Abstract
Background
Drug survival is a marker for treatment sustainability in chronic diseases such as psoriasis.
Objective
The aim of these analyses was to assess survival of biologic treatments in the PSOriasis Longitudinal Assessment and Registry (PSOLAR).
Methods
PSOLAR is a large, prospective, international, disease‐based registry of patients with psoriasis receiving (or eligible for) systemic therapy in a real‐world setting. Drug survival is defined as the time from initiation to discontinuation (stop/switch) of biologic therapy on registry. The number of patients who discontinued each treatment and the duration of therapy were recorded. Using Kaplan–Meier survival curves and Cox‐regression analyses [hazard ratios (HR) and 95% confidence intervals (CIs)], time to discontinuation was compared across cohorts undergoing first‐, second‐ or third‐line treatment with ustekinumab, infliximab, adalimumab or etanercept.
Results
As of the 2013 data cut, 12 095 patients with psoriasis were enrolled in PSOLAR. Of the 4000 patients initiating any new biologic therapy, approximately 3500 started a first‐line, second‐line or third‐line biologic therapy during the registry. Lack of effectiveness was the most common reason for discontinuation across biologic therapies. Based on the multivariate analysis, significantly shorter times to discontinuation were observed for infliximab [HR (95%CI) = 2.73 (1.48–5.04), P = 0.0014]; adalimumab [4.16 (2.80–6.20), P < 0.0001]; and etanercept [4.91 (3.28–7.35) P < 0.0001] compared with ustekinumab [reference treatment]) for first‐line biologic use; results were similar for treatment effects for second/third‐line therapies. Although limited in power, analyses in patients with concurrent psoriatic arthritis confirmed by a rheumatologist reflect observations in the overall psoriasis population.
Conclusion
Drug survival was superior for ustekinumab compared with infliximab, adalimumab and etanercept in patients with psoriasis.
Introduction
The introduction of biologic agents over the past decade has led to a significant shift in the treatment paradigm for both psoriasis and psoriatic arthritis (PsA), chronic disorders that invariably require long‐term therapy to maintain clinical response. The trend towards long‐term use of biologics for maintenance treatment underscores the need to understand drug survival (i.e. how long a patient stays on a given therapy). Drug survival can be influenced by a range of factors,1 including safety, treatment effectiveness (possibly relating to the development of antibodies to individual drugs),2 side‐effects, cost, convenience, quality‐of‐life, access and other patient‐oriented factors. Biologics have been shown to be highly effective for the initial treatment of psoriasis; however, response may wane over time and lead to stopping/switching of treatment.3 In fact, the most common reason for discontinuing biologics is lack of effectiveness.3, 4, 5, 6
Recent observational studies of treatment persistence have reported variable results for tumour necrosis factor‐alpha (TNF‐α) inhibitors, (infliximab, adalimumab and etanercept), ranging from 40% to 80% after at least 1 year of treatment.7, 8, 9 Drug survival rates as high as 80% have been reported for etanercept and ustekinumab through up to 5 years of treatment, and small observational studies indicate that treatment with ustekinumab has significantly longer survival compared with TNF‐α inhibitors.10, 11, 12 However, there are few evaluations of drug survival in large populations within real‐world settings.4, 5, 13
PSOLAR (PSOriasis Longitudinal Assessment and Registry) is a large international registry initially undertaken to address a postmarketing commitment to capture safety outcomes for infliximab and ustekinumab (Janssen Scientific Affairs, LLC, Horsham, PA, USA). The disease‐based design of the registry also allows the capture of safety and clinical outcomes across a spectrum of agents used to treat psoriasis (ustekinumab, infliximab, adalimumab and etanercept). The purpose of this report was to present results of analyses based solely on the longevity of biologic therapies received in PSOLAR. Analyses of comparative effectiveness for these treatments, a key element of drug survival, is presented separately.14
Materials and methods
Details of the eligibility criteria and study design of PSOLAR have been reported.15, 16 Briefly, this multicentre, prospective, observational registry was developed to monitor safety outcomes in approximately 12 000 patients receiving, or eligible to receive, treatment with systemic therapies (including phototherapy) for psoriasis. Registry data reported herein are based on data collected from 20 June 2007 to 23 August 2013. Physicians prescribed treatments as they would in their usual clinical routine; the use of concomitant medications was not restricted. Data were collected at enrolment and approximately every 6 months after the initial visit. Most demographic and disease characteristics were collected at enrolment and, again, at the time of any biologic start on registry. Patients were asked at enrolment if they had a concomitant diagnosis of PsA and if it had been confirmed by a rheumatologist. Adverse events, disease assessments and psoriasis medications were documented at interval visits. Per the study design, start and stop dates were recorded and analysed uniformly for biologic agents administered during the registry.
The current analyses are based on a subset of PSOLAR patients who initiated ustekinumab, infliximab, adalimumab, and/or etanercept as first‐, second‐ or third‐line therapy during the registry. While the patient cohorts within each line of therapy were mutually exclusive, the same patients could be included in more than one line of therapy, if they started more than one biologic treatment over the course of their participation in the registry. Therapy was considered ‘first‐line’ for biologic‐naive patients who initiated their first biologic ever after enrolment in PSOLAR. Second‐ and third‐line therapies included patients who initiated their second and third biologic, respectively, after enrolment; they could have initiated/discontinued their previous biologic either before or after enrolment. Patients initiating treatment on registry with a biologic to which they were previously exposed were not included in these analyses. For the first‐, second‐ and third‐line biologic cohorts, duration of exposure was defined as the time (days) between the date of administration of the first dose of the cohort‐defining therapy and the earlier date of the following: (i) discontinuation of biologic therapy, (ii) initiation of new biologic therapy, (iii) withdrawal from the registry or death or (iv) annual database cutoff (23 August 2013). The reasons for discontinuation were summarized for each biologic agent.
Kaplan–Meier time‐to‐event analyses were conducted to estimate time to treatment discontinuation. ‘Event’ was defined as stopping or switching a biologic therapy. The ‘event date’ was defined as the date of treatment discontinuation. A patient was censored if that patient had not discontinued treatment at the time of withdrawal from the registry, loss to follow‐up, or the date of annual data cut. In addition, Cox proportional hazard regression analyses with adjustment for covariates collected either at entry into the registry or prior to initiation of new therapy (Table 1) were performed to compare time to discontinuation of ustekinumab treatment with that of each anti‐TNF inhibitor. Adjusted hazard ratios (HRs), 95% confidence intervals (95% CIs) and corresponding P‐values (Wald Chi‐square test) were calculated for each clinical characteristic for comparison between given treatment groups and a defined reference group. Missing values for covariates in the Cox model were imputed (i.e. mean for continuous factors and median for categorical factors).
Table 1.
Covariates in the multivariate analyses of predictors of time to discontinuation
| Collected at entry into the registry | Collected prior to initiation of new therapy |
|---|---|
| • Gender | • Agea |
| • Ethnicity | • Types of insurance |
| • Body mass indexb | • Prior biologic therapies usedc |
| • Familial psoriasis history | • Reasons for discontinuation of prior biologicc |
| • Smoking status | • Physician's Global Assessment |
| • Alcohol use status | • Concomitant methotrexate use |
| • Duration of psoriasisa | |
| • Age at psoriasis diagnosis | |
| • Diagnosis of psoriatic arthritis | |
| • Study site/geographic region | |
| • History of immunomodulator use |
The distribution of continuous variables was rescaled to facilitate clinical interpretation (e.g. divided by 10 for age and by 5 for duration of psoriasis.
Based upon National Heart, Lung and Blood Institute Obesity Education Initiative criteria: Underweight/normal = body mass index (BMI) <25, Overweight/obesity class I BMI ≥25 and <35 and Obesity class II–III = BMI ≥ 35.
For second‐ and third‐line therapies only.
Subgroup analyses for the three lines of therapy were also performed for the PsA subpopulation (i.e. patients with PsA confirmed by a rheumatologist) at registry entry. These analyses did not include patients who developed PsA during registry follow‐up, as these data were not systematically captured. The methodology for the PsA subgroup analyses mirrored that for all psoriasis patients.
Results
Overall psoriasis population
Baseline characteristics of the overall PSOLAR population have been described previously.16
Among the subset of psoriasis patients who initiated any new biologic agent on registry, a total of 4000 new treatment starts/switches occurred (1833 for ustekinumab, 1303 for adalimumab, 537 for etanercept and 327 for infliximab); these totals include all new starts (i.e. first‐line through seventh‐line starts/switches; Table 2). Only the 1115 first‐line, 1436 second‐line and 922 third‐line starts reported during PSOLAR are studied here. New starts among bio‐naïve patients were highest for adalimumab, followed by ustekinumab, etanercept and infliximab; etanercept was initiated most often as first‐line therapy, ustekinumab and adalimumab as second‐line therapy and infliximab as third‐line therapy (Table 2).
Table 2.
Summary of new starts for first‐ through seventh‐lines of therapy included in the current analyses; patients with psoriasis initiating new therapy during the registry
| Ustekinumab | Infliximab | Adalimumab | Etanercept | Total | |
|---|---|---|---|---|---|
| New therapy starts, N | 1833 | 327 | 1303 | 537 | 4000 |
| First‐line therapya | 361 (19.7) | 63 (19.3) | 402 (30.9) | 289 (53.8) | 1115 |
| Second‐line therapy | 566 (30.9) | 93 (28.4) | 622 (47.7) | 155 (28.9) | 1436 |
| Third‐line therapy | 551 (30.1) | 103 (31.5) | 197 (15.1) | 71 (13.2) | 922 |
| Fourth‐line therapy | 248 (13.5) | 49 (15.0) | 71 (5.4) | 16 (3.0) | 384 |
| Fifth‐line therapy | 84 (4.6) | 14 (4.3) | 9 (0.7) | 6 (1.1) | 113 |
| Sixth‐line therapy | 21 (1.1) | 5 (1.5) | 2 (0.2) | 0 | 28 |
| Seventh‐line therapy | 2 (0.1) | 0 | 0 | 0 | 2 |
Data are presented as number of patients (%).
For lines of therapy beyond first‐line, the prior biologic may have been any of the four biologics included in these analyses or other biologics not approved for psoriasis (golimumab), those no longer available for treating psoriasis (efalizumab and alefacept) or those received via participation in a clinical study but not available for treatment of psoriasis (briakinumab).
The majority of patients were enrolled at North American sites (79.2% for first‐line therapy, 91.0% for second‐line therapy and 92.8% for third‐line therapy). The proportion of patients initiating first‐line therapy from European sites (20.1%) was higher compared with the proportions of second‐ and third‐line therapies (8.4% and 6.6% respectively); the remaining patients were enrolled in Latin America (0.7%, 0.6% and 0.5%). Just over half of all biologic starters were male (56.7%); mean age was 46.9 years and mean body mass index (BMI) was 30.4 (Table 3). Demographic, disease and treatment characteristics prior to first‐line therapy were comparable across treatment groups. The duration of psoriasis ranged from 11.8 to 16.6 years, and the percent body surface area (BSA) affected with psoriasis ranged from 18.2 to 27.9. Although Physician's Global Assessment (PGA) scores were generally similar across biologic groups, (mean score, 2.8), the proportion of patients with marked/severe psoriasis (PGA 4/5) varied from 17.0% for etanercept to 26.6% for ustekinumab (Table 3). Patient and disease characteristics collected before initiating second‐ and third‐line use of biologic therapies (data not shown) were generally consistent with those reported for first‐line treatment, although the proportions of patients receiving methotrexate (MTX) prior to first‐and second‐line therapies (30.5% and 37.6% respectively) were lower compared with third‐line therapy (50.3%). During first‐line therapy, concomitant MTX use was reported in higher proportions of patients receiving etanercept (46.6%) and adalimumab (25.1%) compared with infliximab (13.8%) and ustekinumab (10.5%); proportions varied during second‐line [adalimumab (49.2%), etanercept (29.3%), infliximab (25.2%) and ustekinumab (20.0%)] and third‐line [infliximab (36.6%), ustekinumab (34.5%), etanercept (19.5%) and adalimumab (6%)] treatment.
Table 3.
Demographics and disease characteristics prior to first‐line therapy; patients with psoriasis initiating new therapy during the registry
| Ustekinumab | Infliximab | Adalimumab | Etanercept | All | |
|---|---|---|---|---|---|
| Patients initiating first‐line therapy | 361 | 63 | 402 | 289 | 1115 |
| Age (years), N | 361 | 63 | 402 | 289 | 1115 |
| Mean ± SD | 46.7 ± 14.41 | 49.9 ± 14.16 | 47.3 ± 13.84 | 46.0 ± 14.89 | 46.9 ± 14.33 |
| Gender, N | 361 | 63 | 402 | 289 | 1115 |
| Male | 204 (56.5) | 39 (61.9) | 230 (57.2) | 159 (55.0) | 632 (56.7) |
| Race, N | 361 | 63 | 402 | 289 | 1115 |
| White | 324 (89.8) | 59 (93.7) | 307 (76.4) | 232 (80.3) | 922 (82.7) |
| BMI (kg/m2), N | 357 | 62 | 400 | 287 | 1106 |
| Mean ± SD | 29.5 ± 6.54 | 31.3 ± 7.42 | 31.1 ± 7.05 | 30.14 ± 7.68 | 30.4 ± 7.11 |
| Years since psoriasis diagnosis, N a | 359 | 61 | 399 | 289 | 1108 |
| Mean ± SD | 16.4 ± 13.44 | 16.6 ± 12.43 | 11.8 ± 11.69 | 12.9 ± 12.90 | 13.9 ± 21.79 |
| Percent BSA, N | 356 | 61 | 398 | 286 | 1101 |
| Mean ± (SD) | 22.6 ± 21.61 | 27.9 ± 23.59 | 20.1 ± 22.40 | 18.2 ± 19.84 | 20.9 ± 21.68 |
| PGA score, N | 350 | 60 | 382 | 276 | 1068 |
| Mean ± SD | 2.9 ± 0.98 | 2.8 ± 0.96 | 2.8 ± 1.07 | 2.6 ± 1.07 | 2.8 ± 1.04 |
| PGA = 4/5b | 93 (26.6) | 14 (23.3) | 80 (21.0) | 47 (17.0) | 234 (21.9) |
| Prior therapy, N a | 361 | 63 | 402 | 289 | 1115 |
| Phototherapy | 182 (50.4) | 34 (54.0) | 151 (37.6) | 115 (39.8) | 482 (43.2) |
| Immunomodulators | 166 (46.0) | 33 (52.4) | 145 (36.1) | 100 (34.6) | 444 (39.8) |
| Methotrexate | 123 (34.1) | 22 (34.9) | 118 (29.4) | 77 (26.6) | 340 (30.5) |
| Cyclosporine | 70 (19.4) | 11 (17.5) | 42 (10.4) | 26 (9.0) | 149 (13.4) |
Data are presented as number of patients (%), unless otherwise indicated.
Duration of psoriasis and prior therapy were collected at entry into the registry.
PGA score of 4 or 5 indicates marked or severe psoriasis.
BMI, body mass index; BSA, body surface area; covered with psoriasis, 1 hand is ≈1%; PGA, Physician's Global Assessment; SD, standard deviation.
During first‐line therapy, the most common dose and dosing interval was 45 mg every 12 weeks for ustekinumab (57.5%), 5 mg/kg every 8 weeks for infliximab (44.4%), 40 mg every other week for adalimumab (80.9%), and 50 mg weekly (57.9%) (Table 4). In addition, notable proportions of doses were given as 90 mg every 12 weeks for ustekinumab, 5 mg/kg every 6 weeks for infliximab and 50 mg twice weekly for etanercept, whereas smaller proportions were recorded at other doses and intervals. Ustekinumab and infliximab were typically administered at a physician's office or hospital location (83.1% and 73.3% of doses respectively), while adalimumab and etanercept were administered in such settings in a small minority of administrations (7.1% and 3.0% of doses, respectively). Generally similar dosing patterns were observed for each biologic agent when prescribed as second‐ or third‐line therapy (Table 4). The median duration of registry follow‐up varied among biologics, possibly due to differences in approval dates, regional market availability and prescribing tendencies (Table 5).
Table 4.
Most common maintenance doses and dose frequencies by treatment for first‐, second‐ and third‐line therapies; doses in patients with psoriasis initiating new therapy during the registry
| Ustekinumab | Infliximab | Adalimumab | Etanercept | |
|---|---|---|---|---|
| First‐line therapy, N | 1412 | 270 | 1274 | 1002 |
| Dose | 45 mga | 5 mg/kg | 40 mg | 50 mg |
| Frequency | Every 12 weeks | Every 8 weeksb | Every other week | Weeklyc |
| n (%) | 812 (57.5) | 120 (44.4) | 1031 (80.9) | 580 (57.9) |
| Second‐line therapy, N | 2081 | 281 | 2113 | 397 |
| Dose | 45 mga | 5 mg/kg | 40 mg | 50 mg |
| Frequency | Every 12 weeks | Every 8 weeksb | Every other week | Weeklyc |
| n (%) | 956 (45.9) | 104 (37.0) | 1743 (82.5) | 195 (49.1) |
| Third‐line therapy, N | 2040 | 358 | 621 | 177 |
| Dose | 45 mga | 5 mg/kg | 40 mg | 50 mg |
| Frequency | Every 12 weeks | Every 8 weeksb | Every other week | Twice Weeklyc |
| n (%) | 803 (39.4) | 115 (32.1) | 487 (78.4) | 92 (52.0) |
Data are reported as number of doses administered (%). N = total number of administrations for each line of therapy.
In addition, a large proportion of 90‐mg doses of ustekinumab every 12 weeks were administered, including 375/1412 (26.6%) during first‐line therapy, 733/2081 (35.2%) during second‐line therapy and 780/2040 (38.2%) during third‐line therapy.
Infliximab 5 mg/kg was administered every 6 weeks (35/270 [13.0%]) and at ‘other’ dosing intervals (32/270 (11.9%) during first‐line therapy; corresponding frequencies were 41/281 (14.6%) and 48/281 (17.1%) during second‐line therapy and 55/358 (15.4%) and 62/358 (17.3%) during third‐line therapy.
A large proportion of etanercept 50‐mg doses were administered twice weekly during first‐line therapy [375/1002 (37.4%)] and second‐line therapy [170/397 (42.8%)] and weekly during third‐line therapy (76/177 [42.9%]).
Table 5.
Median duration of registry follow‐up until stop/switch for first‐line, second‐line and third‐line treatments; patients with psoriasis initiating new therapy during the registry
| Ustekinumab | Infliximab | Adalimumab | Etanercept | All | |
|---|---|---|---|---|---|
| First‐line therapy starts, N | 361 | 63 | 402 | 289 | 1115 |
| Median, Years | 1.98 | 2.73 | 2.70 | 3.08 | 2.50 |
| Second‐line therapy starts, N | 566 | 93 | 622 | 155 | 1436 |
| Median, Years | 2.88 | 3.04 | 3.77 | 3.15 | 3.24 |
| Third‐line therapy starts, N | 551 | 103 | 197 | 71 | 922 |
| Median, Years | 3.49 | 3.34 | 3.92 | 3.22 | 3.52 |
For first‐line users who discontinued biologic therapy, median duration of treatment was 676 days for infliximab, 613 days for ustekinumab, 569 days for adalimumab and 565 days for etanercept (Table 6). In addition, ustekinumab was received for 621 days and 510, 446 and 317 days for adalimumab, infliximab and etanercept, respectively, for second‐line therapy; corresponding days for third‐line therapy were 592, 457, 416 and 337 days. Across all three lines of therapy, numerically lower proportions of patients discontinued ustekinumab compared with each anti‐TNF agent (Table 7). For first‐line use, 8.6% of patients discontinued ustekinumab compared with 25.4% (infliximab), 37.6% (adalimumab) and 43.9% (etanercept) (Table 7). Trends were generally similar for second‐line and third‐line therapies, although the proportions of patients who discontinued treatment were numerically higher than those for first‐line therapy. The most common reason for discontinuing treatment was lack of effectiveness for each treatment cohort and for each line of therapy (Table 7).
Table 6.
Summary of median days on first‐, second‐ and third‐line therapy; patients with psoriasis who initiated and discontinued new therapy during the registry
| Ustekinumab | Infliximab | Adalimumab | Etanercept | |
|---|---|---|---|---|
| First‐line therapy | ||||
| All patients, N | 361 | 63 | 402 | 289 |
| Days on therapy | 613 (448–894) | 676 (309–1034) | 569 (239–894) | 565 (246–1024) |
| Discontinued, n (%) | 31 (8.6) | 16 (25.4) | 151 (37.6) | 127 (43.9) |
| Days on therapy | 316 (199–589) | 305 (188–758) | 258 (129–537) | 215 (120–413) |
| Second‐line therapy | ||||
| All patients, N | 566 | 93 | 622 | 155 |
| Days on therapy | 621 (365–947) | 446 (213–779) | 510 (230–981) | 317 (121–738) |
| Discontinued, n (%) | 106 (18.7) | 51 (54.8) | 250 (40.2) | 75 (48.4) |
| Days on therapy | 302 (183–490) | 316 (162–821) | 244 (127–475) | 153 (53–281) |
| Third‐line therapy | ||||
| All patients, N | 551 | 103 | 197 | 71 |
| Days on therapy | 592 (323–985) | 416 (242–769) | 457 (185–841) | 337 (128–679) |
| Discontinued, n (%) | 129 (23.4) | 49 (47.6) | 90 (45.7) | 35 (49.3) |
| Days on therapy | 287 (155–476) | 312 (172–513) | 204 (95–414) | 166 (74–309) |
Days on therapy are presented as median (interquartile range).
Table 7.
Proportion of patients who discontinued and reasons for discontinuation of first‐, second‐ and third‐line therapy; patients with psoriasis initiating new therapy during the registry
| Ustekinumab | Infliximab | Adalimumab | Etanercept | |
|---|---|---|---|---|
| First‐line therapy, N | 361 | 63 | 402 | 289 |
| Number (%) discontinued | 31 (8.6) | 16 (25.4) | 151 (37.6) | 127 (43.9) |
| Reasons for discontinuation | ||||
| Lack of effectiveness | 12 (38.7) | 9 (56.3) | 61 (40.4) | 65 (51.2) |
| Patient choice | 5 (16.1) | ‐ | 38 (25.2) | 16 (12.6) |
| Insurance/reimbursement | 5 (16.1) | 1 (6.3) | 23 (15.2) | 16 (12.6) |
| Other | 4 (12.9) | 1 (6.3) | 25 (16.6) | 23 (18.1) |
| Adverse event | 2 (6.5) | 5 (31.3) | 15 (9.9) | 18.1) |
| Second‐line therapy, N | 566 | 93 | 622 | 155 |
| Number (%) discontinued | 106 (18.7) | 51 (54.8) | 250 (40.2) | 75 (48.4) |
| Reasons for discontinuation | ||||
| Lack of effectiveness | 53 (50.0) | 17 (33.3) | 121 (48.4) | 36 (48.0) |
| Other | 18 (17.0) | 11 (21.6) | 30 (12.0) | 7 (9.3) |
| Patient choice | 16 (15.1) | 3 (5.9) | 33 (13.2) | 12 (16.0) |
| Insurance/reimbursement | 6 (5.7) | 4 (7.8) | 26 (10.4) | 14 (18.7) |
| Adverse event | 4 (3.8) | 14 (27.5) | 25 (10.0) | 8 (10.7) |
| Third‐line therapy, N | 551 | 103 | 197 | 71 |
| Number (%) discontinued | 129 (23.4) | 49 (47.6) | 90 (45.7) | 35 (49.3) |
| Reasons for discontinuation | ||||
| Lack of effectiveness | 73 (56.6) | 25 (51.0) | 46 (51.1) | 24 (68.6) |
| Patient choice | 15 (11.6) | 5 (10.2) | 11 (12.2) | 5 (14.3) |
| Other | 13 (10.1) | 7 (14.3) | 10 (11.1) | 4 (11.4) |
| Insurance/reimbursement | 12 (9.3) | 2 (4.1) | 9 (10.0) | ‐ |
| Adverse event | 6 (4.7) | 8 (16.3) | 8 (8.9) | 3 (8.6) |
Data are listed in ascending order based on first‐line ustekinumab therapy.
For first‐line therapy, Kaplan–Meier survival curves showed the time to discontinuation was longer for ustekinumab compared with each TNF‐α inhibitor (Fig. 1a). Similar results were observed among second‐ and third‐line therapies. Also, the survival curves for the TNF‐α inhibitor groups initiating second‐ and third‐line therapies declined more rapidly compared with ustekinumab and were overlapping (Fig. 1b,c).
Figure 1.
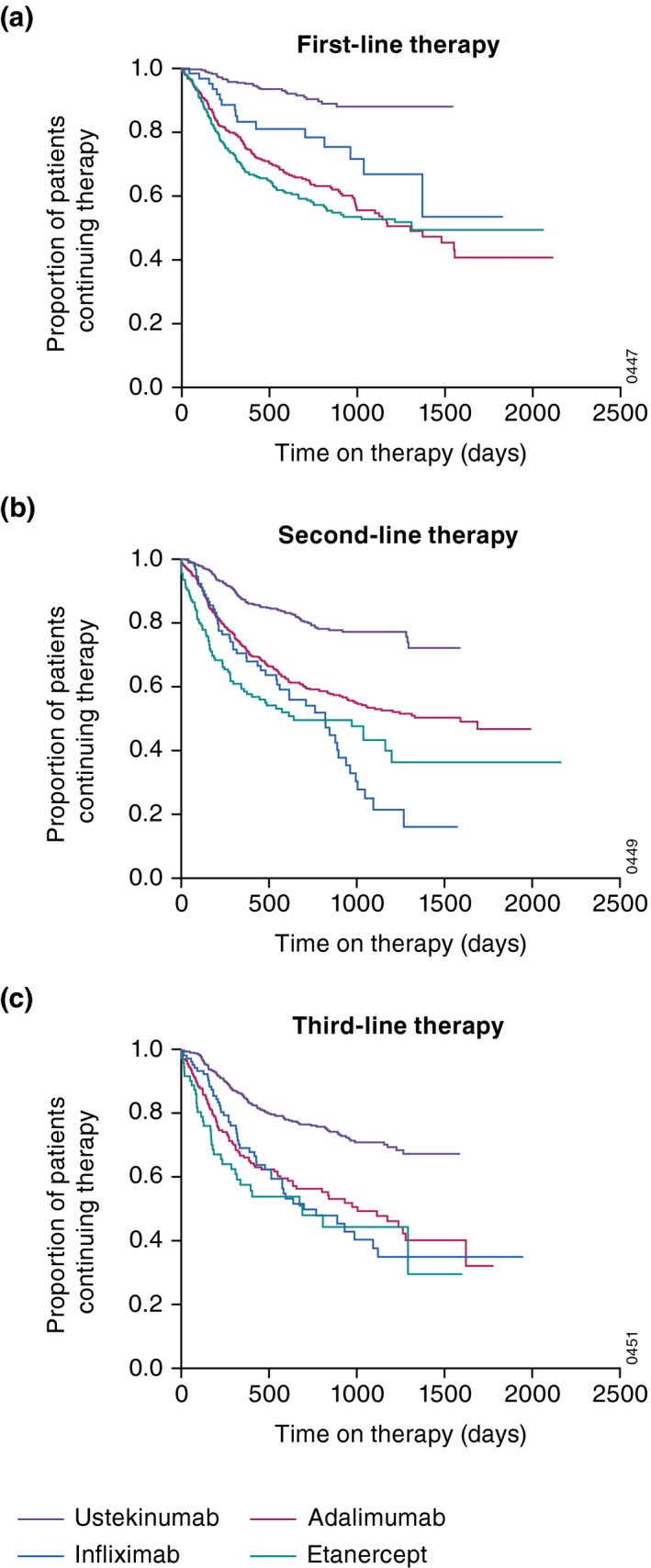
Kaplan–Meier survival curve of time on therapy (days) among all patients with psoriasis initiating any new therapy while enrolled in the registry: (a) first‐line therapy, (b) second‐line therapy and (c) third‐line therapy.
Multivariate analysis revealed significantly shorter times to discontinuation of first‐line use of infliximab, adalimumab and etanercept compared with ustekinumab (reference treatment); the same was true for second‐ and third‐line biologic therapy (Table 8). Factors significantly affecting time to treatment discontinuation varied across first‐, second‐ and third‐line lines of therapy. For first‐line therapy only, concomitant MTX use was independently associated with a shorter time to discontinuation of biologic therapy compared with no use of MTX. Additional factors associated with time to discontinuation of biologic therapy included gender (i.e. lower likelihood of stopping/switching for males for first‐ and third‐line biologic therapy) and geographical differences (e.g. lower likelihood of discontinuing treatment in Europe vs. North America) for first‐line therapy and higher likelihood in Latin America vs. North America for second‐line therapy). Other significant covariates (i.e. age, alcohol use and discontinuation due to insurance) are listed in Table 8. The remaining covariates evaluated (listed in Table 1, but not Table 8) were not found to be significantly associated with drug survival. Of note, the presence of PsA did not significantly affect time to discontinuation for first‐line (HR: 0.886, CI: 0.90, 1.138, P = 0.3445), second‐line (HR: 0.878, CI: 0.722, 1.068, P = 0.1928) or third‐line (HR: 1.064, CI: 0.832, 1.360, P = 0.6234) therapies. Results presented here represent the imputed model, in which there were very few patients with missing data (except for age at diagnosis of psoriasis; Table 8).
Table 8.
Cox hazard regression analysis: treatment effects and other factors significantly affecting time to discontinuation of biologic therapy; patients with psoriasis initiating new therapy during the registry
| First‐line therapy | Second‐line therapy | Third‐line therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P‐ valuea | Missing patients (N = 1115) | Hazard ratio | 95% CI | P‐ valuea | Missing patients (N = 1436) | Hazard ratio | 95% CI | P‐ valuea | Missing patients (N = 922) | |
| Infliximab vs. ustekinumab | 2.729 | 1.476, 5.044 | 0.0014 | 0 | 3.350 | 2.377, 4.723 | <0.0001 | 0 | 2.543 | 1.779, 3.635 | <0.0001 | 0 |
| Adalimumab vs. ustekinumab | 4.164 | 2.796, 6.204 | <0.0001 | 0 | 2.296 | 1.814, 2.907 | <0.0001 | 0 | 2.464 | 1.836, 3.307 | <0.0001 | 0 |
| Etanercept vs. ustekinumab | 4.911 | 3.282, 7.348 | <0.0001 | 0 | 3.219 | 2.349, 4.411 | <0.0001 | 0 | 3.041 | 2.011, 4.599 | <0.0001 | 0 |
| Prior immunomodulator use (Yes vs. No) | 1.119 | 0.851, 1.472 | 0.4209 | 4 | 1.009 | 0.820, 1.242 | 0.9294 | 2 | 1.499 | 1.144, 1.965 | 0.0033 | 1 |
| Concomitant methotrexate (Yes vs. No) | 1.555 | 1.156, 2.091 | 0.0035 | 0 | 1.082 | 0.863, 1.357 | 0.4928 | 0 | 0.941 | 0.722, 1.227 | 0.6518 | 0 |
| Prior TNF‐α inhibitor vs. prior ustekinumab | NA | NA | NA | NA | 0.932 | 0.609, 1.428 | 0.7471 | 0 | 1.168 | 0.776, 1.757 | 0.4574 | 0 |
| Prior other treatment vs. prior ustekinumab | NA | NA | NA | NA | 0.702 | 0.429, 1.147 | 0.1577 | 0 | 1.258 | 0.754, 2.100 | 0.3799 | 0 |
| Male vs. Female | 0.781 | 0.619, 0.986 | 0.0378 | 0 | 0.866 | 0.714, 1.049 | 0.1418 | 0 | 0.717 | 0.564, 0.912 | 0.0068 | 0 |
| Age at PsO diagnosis/10 yearsb | 1.102 | 0.997, 1.219 | 0.0580 | 133b | 1.084 | 1.004, 1.171 | 0.0391 | 183b | 1.017 | 0.916, 1.130 | 0.7501 | 135b |
| Age at biologic start/10 yearsb | 0.916 | 0.816, 1.029 | 0.1386 | 0 | 0.984 | 0.900, 1.076 | 0.7280 | 0 | 0.999 | 0.890, 1.121 | 0.9852 | 0 |
| Non‐white vs. White | 1.120 | 0.847, 1.481 | 0.4257 | 0 | 0.932 | 0.733, 1.184 | 0.5631 | 0 | 1.262 | 0.928, 1.718 | 0.1383 | 0 |
| Overweight/obesity class I (25≤BMI<35 vs. <25) | 1.165 | 0.859, 1.580 | 0.3248 | 14 | 1.123 | 0.866, 1.458 | 0.3820 | 15 | 1.090 | 0.758, 1.567 | 0.6418 | 9 |
| Obesity class II/III (BMI≥ 35 vs. <25) | 1.289 | 0.912, 1.822 | 0.1510 | 14 | 1.207 | 0.905, 1.609 | 0.1998 | 15 | 1.140 | 0.780, 1.667 | 0.4987 | 9 |
| Alcohol (current/past vs. never) | 1.171 | 0.897, 1.528 | 0.2465 | 1 | 0.766 | 0.616, 0.952 | 0.0163 | 1 | 1.203 | 0.899, 1.611 | 0.2140 | 1 |
| Smoking (current/past vs. never) | 1.099 | 0.868, 1.392 | 0.4329 | 1 | 1.125 | 0.928, 1.364 | 0.2293 | 0 | 0.809 | 0.638, 1.026 | 0.0800 | 0 |
| Family history of PsO vs. no history of PsO | 1.116 | 0.888, 1.402 | 0.3456 | 0 | 1.162 | 0.965, 1.398 | 0.1123 | 0 | 0.854 | 0.672, 1.085 | 0.1958 | 0 |
| Baseline PGA 2,3 vs. 0,1 | 0.919 | 0.652, 1.295 | 0.6300 | 16 | 0.865 | 0.692, 1.080 | 0.1995 | 24 | 1.083 | 0.803, 1.461 | 0.5996 | 9 |
| Baseline PGA 4,5 vs. 0,1 | 1.080 | 0.716, 1.628 | 0.7146 | 16 | 0.877 | 0.641, 1.198 | 0.4094 | 24 | 1.358 | 0.909, 2.029 | 0.1348 | 9 |
| PsA vs. no PsA | 0.886 | 0.690, 1.138 | 0.3445 | 0 | 0.878 | 0.722, 1.068 | 0.1928 | 2 | 1.064 | 0.832, 1.360 | 0.6234 | 0 |
| Europec vs. North Americad | 0.523 | 0.315, 0.869 | 0.0123 | 0 | 0.751 | 0.480, 1.173 | 0.2083 | 0 | 0.877 | 0.457, 1.684 | 0.6944 | 0 |
| Latin Americae vs. North Americad | 2.295 | 0.696, 7.572 | 0.1725 | 0 | 2.586 | 1.000, 6.684 | 0.0499 | 0 | 2.677 | 0.814, 8.806 | 0.1051 | 0 |
| Disc: efficacy vs. insurance | NA | NA | NA | NA | 0.839 | 0.621, 1.133 | 0.2523 | 0 | 0.840 | 0.552, 1.278 | 0.4149 | 0 |
| Disc: adverse event vs. insurance | NA | NA | NA | NA | 1.226 | 0.807, 1.864 | 0.3397 | 0 | 0.786 | 0.434, 1.423 | 0.4267 | 0 |
| Disc: patient choice vs. insurance | NA | NA | NA | NA | 0.856 | 0.612, 1.197 | 0.3624 | 0 | 1.288 | 0.823, 2.015 | 0.2684 | 0 |
| Disc: reason unknown vs. due to insurance | NA | NA | NA | NA | 2.706 | 1.883, 3.891 | <0.0001 | 0 | 3.515 | 2.180, 5.667 | <0.0001 | 0 |
| Disc: other reasonf vs. insurance | NA | NA | NA | NA | 0.839 | 0.556, 1.264 | 0.4005 | 0 | 0.759 | 0.436, 1.321 | 0.3299 | 0 |
| Ins: Gov/Public vs. None | 0.962 | 0.585, 1.581 | 0.8791 | 0 | 1.358 | 0.856, 2.154 | 0.1942 | 0 | 0.659 | 0.353, 1.231 | 0.1908 | 0 |
| Ins: Private vs. None | 1.125 | 0.756, 1.675 | 0.5612 | 0 | 1.186 | 0.785, 1.793 | 0.4182 | 0 | 0.874 | 0.508, 1.504 | 0.6264 | 0 |
| Ins: Gov/Public & Private vs. None | 1.081 | 0.596, 1.961 | 0.7971 | 0 | 1.413 | 0.841, 2.376 | 0.1917 | 0 | 1.193 | 0.571, 2.489 | 0.6390 | 0 |
P‐value derived from Wald Chi‐square test.
Results presented here represent the imputed model. When the non‐imputed model was calculated, results differed due to the large number of missing patients for age at diagnosis. When the non‐imputed model was re‐run without this factor, the results without imputation were consistent with the imputed data reported here.
Europe includes Austria, Belgium, Czech Republic, Greece, The Netherlands, Portugal, Slovakia, Slovenia, Ukraine and Israel.
North America includes the United States and Canada.
Latin America includes Chile, Columbia, Argentina and Mexico.
Other reasons for discontinuation (e.g. adverse events, lack of effectiveness, patient choice) were associated with time to stop/switch.
BMI, body mass index; CI, confidence interval; Disc, discontinuation; Gov, government; Ins, insurance; NA, not applicable, PGA, Physician's Global Assessment; PsA, psoriatic arthritis; PsO, psoriasis; TNF, tumour necrosis factor.
Psoriatic arthritis subgroup
While 119 of the 1115 psoriasis patients (10.6%) starting first‐line therapy reported a concurrent diagnosis of PsA confirmed by a rheumatologist, corresponding proportions for second‐ and third‐line therapies were 192/1436 (13.4%) and 141/922 (15.3%). A total of 539 new biologic starts (first‐ through seventh‐line) were identified in the PsA subgroup (Table S1). Demographic and disease characteristics of the PsA subgroup were comparable across first‐, second‐ and third‐line therapies and varied from those of the overall psoriasis population (Table S2). Specifically, patients in the PsA subgroup were older, had been diagnosed with psoriasis for a longer period of time, and had more severe psoriasis (i.e. higher %BSA and higher mean PGA score) compared with the overall psoriasis group; higher proportions of patients had received phototherapy and immunomodulators as well.
The patterns for most common dose and dosing interval for each biologic agent in the PsA subgroup were generally comparable to those in the overall psoriasis population (Table S3). As in the overall psoriasis population, the median duration of registry follow‐up in the PsA subgroup varied across treatment groups (Table S4) and median days on therapy for each line of therapy were generally higher in the ustekinumab group compared with the other treatment groups (Table S5). As in the overall psoriasis population, the proportion of patients in the PsA subgroup who discontinued therapy was lower for patients treated with ustekinumab compared with each anti‐TNF agent across the first‐, second‐ and third‐line cohorts (Table S6, Fig. S1).
Multivariate analyses showed that comparisons of drug survival did not reach statistical significance for infliximab, adalimumab or etanercept for first‐line therapy; however, the comparison between ustekinumab and all three biologics for second‐line therapy and between ustekinumab and etanercept for third‐line therapy showed significantly better survival in the PsA subgroup (Table S7). Again, other significant predictors affecting time to treatment discontinuation varied across first‐, second‐ and third‐line lines of therapy in the PsA subgroup, and the evaluation was limited by cohort sizes (Table S7).
Discussion
In this analysis of PSOLAR, the drug survival of biologics for the treatment of psoriasis was evaluated, while considering whether patients initiated treatment as first‐, second‐ or third‐line therapy. Based on multiple measures, including proportion of patients who discontinued therapy and time to discontinuation, ustekinumab had better drug survival compared with TNF‐α inhibitors among patients with psoriasis across all three lines of therapy and generally among those with concurrent PsA across second‐ and third‐line therapies. This is in keeping with the recently published results from another registry of 3523 bionaive patients (BADBIR), which also found that ustekinumab had the highest drug survival compared with infliximab, adalimumab and etanercept.17 Importantly, our evaluation included a large number of patients who initiated a new biologic therapy at, or after, enrolment in PSOLAR. While each biologic was most frequently administered at a dosage consistent with the product labels,18, 19, 20, 21 dosing was maximized in some patients. We believe the findings presented here represent patterns of treatment and drug survival in real‐world dermatologic practice.
The Kaplan–Meier survival curves generated from these analyses showed ustekinumab had the longest time to discontinuation compared with the other biologics; results were generally comparable to those from other studies that included ustekinumab and TNF‐α inhibitors.4, 5, 10, 12, 22 The overall decline in drug survival observed within each treatment cohort among patients receiving second‐ or third‐line therapies relative to first‐line therapy suggests that discontinuation of prior biologic therapies may be predictive of lower drug survival with subsequent biologics, as reported elsewhere.3, 4, 12, 23, 24 This suggests that patients who discontinued one or more biologic may have more refractory disease or perhaps alterations in their immune system related to prior therapy. The common trend towards initially steeper curves followed by a flattening over time may reflect a well‐motivated group of patients who adapted to initial challenges and were less likely to discontinue therapy later. Declining drug survival may also, in part, be related to treatment effectiveness, which was slightly diminished in patients receiving biologics as later‐line therapy compared with those receiving their first biologic.
Multivariate analyses validated the finding of better treatment survival with ustekinumab based on significant differences in time to discontinuation between ustekinumab and each TNF‐α inhibitor during first‐, second‐ and third‐line therapy. Also, male gender was favourably associated with drug survival, as documented in other studies.9, 12, 25, 26 Of note, patients receiving MTX were significantly more likely to discontinue biologic treatment compared with those without concomitant MTX use in bionaive (i.e. first‐line therapy), but not in biologic‐experienced, patients; the reason for these findings is unclear. Also, MTX usage increased with each subsequent line of therapy. As antibody formation has been shown to diminish response to some biologic therapies,2 it is possible that MTX was used more often with subsequent therapies to prevent antibody formation and potentially increase effectiveness. Finally, it is noteworthy that the presence of PsA was not significantly associated with treatment discontinuation in our analyses.
Lack of response was the most common reason observed for discontinuation of treatment in PSOLAR, which is in keeping with prior reports.3, 4, 5, 6 The proportions of patients who discontinued treatment due to lack of effectiveness in PSOLAR tended to be somewhat higher with second‐ and third‐line biologic use, although the numbers are not large enough to draw definitive conclusions. Other factors that could lead to discontinuation of treatment include lack of tolerability, development of an adverse event requiring cessation of treatment, or lack of access (e.g. change or loss of health insurance). Moreover, factors related to dosing interval or convenience of administration may influence drug survival. For example, both infliximab and ustekinumab are generally administered by a health care provider, which could, in turn, promote better adherence to the prescribed treatment regimen; however, infliximab was not associated with better drug survival on a consistent basis.
In the current analyses, the proportion of first‐line users of biologics with concomitant PsA was somewhat lower compared with second‐ and third‐line therapy (10.6%, 13.4% and 15.3%, respectively), suggesting that patients with PsA may be more likely to be treated with sequential biologics. This is in keeping with other reports that indicated response to treatment may be reduced in bio‐experienced patients with PsA and rheumatoid arthritis, often leading to a switch in treatment to achieve a better response.27, 28, 29, 30 Consistent with overall psoriasis patients, the proportion of confirmed PsA patients who discontinued treatment was lower and the time to discontinuation was longer for the ustekinumab cohort compared with the infliximab, adalimumab and etanercept groups. While the power of the analysis was limited by smaller cohort sizes in the confirmed PsA subgroup, the hazard ratios for drug survival for ustekinumab compared with the TNF‐α inhibitors were notably favourable for all comparisons (except for first‐line use) among PsA patients. Therefore, taking into account the limitations of the analysis, the overall findings suggest that drug survival for ustekinumab in patients with concurrent PsA reflect observations among the overall psoriasis population.
Our analyses were designed to reduce the impact of prior exposure to other biologic treatments on the evaluation of drug survival. Specifically, the cohorts were defined by whether newly initiated treatments represented first‐, second‐ or third‐line biologic use, which may have prevented potential biases and confounding factors associated with an analysis of all new users. However, the results reported herein may be subject to limitations inherent to observational studies, such as lack of randomization of patients to different biologic agents as well as various factors that affect patient access to therapy. Limitations with regard to data capture include that dose escalation data were not consistently available for all biologic cohorts preventing stratification based on this factor, the number of patients with Psoriasis Area and Severity Index data was too small to allow meaningful interpretation, and specific details with regard to dose and duration of MTX usage were not available. Also, the numbers of patients initiating ustekinumab and adalimumab in our analyses were higher than those for etanercept and infliximab. The large majority of patients received ustekinumab and infliximab in a medical setting while adalimumab and etanercept were self‐administered in most cases. Finally, the subset of PsA patients was limited to only those who were diagnosed before entering the registry.
In conclusion, our results indicate that drug survival of ustekinumab is better than that of TNF‐α inhibitors for both biologic‐naive and biologic‐experienced patients with psoriasis. Findings among patients with a concurrent diagnosis of PsA were limited by population size, but they suggest no difference from those reported for all psoriasis patients, which supports the sustainability of chronic therapy with ustekinumab for both conditions. Additional studies of comparative effectiveness and quality of life among biologics in PSOLAR will help evaluate the reasons for differences in drug survival reported here.
Supporting information
Fig. S1. Kaplan–Meier survival curve of time on therapy (days) among patients with confirmed psoriatic arthritis initiating any new therapy while enrolled in the registry: (a) first‐line therapy, (b) second‐line therapy and (c) third‐line therapy.
Table S1. Summary of new starts for first‐ through seventh‐lines of therapy included in the current analyses; patients with psoriatic arthritis initiating new therapy during the registry.
Table S2. Demographics and disease characteristics prior to first‐line therapy; patients with psoriatic arthritis initiating new therapy during the registry.
Table S3. Most common maintenance doses and dose frequencies by treatment for first‐, second‐, and third‐line therapies; doses in patients with psoriatic arthritis initiating new therapy during the registry.
Table S4. Median duration of registry follow‐up until stop/switch for first‐line, second‐line, and third‐line treatments; patients with psoriatic arthritis initiating new therapy during the registry.
Table S5. Summary of median days on first‐, second‐, and third‐line therapy; patients with psoriatic arthritis who initiated and discontinued new therapy during the registry.
Table S6. Proportion of patients who discontinued and reasons for discontinuation of first?, second‐, and third‐line therapy; patients with psoriatic arthritis initiating new therapy during the registry.
Table S7. Cox hazard regression analysis: treatment effects and other factors significantly affecting time to discontinuation of biologic therapy; patients with psoriatic arthritis initiating new therapy during the registry.
Acknowledgements
The authors thank Cynthia Arnold, Samantha Simpson and Michelle Perate (Janssen Scientific Affairs, LLC; Spring House, PA, USA) for their editorial assistance and writing support of this manuscript as well as Kezhen L. Tang, PhD (Janssen Research & Development, Inc., Horsham, PA, USA) for her statistical support.
Conflicts of interest
A. Menter has received grants and/or honoraria as a consultant, speaker, investigator and/or advisory board member for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Convoy Therapeutics, Eli Lilly, Genentech, Janssen Biotech, Leo Pharma, Merck, Novartis, Pfizer, Symbio/Maruho, Syntrix, Vitae, Wyeth and XenoPort; K. Papp has received grants and/or honoraria as a consultant, speaker, and/or advisory board member from Abbott, Amgen., Anacor, Astellas, Boehringer Ingelheim, Celgene, Celtic Pharma, Dow Pharma, Eli Lilly, Galderma, Janssen Biotech, MSD, Merck‐Serono, Pfizer and UCB; M. Gooderham grants and/or honoraria as a consultant, speaker, investigator and/or advisory member from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Galderma, Eli Lilly, Leo Pharma, Janssen Biotech, Merck, Novartis and Pfizer; D. Pariser has received has received grants and/or honoraria as a consultant, investigator, and/or advisory board member from AbbVie, Amgen, Celgene, Eli Lilly and Company, Galderma, Janssen Biotech, Inc., Merck, Novartis and Pfizer; M. Augustin has received grants and/or honoraria as a consultant, speaker, and/or advisory board member from Abbvie, Almirall, Amgen, Astellas, Biogen, Celgene, Eli Lilly and Company, Janssen Biotech, Leo Pharma, MSD, Merck‐Serono, Mundipharma, Novartis, Pfizer, Pohl‐Boskamp, UCB and Xenoport; F.A. Kerdel has received grants, participated on advisory boards, and/or was a speaker for AbbVie, Actelian, Amgen, Celgene, Galderma, Janssen, Novartis, Pfizer and Valeant; S. Fakharzadeh, K. Goyal and S. Calabro are employees of Janssen Scientific Affairs, LLC; W. Langholff and S. Chavers are employees of Janssen Research & Development, LLC. D. Naessens and J. Sermon are employees of Janssen‐Cilag NV. G. Krueger has received fees as a consultant or advisory board member for Abbott, Amgen, ApoPharma, Astellas, Boehringer Ingleheim, Bristol Myers Squibb, Celgene, Idera, Isis, Janssen, Eli Lilly, L'Oreal, Novartis, Pfizer, Vascular Biologics Limited and UCB; has received lecture fees from Abbott, Amgen, Celgene, Janssen and Novartis in the last 12 months; and has received stipend support for a clinical research fellowship from Abbott, Amgen and Janssen.
Funding sources
The PSOLAR registry is sponsored by Janssen Scientific Affairs, LLC, Horsham, PA, USA.
References
- 1. Yeung H, Wan J, van Voorhees AS et al Patient‐reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol 2013; 68: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu L, Snodgrass BT, Armstrong AW. Antidrug antibodies in psoriasis: a systematic review. Br J Dermatol 2014; 170: 261–273. [DOI] [PubMed] [Google Scholar]
- 3. Levin AA, Gottlieb AB, Au SC. A comparison of psoriasis drug failure rates and reasons for discontinuation in biologics vs. conventional systemic therapies. J Drugs Dermatol 2014; 13: 848–853. [PubMed] [Google Scholar]
- 4. Umezawa Y, Nobeyama Y, Hayashi M et al Drug survival rates in patients with psoriasis after treatment with biologics. J Dermatol 2013; 40: 1008–1013. [DOI] [PubMed] [Google Scholar]
- 5. van den Reek JMPA, Zweegers J, Kievit W et al ‘Happy’ drug survival of adalimumab, etanercept and ustekinumab in psoriasis in daily practice care: results from the BioCAPTURE network. Br J Dermatol 2014; 71: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 6. Menting SP, Sitaram AS, Bonnerjee‐van der Stok HM et al Drug survival not significantly different between biologics in patients with psoriasis vulgaris: a single center database analysis. Br J Dermatol 2014; 171: 875–883. [DOI] [PubMed] [Google Scholar]
- 7. Bonafede M, Johnson BH, Fox KM et al Treatment patterns with etanercept and adalimumab for psoriatic diseases in a real‐world setting. J Dermatol Treat 2013; 24: 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gniadecki R, Kragballe K, Dam TN, Skov L. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol 2011; 164: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 9. Esposito M, Gisondi P, Cassano N et al Survival rate of antitumour necrosis factor‐α treatments for psoriasis in routine dermatological practice: a multicentre observational study. Br J Dermatol 2013; 169: 666–672. [DOI] [PubMed] [Google Scholar]
- 10. Cao Z, Carter C, Wilson KL, Schenkel B. Ustekinumab dosing, persistence, and discontinuation patterns in patients with moderate‐to‐severe psoriasis. J Dermatol Treat 2015; 26: 113–120. [DOI] [PubMed] [Google Scholar]
- 11. Herrera‐Acosta E, Suarez‐Perez JA, Aguilera J et al Survival rate of etanercept for psoriasis in real life: a multicentre observational study. Eur J Dermatol 2014; 24: 619–620. [DOI] [PubMed] [Google Scholar]
- 12. Gniadecki R, Bang B, Bryld LE et al Comparison of long‐term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015; 172: 244–252. [DOI] [PubMed] [Google Scholar]
- 13. Levin EC, Gupta R, Brown G et al Biologic fatigue in psoriasis. J Dermatolog Treat 2014; 25: 78–82. [DOI] [PubMed] [Google Scholar]
- 14. Strober BE, Bissonnette R, Fiorentino D et al Comparative effectiveness of biologic agents for the treatment of psoriasis in a real world setting: results from a large, prospective, observational study (PSOLAR). J Am Acad Dermatol. doi: 10.1016/j.jaad.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 15. Papp KA, Strober B, Augustin M et al PSOLAR: design, utility, and preliminary results of a prospective, international, disease‐based registry of patients with psoriasis who are receiving, or are candidates for, conventional systemic treatments or biologic agents. J Drugs Dermatol 2012; 11: 1210–1217. [PubMed] [Google Scholar]
- 16. Kimball AB, Leonardi C, Stahle M et al Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicenter, prospective, disease‐based registry (PSOLAR). Br J Dermatol 2014; 171: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warren RB, Smith CH, Yiu ZZ et al Differential Drug Survival of Biologic Therapies for the Treatment of Psoriasis: A Prospective Observational Cohort Study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015; 135: 2632–2640. [DOI] [PubMed] [Google Scholar]
- 18. Stelara® . (ustekinumab) [Package Insert]. Janssen Biotech, Inc, Horsham, PA, 2013. [Google Scholar]
- 19. Remicade® . (infliximab) [Package Insert]. Janssen Biotech, Inc, Horsham, PA, 2013. [Google Scholar]
- 20. Humira . (adalimumab) [Package Insert]. AbbVie Inc, North Chicago, IL, 2013. [Google Scholar]
- 21. Enbrel . (etanercept) [Package Insert]. Immunex Corporation, Thousand Oaks, CA, 2013. [Google Scholar]
- 22. Clemmenson A, Spon M, Skov L et al Responses to ustekinumab in the anti‐TNF agent‐naïve vs. anti‐TNF agent‐exposed patients with psoriasis vulgaris. J Eur Acad Dermatol Venereol 2011; 25: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 23. López‐Ferrer A, Vilarrasa E, Gich IJ, Puig L. Adalimumab for the treatment of psoriasis in real life: a retrospective cohort of 119 patients at a single Spanish centre. Br J Dermatol 2013; 169: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 24. Ruis Salas V, Puig L, Alomar A. Ustekinumab in clinical practice: response depends on dose and previous treatments. J Eur Acad Dermatol Venereol 2012; 26: 508–513. [DOI] [PubMed] [Google Scholar]
- 25. van den Reek JMPA, van Lumig PPM, Driessen RJB et al Determinants of drug survival for etanercept in a long‐term daily practice cohort of patients with psoriasis. Br J Dermatol 2014; 170: 415–424. [DOI] [PubMed] [Google Scholar]
- 26. van den Reek JMPA, Tummers M, Zweegers J et al Predictors of adalimumab drug survival in psoriasis differ by reason for discontinuation: long‐term results from the Bio‐CAPTURE registry. J Eur Acad Dermatol Venereol 2015; 29: 560–565. [DOI] [PubMed] [Google Scholar]
- 27. Ritchlin C, Rahman P, Kavanaugh A et al Efficacy and safety of the anti‐IL‐12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non‐biological and biological anti‐tumour necrosis factor therapy: 6‐mon and 1‐year results of the phase 3, multicentre, double‐blind, placebo‐controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014; 73: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smolen JS, Kay J, Matteson EL et al Insights into the efficacy of golimumab plus methotrexate in patients with active rheumatoid arthritis who discontinued prior anti‐tumour necrosis factor therapy: post‐hoc analyses from the GO‐AFTER study. Ann Rheum Dis 2014; 73: 1811–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mease PJ, Fleischmann R, Deodhar AA et al Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24‐week results of a Phase 3 double‐blind randomised placebo‐controlled study (RAPID‐PsA). Ann Rheum Dis 2014; 73: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rudwaleit M, Van den Bosch F, Kron M et al Effectiveness and safety of adalimumab in patients with ankylosing spondylitis or psoriatic arthritis and a history of anti‐tumor necrosis factor therapy. Arthritis Res Ther 2010; 12: R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Kaplan–Meier survival curve of time on therapy (days) among patients with confirmed psoriatic arthritis initiating any new therapy while enrolled in the registry: (a) first‐line therapy, (b) second‐line therapy and (c) third‐line therapy.
Table S1. Summary of new starts for first‐ through seventh‐lines of therapy included in the current analyses; patients with psoriatic arthritis initiating new therapy during the registry.
Table S2. Demographics and disease characteristics prior to first‐line therapy; patients with psoriatic arthritis initiating new therapy during the registry.
Table S3. Most common maintenance doses and dose frequencies by treatment for first‐, second‐, and third‐line therapies; doses in patients with psoriatic arthritis initiating new therapy during the registry.
Table S4. Median duration of registry follow‐up until stop/switch for first‐line, second‐line, and third‐line treatments; patients with psoriatic arthritis initiating new therapy during the registry.
Table S5. Summary of median days on first‐, second‐, and third‐line therapy; patients with psoriatic arthritis who initiated and discontinued new therapy during the registry.
Table S6. Proportion of patients who discontinued and reasons for discontinuation of first?, second‐, and third‐line therapy; patients with psoriatic arthritis initiating new therapy during the registry.
Table S7. Cox hazard regression analysis: treatment effects and other factors significantly affecting time to discontinuation of biologic therapy; patients with psoriatic arthritis initiating new therapy during the registry.


