Abstract
Objective
To assist policymakers as they reflect on treatment protocols and approaches for the efficient delivery of medical care for multiple sclerosis (MS) patients in Japan.
Methods
We analyzed data from a large Japanese health insurance claims database. Using an algorithm based on diagnosis codes, all patients with a diagnosis of MS were identified; patients having a non‐MS demyelinating disease were excluded from the population. MS patient data were used for cross‐sectional analysis carried out on the data collected at a certain period. We identified a total of 1808 MS patients, and we analyzed data for 1133 patients with an observation period of ≥6 months from October 2013 to September 2014. Newly diagnosed MS patients were identified within the MS patients, and their data were used for longitudinal analysis, tracking each patient over a period of time.
Results
The total per patient per month cost for MS was ¥93 542 (US$781, €695 as of October 2015). Disease‐modifying therapy drugs costs constituted half of the overall medical costs. For newly diagnosed MS patients, hospitalization costs were the largest component in the initial month, while drug costs were the largest component more than several months after the initial visit. There was a positive correlation between relapse frequency and medical cost.
Conclusions
These results provide up‐to‐date information on the demographics, medical treatment and cost status of MS in almost real‐time by using a claims database. They suggest that claims data analysis can effectively support medical policymaking.
Keywords: claims database, disease‐modifying therapy, health economics, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic, progressive, inflammatory, demyelinating disease of the central nervous system that often leads to physical disability.1 MS is one of the common neurological diseases of young adults in Europe and the USA. Its functional symptoms significantly affect the long‐term quality of life of patients.2, 3, 4 Although the prevalence of MS in Japan is low compared with that in Europe and the USA, the number of cases has been steadily increasing in recent years.5 Because the medical costs for intractable diseases are increasing, the medical cost structure has been highlighted in Japan by the Intractable Diseases Treatment Research Program of the Ministry of Health, Labor and Welfare. Among these intractable diseases, MS is one of the costly diseases.
Treatment for MS focuses on disease‐modifying therapy (DMT) drugs, which are drugs that are used to prevent relapse. Over the past 15 years, several DMT drugs for MS have been launched in Japan: interferon (IFN)‐β1b in 2000, IFN‐β1a in 2006, fingolimod in 2011 and natalizumab in 2014. Each drug costs approximately ¥2 million to ¥3 million per patient per year. Other drugs used in MS might include relapse treatment drugs to treat relapse symptoms. Because other costs are also required for MS treatment, overall medical costs are high compared with other diseases.
Because the prevalence of MS is low in Japan compared with Western countries, the number of patients per hospital is limited. In addition, treatment protocols for MS patients vary among hospitals. As such, in order to gain a comprehensive understanding of national trends in medical costs and treatment of MS in Japan, it is essential to have access to health insurance claims data and to establish appropriate methodologies to analyze this data.
We have extensive experience analyzing data collected by individual insurers. However, databases constructed from a single source are not suitable for understanding the status of diseases nationwide because of variations in demographic factors, such as age and occupation. Analyzing data from multiple insurers and health plans is desirable as a more complete and useful basis for policymaking. The Japanese government has initiated the compilation of a National Database and has provided the data externally since 2011. However, use of the National Database is currently restricted to a very limited numbers of researchers.
Another approach is to utilize data from a private company that has accumulated data through purchase from various sources. Because this is a relatively new phenomenon, private databases do not have a long history; however, the volume of data has grown dramatically in recent years. The availability of health insurance claims data offers significant value for analyzing the profile of patients, treatments, and costs across a wide spectrum of medical conditions and services.
In the present study, we used a health insurance claims database provided by Medical Data Vision (MDV), one of several private companies that have assembled such databases. DPC hospitals have adopted the Diagnosis Procedure Combination/Per‐Diem Payment System (DPC/PDPS), the bundled payment system of medical expenses provided by Ministry of Health, Labor and Welfare since 2003. Under this system, reimbursement for hospitalization (basic hospital stays, laboratory testing, diagnostic imaging, medication and injections, and basic treatment) is a flat rate payment per day based on the diagnosis group. The amount of payment per day in DPC/PDPS decreases incrementally over three levels as the number of days of hospitalization increases. DPC/PDPS has been adopted mainly at larger Japanese hospitals, which are required to submit data compliant with a format specified by the Ministry of Health, Labor and Welfare. The database includes health insurance claims data collected from DPC hospitals that contribute data from all insurers; that is, medical care insurance and the Advanced Elderly Medical Service System, a healthcare social security system for people aged 75 years and over.
We propose a new method to analyze health insurance claims data for accurate and quantitative understanding of current MS treatment status and medical cost. Our method might help enhance diagnosis and improve MS treatment, as well as aid the development of measures to reduce medical costs.
Methods
Data sources
Health insurance claims data provided by MDV were used in the present study. The database covers 10.5 million patients treated in any of 192 DPC hospitals during the study period of April 2008 to September 2014. These hospitals account for nearly 11% of approximately 1600 DPC hospitals in Japan (as of May 2015), and include seven university hospitals and five hospitals in the National Hospital Organization. The number of patients in the MDV database has increased rapidly in recent years as the number of DPC hospitals has increased, especially since 2010.
The types of data included in the MDV database are as follows: diagnoses coded according to the International Classification of Diseases 10th revision coding scheme produced by World Health Organization, disease name coded using Japanese Disease Name Codes, procedures coded using Japanese Procedure Codes and prescriptions containing drug general names submitted on health insurance claims. The MDV database includes not only hospitalization data, but also outpatient and prescription data collected subsequent to a hospital visit, unless the patient has transferred to another hospital.
We defined the observation period for each patient as the period from the first claim date to the last claim date in the dataset. The observation period was used as the denominator for the cost per patient per month (PPPM).
Statistical analysis
We carried out analyses by two analytic methods: cross‐sectional analysis and longitudinal analysis. Cross‐sectional analysis is a type of analysis used in observational studies, and it is carried out on data collected during a certain period. A cross‐sectional analysis provides a snapshot view of a well‐mixed population of patients, which can be expected to reflect actual status. In the present study, cross‐sectional analysis was carried out to examine the profile and cost of MS patients who could be observed for at least 6 months during the year 1 October 2013 to 30 September 2014. Longitudinal analysis is used to analyze a change over time in an observational study, and is carried out on data collected at several points for each patient over a period of time. Longitudinal analysis allows the same patients to be followed over multiple observation periods, usually several years. In the present study, longitudinal analysis was carried out to examine the profile, treatment status and status of relapse of newly diagnosed MS patients. Statistical analysis was carried out using sas version 9.2 (SAS institute, Cary, NC, USA). The significance of differences was analyzed by t‐test.
Identification of MS patients
Patients included in the study were determined to have MS as defined by a diagnosis of MS under the International Classification of Diseases 10th revision code G35, multiple sclerosis.6 We extracted claims data from all patients having at least one claim with an MS diagnosis during the study period. We used the following algorithm to select patients to include in our analysis (Fig. S1):
Identify patients with at least one claim with a definitive MS diagnosis.
Exclude those who had at least one claim with neuromyelitis optica (NMO) coded as 3410003 by Japanese Disease Name Codes.7
- Include as MS study patients those who met any of the following criteria:
- Had at least one hospitalization claim with a definitive MS diagnosis.
- Had at least one outpatient claim with a definitive MS diagnosis and had at least one DMT claim.
- Had at least one outpatient claim with a definitive MS diagnosis and a diagnostic start date claim before the observation period. Here, the diagnostic start date is a data item associated with the outpatient claim that records the start of the disease.
- Had at least three outpatient claims with a definitive MS diagnosis.
In this algorithm, “definitive diagnosis” means the diagnosis is not a “suspected diagnosis.” A suspected diagnosis is given by a doctor first if a certain disease or condition is thought to be present, and a definitive diagnosis is given after determination according to criteria through medical examination and testing. We used this algorithm to exclude cases of inaccurate diagnosis, because the patients who underwent examination, laboratory testing and diagnostic imaging did not necessarily have MS. The MDV database has the diagnosis on a claim, but does not have information regarding the Japanese medical expense subsidy system for intractable diseases that would facilitate the identification of MS patients. Because a previous study showed low specificity of coding of MS in outpatient claims data,8 we excluded some patients with only one or two outpatient claims with MS diagnosis, following the algorithm described above.
Identification of newly diagnosed MS patients
We defined newly diagnosed MS patients as those patients with MS (as defined in the previous section) whose diagnostic start date was in the month of the earliest diagnosis of MS or who had a claim for computed tomography or magnetic resonance imaging within 100 days after the earliest date of the MS diagnosis (Fig. S2). Table S1 provides the definitions for the initial MS date and Table S2 provides the definitions of computed tomography and magnetic resonance imaging.
Classification of medical costs
Medical costs were classified into three categories: inpatient, outpatient and prescription drugs. We further classified the prescription drugs into three groups by general name: relapse treatment drugs (prednisolone sodium succinate, dexamethasone phosphate ester sodium and methylprednisolone sodium ester succinate), DMT drugs (IFN‐β1a, IFN‐β1b, fingolimod hydrochloride, prednisolone, cyclophosphamide hydrate, tacrolimus hydrate and azathioprine) and any other drugs.
Definition of the date of relapse
The date of relapse of newly diagnosed MS patients was defined as the date meeting either of the following two criteria:
The first injection of relapse treatment drugs occurring at least 30 days after the initial MS date.
In the case of multiple claims, the earliest date of a group of claims for injections of drugs to treat relapses. Individual claims for injections of drugs for relapse were combined into a group if the dates of injection were within 30 days of each other. The cumulative number of relapses per patient in a given month was defined as the cumulative total number of relapses from the initial MS date to a certain month divided by the sum of the number of months from the initial MS date to a certain month.
Definition of initial DMT treatment
A patient was considered to have received an initial DMT treatment if a DMT treatment was received at least once in the first 4 months after first diagnosis.
Results
Profile of MS patients and their medical costs by cross‐sectional analysis
There were 2216 patients who had at least one claim with a definitive MS diagnosis during the study period 1 April 2008 to 30 September 2014. Of this group, 185 patients had at least one claim with NMO and were excluded. From the remaining 2031 MS patients, 223 were excluded because they did not meet any of the criteria listed in the Methods section. The resulting 1808 patients were identified as MS patients (Table S3).
Figure 1 shows the number of MS patients over time in the MDV database. Among the 1808 MS patients, 1368 patients had an observation period in the most recent year, from 1 October 2013 to 30 September 2014. Among them, 1133 patients (63% of the total) had an observation period of at least 6 months in this period. Because this difference, between 1368 and 1133, was small, we believe that patients with an observation period of <6 months were likely to have changed hospitals, and they were excluded from the cross‐sectional analysis. Therefore, 1133 patients were used for cross‐sectional analysis of the cost status of MS patients.
Figure 1.
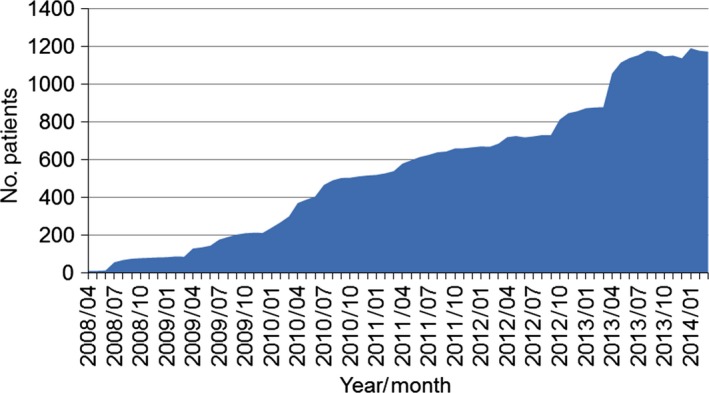
Transition of the number of multiple sclerosis patients in the Medical Data Vision database.
The mean, median, and standard deviation (SD) of distribution of patient age were 50.4 years, 49.0 years and 16.5 years, respectively. The age range with the most patients was 40–49 years, for both men and women (Fig. 2). The mean, median, and SD of the observation period were 10.9 months, 11.0 months and 1.6 months, respectively. The medical cost for an MS patient PPPM was calculated by dividing total cost over the observation period by the number of months of the observation period. Table 1 shows that mean PPPM ± SD was ¥93 542 ± ¥131 751 (US$781 ± US$1100, €695 ± €978 as of October 2015), and median PPPM was ¥27 700 (US$231, €206 as of October 2015). The percentage of patients with at least one hospitalization during 6 months was <24%, therefore the cost for 25th percentile, median and 75th percentile were ¥0 (Table 1). Similarly, the percentage of patients with at least one DMT prescription was <47%, and costs for 25th percentile and median of prescription DMT drugs (inpatient + outpatient) were ¥0.
Figure 2.
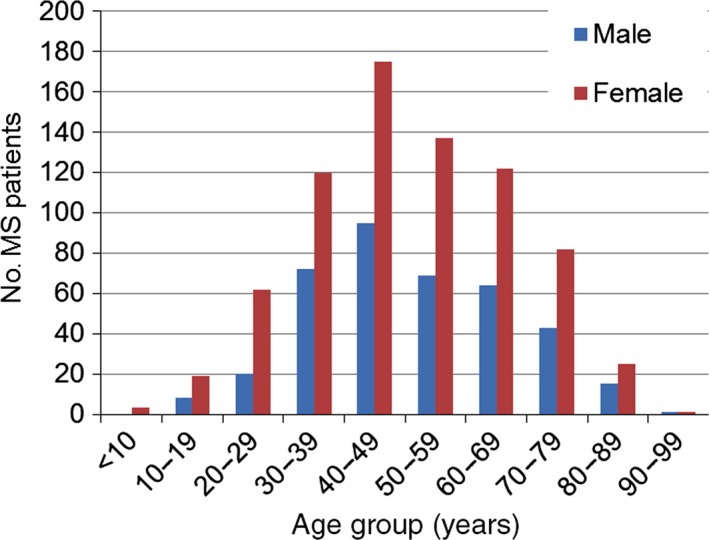
Distribution of age and sex of 1133 patients with an observation period of at least 6 months from October 2013 to September 2014.
Table 1.
Detailed classification of the monthly medical costs for 1133 patients with an observation period of at least 6 months from October 2013 to September 2014
| Mean | 25th percentile | Median | 75th percentile | SD | |
|---|---|---|---|---|---|
| Observation months | 10.9 | 10 | 11 | 12 | 1.6 |
| Medical cost PPPM | ¥93 542 | ¥8522 | ¥27 700 | ¥163 465 | ¥131 751 |
| Inpatient | |||||
| Medical procedure | ¥24 427 | ¥0 | ¥0 | ¥0 | ¥87 004 |
| Prescription drug (relapse treatment) | ¥188 | ¥0 | ¥0 | ¥0 | ¥835 |
| Prescription drug (DMT) | ¥1158 | ¥0 | ¥0 | ¥0 | ¥5469 |
| Prescription drug (others) | ¥2583 | ¥0 | ¥0 | ¥0 | ¥14 029 |
| Outpatient | |||||
| Medical procedure | ¥10 143 | ¥3614 | ¥6618 | ¥12 014 | ¥20 173 |
| Prescription drug (relapse treatment) | ¥99 | ¥0 | ¥0 | ¥0 | ¥536 |
| Prescription drug (DMT) | ¥45 284 | ¥0 | ¥0 | ¥69 796 | ¥79 975 |
| Prescription drug (others) | ¥9661 | ¥752 | ¥3785 | ¥11 842 | ¥18 120 |
| Medical procedure (inpatient + outpatient) | ¥34 570 | ¥4025 | ¥7795 | ¥20 021 | ¥91 369 |
| Prescription drug (inpatient + outpatient) | ¥58 971 | ¥2108 | ¥12 164 | ¥111 469 | ¥83 037 |
| Relapse treatment | ¥287 | ¥0 | ¥0 | ¥0 | ¥1007 |
| DMT | ¥46 441 | ¥0 | ¥0 | ¥76 152 | ¥81 441 |
| Ratio of DMT to the overall medical cost | 50% | 0% | 0% | 47% | N/A |
DMT, disease‐modifying therapy; MS, multiple sclerosis; PPPM, per patient per month; SD, standard deviation.
Figure 3 shows the medical cost PPPM by demographic segment. The cost for patients aged <50 years was ¥112 000 (n = 574), which was significantly higher than for patients aged ≥50 years, ¥74 000 (n = 559; P < 0.001). Among the medical costs, inpatient cost for the patients aged <50 years was ¥25 000, which was lower than for patients aged ≥50 years, ¥29 000, although this was not statistically significant. In contrast, DMT cost for patients aged <50 years was ¥70 000, which was significantly higher than for patients aged ≥50 years, ¥22 000 (P < 0.001). Male patients aged <30 years had significantly higher medical costs, ¥147 000, compared with all other patients, ¥92 000 (P = 0.0024). Although there were just 28 patients in this category, this difference was highly statistically significant. Within this category, inpatient cost PPPM was ¥31 000, which was slightly higher than for all other patients (¥27 000); however, this difference did not reach statistical significance. In contrast, DMT cost PPPM was ¥101 000, which was significantly higher than for all other patients, ¥45 000 (P < 0.001).
Figure 3.
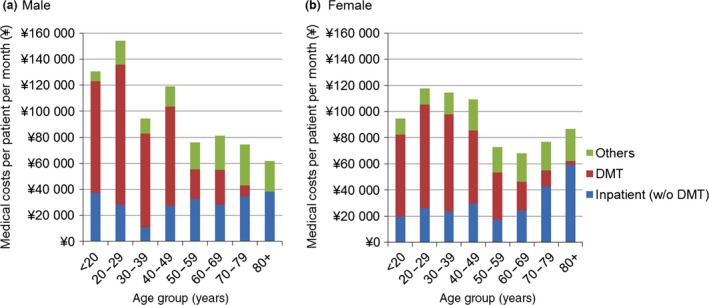
Medical costs per patient per month by sex and age groups for 1133 patients with an observation period of at least 6 months from October 2013 to September 2014. DMT, disease‐modifying therapy.
Prescription drug costs accounted for 63% of overall costs, with DMT costs accounting for 50% of total costs (Table 1). After prescription drug costs, hospitalization accounted for the largest proportion of total costs, followed by examination/pathology and diagnostic imaging. IFN‐β (¥27 919: IFN‐β1b, ¥17 072; IFN‐β1a, ¥10 847) and fingolimod (¥17 617) accounted for the highest average cost among DMT drugs (Table S4). Costs varied significantly by patient (Fig. 4). One‐third of patients had medical costs >¥100 000 PPPM, while more than half of the patients had costs <¥40 000 PPPM.
Figure 4.
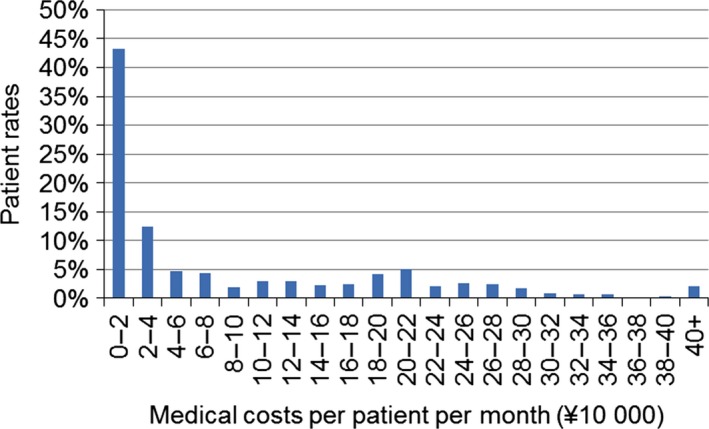
Patient rates among the 1133 patients with an observation period of at least 6 months from October 2013 to September 2014 by medical cost per patient per month.
Newly diagnosed MS patients: Profile and treatment status by longitudinal analysis
We identified 547 newly diagnosed MS patients in the MDV database during the study period. The male‐to‐female ratio was 1:1.69, and the mean age of newly diagnosed MS patients was 47.0 ± 17.5 (mean ± SD), approximately 3.4 years younger than that of the overall patient group. In this analysis, we focused on the 356 patients aged 18 years or older as of the earliest date of MS diagnosis, and who had an observation period of at least 6 months (Table S5). The mean age of this group of newly diagnosed MS patients was 47.0 ± 15.8 (mean ± SD).
Figure 5 shows the medical cost PPPM of newly diagnosed MS patients aged 18 years or older by elapsed month subsequent to the initial MS date. Hospitalization costs peaked during the first month and decreased significantly during the following few months, whereas drug costs remained level. Thus, while hospitalization costs accounted for the largest proportion of costs during the initial stage of treatment, drug costs accounted for the largest proportion after several months of observation.
Figure 5.
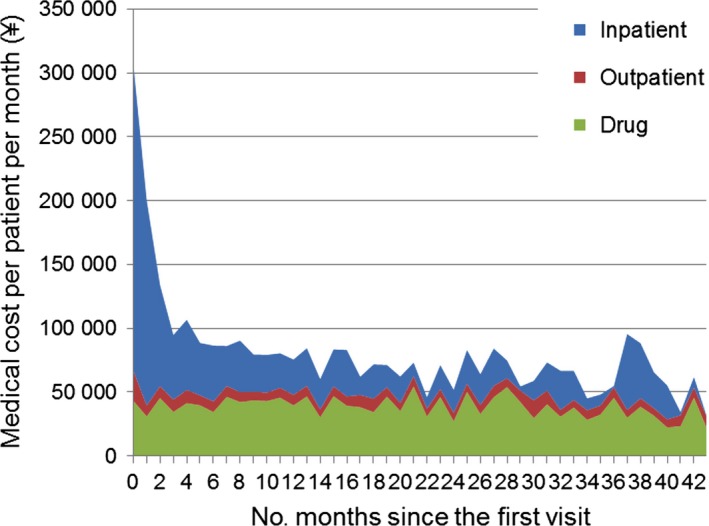
Medical cost per patient per month for 356 newly diagnosed multiple sclerosis patients by month since the initial diagnosis date.
Status of relapse by longitudinal analysis
Figure 6 shows the cumulative number of relapses per patient by sex and duration after the initial MS diagnosis date. The rate of cumulative number of relapses per MS patient gradually decreased, consistent with the decreased relapse rate per year from the first month (Fig. S3). There was no difference between sexes. Among 356 newly diagnosed MS patients, 255 (71.6%) did not experience a relapse during the observation period (Table S6).
Figure 6.
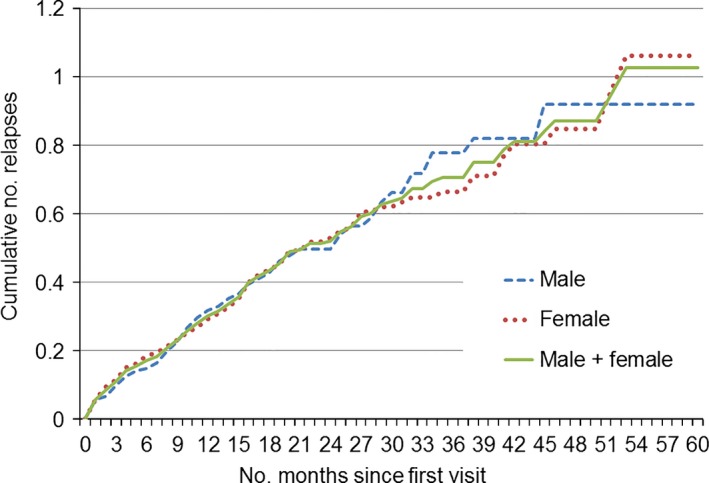
Transition of the cumulative number of relapses per patient for 121 male and 235 female newly diagnosed multiple sclerosis patients.
Table 2 shows the relationship between the mean medical cost PPPM and the annual number of relapses (number of relapses divided by observation period in years), for 356 newly diagnosed MS patients. There was a positive correlation between the frequency of relapses and the PPPM medical cost.
Table 2.
Relationship between the number of annual relapses and medical cost per patient per month for 356 newly diagnosed multiple sclerosis patients
| Group of relapse frequency by year | No. patients | Average observation period after the initial visit | No. relapses | Average no. relapses per year | Medical costs per patient per month | No. patients with initial DMT treatment | Rate of patients with initial DMT treatment |
|---|---|---|---|---|---|---|---|
| 0 | 255 | 22.5 | 0 | 0.00 | ¥72 913 | 106 | 42% |
| >0–0.5 | 38 | 41.7 | 44 | 0.33 | ¥105 222 | 20 | 53% |
| >0.5–1.0 | 36 | 23.6 | 54 | 0.76 | ¥111 528 | 23 | 64% |
| >1.0 | 27 | 20.2 | 80 | 1.76 | ¥246 092 | 17 | 63% |
| Total | 356 | 24.5 | 178 | 0.25 | ¥93 390 | 166 | 47% |
DMT, disease‐modifying therapy.
Discussion
MS is a costly disease, with a mean PPPM cost of ¥93 542, approximately 3.7‐fold the mean PPPM for the general population of Japan, ¥25 625, which was calculated based on total Japanese expenditures on medical treatment divided by total population.9 It is also found that the cost for MS patients is more expensive than mean medical cost for all diseases patients, ¥34 200, which was calculated by dividing total costs consisting of inpatient, outpatient, and drugs by numbers of inpatient and outpatient claims in each month.10
We found a significant difference in costs for different demographic subgroups. Specifically, we found extremely high costs for male patients under the age of 30 years, and patients under the age of 50 years were more costly than older patients. These differences were largely because of costs associated with DMT drugs, reflecting differences in treatment approaches to these subgroups. To what extent these are as a result of patient preferences and to disease activity is unknown, because of the limits of our data sources. However, the results are clinically plausible. Potential reasons for differences of DMT drug costs by sex and age groups include: (i) younger patients might have shorter terms after onset of MS and prefer DMT treatment; (ii) younger patients, especially male patients, wished to continue working and requested aggressive treatment; (iii) some older patients might have progressive forms of MS, for which DMT treatment is not appropriate; and (iv) some older patients might not require any treatment, because their neurological status has plateaued.11 Additional analyses showed that relapse frequency in male patients under the age of 30 years was relatively higher than that in other patients, which might relate to the higher costs in male patients under the age of 30 years as well.
To confirm that the prevalence of MS in our MDV database accurately reflects the prevalence of MS in the Japanese population, we compared the number of MS patients in 2014 in the MDV database with recent data on MS patient numbers available from the Japan Intractable Diseases Research Foundation/Japan Intractable Diseases Information Center (JIDRF/JIDIC). The national foundation reported 17 073 persons in 2012, and 18 082 persons in 2013, nationwide, who were certified to receive subsidies for medical treatment of MS.5 The ratio between the number of MS patients (1368) on our database in the most recent year and the national numbers (17 073 for 2012 and 18 082 for 2013) is approximately 8%, which is smaller than the coverage ratio, approximately 11%, of the MDV database to all DPC hospitals. Most MS patients use DPC hospitals, but not all the patients. Also, the number of MS patients reported by JIDRF/JIDIC might include some patients with NMO, so the number of MS patients in JIDRF/JIDIC data might be overestimated. Further study is required to determine the actual prevalence rate based on the health insurance claims database.
In addition, we focused on the hospitalization and outpatient costs among medical costs for MS, and compared them with a previous report. We calculated the hospitalization cost for one hospitalization and the outpatient cost for one outpatient. The mean cost of hospitalization in the present study was ¥570 000 per hospitalization, in agreement with National Health Insurance in Japan (November 2009 to January 2010) and Employees Health Insurance in Japan (November 2009 to January 2010), both of which were ¥550 000.8 The mean cost for outpatients in the present study was ¥65 000 per outpatient, and those in the previous study were ¥37 000 from National Health Insurance in Japan and ¥49 000 from Employees Health Insurance in Japan, respectively.8 It is reasonable that the outpatient cost in the present study was higher than those in the previous studies, because the MDV database used in the present study consisted of data from DPC hospitals only, whereas previous studies included various types of hospitals. These results are consistent with previous reports, suggesting the MDV database is a useful source of information for healthcare costs in Japan.
We compared the male‐to‐female ratio of newly diagnosed MS patients in the present study with a nationwide survey carried out in 2004. In both studies, the population contained more female MS patients than male patients. The ratio of males to females is 1:1.69 in the present study, whereas it was 1:2.9 in the previous study.12 We believe this is caused by changes in the classification of MS that took place between studies. At the time of the previous study, optic‐spinal MS was considered to be a subcategory of MS, whereas it is now considered to be a subcategory of NMO, specifically excluded from the present study. Furthermore, the male‐to‐female ratio in optic‐spinal MS was 1:4.5.12
The mean age of newly diagnosed MS patients in the present study, 47.0 ± 17.5 years, was old in comparison with the onset age of MS, 32 ± 13 years in a previous study.12 This result is expected, because the age of onset was obtained by questionnaire in the previous study, and the onset of symptoms is always before the date of diagnosis.
In order to investigate medical costs during the early period of illness, we tried to identify newly diagnosed MS patients. The profile of the newly diagnosed MS patients resembled that of the overall MS patient group, although it showed a lower mean age and smaller coefficient of variation. In addition, cost analysis in the MS patient population showed that hospitalization costs are prominent in the first month, which is expected for newly diagnosed MS patients (Fig. 5).
Medical costs clearly increased parallel to the relapse frequency in the newly diagnosed MS patients (Table 2). Of note, just 47% of MS patients in Japan were treated with DMT within 4 months after the first diagnosis. Also, it is interesting to note that 37% of patients with a relapse frequency of more than once per year did not receive DMT drugs treatment during the first 4 months. It is possible that relapse frequency in this group could have been decreased by initial treatment with DMT drugs.
Data gathered in the course of actual medical practice are referred to as “real‐world data.” This includes information compiled from hospital records, health insurance claims, personal health records and other sources. Real‐world data is increasingly available and important to biomedical research. Retrospective studies based on these data can be completed quickly and at relatively low cost. Such analysis can supplement and facilitate other research, including randomized controlled trials. In the present study, we used real‐world data to understand trends in the medical treatment of MS in Japan. We believe the present study illustrates the value of real‐world data analytics. However, caution must be exercised in the use of these techniques. Even using a very large database, it might be difficult to eliminate or understand the effect of confounding factors. It is often easy to find correlations among variables in a database. Our discoveries might be of interest if we can give an affirmative answer to the following questions: Are the correlations likely to be observed in other studies? Are they likely to persist over time? Are the correlations clinically meaningful? And, in particular, is there likely to be a causal relationship? In the present study, higher relapse frequency in MS was associated with higher medical costs. Thus, there is a correlation between relapse frequency and medical cost, but causality is not determined. In some situations, real‐world data might be used to support a hypothetical causal relationship. In other cases, real‐world data can aid insights or develop an initial hypothesis that will lead to future avenues of research.
In conclusion, we examined the status of current MS treatment and medical costs in Japan by analyzing health insurance claims data provided by MDV. The mean monthly medical cost for treating an MS patient in Japan is more than threefold that of the medical cost for the general population. Thus, this analysis clearly shows the high costs associated with MS treatment in Japan. Cost varies significantly by patient. Approximately 30% of patients had PPPM in excess of ¥100 000, while half were <¥40 000. We have not attempted to evaluate the extent to which this deviation is due to patient attributes or treatment protocols. However, given the high and increasing cost associated with MS treatment in Japan, this deviation represents a worthy subject for future research. Initial hospital costs account for high treatment costs immediately after diagnosis. Higher relapse rates are correlated with higher costs; future avenues of research should evaluate the potential for cost reductions through outpatient treatments, as well as the potential for cost savings through early DMT interventions. The hospitalization cost in the present study (1 October 2013 to 30 September 2014) shows a good agreement with those from the data of National Health Insurance in Japan and Employees Health Insurance in Japan (November 2009 to January 2010). Other data obtained in the present study are also confirmed to be reasonable in comparison with other reports. The findings of the present study provide up‐to‐date information on medical treatment and cost status of MS in Japan. Our findings also suggest that MS treatment status and costs fluctuate rapidly with changes in the treatment landscape. Health insurance claims data analyses can assist policymakers and medical professionals with insights to help develop policy and protocols.
To carry out the analysis in the present study, MDV provided DPC data comprised of administrative data from its contracted 192 hospitals, 11% of the DPC hospitals in Japan. This sample might not be reflective of MS care on a national level in Japan, because the database primarily includes large hospitals, providing little information for small‐scale hospitals or clinics. Additionally, the rapid increase in the number of patients covered by the MDV data could lead to biased results. Results from analysis of the MDV database are similar to “The Report on distribution ratio of discharged patient based on MDC (Major Diagnostic Category) in 2012”.13 An improvement opportunity would be to establish a database that includes more hospitals and overall carrying out studies covering all DPC hospitals.
In the MDV database, patient data are anonymized before receipt, and cannot be tied back to either paper‐chart‐based medical records or electronic medical records. Also, individual treatment histories are not available before the first visit, and cannot be tracked if a patient transfers from one hospital to another. Furthermore, because patient chart data is not included, we cannot gain a deep understanding of each case, and cannot evaluate the accuracy of diagnosis, existence of relapses and severity. Theoretically, we should use the insurance coverage period as the denominator of medical costs; however, it is not available in MDV data, and we used the observation period as the denominator. As the observation period must be included in the insurance coverage period, the cost PPPM might be overestimated. To evaluate the relationship between medical costs and long‐term outcomes, surrogate indices that correlate with severity or changes in severity are required. Despite these limitations, the results and preliminary conclusions of this analysis are interesting, and are suggestive of further lines of research.
Conflict of interest
Takeda Pharmaceutical Company Limited has financially supported this work. Mieko Ogino has received funding for travel from Takeda Pharmaceutical Company Limited. Izumi Kawachi has received funding for travel and/or speaker honoraria from Novartis Pharma, Biogen, Bayer Yakuhin Limited, Mitsubishi Tanabe Pharma, Takeda Pharmaceutical Company Limited and Astellas Pharma, and is an advisory board member for Biogen and Takeda Pharmaceutical Company Limited. Kazuyoshi Otake, Hiroyuki Ohta and Shinzo Hiroi are employees of Takeda Pharmaceutical Company Limited. Kosuke Iwasaki and Yujiro Otsuka are employees of Milliman. Financial support for writing and editorial assistance was provided by Takeda Pharmaceutical Company Limited.
Supporting information
Figure S1. Algorithm for the identification of multiple sclerosis patients.
Figure S2. Algorithm for the identification of newly diagnosed multiple sclerosis patients.
Figure S3. Annual relapse rate for newly diagnosed multiple sclerosis patients.
Table S1. Codes used for definition of the initial multiple sclerosis date.
Table S2. Codes used for definition of computed tomography and magnetic resonance imaging.
Table S3. Detailed classification of multiple sclerosis patients.
Table S4. Breakdown of disease‐modifying therapy drugs and relapse treatment drugs.
Table S5. Demographic details of newly diagnosed multiple sclerosis patients.
Table S6. Distribution number of relapses for newly diagnosed multiple sclerosis patients.
Acknowledgements
The authors thank Ms Kaoru Yamabe at Takeda Pharmaceutical Company Limited for useful comments, and Dr Helen Blumen, Dr Hideyuki Arata, and Dr Tomomi Takeshima at Milliman for writing and editorial support.
References
- 1. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long‐term disability. Brain. 2010; 133: 1914–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naci H, Fleurence R, Birt J, et al. Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics. 2010; 28: 363–79. [DOI] [PubMed] [Google Scholar]
- 3. Deshmukh VA, Tardif V, Lyssiotis CA, et al. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013; 502: 327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uitdehaag BM. Clinical outcome measures in multiple sclerosis. Handb Clin Neurol. 2014; 122: 393–404. [DOI] [PubMed] [Google Scholar]
- 5. Japan Intractable Diseases Information Center . Number of patients who were certified to receive subsidies for the medical treatment of multiple sclerosis (In Japanese). [Cited 8 Jul 2015.] Available from URL: http://www.nanbyou.or.jp/entry/1356
- 6. Ministry of Health, Labour and Welfare (Japan) . Statistical classification of diseases and cause of death (In Japanese). [Cited 5 Oct 2015.] Available from URL: http://www.mhlw.go.jp/toukei/sippei/
- 7. Ministry of Health, Labour and Welfare (Japan) . Various information of medical fee (In Japanese). [Cited 25 Nov 2015.] Available from URL: http://www.iryohoken.go.jp/shinryohoshu/searchMenu/doSearchInputBp
- 8. Ogino M. Study on medical cost structure of intractable diseases (In Japanese). Report of the Grant‐in‐Aid for Health and Labor Sciences Research for Intractable Diseases Treatment Research Program, Fiscal Year 2008–2010 summary and shared research report.
- 9. Ministry of Health, Labour and Welfare (Japan) . Medical cost of the general population in Japan in 2012 (In Japanese). [Cited 5 Oct 2015.] Available from URL: http://www.e-stat.go.jp/SG1/estat/List.do?lid=000001127463
- 10. Ministry of Health, Labour and Welfare (Japan) . Estimated medical cost database (In Japanese). [Cited 28 Jan 2016.] Available from URL: http://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken03/01.html
- 11. Mattson DH. Alphabet soup: a personal, evolving, mostly evidence‐based and logical, sequential approach to the “ABCNR” drugs in multiple sclerosis. Semin Neurol. 2002; 22: 17–25. [DOI] [PubMed] [Google Scholar]
- 12. Osoegawa M, Kira J, Fukazawa T, et al. Temporal changes and geographical differences in multiple sclerosis phenotypes in Japanese: nationwide survey results over 30 years. Mult. Scler. 2009; 15: 159–73. [DOI] [PubMed] [Google Scholar]
- 13. Ministry of Health, Labour and Welfare (Japan) . The report of discharged patient in 2012 (In Japanese). [Cited 10 Nov 2015.] Available from URL: http://www.mhlw.go.jp/stf/shingi/0000023522.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Algorithm for the identification of multiple sclerosis patients.
Figure S2. Algorithm for the identification of newly diagnosed multiple sclerosis patients.
Figure S3. Annual relapse rate for newly diagnosed multiple sclerosis patients.
Table S1. Codes used for definition of the initial multiple sclerosis date.
Table S2. Codes used for definition of computed tomography and magnetic resonance imaging.
Table S3. Detailed classification of multiple sclerosis patients.
Table S4. Breakdown of disease‐modifying therapy drugs and relapse treatment drugs.
Table S5. Demographic details of newly diagnosed multiple sclerosis patients.
Table S6. Distribution number of relapses for newly diagnosed multiple sclerosis patients.


