severity.
Supportive/Symptomatic Pain Control
Pain management strategy
As a consequence of the heterogeneous nature of pain in FD, patients require individual pain‐management strategies alongside ERT. Advice on lifestyle modifications, such as the use of air conditioning to avoid overheating, taking off shoes and socks during pain attacks, rapid treatment of fever or infections, and maintaining good levels of hydration, may help to avoid pain triggers and should be included in the patient's pain‐management plan as appropriate. It is important to consider the underlying mechanism of pain for the patient's symptoms and, where possible, use a targeted approach tailored to the type of pain involved. Importantly, patients should be counseled that it is not always possible to eliminate pain, but rather that the treatment goal should be to make pain manageable. In this context a pain diary may be useful, particularly during changes in pain medication, in order to document pain development as well as the level of pain control achieved. With good pain management, functional improvement may precede a decline in pain perception; therefore, changes in function should also be a guide regarding efficacy of ERT.
To reduce the likelihood of side effects from polypharmacy, the dosage of each drug prescribed should be titrated to the highest tolerated dose providing significant pain control (with appropriate consideration of any possible cardiac and renal contraindications) before other pain‐modulating agents are added. Some patients, particularly the elderly, can present with other comorbidities such as osteopenia or osteoporosis 65, which also cause pain, or with conditions that are contraindications for certain adjunctive anti‐pain medications. For example, tricyclic antidepressants can increase the risk of cardiac arrhythmia and, therefore, should be avoided in elderly patients.
Neuropathic pain control agents
A recent survey found that frequently used acute pain medications among patients with FD include the NSAIDs and non‐opioid analgesics, with few patients using neuropathic pain‐control agents 16. This could be because there are limited comparative clinical data regarding the use of neuropathic pain‐control agents in patients with FD as a specific group. Nevertheless, case reports of small numbers of patients with FD have shown that pain can be reduced with the administration of carbamazepine, gabapentin, phenytoin, and intravenous lidocaine 66, 67, 68, 69 (Tables 2 and 3).
Table 2.
Recommended analgesic drugs for supportive treatment of chronic neuropathic pain in Fabry disease
| Agent | Dose | Expert panel comment | Cardiac restrictions | Renal restrictions | Clinical evidence |
|---|---|---|---|---|---|
| Carbamazepine | 250–800 mg/day | Good clinical experience | May interfere with activity of other drugs, e.g., warfarin | None | Filling‐Katz et al. 66 |
| Gabapentin | Slowly titrated from 100 mg/day to max. 2400 mg/day | Good clinical experience | None | Yes (with precaution in cases of renal insufficiency) | Ries et al. 67 |
| Phenytoin | 300 mg/day | Good clinical experience | None | None | Lockman et al. 68 |
| Pregabalin | 75–300 mg/day | None | Yes (with precaution in cases of renal insufficiency) | Expert panel clinical experience | |
| Tricyclic antidepressants | 12.5–150 mg | Avoid due to risk of arrhythmias | Unknown | Expert panel clinical experience | |
| Serotonin‐norepinephrine reuptake inhibitors | Sommer et al. 73; Finnerup et al. 70 | ||||
| Duloxetine | 60–120 mg/day | None | None | ||
| Venlafaxine | 150–225 mg ER/day | QT‐interval prolongation | Dose adjustment according to renal function |
Relevant studies are listed where available; however, as limited clinical evidence has been published, recommendations are based on the clinical experience of the authors. Table has been adapted from Sommer et al. 73.
ER, extended release.
Table 3.
Recommended analgesic drugs for supportive treatment of acute pain in Fabry disease
| Agent | Dose | Expert panel comment | Cardiac restrictions | Renal restrictions | Clinical evidence |
|---|---|---|---|---|---|
| Intravenous lidocaine | 2–5 mg/kg | Good clinical response | None | None | Politei 69 |
| Tramadol | 100–400 mg/day | Caution with concomitant use of SSRIs, SNRIs, or TCAs | None | Caution in patients with renal insufficiency and epilepsy | O'Connor & Dworkin 74 |
| Morphine | Titration of 30–120 mg every 12 h | Monitor for addiction; constipation requires concurrent bowel regimen control | None | None | Gordon et al. 75 |
| Oxycodone | Titration of 20–60 mg every 12 h | Monitor for addiction; constipation requires concurrent bowel regimen control | None | None | |
| Ibuprofen | 400–2400 mg/day | Use lowest effective dose to reduce risk of gastrointestinal bleeding | None | None | |
| Diclofenac | 50–150 mg/day | Use lowest effective dose to reduce risk of gastrointestinal bleeding | None | Caution in patients with renal insufficiency |
Relevant studies are listed where available; however, as limited clinical evidence has been published, recommendations are based on the clinical experience of the authors. Table has been adapted from Sommer et al. 73.
SNRIs, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants.
Current pain‐management strategies are founded on clinical experience or follow national and international guidelines for the management of neuropathic pain 51, 70, 71. Based on a systematic literature review and meta‐analysis of pharmacotherapy for the management of neuropathic pain in adults, tricyclic antidepressants, serotonin and norepinephrine reuptake inhibitors (e.g., duloxetine, venlafaxine), carbamazepine, gabapentin, and the gabapentinoid pregabalin should be considered as first‐line options; lidocaine patches, topical capsaicin (8%) patches, and tramadol should be considered as second‐line options; strong opioids should be considered as third‐line options 70. Controlled‐release opioids are preferred over short‐acting opioids. Cannabinoids are also now recommended as third‐line treatments. Fourth‐line treatments may include methadone (with both N‐methyl‐d‐aspartate and opioid receptor effects), anticonvulsants with lesser evidence of efficacy (e.g., lamotrigine, lacosamide), tapentadol, and botulinum toxin 72.
Evidence regarding the use of neuropathic pain control agents specifically in patients with FD has been summarized in Table 2 (chronic pain) 66, 67, 68, 70, 73 and Table 3 (acute pain) 69, 73, 74, 75. Lidocaine and capsaicin plasters may be useful to control localized pain as well as during pain crises. However, the mostly episodic nature of pain in FD reduces the appropriateness of these drugs. Furthermore, in FD, the area affected by pain crises is too large for plasters. Based on our clinical experience, lidocaine or capsaicin cream may be used as acute‐pain prophylaxis before physical activity. There is some evidence to suggest that phenytoin may be useful during pain attacks 68. More recently, intravenous lidocaine has been used to successfully treat FD pain crises 69. Opioid agonists may also be effective during pain crises, but should be used only when anticonvulsant agents are ineffective in order to avoid worsening of gut motility problems 76 and reduce the risk of dependency 17. Ketamine has been used to effectively treat pain with a neuropathic component; however, because of its adverse events and potential addiction risks, its use should be restricted to therapy‐resistant severe neuropathic pain until definite proof that its benefits outweigh the risks and costs of treatment 77. Finally, although the potential of alpha‐2 adrenoceptor agonists for the management of chronic neuropathic pain has also been explored, further clinical trials in this area are probably required 78. Because of the distinct phenotype and comorbidities in FD patients and since evidence for the use of analgesic treatment in FD is scarce, individual recommendations will be necessary in most cases.
Clinical Monitoring of Pain in Fabry Disease
Due to the progressive nature of FD pathology, regular monitoring of peripheral nervous system symptoms and autonomic function is required throughout treatment. For peripheral nervous system symptoms, a comprehensive neurologic assessment should be conducted at diagnosis, at ERT initiation (if not at the same time as diagnosis), and prior to the start of supportive analgesic treatment. Follow‐up assessments with the patient's primary care physician or neurologist should be carried out regularly to review doses of analgesics and the level of pain control. Analgesic drugs only provide symptomatic treatment of pain. Therefore, it is also important to ensure that the patients, caregivers, and the multidisciplinary physician team appreciate that disease‐specific therapy with ERT is necessary to control the progression of the underlying FD pathology.
Conclusion
Pain occurs frequently in the majority of male and female patients with FD. In fact, pain should be considered a very early red flag, useful to confirm a diagnosis of FD. When treatment is indicated before renal function decline, ERT may improve small nerve fiber function and help decrease pain; there is some evidence suggesting that ERT dose is relevant. Adjunctive pain management, using a combination of analgesics, is targeted at the underlying pain mechanisms for chronic neuropathic and acute nociceptive, and inflammatory or mixed pain. In conclusion, early initiation of ERT and good supportive pain management using adjunctive therapies may improve the patient's quality of life as well as reduce or delay the likelihood of life‐threatening renal and cardiac complications 52.
Disclosure
Juan M. Politei, M.D., has received honoraria for lectures on Fabry disease from Genzyme, Shire HGT, Amicus Therapeutics, and Protalix Corp. Didier Bouhassira, M.D., Ph.D., has received honorarium from Genzyme for participation in an advisory board. Dominique P. Germain, M.D., Ph.D., has received travel grants and honoraria for lectures on Fabry disease from Genzyme, Shire, and Amicus Therapeutics. Cyril Goizet, M.D., Ph.D., has received honoraria for participation in an advisory board on Fabry disease from Genzyme, for lectures on Fabry disease from Genzyme and Shire HGT, financial support for research activities on Fabry disease from Genzyme and Shire HGT, and travel grants from Genzyme and Shire HGT. Antonio Guerrero‐Sola, M.D., has received travel grants and honoraria for lectures on Fabry disease from Genzyme. Max J. Hilz, M.D., has received travel grants and honoraria for lectures on Fabry disease from Genzyme, and research support from Novartis Germany (not related to Fabry disease) and Bayer Health Care, Germany (not related to Fabry disease). Elspeth J. Hutton, M.D., has received travel grants and honoraria for lectures on Fabry disease from Genzyme. Amel Karaa, M.D., has received honoraria from Genzyme for consulting services and lectures, and grant support for research. She is also on the scientific advisory board of Stealth Biotherapeutics. Rocco Liguori, M.D., has received honorarium from Genzyme (participation in an advisory board), and from HPS Health Publishing and Services, Biomarin Europe Ltd, and Ad Hoc Eventi (not specifically related to Fabry disease). Nurcan Üçeyler, M.D., has received travel grants and honoraria for lectures on Fabry disease from Genzyme and Shire HGT. Lonnie K. Zeltzer, M.D., has received honoraria for lectures on pain in Fabry disease from Genzyme. Alessandro Burlina, M.D., Ph.D., is a member of the European Advisory Board of the Fabry Registry, which is sponsored by Genzyme. He has received travel grants and honoraria for lectures on Fabry disease from Genzyme.
Acknowledgment
The Expert Panel on Peripheral Neuropathy in Fabry disease (Rome, Italy, March 2014) was sponsored by Genzyme, a Sanofi company. The authors received editorial/writing support in the preparation of this manuscript from Alessia Piazza, PhD, of Excerpta Medica, funded by Genzyme. The authors were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
References
- 1. Germain DP. Fabry disease. Orphanet J Rare Dis 2010;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aerts JM, Groener JE, Kuiper S, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A 2008;105:2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thurberg BL, Politei JM. Histologic abnormalities of placental tissues in Fabry disease: A case report and review of the literature. Hum Pathol 2012;43:610–614. [DOI] [PubMed] [Google Scholar]
- 4. Germain DP. A new phenotype of Fabry disease with intermediate severity between the classical form and the cardiac variant. Contrib Nephrol 2001;136:234–240. [DOI] [PubMed] [Google Scholar]
- 5. Echevarria L, Benistan K, Toussaint A, et al. X‐chromosome inactivation in female patients with Fabry disease. Clin Genet 2015;89:44–54. [DOI] [PubMed] [Google Scholar]
- 6. Ries M, Ramaswami U, Parini R, et al. The early clinical phenotype of Fabry disease: A study on 35 European children and adolescents. Eur J Pediatr 2003;162:767–772. [DOI] [PubMed] [Google Scholar]
- 7. Hopkin RJ, Bissler J, Banikazemi M, et al. Characterization of Fabry disease in 352 pediatric patients in the Fabry Registry. Pediatr Res 2008;64:550–555. [DOI] [PubMed] [Google Scholar]
- 8. Wilcox WR, Oliviera JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: Lessons from the Fabry Registry. Mol Genet Metab 2008;93:112–128. [DOI] [PubMed] [Google Scholar]
- 9. Laney DA, Peck DS, Atherton AM, et al. Fabry disease in infancy and early childhood: A systematic literature review. Genet Med 2015;17:323–330. [DOI] [PubMed] [Google Scholar]
- 10. Hoffman B, Beck M, Sunder‐Plassmann G, et al. Nature and prevalence of pain in Fabry disease and its response to enzyme replacement therapy–a retrospective analysis from the Fabry Outcome Survey. Clin J Pain 2007;23:535–542. [DOI] [PubMed] [Google Scholar]
- 11. Watt T, Burlina AP, Cazzorla C, et al. Agalsidase beta treatment is associated with improved quality of life in patients with Fabry disease: Findings from the Fabry Registry. Genet Med 2010;12:703–712. [DOI] [PubMed] [Google Scholar]
- 12. Biegstraaten M, Hollak CE, Bakkers M, Faber CG, Aerts JM, van Schaik IN. Small fiber neuropathy in Fabry disease. Mol Genet Metab 2012;106:135–141. [DOI] [PubMed] [Google Scholar]
- 13. Eng CM, Germain DP, Banikazemi M, et al. Fabry disease: Guidelines for the evaluation and management of multi‐organ system involvement. Genet Med 2006;8:539–548. [DOI] [PubMed] [Google Scholar]
- 14. Eng CM, Fletcher J, Wilcox WR, et al. Fabry disease: Baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis 2007;30:184–192. [DOI] [PubMed] [Google Scholar]
- 15. Ramaswami U, Whybra C, Parini R, et al. Clinical manifestations of Fabry disease in children: Data from the Fabry Outcome Survey. Acta Paediatr 2006;95:86–92. [DOI] [PubMed] [Google Scholar]
- 16. Üçeyler N, Ganendiran S, Kramer D, Sommer C. Characterization of pain in Fabry disease. Clin J Pain 2014;30:915–920. [DOI] [PubMed] [Google Scholar]
- 17. Burlina AP, Sims KB, Politei JM, et al. Early diagnosis of peripheral nervous system involvement in Fabry disease and treatment of neuropathic pain: The report of an expert panel. BMC Neurol 2011;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laaksonen SM, Röyttä M, Jääskeläinen SK, Kantola I, Penttinen M, Falck B. Neuropathic symptoms and findings in women with Fabry disease. Clin Neurophysiol 2008;119:1365–1372. [DOI] [PubMed] [Google Scholar]
- 19. Ghali J, Murugasu A, Day T, Nicholls K. Carpal tunnel syndrome in Fabry disease. JIMD Rep 2012;2:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolsover FE, Murphy E, Cipolotti L, Werring DJ, Lachmann RH. Cognitive dysfunction and depression in Fabry disease: A systematic review. J Inherit Metab Dis 2014;37:177–187. [DOI] [PubMed] [Google Scholar]
- 21. Cole AL, Lee PJ, Hughes DA, Deegan PB, Waldek S, Lachmann RH. Depression in adults with Fabry disease: A common and under‐diagnosed problem. J Inherit Metab Dis 2007;30:943–951. [DOI] [PubMed] [Google Scholar]
- 22. Hoffmann B, Schwarz M, Mehta A, Keshav S. Gastrointestinal symptoms in 342 patients with Fabry disease: Prevalence and response to enzyme replacement therapy. Clin Gastroenterol Hepatol 2007;5:1447–1453. [DOI] [PubMed] [Google Scholar]
- 23. Kahn P. Anderson‐Fabry disease: A histopathological study of three cases with observations on the mechanism of production of pain. J Neurol Neurosurg Psychiatry 1973;36:1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gadoth N, Sandbank U. Involvement of dorsal root ganglia in Fabry's disease. J Med Genet 1983;20:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Üçeyler N, Kahn AK, Kramer D, et al. Impaired small fiber conduction in patients with Fabry disease: A neurophysiological case‐control study. BMC Neurol 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liguori R, Di Stasi V, Bugiardini E, et al. Small fiber neuropathy in female patients with Fabry disease. Muscle Nerve 2010;41:409–412. [DOI] [PubMed] [Google Scholar]
- 27. Hilz MJ, Brys M, Marthol H, Stemper B, Dütsch M. Enzyme replacement therapy improves function of C‐, Adelta‐, and Abeta‐nerve fibers in Fabry neuropathy. Neurology 2004;62:1066–1072. [DOI] [PubMed] [Google Scholar]
- 28. Lakomá J, Rimondini R, Donadio V, Liguori R, Caprini M. Pain related channels are differentially expressed in neuronal and non‐neuronal cells of glabrous skin of Fabry knockout male mice. PLoS One 2014;9:e108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi L, Vernon J, Kopach O, et al. The Fabry disease‐associated lipid Lyso‐Gb3 enhances voltage‐gated calcium currents in sensory neurons and causes pain. Neurosci Lett 2015;594:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clauw DJ. Fibromyalgia and related conditions. Mayo Clin Proc 2015;90:680–692. [DOI] [PubMed] [Google Scholar]
- 31. Germain DP, Poenaru L. Fabry disease: Identification of novel alpha‐galactosidase A mutations and molecular carrier detection by use of fluorescent chemical cleavage of mismatches. Biochem Biophys Res Commun 1999;257:708–713. [DOI] [PubMed] [Google Scholar]
- 32. Ramaswami U, Stull DE, Parini R, et al. Measuring patient experiences in Fabry disease: Validation of the Fabry‐specific Pediatric Health and Pain Questionnaire (FPHPQ). Health Qual Life Outcomes 2012;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Üçeyler N, Magg P, Thomas S, Wiedmann S, Heuschmann P, Sommer C. A comprehensive Fabry‐related pain questionnaire for adult patients. Pain 2014;155:2301–2305. [DOI] [PubMed] [Google Scholar]
- 34. Magg B, Riegler C, Wiedmann S, Heuschmann P, Sommer C, Üçeyler N. Self‐administered version of the Fabry‐associated pain questionnaire for adult patients. Orphanet J Rare Dis 2015;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004;108:248–257. [DOI] [PubMed] [Google Scholar]
- 36. Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain 2003;19:306–314. [DOI] [PubMed] [Google Scholar]
- 37. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- 38. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–1920. [DOI] [PubMed] [Google Scholar]
- 39. Bennett M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain 2001;92:147–157. [DOI] [PubMed] [Google Scholar]
- 40. Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin 2006;22:1555–1565. [DOI] [PubMed] [Google Scholar]
- 41. Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti‐oxidant alpha‐lipoic acid. A 3‐week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995;38:1425–1433. [DOI] [PubMed] [Google Scholar]
- 42. Arning K, Naleschinski D, Maag R, et al. FabryScan: A screening tool for early detection of Fabry disease. J Neurol 2012;259:2393–2400. [DOI] [PubMed] [Google Scholar]
- 43. Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur J Pain 2006;10:77–88. [DOI] [PubMed] [Google Scholar]
- 44. Lauria G, Cazzato D, Porretta‐Serapiglia C, et al. Morphometry of dermal nerve fibers in human skin. Neurology 2011;77:242–249. [DOI] [PubMed] [Google Scholar]
- 45. Dyck PJ, Curtis DJ, Bushek W, Offord K. Description of “Minnesota Thermal Disks” and normal values of cutaneous thermal discrimination in man. Neurology 1974;24:325–330. [DOI] [PubMed] [Google Scholar]
- 46. Hilz MJ, Stemper B, Kolodny EH. Lower limb cold exposure induces pain and prolonged small fiber dysfunction in Fabry patients. Pain 2000;84:361–365. [DOI] [PubMed] [Google Scholar]
- 47. Dütsch M, Marthol H, Stemper B, Brys M, Haendl T, Hilz MJ. Small fiber dysfunction predominates in Fabry neuropathy. J Clin Neurophysiol 2002;19:575–586. [DOI] [PubMed] [Google Scholar]
- 48. Hilz MJ. Evaluation of peripheral and autonomic nerve function in Fabry disease. Acta Paediatr Suppl 2002;91:38–42. [DOI] [PubMed] [Google Scholar]
- 49. Hilz MJ, Axelrod FB, Hermann K, Haertl U, Duetsch M, Neundörfer B. Normative values of vibratory perception in 530 children, juveniles and adults aged 3‐79 years. J Neurol Sci 1998;159:219–225. [DOI] [PubMed] [Google Scholar]
- 50. Schiffmann R, Hauer P, Freeman B, et al. Enzyme replacement therapy and intraepidermal innervation density in Fabry disease. Muscle Nerve 2006;34:53–56. [DOI] [PubMed] [Google Scholar]
- 51. Zeltzer LK, Palermo T, Krane E. Pain management In: Kliegman R, Stanton B, St. Geme J, Schor N, editors. Nelson Textbook of Pediatrics, 20th edn, Philadelphia: Elsevier, Inc., 2015;Vol. 1, Ch. 62: 430–446. [Google Scholar]
- 52. Germain DP, Charrow J, Desnick RJ, et al. Ten‐year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet 2015;52:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tøndel C, Bostad L, Larsen KK, et al. Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol 2013;24:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Germain DP, Weidemann F, Abiose A, et al. Analysis of left ventricular mass in untreated men and in men treated with agalsidase‐β: Data from the Fabry Registry. Genet Med 2013;15:958–965. [DOI] [PubMed] [Google Scholar]
- 55. Eng CM, Banikazemi M, Gordon RE, et al. A phase 1/2 clinical trial of enzyme replacement in Fabry disease: Pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet 2001;68:711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schiffmann R, Kopp JB, Austin HA 3rd, et al. Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 2001;285:2743–2749. [DOI] [PubMed] [Google Scholar]
- 57. Wilcox WR, Banikazemi M, Guffon N, et al. Long‐term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet 2004;75:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schiffmann R, Floeter MK, Dambrosia JM, et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve 2003;28:703–710. [DOI] [PubMed] [Google Scholar]
- 59. Schiffmann R, Martin RA, Reimschisel T, et al. Four‐year prospective clinical trial of agalsidase alfa in children with Fabry disease. J Pediatr 2010;156:832–837. [DOI] [PubMed] [Google Scholar]
- 60. Borgwardt L, Feldt‐Rasmussen U, Rasmussen AK, Ballegaard M, Meldgaard Lund A. Fabry disease in children: Agalsidase‐beta enzyme replacement therapy. Clin Genet 2013;83:432–438. [DOI] [PubMed] [Google Scholar]
- 61. Politei J, Schenone AB, Cabrera G, Heguilen R, Szlago M. Fabry disease and enzyme replacement therapy in classic patients with the same mutation: Different formulations ‐ different outcome? Clin Genet 2016;89:88–92. [DOI] [PubMed] [Google Scholar]
- 62. Weidemann F, Krämer J, Duning T, et al. Patients with Fabry disease after enzyme replacement therapy dose reduction versus treatment switch. J Am Soc Nephrol 2014;25:837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sirrs SM, Bichet DG, Casey R, et al. Outcomes of patients treated through the Canadian Fabry disease initiative. Mol Genet Metab 2014;111:499–506. [DOI] [PubMed] [Google Scholar]
- 64. Lenders M, Canaan‐Kühl S, Krämer J, et al. Patients with Fabry disease after enzyme replacement therapy dose reduction and switch‐2‐year follow‐up. J Am Soc Nephrol 2016;27:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Germain DP, Benistan K, Boutouyrie P, Mutschler C. Osteopenia and osteoporosis: Previously unrecognized manifestations of Fabry disease. Clin Genet 2005;68:93–95. [DOI] [PubMed] [Google Scholar]
- 66. Filling‐Katz MR, Merrick HF, Fink JK, Miles RB, Sokol J, Barton NW. Carbamazepine in Fabry's disease: Effective analgesia with dose‐dependent exacerbation of autonomic dysfunction. Neurology 1989;39:598–600. [DOI] [PubMed] [Google Scholar]
- 67. Ries M, Mengel E, Kutschke G, et al. Use of gabapentin to reduce chronic neuropathic pain in Fabry disease. J Inherit Metab Dis 2003;26:413–414. [DOI] [PubMed] [Google Scholar]
- 68. Lockman LA, Hunninghake DB, Krivit W, Desnick RJ. Relief of pain of Fabry's disease by diphenylhydantoin. Neurology 1973;23:871–875. [DOI] [PubMed] [Google Scholar]
- 69. Politei JM. [Intravenous lidocaine as treatment for the painful episodes in Fabry's disease]. Rev Neurol 2009;49:166–167. Spanish. [PubMed] [Google Scholar]
- 70. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta‐analysis. Lancet Neurol 2015;14:162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010;17:1113–e88. [DOI] [PubMed] [Google Scholar]
- 72. Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: Revised consensus statement from the Canadian Pain Society. Pain Res Manag 2014;19:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sommer C, Uçeyler N, Duning T, et al. [Pain therapy for Fabry's disease]. Internist (Berl) 2013;54:121–130. German. [DOI] [PubMed] [Google Scholar]
- 74. O'Connor AB, Dworkin RH. Treatment of neuropathic pain: An overview of recent guidelines. Am J Med 2009;122(10 Suppl):S22–S32. [DOI] [PubMed] [Google Scholar]
- 75. Gordon KE, Ludman MD, Finley GA. Successful treatment of painful crises of Fabry disease with low dose morphine. Pediatr Neurol 1995;12:250–251. [DOI] [PubMed] [Google Scholar]
- 76. Schiffmann R, Scott LJ. Pathophysiology and assessment of neuropathic pain in Fabry disease. Acta Paediatr Suppl 2002;91:48–52. [DOI] [PubMed] [Google Scholar]
- 77. Niesters M, Martini C, Dahan A. Ketamine for chronic pain: Risks and benefits. Br J Clin Pharmacol 2014;77:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Teasell RW, Arnold JM. Alpha‐1 adrenoceptor hyperresponsiveness in three neuropathic pain states: Complex regional pain syndrome 1, diabetic peripheral neuropathic pain and central pain states following spinal cord injury. Pain Res Manag 2004;9:89–97. [DOI] [PubMed] [Google Scholar]


